Abstract
Accumulating isotopic evidence from fossil hominin tooth enamel has provided unexpected insights into early hominin dietary ecology. Among the South African australopiths, these data demonstrate significant contributions to the diet of carbon originally fixed by C4 photosynthesis, consisting of C4 tropical/savannah grasses and certain sedges, and/or animals eating C4 foods. Moreover, high-resolution analysis of tooth enamel reveals strong intra-tooth variability in many cases, suggesting seasonal-scale dietary shifts. This pattern is quite unlike that seen in any great apes, even ‘savannah’ chimpanzees. The overall proportions of C4 input persisted for well over a million years, even while environments shifted from relatively closed (ca 3 Ma) to open conditions after ca 1.8 Ma. Data from East Africa suggest a more extreme scenario, where results for Paranthropus boisei indicate a diet dominated (approx. 80%) by C4 plants, in spite of indications from their powerful ‘nutcracker’ morphology for diets of hard objects. We argue that such evidence for engagement with C4 food resources may mark a fundamental transition in the evolution of hominin lineages, and that the pattern had antecedents prior to the emergence of Australopithecus africanus. Since new isotopic evidence from Aramis suggests that it was not present in Ardipithecus ramidus at 4.4 Ma, we suggest that the origins lie in the period between 3 and 4 Myr ago.
Keywords: carbon isotopes, enamel, C4 resources, australopiths
1. Introduction
Diet is a fundamental feature of a species' biology, strongly influencing basic body size and morphology, life-history strategies for survival of the young in particular, social organization and the manner of its adaptations to its environment. Consequently, the nature of ancestral diets remains one of the most active topics of research in human evolution. Many years after Dart first puzzled over how the newly discovered ‘man-like apes’ (in reference to the Taung child and its kind) had survived in Taung's xeric, open, Kalahari environment, so alien to all the other forest-loving great apes (Dart 1925, 1926), we are still actively debating these issues today (e.g. White et al. 2009a). Dart could not have comprehended, at that time, the full scale of environmental shifts that occurred in Africa in the past several millions years, or indeed the depth of time encompassed. But he made some surprisingly prescient suggestions, including the likely expansion of the conventional frugivorous and folivorous hominid diet to include roots, bulbs and animal foods such as insects, scorpions, lizards, bird's eggs and the young of small antelope (Dart 1926). Effectively, it was a pre-statement of the ‘Dietary Breadth’ hypothesis. These debates have continued apace, but although our understanding of the nature of environments and preferred habitats has advanced substantially, the fossil record has grown and more sophisticated methods have been applied to study morphology and the biomechanics of food processing, our comprehension of the important dietary shifts that must have occurred during the early emergence of hominins in the Pliocene is still uncertain. Such challenges have encouraged the development of new methods for examining dietary ecology.
In an earlier Royal Society meeting on human evolution almost 30 years ago, Alan Walker set out a series of what he considered to be the more promising emerging avenues in early hominin dietary research (Walker 1981). Among other candidates, Walker suggested the inspection of tooth microwear, and carbon isotope and trace element analysis of fossil bones (Walker 1981, p. 58). It was evident at the 2009 meeting that the first two methods have undergone considerable development and progress since that time, in opening new windows on hominin dietary ecology. Walker's predictions followed closely on a flurry of pioneering studies in the late 1970s that began to explore the systematics of these three approaches. In the case of stable isotopes, studies demonstrated the direct relationship between carbon isotopes in the diet and in animal tissues (DeNiro & Epstein 1978a), others that these distinctions held for wild grazers and browsers (DeNiro & Epstein 1978b; Vogel 1978) and finally that it provided a new approach for addressing an archaeological question about the introduction of maize agriculture (van der Merwe & Vogel 1978). Other studies hinted that these methods might be extended to fossils at greater time depth if based on the mineral rather than the organic phase of bone (e.g. Ericson et al. 1981; Sullivan & Krueger 1981).
At that stage, almost all chemical studies were based on bone collagen or bone mineral (a biological apatite), but the latter is an extremely unstable structure, which is vulnerable to diagenesis. It was not until the potential of the more crystalline, stable enamel phase was explored and demonstrated (Lee-Thorp & van der Merwe 1987) that the full potential of the method to older fossils could be realized. Following earlier work (Parker & Toots 1980; Elias et al. 1982), the potential of trace elements, especially strontium to calcium ratios (Sr/Ca), as trophic indicators in fossil foodwebs was explored extensively (Sillen 1988, 1992), but these efforts have largely stalled. This may be a reflection of the former strong focus on fossil bone, with its attendant problems of diagenesis. A single, broader enamel-based study in the South African hominin sites was unable to replicate the trophic patterns seen earlier for Paranthropus robustus (Sponheimer & Lee-Thorp 2006); instead, it was suggested that Sr/Ca and Ba/Ca, in combination, may be more informative about plant resources. That suggestion awaits further exploration, and trace elemental analysis will not be discussed further here.
Our main purposes in this paper are to review the evidence for the shift to incorporate C4 resources in early hominin diets, to present new data for temporal variability in C4 consumption in the earlier South African australopith, Australopithecus africanus, which is comparable to that of P. robustus (Sponheimer et al. 2006b), and to make some predictions about the origins and inferences of such an adaptation.
