Abstract
Mutations in the acceptor stem, the 5-methyluridine-pseudouridine-cytidine (TFC) arm, and the anticodon of Salmonella tRNA2Gly can cause -1 frameshifting. The potential for standard base pairing between acceptor stem positions 1 and 72 is disrupted in the mutant sufS627. This disruption may interfere with the interaction of the tRNA with elongation factor-Tu.GTP or an as-yet-unspecified domain of the ribosome. The potential for standard base pairing in part of the TFC stem is disrupted in mutant sufS625. The nearly universal C-61 base of the TFC stem is altered in mutant sufS617, and the TFC loop is extended in mutant sufS605. These changes are expected to interfere with the stability of the TFC loop and its interaction with the D arm. The mutation in mutant sufS605, and possibly other mutants, alters nucleoside modification in the D arm. Three mutants, sufS601, sufS607, and sufS609, have a cytidine substituted for the modified uridine at position 34, the first anticodon position. None of the alterations grossly disrupts in-frame triplet decoding by the mutant tRNAs. The results show that -1 frameshifting in vivo can be caused by tRNAs with normal anticodon loop size and suggest that alternative conformational states of the mutant tRNAs may allow them to read a codon in frame or to shift reading frame.
Full text
PDF
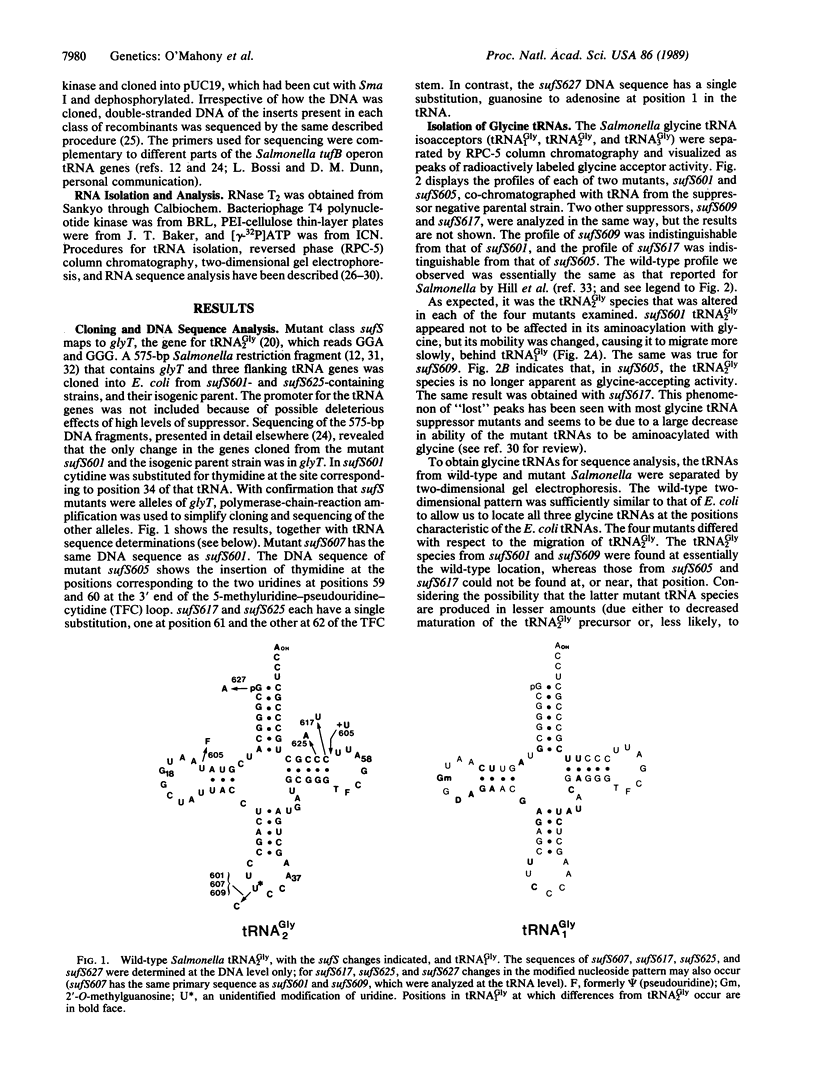


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Menninger J. R. Tests of the ribosome editor hypothesis. III. A mutant Escherichia coli with a defective ribosome editor. Mol Gen Genet. 1987 Sep;209(2):313–318. doi: 10.1007/BF00329659. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Gesteland R. F., Reid B. R., Anderson C. W. Normal tRNAs promote ribosomal frameshifting. Cell. 1979 Dec;18(4):1119–1131. doi: 10.1016/0092-8674(79)90225-3. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Nichols B. P., Thompson S. The nucleotide sequence of the first externally suppressible--1 frameshift mutant, and of some nearby leaky frameshift mutants. EMBO J. 1983;2(8):1345–1350. doi: 10.1002/j.1460-2075.1983.tb01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Coulson A. R., McClain W. H. Nucleotide sequence of a glycine transfer RNA coded by bacteriophage T4. FEBS Lett. 1973 Nov 15;37(1):64–69. doi: 10.1016/0014-5793(73)80427-2. [DOI] [PubMed] [Google Scholar]
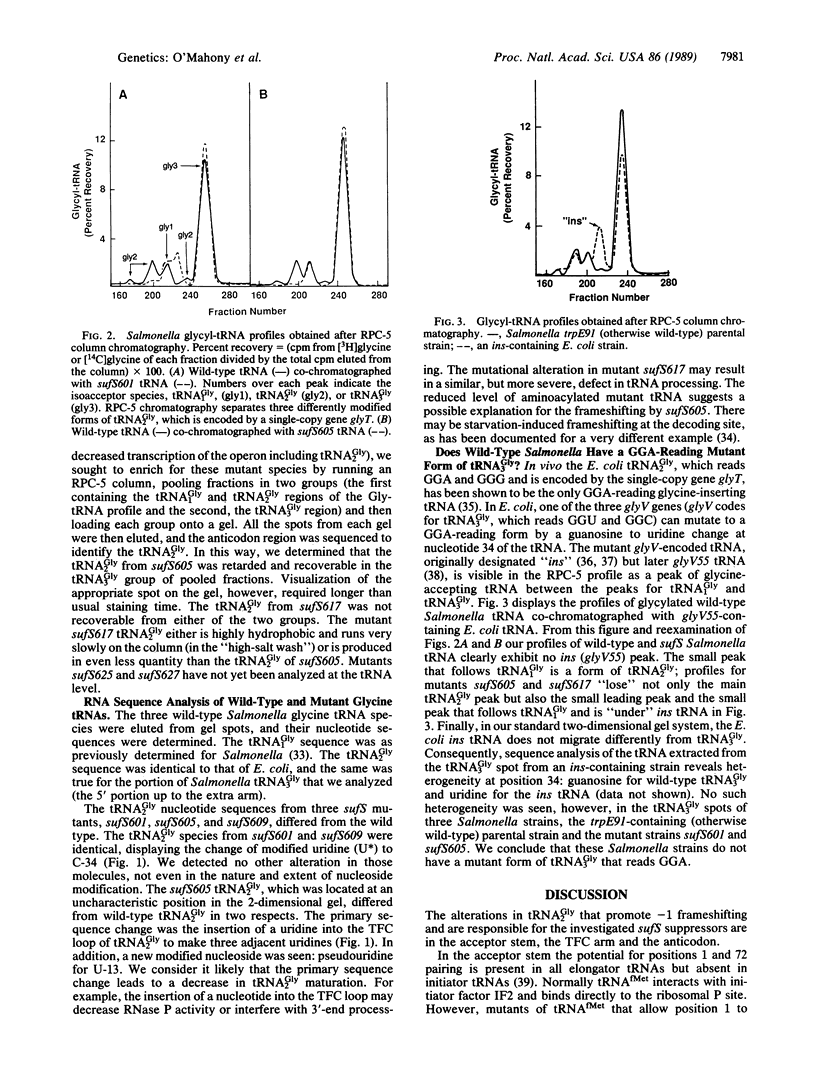
- Beremand M. N., Blumenthal T. Overlapping genes in RNA phage: a new protein implicated in lysis. Cell. 1979 Oct;18(2):257–266. doi: 10.1016/0092-8674(79)90045-x. [DOI] [PubMed] [Google Scholar]
