Abstract
The niche concept is central to ecology but is often depicted descriptively through observing associations between organisms and habitats. Here, we argue for the importance of mechanistically modelling niches based on functional traits of organisms and explore the possibilities for achieving this through the integration of three theoretical frameworks: biophysical ecology (BE), the geometric framework for nutrition (GF) and dynamic energy budget (DEB) models. These three frameworks are fundamentally based on the conservation laws of thermodynamics, describing energy and mass balance at the level of the individual and capturing the prodigious predictive power of the concepts of ‘homeostasis’ and ‘evolutionary fitness’. BE and the GF provide mechanistic multi-dimensional depictions of climatic and nutritional niches, respectively, providing a foundation for linking organismal traits (morphology, physiology, behaviour) with habitat characteristics. In turn, they provide driving inputs and cost functions for mass/energy allocation within the individual as determined by DEB models. We show how integration of the three frameworks permits calculation of activity constraints, vital rates (survival, development, growth, reproduction) and ultimately population growth rates and species distributions. When integrated with contemporary niche theory, functional trait niche models hold great promise for tackling major questions in ecology and evolutionary biology.
Keywords: functional traits, climatic niche, nutritional niche, dynamic energy budget, geometric framework, biophysical ecology
1. Introduction
In ecology, strong patterns exist with respect to body size, geographical distributions, abundances, species diversity and community structure at coarse spatio-temporal scales (Brown 1995). These ‘macroecological’ patterns suggest that there are general ecological laws to be discovered that could form the basis of a more strongly predictive science. Yet, such laws, if they exist, remain elusive, with the consequence that ecology has been criticized for stalling at the ‘What’ stage rather than progressing, as have other life sciences, further into the ‘Why’ and ‘How’ domains (O'Connor 2000).
We believe that a relevant distinction between ecology and the more strongly predictive functional life sciences is that ecology lacks a teleonomic framework (Thompson 1987): it has no credible equivalent to the notions of ‘design’ (adaptation) and ‘goal-directedness’ (homeostasis) that tightly constrain the expected behaviour of physiological systems, and so limit the range of outcomes that can reasonably be expected (de Laguna 1962). Possibly for this reason, there have been several historical attempts to characterize ecological communities as ‘superorganisms’ with teleonomic properties, but none of these stand up to critical scrutiny (McIntosh 1998).
We agree with McIntosh that analogies between ecological communities and organisms are weak, but we do not believe that this should exclude the notions of adaptation and homeostasis from ecological models. These principles are deeply embedded within the patterns that ecologists describe, and should therefore provide a baseline to aid prediction in ecology. The challenge, however, is to derive an approach for studying the penetrance of functional traits of individual organisms into higher, group-level phenomena. The study of collective behaviour has achieved this in the context of group-level behavioural interactions (Couzin & Krause 2003; Simpson et al. 2010), and the powerful framework of life-history theory exists for linking functional traits to population dynamics (Roff 2002). In ecology, a promising start has been made in the form of ‘metabolic theories in ecology’ (van der Meer 2006), but much remains to be achieved (Kearney & Porter 2006; McGill et al. 2006).
In this article, we compare and contrast three theoretical frameworks that have potential for linking functional traits to community ecology: biophysical ecology (BE), the geometric framework of nutrition (GF) and dynamic energy and mass budget (DEB) models. Our aim is to show how their integration may facilitate the development of a more strongly predictive, mechanistic approach to understanding the ecology and evolution of organisms in changing environments from individuals through to communities and ecosystems. We build our discussion around the ecological niche, because this is the ecological concept that provides the closest interface between the physiology of organisms and their interactions with environment.