2. Isotopic evidence for C4 in hominin diets
The primary distinction in application of stable carbon isotopes to hominin diets is the difference in 13C/12C (expressed as δ13C)1 between C3 and C4 plants. In the African environments they typically occupied, where the growing (wet) season is warm, all carbon dietary sources from trees, bushes, shrubs and forbs are distinctly lower in δ13C compared with those from tropical grasses and some sedges. The primary exception is in tropical forest ecosystems where C3 subcanopy (shaded) plants are even more depleted in 13C (i.e. lower δ13C), while vegetation in clearings and in the canopy (including fruits) is slightly less depleted (van der Merwe & Medina 1989; Cerling et al. 2004). Dietary δ13C values are reflected in all tissues, including enamel, so that fossil enamel δ13C values provide opportunities to test hypotheses about the dietary habits of extinct animals (e.g. Lee-Thorp et al. 1989; Cerling et al. 1997).
Most published isotope hominin dietary research has so far focused on the South African hominins (Lee-Thorp et al. 1994; Sponheimer & Lee-Thorp 1999; Sponheimer et al. 2005, 2006b; Lee-Thorp & Sponheimer 2006), although this situation is beginning to change. Comparisons between the two South African australopiths were also a starting point for the development of occlusal enamel microwear comparisons (Grine 1981, 1986). This and subsequent studies using less subjective, more quantifiable methods (Scott et al. 2005) suggested that, in spite of significant overlap, P. robustus microwear showed subtly more complexity or pitting in occlusal enamel wear compared with Australopithecus africanus. Therefore, the inference is that the former included a higher proportion of harder food items that required more processing and caused more pitting and fewer directional scratches (Grine 1986). These results were thought to be consistent with the widely held view that Paranthropus was a specialist vegetarian (Grine 1986). The microwear distinctions are quite subtle and may also be influenced by differences in enamel prism orientation between the two taxa (Macho & Shimizu 2009).
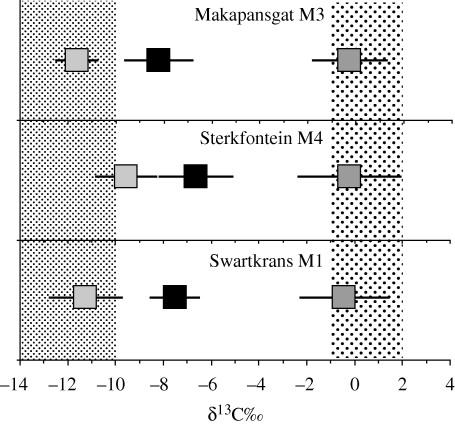
Nevertheless, the microwear findings provide a useful framework for hypothesis-testing using stable isotopes since the data essentially suggest diets that would be classed as C3 (i.e. hard fruits and nuts). The prediction would be that A. africanus and P. robustus should be indistinguishable in their δ13C values from C3 feeders, such as browsers and frugivores. The results, however, flatly contradict this prediction. Analysis of more than 40 hominin specimens from the sites Makapansgat, Sterkfontein, Kromdraai and Swartkrans, spanning a period of about 3–1.5 Ma, demonstrate that δ13C values of both australopiths are indistinguishable from each other, but distinct from that of coexisting C3 consumers (figure 1). Surprisingly, the proportions of C4 in enamel, on average, remain relatively constant in spite of the passage of time and marked shifts in environments, from relatively closed to far more open landscapes, by about 1.8 Ma (Vrba 1985; Reed 1997; Lee-Thorp et al. 2007).
Figure 1.
Data for all the South African hominins are summarized as means δ13C (black boxes) and standard deviations compared with means and standard deviations for the browsing and grazing fauna. The sites are given in sequence from oldest (top) to youngest (bottom). Makapansgat Member 3 is about 2.7–3 Ma, Sterkfontein Member 4 is usually considered to be about 2.2–2.5 Ma and Swartkrans Member 1 is younger than 2 Ma. These ages based on biostratigraphy are imprecise, but sufficient for our purposes. More precise chronometric studies based on Pb/U isotopes have recently been completed (R. Pickering 2009, personal communication), but do not change the overall sequence. All the hominin data show significant C4 contributions compared with C3 feeders, in spite of large shifts, from closed to open, in the environments (Reed 1997). Adapted from Lee-Thorp & Sponheimer (2006).
On average, both taxa obtained 25–35% of their carbon from C4 sources. These resources must have been obtained either directly from grasses or sedges, or indirectly from animals that ate these plants. Since few of the fine scratches characterizing consumption of grass are present in their microwear (Grine 1986; Scott et al. 2005), it was deduced that direct consumption of grass blades was less plausible (although not ruled out) (Lee-Thorp & Sponheimer 2006). C4 sedges, grass rhizomes and the proposed consumption of grass-eating termites (and other small animal foods) may be implicated (Sponheimer et al. 2005; Yeakel et al. 2007), but when examined individually, none of these resources offers a completely satisfactory solution. For instance, it has been shown that in this part of southern Africa the proportion of sedges following the C4 pathway is modest (Stock et al. 2004), and likewise few termite species seem to be C4 specialists (Sponheimer et al. 2005). The most plausible explanation is that they utilized C4 resources quite broadly, including both C4 plant and animal resources. Although the results say little about the rest of the diet (i.e. the major C3 portion), they hint that neither of these australopiths were plant specialists.