- Bossi L., Smith D. M. Suppressor sufJ: a novel type of tRNA mutant that induces translational frameshifting. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6105–6109. doi: 10.1073/pnas.81.19.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I., Boursnell M. E., Binns M. M., Bilimoria B., Blok V. C., Brown T. D., Inglis S. C. An efficient ribosomal frame-shifting signal in the polymerase-encoding region of the coronavirus IBV. EMBO J. 1987 Dec 1;6(12):3779–3785. doi: 10.1002/j.1460-2075.1987.tb02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K. J., Hayashi M. Role of premature translational termination in the regulation of expression of the phi X174 lysis gene. J Mol Biol. 1987 Dec 20;198(4):599–607. doi: 10.1016/0022-2836(87)90203-8. [DOI] [PubMed] [Google Scholar]
- Carbon J., Squires C., Hill C. W. Glycine transfer RNA of Escherichia coli. II. Impaired GGA-recognition in strains containing a genetically altered transfer RNA; reversal by a secondary suppressor mutation. J Mol Biol. 1970 Sep 28;52(3):571–584. doi: 10.1016/0022-2836(70)90420-1. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Clare J. J., Belcourt M., Farabaugh P. J. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty1 transposon. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6816–6820. doi: 10.1073/pnas.85.18.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. F., Yarus M. Reading frame selection and transfer RNA anticodon loop stacking. Science. 1987 Dec 11;238(4833):1545–1550. doi: 10.1126/science.3685992. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Falahee M. B., Weiss R. B., O'Connor M., Doonan S., Gesteland R. F., Atkins J. F. Mutants of translational components that alter reading frame by two steps forward or one step back. J Biol Chem. 1988 Dec 5;263(34):18099–18103. [PubMed] [Google Scholar]
- Feinstein S. I., Altman S. Coding properties of an ochre-suppressing derivative of Escherichia coli tRNAITyr. J Mol Biol. 1977 May 25;112(3):453–470. doi: 10.1016/s0022-2836(77)80192-7. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Weiss-Brummer B. Leaky +1 and -1 frameshift mutations at the same site in a yeast mitochondrial gene. Nature. 1980 Nov 6;288(5786):60–63. doi: 10.1038/288060a0. [DOI] [PubMed] [Google Scholar]
- Goldman E., Hatfield G. W. Use of purified isoacceptor tRNAs for the study of codon-anticodon recognition in vitro with sequenced natural messenger RNA. Methods Enzymol. 1979;59:292–309. doi: 10.1016/0076-6879(79)59092-2. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helk B., Sprinzl M. Interaction of unfolded tRNA with the 3'-terminal region of E. coli 16S ribosomal RNA. Nucleic Acids Res. 1985 Sep 11;13(17):6283–6298. doi: 10.1093/nar/13.17.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Combriato G., Steinhart W., Riddle D. L., Carbon J. The nucleotide sequence of the GGG-specific glycine transfer ribonucleic acid of Escherichia coli and of Salmonella typhimurium. J Biol Chem. 1973 Jun 25;248(12):4252–4262. [PubMed] [Google Scholar]