2. The ecological niche
The niche concept has been defined in many ways throughout the history of ecology (Schoener 1989, 2009; Chase & Leibold 2003). It began with Grinnell and with Elton as qualitative descriptions of species' roles and requirements in communities (Grinnell 1917; Elton 1927). Hutchinson (1957) later proposed a more formal, quantitative concept based on set theory. He conceived of the niche as a hyper-volume in multi-dimensional environmental space delimiting where stable populations can be maintained. When biotic interactions such as predation and competition are included in the calculation of niche space, one obtains the ‘realized niche’, as opposed to the ‘fundamental’ or ‘physiological niche’ that ignores such interactions. Hutchinson's niche concept differed most markedly from that of Grinnell and Elton in being defined as a property of a species rather than as a recess in a community (Schoener 1989). The Hutchinsonian niche hyperspace sits in ‘environmental’ dimensions or axes, typically including physical conditions (habitat temperature, humidity, pH etc.) and resources (e.g. food particle size). The niche in ‘environmental space’ can be transposed to physical space and time, in the context of environmental gradients and other habitat features, to predict survivorship, development, growth, reproduction and ultimately, population dynamics, abundance, distribution and species interactions (Kearney 2006; Holt 2009).
(a). Correlative niche models
The spatio-temporal transposition of the niche is usually estimated in a descriptive or correlative manner. The most common approach at present is to link species-occurrence data to coarse spatial datasets on climate, vegetation, terrain and soil via statistical models (Elith & Leathwick 2009), although finer scale approaches have also been attempted (Green 1971). Such models fit nicely with the multivariate Hutchinsonian niche concept (Soberón 2007), and are increasingly becoming objects of study in analyses of species' niches (Wiens & Graham 2005; Pearman et al. 2008). While such analyses may implicitly represent many different ecological processes, they are ultimately inductively driven local analyses revealing little in the way of causal understanding, and may also have poor predictive power when transposed to novel environments (Davis et al. 1998). The latter issue is of practical significance, as correlative niche models are increasingly applied to forecast the ecological impacts of invasions and future climate change scenarios (Thomas et al. 2004; Schwartz et al. 2006). There are hence strong theoretical and practical reasons to foster the development of mechanistic models of the niche that can be used to forecast future patterns of abundance and distribution (Helmuth 2009).
(b). Mechanistic niche models
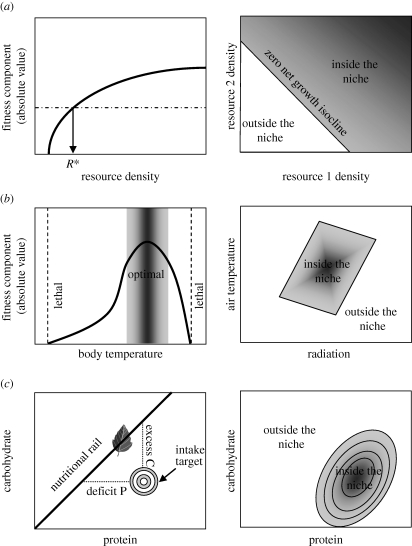
What is the current state of development of mechanistic niche models? In the 1970s and 1980s, there was an intense focus on niches with respect to competition for resources and the extent that species' niches overlapped. The aim was to develop a theory of community ecology that could explain patterns of coexistence and competitive exclusion. Dissatisfied with the descriptive, phenomenological nature of the Lotka–Volterra approach to inferring niche overlap (Macarthur & Levins 1967), a number of ecologists developed more mechanistic frameworks (MacArthur 1972; Maguire 1973; Tilman 1982; Schoener 1986). Schoener (1986) emphasized how the ‘megaparameters’ of population dynamics models could be decomposed into behavioural, physiological and morphological trait parameters of individuals and their environmental interactions; i.e. to ‘functional traits’ for which there is a defined link between the value of that trait and performance/fitness. Most influential of these kinds of mechanistic models was Tilman's resource-dependent isocline approach for depicting competition between species (Tilman 1982), inspired by MacArthur's (1972) consumer-resource models. It provided a way to depict resource-dependent growth rates against mortality rates to infer ‘zero net growth isoclines’ (ZNGIs) for multiple limiting resources (figure 1a). These isoclines could then be represented in multivariate resource space to define niches in a Hutchinsonian manner with respect to requirements and impacts (Leibold 1995; Chase & Leibold 2003). Chase & Leibold (2003) generalized these resource consumption models to other factors such as predation and abiotic stresses. They thus provided a general mechanistic depiction of the niche that included both requirements for resources and impacts on the availability of those resources for other individuals of the same or of different species.