These results were also unexpected because extant great apes consume minimal or no C4 resources even when they live in relatively open habitats. Several studies have shown that even ‘savannah’ chimpanzees, who live in the more open parts of the Pan range, consume few, if any, C4 resources (Schoeninger et al. 1999; Sponheimer et al. 2006a). Most forest-dwelling chimpanzees and gorillas reveal distinct low δ13C values, indicative of the use of resources located in shaded understorey vegetation (Carter 2001; Cerling et al. 2004; Sponheimer et al. 2009; J. A. Lee-Thorp, Y. Warren & G. A. Macho 2009, unpublished data). It is this engagement with C4 resources, which were becoming increasingly available in the Plio-Pleistocene, that indicates a fundamental niche difference between the australopiths and extant apes.
3. Variability between and within individuals
The foregoing discussion relies on broad averages, and we now turn to examine patterns in individuals. Where sufficient australopith δ13C values exist within any one site, or members within that site, the data are quite variable, which suggests a degree of dietary opportunism and flexibility. This observation leads to the question of whether hominins shifted their diets on annual or even seasonal time scales, and whether the C4 contributions observed in bulk tooth enamel measurements mask short-term dietary variability.
Tooth enamel is an incremental tissue that can be sampled to investigate temporal changes in both climate and diet using stable isotope analysis. Developments in laser ablation techniques now permit high-resolution sequential sampling of enamel crowns, with minimal visible damage (Passey & Cerling 2006). However, the sequential chronological resolution that can be attained, no matter how small the sample spot, is severely constrained by overprinting during maturation of the enamel. Enamel maturation occurs for many months, even years, after primary mineralization during prism formation (Suga 1982; Balasse 2002). Recovery of primary dietary signals has been successfully accomplished using a forward and inverse model only in continuously growing teeth and where the growth and enamel maturation parameters are well characterized (Passey et al. 2005). The patterns of enamel maturation in modern human tooth crowns are poorly understood, and those of early hominins even less so. However, it is evident that the nature of crown formation and enamel maturation inevitably produces a mixture of isotope signals, from both the initial primary mineralization and the maturation periods, so that there will be dampening or overprinting of the original signals. Nevertheless, three statements can be made with certainty: (i) the isotope profile from crown to root preserves an ordered time series extending from earlier (crown) to later (root) in time, (ii) the mimimum time duration of any single spot-sample within a tooth is equivalent to the ‘maturation time’, or time required for enamel to cure into its fully mineralized form (probably months in primates), and (iii) the total time duration of an isotopic profile across a tooth is the sum of the crown enamel deposition time (for example, as recorded by perikymata) and the maturation time of the last enamel increment deposited.
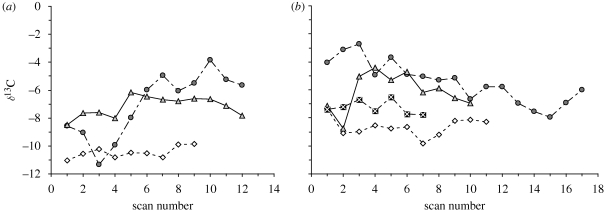
Notwithstanding the constraints imposed by maturation patterns and by lower analytical precision, laser ablation has been applied to sample the external surface along the growth trajectory of four P. robustus tooth crowns (Sponheimer et al. 2006b) (figure 2b). The results showed that δ13C values, and the pattern or temporal change, differed between individuals and, most startling, showed differences of up to 5‰ within a given P. robustus tooth. Given that the signal is attenuated or even scrambled as described above, these data still suggest a very large shift from a diet dominated by C3 to a diet dominated by C4 resources in certain individuals. Variability is observed at several time scales—intra- and inter-annual.
Figure 2.
High-resolution laser ablation δ13C sequences for (a) A. africanus and (b) P. robustus plotted against sample (scan) number. Sample increments were approximately 0.3 mm. The Paranthropus data are from Sponheimer et al. (2006b), where the data were plotted according to a time-sequence model based on perikymata counts. In this case, we avoided the application of a time sequence based on perikymata because the lengthy maturation time introduces not only a longer time period but also more uncertainty. (a) Open diamonds, STS 2518 max RM3; filled circles, STS 31 max RM3; filled triangles, STS 2253 mand RM1. (b) Open diamonds, SKW 6427 M; filled circles, SKW 5939 M; filled triangles, SK 24606 RM2 or 3; squares with crosses, SK 24606 RM3.
Four A. africanus molar crowns from Sterkfontein Member 4 were analysed using the same methods. The results for one tooth were omitted because of concerns about the interference of glue on the surface, observed as puffs of gas during ablation. Although the age of Member 4 is uncertain, biostratigraphic evidence indicates that it is considerably older than Swartkrans, and with several taxa—including A. africanus—in common with the older site of Makapansgat (Vrba 1985). As was the case for the P. robustus crowns, all teeth are lightly to moderately worn molars. The tooth assignments are shown in figure 2, although some in figure 2b were too fragmentary to allow determination other than that they are molars.