- Hudson L., Rossi J., Landy A. Dual function transcripts specifying tRNA and mRNA. Nature. 1981 Dec 3;294(5840):422–427. doi: 10.1038/294422a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D., Atkins J. F., Thompson S. Mutants of elongation factor Tu promote ribosomal frameshifting and nonsense readthrough. EMBO J. 1987 Dec 20;6(13):4235–4239. doi: 10.1002/j.1460-2075.1987.tb02772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D., Thompson S., O'Connor M., Tuohy T., Nichols B. P., Atkins J. F. Genetic characterization of frameshift suppressors with new decoding properties. J Bacteriol. 1989 Feb;171(2):1028–1034. doi: 10.1128/jb.171.2.1028-1034.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. P., Sibler A. P., Schneller J. M., Keith G., Stahl A. J., Dirheimer G. Primary structure of yeast mitochondrial DNA-coded phenylalanine-tRNA. Nucleic Acids Res. 1978 Dec;5(12):4579–4592. doi: 10.1093/nar/5.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall M. D., Leeds P., Fen H., Mathison L., Zwick M., Sleiziz C., Culbertson M. R. Frameshift suppressor mutations affecting the major glycine transfer RNAs of Saccharomyces cerevisiae. J Mol Biol. 1987 Mar 5;194(1):41–58. doi: 10.1016/0022-2836(87)90714-5. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Pagel F. T. Codon recognition by glycine transfer RNAs of Escherichia coli in vivo. J Mol Biol. 1980 Apr 25;138(4):833–844. doi: 10.1016/0022-2836(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Prather N. E., Hadley K. H. Variations among glyV-derived glycine tRNA suppressors of glutamic acid codons. J Bacteriol. 1978 Jun;134(3):801–807. doi: 10.1128/jb.134.3.801-807.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J. tRNA, suppression, and the code. Annu Rev Genet. 1985;19:57–80. doi: 10.1146/annurev.ge.19.120185.000421. [DOI] [PubMed] [Google Scholar]
- O'Mahony D. J., Hughes D., Thompson S., Atkins J. F. Suppression of a -1 frameshift mutation by a recessive tRNA suppressor which causes doublet decoding. J Bacteriol. 1989 Jul;171(7):3824–3830. doi: 10.1128/jb.171.7.3824-3830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather N. E., Murgola E. J., Mims B. H. Nucleotide substitution in the amino acid acceptor stem of lysine transfer RNA causes missense suppression. J Mol Biol. 1984 Jan 15;172(2):177–184. doi: 10.1016/s0022-2836(84)80036-4. [DOI] [PubMed] [Google Scholar]
- Prather N. E., Murgola E. J., Mims B. H. Primary structure of an unusual glycine tRNA UGA suppressor. Nucleic Acids Res. 1981 Dec 11;9(23):6421–6428. doi: 10.1093/nar/9.23.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery L. A., Bermingham J. R., Jr, Yarus M. Mutation in the D arm enables a suppressor with a CUA anticodon to read both amber and ochre codons in Escherichia coli. J Mol Biol. 1986 Aug 5;190(3):513–517. doi: 10.1016/0022-2836(86)90020-3. [DOI] [PubMed] [Google Scholar]
- Riyasaty S., Atkins J. F. External suppression of a frameshift mutant in salmonella. J Mol Biol. 1968 Jun 28;34(3):541–557. doi: 10.1016/0022-2836(68)90179-4. [DOI] [PubMed] [Google Scholar]