Figure 1.
Mechanistic niche concepts. (a) The consumer-resource model (Leibold 1995). In this model, resource-dependent fitness components affecting population growth (solid line) and loss responses (dashed-dotted line) are plotted in relation to individual resources to determine the point of zero net growth R*, and then the intersections of zero net growth isoclines (ZNGIs) are plotted for different resources relative to each other to define regions inside and outside the fundamental niche. (b) The climatic niche. This is defined by a thermal performance curve in relation to body temperature, and then plotting fitness (or fitness components) as a function of body temperature in relation to environmental space. (c) The nutritional niche. The target nutritional state is plotted with respect to nutrient components, together with the nutritional ‘rails’ represented by available foods. Fitness landscapes can be superimposed on this space to represent the consequences of nutrient deficits and excesses. See text for more details.
Such analyses are mechanistic in the sense that they capture population processes of growth rate and mortality explicitly as functional responses to resources and other environmental factors. In these diagrammatic representations, however, the functional traits driving the population responses at the individual level, such as feeding behaviour, digestive systems and thermal tolerances, are included only implicitly. One must study individual responses to determine the ZNGIs (Chase & Leibold 2003). To the extent that these underling traits and their functional linkages with environments can be theoretically formalized, one would have a powerful basis for understanding ecological and evolutionary patterns at different levels of biological organization (Nisbet et al. 2000; Brown et al. 2004).
While some progress is being made (e.g. Rossberg et al. 2009), Schoener's (1986) ‘mechanistic ecologist's utopia’, where a mechanistic programme is realized in its ‘wildest aspirations’, is yet to be attained. The past two decades have instead seen the niche concept applied to the task of associative modelling, riding the wave of research initiated by GIS-based models of species-occurrence data. In partial response, McGill et al. (2006) argued that community ecology needs to harness these rich spatial datasets but use them mechanistically in the context of the functional traits of individuals (see also Violle & Jiang 2009). They thus echoed Schoener's call 20 years later, and since Schoener's paper, a number of important new theories and tools have arisen to aid the task. In particular, integration of the theoretical frameworks of BE, the GF and DEB models may be ideally suited to developing functional trait-based models of ecological niches (Kearney & Porter 2006). In the remainder of this article, we explore in more detail how this could be done, first outlining the different approaches and then discussing how they can be integrated. We illustrate the potential for model integration using two separate case studies. We then discuss how this functional trait-based approach can be included in a general research programme based on the niche concept.
3. Biophysical Ecology and the climatic niche
A mechanistic niche model must account for the ways that aspects of the physical environment interact with the functional traits of the organism to affect fitness. A key pathway for such an interaction is via influences on heat, mass and momentum transfer. BE has long served as a highly effective means of quantifying body temperature and water balance through the application of detailed heat and mass (water) budget equations (Porter & Gates 1969; Gates 1980; Campbell & Norman 1998). In brief, using basic physics, these methods keep track of an organism's heat (or water) inputs, outputs and stores by quantifying patterns such as conduction, convection and radiation (for heat) and evaporation (for both heat and water). In a similar vein, biomechanics approaches keep track of momentum transfer between organisms and their surroundings as a means of estimating probabilities of breakage, dislodgment or impediments to motion (Denny 1988). While here we focus on heat and mass (specifically water) transfer, the potential for biomechanics approaches to contribute to ecological theory holds comparable promise (e.g. Denny et al. 2009).
Such approaches are often computationally and experimentally difficult, as they require extensive information both on environmental parameters and the characteristics of the organism in question. Nevertheless, BE methods have been used to quantify the interactions of organisms with their environment with high fidelity (e.g. Porter et al. 1973, 1994; Spotila et al. 1973; Tracy 1976; Kingsolver 1979; Stevenson 1985; Campbell & Norman 1998; Helmuth 1998; Seebacher et al. 1999; Pincebourde & Casas 2006). Recent integrations of BE methods with spatial environmental data provide a means to infer past, current and future species distribution limits as constrained by heat and water balances (Gilman et al. 2006; Jones et al. 2009; Kearney & Porter 2009).