The analytical methods for the A. africanus molars followed exactly those reported in Sponheimer et al. (2006b). Each tooth was cleaned mechanically and chemically (with acetone), and then thoroughly dried in a low-temperature oven. Samples were purged with helium inside the laser chamber for several minutes or hours as required for the rate of CO2 outgassing to fall below appropriate levels. Small amounts (10–30 nmol) of CO2 were generated using a CO2 laser (10.6 µm) operating at 5–15 W and 8.5 ms pulse duration in a He atmosphere. The CO2 was cryogenically purified and ‘focused’ prior to introduction to a continuous-flow GC-IRMS (MAT 252). Systematic isotope fractionation and fractionation associated with laser ablation production of CO2 were monitored by analyses of injected aliquots of CO2 and by analysis of a suite of internal tooth enamel standards, both calibrated against NBS-19 gas (δ13C = 1.95‰). The laser-carbonate isotope fractionation (ɛ*LASER-carb) for fossil herbivore samples from several South African hominin sites was –1.3 ± 1.5‰ (1σ).
Enamel was sampled at ca 0.3 mm intervals, encompassing about four perikymata for each laser ablation track (or scan) and the space between tracks. The length of each sampling trajectory varied depending on the available tooth surface, with between nine and 12 scans for the three reported molars. Based on a periodicity of 7 days per striae of Retzius (observed externally as perikymata) as calculated in Lacruz et al. (2006), the period of primary mineralization in the sampled area is over a year for two specimens (STS 2253 and STS 31) and just under a year for the third (STS 2518). However, as discussed above, the time represented in the isotope profile is much longer when enamel maturation is taken into account, and additionally the measured values represent an attenuated signal of higher amplitude.
Variability in the proportions of C3 and C4 resources between and within individuals is on a similar scale for A. africanus compared with P. robustus (figure 2). At least one individual, STS 2518, indicates a more or less uniform C3 resource base, while another, STS 31, shows values varying by over 6‰ from a C3 to a predominantly C4 resource base. As emphasized above, it is difficult to ascertain the precise time scales over which this variation occurred. However, given that the enamel underlying the sampling arrays mineralized and matured over a period of over a year in each case, the differences between individuals cannot be ascribed to sampling of a single season in one case and multiple seasons in another.
The results suggest that C4 resources formed an important but highly variable component of hominin diets, extending at least as far back as A. africanus at Sterkfontein. There is no reason to believe that the dietary ecology of A. africanus at the earlier site of Makapansgat Member 3 would be significantly different.
4. Origins and time depth of the C4 pattern in hominin diets
Few analyses have been performed on eastern African material to allow us to definitively address the question of the origins of engagement with C4 resources. Clues from several sources may hint at an origin associated with the emergence of the genus Australopithecus, but at present there are no data to address the period prior to emergence of A. africanus.
Recently published δ13C data for two Paranthropus boisei individuals from Olduvai revealed a strong dependence on C4 resources (van der Merwe et al. 2008). This was unexpected since the similarity of the ‘nutcracker’ dental and masticatory complex to that of South African P. robustus led to the expectation of a broadly similar dietary adaptation. Such high δ13C values cannot be explained by consumption of C4 animal foods alone since they would require a highly unlikely dietary scenario that included almost exclusive consumption of the flesh of grazing (C4) animals. The results demand an alternative explanation. The authors suggested that P. boisei, or at least these two individuals, probably specialized in exploitation of C4 sedges, which are far more abundant in East African wetlands than they are in South Africa (van der Merwe et al. 2008). Additionally, a recent microwear study of P. boisei occlusal enamel showed little evidence for the pitting associated with hard object feeding (Ungar et al. 2008). These data seem inconsistent with their strongly developed masticatory complex, widely considered to be an adaptation for the consumption of hard foods. However, their diets were clearly abrasive when the degree of wear is considered (Tobias 1967). In combination, the high C4 resource consumption and the lack of a hard-object microwear pattern may require that we rethink the functional significance of the australopith masticatory package.
Interestingly, microwear patterns for Australopithecus afarensis and Australopithecus anamensis also lack evidence for hard-object feeding (Ungar 2004; Grine et al. 2006), but rather resemble patterns seen in apes, especially gorillas. These results could also be considered as inconsistent with an evolutionary trajectory for larger molars and thicker enamel, which seem to suggest adaptation to increasingly more xeric habitats (Grine et al. 2006). However, other evidence points in a similar direction. It has been argued that the biomechanical masticatory complex of both A. anamensis (Macho et al. 2005) and A. afarensis (Rak et al. 2007) is more gorilla-like. Furthermore, modern humans and gorillas share life-history characteristics including non-seasonal breeding (Knott 2005), which in turn carries implications for a pattern of seasonal resource exploitation that ensures maximum infant survival. No isotope data exist for these older australopiths that would allow us to test whether C4 exploitation formed part of an increasingly seasonal foraging round as suggested by Macho et al. (2003). If they did engage with such resources, it will be important to understand the seasonal variation.
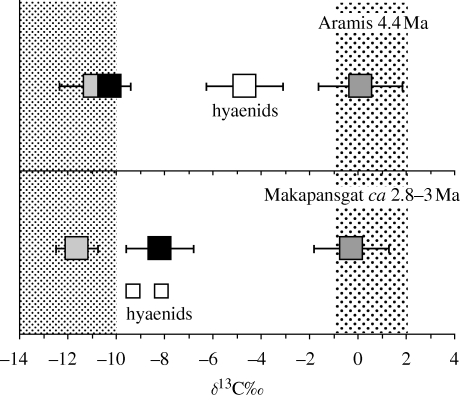
While no isotopic data yet exist for A. anamensis and A. afarensis, recently published data for Aramis suggest that Ardipithecus ramidus, at 4.4 Ma, included few, if any, C4 resources in the diet (White et al. 2009b). Rather their isotopic composition most closely resembles that of savannah chimpanzees which avoid C4 resources, and contrasts with that observed in A. africanus at Makapansgat (figure 3). Although the authors lay stress on the woody nature of the Aramis environment, the δ13C values for the fauna, taken together, show that a good deal of C4 grassy vegetation was present in the environs. Therefore, the important point is not that ‘Ar. ramidus was a denizen of woodland’ (White et al. 2009a), but that Ar. ramidus focused almost exclusively on C3 resources while avoiding nearby C4 resources, which were present. In contrast, the Makapansgat hominins clearly had moved to exploit C4 resources (figure 3), in spite of the relatively closed nature of that environment (Reed 1997). The Aramis data would certainly suggest that, if an engagement with C4 foods marked a fundamental shift in hominin evolution as we have argued, then such a shift occurred post Ar. ramidus, or elsewhere.