- Romby P., Carbon P., Westhof E., Ehresmann C., Ebel J. P., Ehresmann B., Giegé R. Importance of conserved residues for the conformation of the T-loop in tRNAs. J Biomol Struct Dyn. 1987 Dec;5(3):669–687. doi: 10.1080/07391102.1987.10506419. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. The structural basis for the resistance of Escherichia coli formylmethionyl transfer ribonucleic acid to cleavage by Escherichia coli peptidyl transfer ribonucleic acid hydrolase. J Biol Chem. 1975 Jan 25;250(2):542–547. [PubMed] [Google Scholar]
- Seong B. L., Lee C. P., RajBhandary U. L. Suppression of amber codons in vivo as evidence that mutants derived from Escherichia coli initiator tRNA can act at the step of elongation in protein synthesis. J Biol Chem. 1989 Apr 15;264(11):6504–6508. [PubMed] [Google Scholar]
- Smith D., Yarus M. Transfer RNA structure and coding specificity. I. Evidence that a D-arm mutation reduces tRNA dissociation from the ribosome. J Mol Biol. 1989 Apr 5;206(3):489–501. doi: 10.1016/0022-2836(89)90496-8. [DOI] [PubMed] [Google Scholar]
- Squires C., Carbon J. Normal and mutant glycine transfer RNAs. Nat New Biol. 1971 Oct 27;233(43):274–277. doi: 10.1038/newbio233274a0. [DOI] [PubMed] [Google Scholar]
- Stern L., Schulman L. H. The role of the minor base N4-acetylcytidine in the function of the Escherichia coli noninitiator methionine transfer RNA. J Biol Chem. 1978 Sep 10;253(17):6132–6139. [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Base pairing and fidelity in codon-anticodon interaction. Nature. 1976 Sep 23;263(5575):289–293. doi: 10.1038/263289a0. [DOI] [PubMed] [Google Scholar]
- Tucker S. D., Murgola E. J., Pagel F. T. Missense and nonsense suppressors can correct frameshift mutations. Biochimie. 1989 Jun;71(6):729–739. doi: 10.1016/0300-9084(89)90089-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Atkins J. F., Gesteland R. F. Slippery runs, shifty stops, backward steps, and forward hops: -2, -1, +1, +2, +5, and +6 ribosomal frameshifting. Cold Spring Harb Symp Quant Biol. 1987;52:687–693. doi: 10.1101/sqb.1987.052.01.078. [DOI] [PubMed] [Google Scholar]
- Weiss R. B., Dunn D. M., Dahlberg A. E., Atkins J. F., Gesteland R. F. Reading frame switch caused by base-pair formation between the 3' end of 16S rRNA and the mRNA during elongation of protein synthesis in Escherichia coli. EMBO J. 1988 May;7(5):1503–1507. doi: 10.1002/j.1460-2075.1988.tb02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R., Lindsley D., Falahee B., Gallant J. On the mechanism of ribosomal frameshifting at hungry codons. J Mol Biol. 1988 Sep 20;203(2):403–410. doi: 10.1016/0022-2836(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Wikman F. P., Siboska G. E., Petersen H. U., Clark B. F. The site of interaction of aminoacyl-tRNA with elongation factor Tu. EMBO J. 1982;1(9):1095–1100. doi: 10.1002/j.1460-2075.1982.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W., Malim M. H., Mellor J., Kingsman A. J., Kingsman S. M. Expression strategies of the yeast retrotransposon Ty: a short sequence directs ribosomal frameshifting. Nucleic Acids Res. 1986 Sep 11;14(17):7001–7016. doi: 10.1093/nar/14.17.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]