Importantly, these methods measure and model not the ‘environment’ per se, but rather the state (body temperature, water balance) of the organism. This is a key distinction because of the highly nonlinear ways in which the physical environment interacts with organisms to drive thermal and hydric exchange. Organismal body temperature is frequently not the same as standard environmental measurements like air and water temperature, particularly in terrestrial environments and for organisms with strong behavioural and physiological regulatory responses. Nevertheless, it is the body temperature that drives an organism's physiological state, and it is therefore crucial that we develop methods for quantifying patterns of body temperature if we are to link studies conducted under controlled laboratory conditions to those in the field.
The principles of BE provide a robust approach to mechanistically determining what we can call ‘climatic’ niches of organisms. A useful concept in this respect is ‘climate space’ (Porter & Gates 1969; figure 1b), a graphical depiction of the combinations of environmental variables that produce body temperatures suitable for survival and reproduction. Climate space has obvious connections to the Hutchinsonian niche concept. Rather than being a descriptive concept, as in associative habitat-modelling studies, it is instead a reflection of the interaction between functional traits and environment to influence a fitness component. Environmental axes of microclimatic niche space, such as wind speed, are of course not consumable, but they are distributed across space that be consumed, and can be significantly altered by the presence of other organisms; e.g. sessile suspension-feeding organisms often ‘compete for flow’ (Kim & Lasker 1997). Thus, the transposition of climate space onto physical space (i.e. habitats) allows inference of not only the climatic suitability of the site for a focal species (its fundamental niche), but also the potential for interactions between species (realized niche; Porter et al. 1973; Roughgarden et al. 1981; Tracy & Christian 1986).
4. The geometric framework and the nutritional niche
Nutrition is a primary driver of ecological interactions among organisms, and must therefore be captured in a mechanistic niche model. Recent developments in nutritional ecology provide a means for doing so (Raubenheimer et al. 2009; Simpson et al. 2010). The GF is an approach based on state-space geometry for modelling the nutritional interactions between organisms and their environments, which shares much in common with Hutchinson's niche concept. In both approaches, the organism is viewed as inhabiting a multi-dimensional hyper-volume, but in GF, the hyper-volume (referred to as a ‘nutritional space’) is defined specifically in terms of food chemistry (figure 1c). Each axis represents a food component that is functionally relevant to the animal, whether this relevance be for its nutritional, toxic or medicinal properties (Raubenheimer & Simpson 2009).
Under the GF framework, foods—principally other organisms and their derivatives—are represented as open-ended trajectories termed ‘nutritional rails’, which radiate from the origin through the hyper-volume at angles defined by the balance they contain of the defining components. As the animal eats, it ingests the food components in the same balance as they exist in the food, and can thus be modelled as ‘moving’ along the nutritional rail at a rate determined by the rate of ingestion and density of nutrients in the food. By selecting different foods and regulating the rate at which each is eaten, animals can thus navigate through nutritional space, inhabiting those areas that confer fitness advantage and avoiding others. The area of maximal advantage is termed the ‘intake target’. This is not a static area, but moves and changes shape as the animal encounters differential demands for nutrients (e.g. with activity levels, environmental temperature, health, reproductive status etc.). The foraging challenge for the animal is thus to track this moving target, and to the extent that it is constrained by ecological or other factors, realized fitness benefits are inversely proportional to the distance it achieves from the target.