Figure 3.
A summary comparison of the hominin data for Aramis (above), and Makapansgat (below), plotted as means (black boxes) and standard deviations with mean values for C3 and C4 feeders shown for comparison. Data for carnivores (hyaenids) are also shown (white boxes; n = 2 for Makapansgat), because they are effectively integrators for the values of all the fauna they consume. These data are shifted towards values more enriched in 13C in Aramis, suggesting more open, C4 elements in the environment compared with Makapansgat, where the faunal assemblage consists largely of C3 feeders. In spite of this difference, Ar. ramidus remains relatively depleted in 13C, quite unlike the patterns for A. africanus seen at Makapansgat. Data for Aramis are from White et al. (2009b), and for Makapansgat are from Sponheimer (1999).
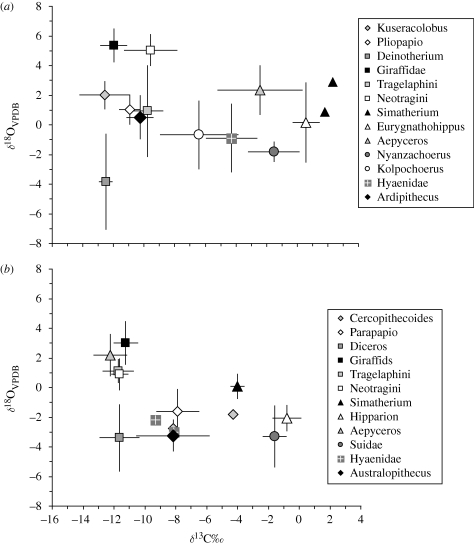
Further evidence for a possible ecological shift between Aramis and Makapansgat may reside in a comparison of the combined δ13C and δ18O data (figure 4). The δ18O composition of enamel provides a potential source of information about dietary ecology because, in addition to the influences of hydrology and isotopic composition of precipitation, an animal's δ18O value is affected by dietary ecology, drinking behaviour and thermophysiology (Bocherens et al. 1996; Kohn 1996; Kohn et al. 1996; Sponheimer & Lee-Thorp 2001). It has been shown that suids, some primates and in particular all faunivores have relatively low δ18O compared with herbivores (Lee-Thorp & Sponheimer 2005). The reasons are not yet clear and almost certainly differ for these groups. In the case of suids, it may reflect reliance on underground storage organs, and for faunivores, a high proportion of dietary lipids and proteins, or a very heavy reliance on drinking water. Australopith δ18O data from Makapansgat overlap with those of carnivores in the same strata (figure 4). Similarly low δ18O values—compared with other taxa in the Aramis faunal assemblage—are not observed for Ar. ramidus. Whatever the underlying contributors to the lower δ18O values for hominins at Makapansgat (and we do not imply that they are necessarily the same), these observations call for further study and explanation.
Figure 4.
Bivariate δ13C and δ18O comparisons of similar taxa in (a) Aramis and (b) Makapansgat shown as means and standard deviations. Several significant differences are observed. On average, δ18O values for Makapansgat fauna are about 2‰ lower than at Aramis, which is consistent with their relative geographical positions and associated values for hydrology. However, the Aramis data are more variable, with some exceptional and unusually low values for Deinotherium in particular. Australopithecus africanus data are relatively enriched in 13C and depleted in 18O, and occupy the same isotopic ‘space’ as the hyaenids, quite unlike Ar. ramidus. The other primate species are also more enriched in 13C, unlike Aramis, but the impala (Aepyceros) has higher δ13C values at Aramis. Although impalas are generally considered to be mixed feeders, a recent study showed high δ13C and almost exclusive grazing habits for Aepyceros in the nutritious grasslands of Rwanda (Copeland et al. 2009). The data for Nyanzochoerus at Aramis are remarkably similar to those for the Suidae at Makapansgat, suggesting a similar ecological niche. Data for Aramis are from White et al. (2009b) and for Makapansgat from Sponheimer (1999).
5. Conclusions
Carbon isotope data have demonstrated that australopiths in South Africa increased their dietary breadth by consuming C4 resources, while in East African P. boisei, this involvement might rather be regarded as a specialization. The exact nature of these C4 resources remains unclear, and cannot be deduced from the δ13C values alone, but they most plausibly included various C4 resources, in varying proportions. Among the South African australopiths at least, consumption of C4 resources varied strongly between individuals and within individuals, in both A. africanus and P. robustus. The australopith pattern is quite unlike that seen in modern chimpanzees, and indeed in early Pliocene Ar. ramidus, and we argue that it represented a fundamental shift in dietary ecology that increased dietary breadth. Additionally, the exact foods that contributed to the observed C4 signals in australopith enamel are not clear. We cannot yet pinpoint when the shift occurred because no published data yet exist for A. anamensis and A. afarensis, but certainly further research should target the period between 4 and 3 Ma.
Acknowledgements
We thank Alan Walker and Chris Stringer for organizing the Royal Society Symposium on the ‘First four million years of human evolution’, the Royal Society for sponsoring the meeting, Debbie Guatelli-Steinberg for her work on the perikymata and many colleagues for their assistance and helpful discussions over the course of many years spent exploring the fascinating discipline of isotope ecology.
Endnote
By convention, 13C/12C ratios are expressed in the delta (δ) notation relative to the PDB standard, as follows: δ13C (‰) = (Rsample/Rstandard − 1)×1000, where R = 13C/12C, and similarly, 18O/16O ratios are expressed as δ18O relative to PDB or SMOW (we use PDB in this paper).
One contribution of 14 to a Discussion Meeting Issue ‘The first four million years of human evolution’.
References
- Balasse M.2002Reconstructing dietary and environmental history from enamel isotopic analysis: time resolution of intra-tooth sequential sampling. Int. J. Osteoarch. 12, 155–165 (doi:10.1002/oa.601) [Google Scholar]
- Bocherens H., Koch P. L., Mariotti A., Geraads D., Jaeger J.1996Isotopic biogeochemistry (13C, 18O) of mammalian enamel from African Pleistocene hominid sites. PALAIOS 11, 306–318 (doi:10.2307/3515241) [Google Scholar]
- Carter M. L.2001Sensitivity of stable isotopes (13C, 15N, and 18O) in bone to dietary specialization and niche separation among sympatric primates in Kibale National Park, Uganda. PhD dissertation, University of Chicago [Google Scholar]
- Cerling T. E., Harris J. M., MacFadden B. J., Leakey M. G., Quade J., Eisenman V., Ehleringer J. R.1997Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158 (doi:10.1038/38229) [Google Scholar]
- Cerling T. E., Hart J. A., Hart T. B.2004Stable isotope ecology in the Ituri Forest. Oecologia 138, 5–12 (doi:10.1007/s00442-003-1375-4) [DOI] [PubMed] [Google Scholar]
- Copeland S. R., Sponheimer M., Spinage C. A., Lee-Thorp J. A., Codron D., Codron J., Reed K.2009Stable isotope evidence for impala Aepyceros melampus diets at Akagera National Park, Rwanda. Afr. J. Ecol. 47, 490–501 (doi:10.1111/j.1365-2028.2008.00969.x) [Google Scholar]
- Dart R. A.1925Australopithecus africanus: the man-ape of South Africa. Nature 115, 195–199 (doi:10.1038/115195a0) [Google Scholar]
- Dart R. A.1926Taungs and its significance. Nat. Hist. 26, 315–327 [Google Scholar]
- DeNiro M. J., Epstein S.1978aInfluences of diet on the carbon isotope distribution in animals. Geochim. Cosmochim. Acta 42, 495–506 (doi:10.1016/0016-7037(78)90199-0) [Google Scholar]
- DeNiro M. J., Epstein S.1978bCarbon isotopic evidence for different feeding habits in two hyrax species occupying the same habitat. Science 201, 906–908 (doi:10.1126/science.201.4359.906) [DOI] [PubMed] [Google Scholar]
- Elias R. W., Hirao Y., Patterson C. C.1982The circumvention of the natural biopurification of calcium along nutrient pathways by atmospheric inputs of industrial lead. Geochim. Cosmochim. Acta 46, 2561–2580 (doi:10.1016/0016-7037(82)90378-7) [Google Scholar]
- Ericson J. E., Sullivan C. H., Boaz N. T.1981Diets of Pliocene mammals from Omo deduced from carbon isotopic ratios in tooth apatite. Palaeogeogr. Palaeoclimatol. Palaeoecol. 36, 69–73 (doi:10.1016/0031-0182(81)90049-3) [Google Scholar]
- Grine F. E.1981Trophic differences between gracile and robust australopithecines. South Afr. J. Sci. 77, 203–230 [Google Scholar]
- Grine F. E.1986Dental evidence for dietary differences in Australopithecus and Paranthropus. J. Hum. Evol. 15, 783–822 (doi:10.1016/S0047-2484(86)80010-0) [Google Scholar]
- Grine F. E., Ungar P. S., Teaford M. F., El-Zaatari S.2006Molar microwear in Praeanthropus afarensis: evidence for dietary stasis through time and under diverse paleoecological conditions. J. Hum. Evol. 51, 297–319 (doi:10.1016/j.jhevol.2006.04.004) [DOI] [PubMed] [Google Scholar]
- Knott C. D.2005Energetic responses to food availability in the great apes: implications for hominin evolution. In Seasonality in primates (eds Brockman D. K., van Schaik C. P.), pp. 351–378 Cambridge, UK: Cambridge University Press [Google Scholar]
- Kohn M. J.1996Predicting animal δ18O: accounting for diet and physiological adaptation. Geochim. Cosmochim. Acta 60, 4811–4829 (doi:10.1016/S0016-7037(96)00240-2) [Google Scholar]
- Kohn M. J., Schoeninger M. J., Valley J. W.1996Herbivore tooth oxygen isotope composition: effects of diet and physiology Geochim. Cosmochim. Acta 60, 3889–3896 (doi:10.1016/0016-7037(96)00248-7) [Google Scholar]