In this model, the nutritional niche can be defined as that region of nutrient space delimiting where the life cycle of the organism can be sustained. Transposing this niche space onto real environments requires information on both the availability of the food in a given habitat and the regulatory decisions of the organism. An important biological attribute that is highlighted by this approach is the mathematical function relating aspects of performance (ultimately evolutionary fitness, but also components thereof) to geometric distance from the intake target (Simpson et al. 2004; Cheng et al. 2008). This function includes the independent and interactive costs of excesses and deficits of nutrients and other dietary components relative to the intake target. The function defines the geometric shape and breadth of the niche (e.g. Warbrick-Smith et al. 2009; figure 1c), and constitutes important information for explaining and predicting the homeostatic, ecological and evolutionary responses of animals under different dietary regimes. For example, in recent studies on insects, the shape of underlying performance surfaces has been mapped in detail for various different life-history traits (e.g. growth rate, body composition, immunity, lifespan, reproductive effort), and related to the homeostatic responses of the insects (Lee et al. 2008; Maklakov et al. 2008).
One example of GF applied in the context of niche theory considered how seven species of generalist grasshoppers coexist despite the fact that they eat a broadly overlapping spectrum of plants (Behmer & Joern 2008). These authors reasoned that the extensive dietary overlap among these species is at odds with the standard resource partitioning framework, and argued that an explanation for their coexistence might be found by characterizing the niche in terms of macronutrients (e.g. protein, carbohydrate, lipid) rather than foods. They demonstrated experimentally that the macronutrient requirements differed among the grasshopper species, providing the possibility for the seven species to coexist in the same habitat within separate nutritional niches.
5. Deb theory and the modelling of energy and mass budgets
An individual-level mechanistic niche model must be founded on a budget of energy and matter as these currencies flow through the organism and are allocated to development, growth, maintenance and reproduction. Traditionally, approaches to modelling climatic and nutritional niches have incorporated ‘static’ energy and mass budgets. Such budgets consider a series of steady-state snapshots of income (assimilation) versus expenditure (maintenance), the difference being the ‘scope for growth’ or ‘discretionary’ energy and mass (Widdows & Johnson 1988). These balances are tallied through time to estimate the potential for growth or reproduction. A cornerstone of such analyses is the use of an allometric equation relating body mass and temperature to maintenance energy costs. The use of such statistical descriptions is of course not ideal when one is attempting to develop a maximally mechanistic niche model. Moreover, static budgets do not quantify overhead costs associated with growth and reproduction, thus potentially misinterpreting these production overheads as losses.
The DEB theory of Kooijman (Sousa et al. 2008; Kooijman 2010) is a mechanistic model for how organisms take up and use energy and matter through their life cycle. It uses surface area and volume relationships to keep track of two (indirectly measurable) state variables, the reserve density and the structural volume. Energy and matter are assimilated proportional to the surface area, and directed first to the reserve pool of the organism. As with the GF, DEB models can deal with variable stoichiometry because reserves and structure can have different chemical compositions. The reserves, which may consist of, for example, fat, carbohydrate and amino acids scattered across the body, are used and replenished, hence do not require storage maintenance. The structure is the ‘permanent’ biomass such as proteins and membranes, and requires energy for its maintenance (protein turnover and the maintenance of concentration gradients and ionic potentials) in direct proportion to structural volume. Development, growth and reproduction are predicted dynamically according to the κ-rule whereby a fixed fraction κ of the energy/matter in the reserves flows to growth and somatic maintenance, the rest to increasing and maintaining the level of maturity and to reproduction. Allometrically observed scaling of ‘metabolic rate’ with body mass, as inferred via indirect calorimetry such as oxygen consumption rate, then follows naturally from the relative amounts of reserve and structure, and from other costs such as growth overheads and endothermic heating (Kooijman 2010). A fundamental construct within the DEB theory is the ‘synthesizing unit’ (SU), which is a generalization of the classical enzyme concept to complex reactions involving more than one potentially limiting substrate (Kooijman 2010; Poggiale et al. 2010). SU kinetics is used in DEB theory to model the process whereby ingested substrates are transformed into ‘reserves’ (i.e. ‘assimilation’) that are in turn transformed for growth and metabolic functions.
The κ-rule dynamic energy budget (κ-DEB) model is an attractive platform for a functional trait-based niche model because of its capacity to be used in a ‘supply-side’ context. While alternative approaches to mechanistic energy and mass budgets exist (Brown et al. 2004; van der Meer 2006), the κ-rule DEB model provides the most direct link among food density, food quality and feeding behaviour, as we discuss further below. This provides a strong basis for linking individuals and their functional traits to population (Klanjscek et al. 2006) and higher level processes (Nisbet et al. 2000).