- Lacruz R. S., Rozzi F. R., Bromage T. G.2006Variation in enamel development of South African fossil hominids. J. Hum. Evol. 51, 580–590 (doi:10.1016/j.jhevol.2006.05.007) [DOI] [PubMed] [Google Scholar]
- Lee-Thorp J. A., Sponheimer M.2005Opportunities and constraints for reconstructing palaeoenvironments from stable light isotope ratios in fossils. Geol. Q. 49, 195–204 [Google Scholar]
- Lee-Thorp J. A., Sponheimer M.2006Biogeochemical approaches to investigating hominin diets. Yrbk Phys. Anthropol. 49, 131–148 [DOI] [PubMed] [Google Scholar]
- Lee-Thorp J. A., van der Merwe N. J.1987Carbon isotope analysis of fossil bone apatite. South Afr. J. Sci. 83, 712–715 [Google Scholar]
- Lee-Thorp J. A., van der Merwe N. J., Brain C. K.1989Isotopic evidence for dietary differences between two extinct baboon species from Swartkrans. J. Hum. Evol. 18, 183–190 [Google Scholar]
- Lee-Thorp J. A., van der Merwe N. J., Brain C. K.1994Diet of Australopithecus robustus at Swartkrans deduced from stable carbon isotope ratios. J. Hum. Evol. 27, 361–372 (doi:10.1006/jhev.1994.1050) [Google Scholar]
- Lee-Thorp J. A., Sponheimer M., Luyt J. C.2007Tracking changing environments using stable carbon isotopes in fossil tooth enamel: an example from the South African hominin sites. J. Hum. Evol. 53, 595–601 (doi:10.1016/j.jhevol.2006.11.020) [DOI] [PubMed] [Google Scholar]
- Macho G. A., Shimizu D.2009Dietary adaptations of South African australopiths: inference from enamel prism attitude. J. Hum. Evol. 57, 241–247 (doi:10.1016/j.jhevol.2009.05.003) [DOI] [PubMed] [Google Scholar]
- Macho G. A., Leakey M. G., Williamson D. K., Jiang Y.2003Palaeoenvironmental reconstruction: evidence for seasonality at Allia Bay, Kenya, at 3.9 million years. Palaeogeogr. Palaeoclimatol. Palaeoecol. 199, 17–30 (doi:10.1016/S0031-0182(03)00483-8) [Google Scholar]
- Macho G. A., Shimizu D., Jiang Y., Spears I. R.2005Australopithecus anamensis: a finite-element approach to studying the functional adaptations of extinct hominins. Anat. Rec. 283A, 310–318 (doi:10.1002/ar.a.20175) [DOI] [PubMed] [Google Scholar]
- Parker R., Toots H.1980Trace elements in bones as paleobiological indicators. In Fossils in the making (eds Behrensmeyer A. K., Hill A.), pp. 197–207 Chicago, IL: University of Chicago Press [Google Scholar]
- Passey B. H., Cerling T. E.2006In situ stable isotope analysis (δ13C, δ18O) of very small teeth using laser ablation GC/IRMS. Chem. Geol. 235, 238–249 (doi:10.1016/j.chemgeo.2006.07.002) [Google Scholar]
- Passey B. H., Cerling T. E., Schuster G. T., Robinson T. F., Roeder B. L., Krueger S. K.2005Inverse methods for estimating primary input signals from time-averaged isotope profiles. Geochim. Cosmochim. Acta 69, 4101–4116 (doi:10.1016/j.gca.2004.12.002) [Google Scholar]
- Rak Y., Ginzburg A., Geffen E.2007Gorilla-like anatomy on Australopithecus afarensis mandibles suggests Au. afarensis link to robust australopiths. Proc. Natl Acad. Sci. USA 104, 6568–6572 (doi:10.1073/pnas.0606454104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.1997Early hominid evolution and ecological change through the African Plio-Pleistocene. J. Hum. Evol 32, 289–322 (doi:10.1006/jhev.1996.0106) [DOI] [PubMed] [Google Scholar]
- Schoeninger M. J., Moore J., Sept M.1999Subsistence strategies of two ‘savanna’ chimpanzee populations: the stable isotope evidence. Am. J. Primatol. 49, 297–314 (doi:10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- Scott R. S., Ungar P. S., Bergstrom T. S., Brown C. A., Grine F. E., Teaford M. F., Walker A.2005Dental microwear texture analysis shows within-species dietary variability in fossil hominins. Nature 436, 693–695 (doi:10.1038/nature03822) [DOI] [PubMed] [Google Scholar]
- Sillen A.1988Elemental and isotopic analysis of mammalian fauna from southern Africa and their implications for paleodietary research. Am. J. Phys. Anthropol. 76, 49–60 (doi:10.1002/ajpa.1330760106) [Google Scholar]
- Sillen A.1992Strontium–calcium ratios (Sr/Ca) of Australopithecus robustus and associated fauna from Swartkrans. J. Hum. Evol. 23, 495–516 (doi:10.1016/0047-2484(92)90049-F) [Google Scholar]
- Sponheimer M.1999Isotopic ecology of the Makapansgat Limeworks fauna. PhD dissertation, Rutgers University [Google Scholar]
- Sponheimer M., Lee-Thorp J. A.1999Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science 283, 368–370 (doi:10.1126/science.283.5400.368) [DOI] [PubMed] [Google Scholar]
- Sponheimer M., Lee-Thorp J. A.2001The oxygen isotope composition of mammalian enamel carbonate from Morea Estate, South Africa. Oecologia 126, 153–157 (doi:10.1007/s004420000498) [DOI] [PubMed] [Google Scholar]
- Sponheimer M., Lee-Thorp J. A.2006Enamel diagenesis at South African Australopith sites: implications for paleoecological reconstruction with trace elements. Geochim. Cosmochim. Acta 70, 1644–1654 (doi:10.1016/j.gca.2005.12.022) [Google Scholar]
- Sponheimer M., Lee-Thorp J. A., de Ruiter D., Codron D., Codron J., Baugh A. T., Thackeray J. F.2005Hominins, sedges, and termites: new carbon isotope data from the Sterkfontein valley and Kruger National Park. J. Hum. Evol. 48, 301–312 (doi:10.1016/j.jhevol.2004.11.008) [DOI] [PubMed] [Google Scholar]
- Sponheimer M., Loudon J. E., Codron D., Howells M. E., Pruetz J. D., Codron J., de Ruiter D., Lee-Thorp J. A.2006aDo ‘savanna’ chimpanzees consume C4 resources? J. Hum. Evol. 51, 128–133 (doi:10.1016/j.jhevol.2006.02.002) [DOI] [PubMed] [Google Scholar]
- Sponheimer M., Passey B. H., de Ruiter D. J., Guatelli-Steinberg D., Cerling T. E., Lee-Thorp J. A.2006bIsotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science 314, 980–982 (doi:10.1126/science.1133827) [DOI] [PubMed] [Google Scholar]
- Sponheimer M., Codron D., Passey B. H., De Ruiter D. J., Cerling T. E., Lee-Thorp J. A.2009Using carbon isotopes to track dietary change in modern, historical, and ancient primates. Am. J. Phys. Anthropol. 140, 661–670 (doi:10.1002/ajpa.21111) [DOI] [PubMed] [Google Scholar]
- Stock W. D., Chuba D. K., Verboom G. A.2004Distribution of South African C-3 and C-4 species of Cyperaceae in relation to climate and phylogeny. Aust. Ecol. 29, 313–319 (doi:10.1111/j.1442-9993.2004.01368.x) [Google Scholar]
- Suga S.1982Progressive mineralization pattern of developing enamel during the maturation stage. J. Dent. Res. 61, 1532–1542 [PubMed] [Google Scholar]
- Sullivan C. H., Krueger H. W.1981Carbon isotope analysis of separate chemical phases in modern and fossil bone. Nature 292, 333–335 (doi:10.1038/292333a0) [DOI] [PubMed] [Google Scholar]
- Tobias P. V.1967The cranium and maxillary dentition of Australopithecus (Zinjanthropus) boisei. Olduvai Gorge, vol. 2 Cambridge, UK: Cambridge University Press [Google Scholar]
- Ungar P. S.2004Dental topography and diets of Australopithecus afarensis and early Homo. J. Hum. Evol. 46, 605–622 (doi:10.1016/j.jhevol.2004.03.004) [DOI] [PubMed] [Google Scholar]
- Ungar P. S., Grine F. E., Teaford M. F.2008Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS ONE 3, e2044 (doi:10.1371/journal.pone.0002044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe N. J., Medina E.1989Photosynthesis and 13C/12C ratios in Amazonian rain forests. Geochim. Cosmochim. Acta 53, 1091–1094 [Google Scholar]
- van der Merwe N. J., Vogel J. C.1978Content of human collagen as a measure of prehistoric diet in Woodland North America. Nature 276, 815–816 (doi:10.1038/276815a0) [DOI] [PubMed] [Google Scholar]
- van der Merwe N. J., Masao F. T., Bamford R. J.2008Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. S. Afr. J. Sci. 104, 153–155 [Google Scholar]
- Vogel J. C.1978Isotopic assessment of the dietary habits of ungulates. S. Afr. J. Sci. 74, 298–301 [Google Scholar]
- Vrba E. S.1985Early hominids in southern Africa: updated observations on chronological and ecological background. In Hominid evolution: past, present and future (ed. Tobias P. V.), pp. 195–200 New York, NY: Alan R. Liss [Google Scholar]
- Walker A.1981Dietary hypotheses and human evolution. Phil. Trans. R. Soc. Lond. B 292, 57–64 (doi:10.1098/rstb.1981.0013) [DOI] [PubMed] [Google Scholar]
- White T. D., Asfaw B., Beyene Y., Haile-Selassie Y., Lovejoy C. O., Suwa G., WoldeGabriel G.2009aArdipithecus ramidus and the paleobiology of early hominids. Science 326, 64 (doi:10.1126/science.1175802) [PubMed] [Google Scholar]
- White T. D., et al. 2009bMacrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science 326, 87–93 See www.sciencemag.org/cgi/content/full/326/5949/67/DC1 [PubMed] [Google Scholar]
- Yeakel J. D., Bennett N. C., Koch P. L., Dominy N. J.2007The isotopic ecology of African mole rats informs hypotheses on the evolution of human diet. Proc. R. Soc. B 274, 1723–1730 (doi:10.1098/rspb.2007.0330) [DOI] [PMC free article] [PubMed] [Google Scholar]