6. Connecting the three modelling approaches
The three approaches just described are similar in many respects as they are all fundamentally based on the first law of thermodynamics, i.e. the conservation of energy and mass, and the principle of homeostasis. BE and GF models have to date been implemented in their static form, integrated over a period during the life of the organism. Dynamic formulations would allow the modelling of physiological rates, including growth and reproduction, across the life cycle under fluctuating food densities. DEB and GF models have been linked to abiotic environmental gradients only in a simplified manner, restricting their coupling with spatial environmental datasets (but see Thomas et al. 2006). DEB and BE models have included only simple foraging behaviours and have largely ignored the fitness consequences of nutritional status, including nutrient excesses (but see Kuijper et al. 2004a,b). Given these similarities and complementarities, we now explore the extent to which the three approaches could be integrated to produce a general mechanistic model of the niche. The linkages between the models are represented schematically in figure 2.
Figure 2.
A functional trait-based model of the niche derived by integrating a biophysical model and a nutritional state-space model (the geometric framework) with a DEB model. The biophysical model is represented as a climate space diagram, while the geometric framework is represented as a macronutrient space diagram. In the case illustrated, there are three dimensions for each: radiation, air temperature and wind speed for the climate space and protein, carbohydrate and water for the nutrient space. However, any number of dimensions can be accommodated. Organisms can survive within a subset of this space and behaviourally regulate around a target state within the survivable space. The efficiency with which the organism regulates around the target state depends on both the availability of the environments in the habitat (e.g. shade availability or prey types) and the costs and benefits of the regulatory behaviour. The outcome of the regulatory behaviours interacting with the habitat-specific availability of the climatic/food environments is a temporal sequence of body temperature (or heating/cooling costs for endotherms), water balance and food consumed. These act as driving variables for the DEB model that dictates the rates of growth, development, reproduction and ageing. Feedbacks exist between all three model components. For example, the target states in climatic and nutritional space depend on size, shape, stage and composition of the organism, as dictated by the DEB model, while feeding rates and water balance affect food consumption. See text for further details.
The BE and GF frameworks are both structured to link the environment (modelled as axes) to fitness (modelled as targets) via functional responses (behaviour and physiology). There are thus strong parallels in how the BE and GF models operate. Both depict in multi-dimensional space (climatic and nutritional, respectively) the ways that organisms respond to the environment, the changes that result in the animal's state as a consequence, the extent to which the organism achieves a target state (body temperature, nutritional status) and the consequences of failing to do so. The BE model links to environmental gradients through the spatio-temporal changes in weather, terrain, vegetation, soil etc., while GF does so through spatio-temporal changes in the availability of different food types (and water; Raubenheimer & Gäde 1994).
The DEB model, by contrast, provides a dynamic budgetary approach for modelling the physiological and developmental events that link nutritional and thermal status to organismal fitness. Given the food type chosen and the microclimate selected (and thus body temperature and water loss rate), the DEB model can be used to determine the consequences for growth, reproduction, development and storage, with appropriate feedbacks. Thus, the GF and BE models provide the overarching framework for tracking the individual's interactions with the environment and how these impact upon the animal's state (in terms of temperature, nutrient and water balance), while DEB models the way that nutritional and thermal status translates to growth, development and reproduction. We now explore the linkages between the models in more detail.
(a). Linkages between biophysical ecology and the geometric framework
Feeding interacts extensively with water balance and heat exchange in the behavioural and physiological ecology of animals. Examples at the ecological level include the influence that foraging has on where the animal places itself in its habitat, and also the influence that availability of water has on the foraging ranges of many animals. At the behavioural level, water status can be a fundamental constraint on feeding, and feeding in turn influences water status (Raubenheimer & Gäde 1994). Both nutritional and water status can influence patterns of activity (Raubenheimer & Gäde 1996), which in turn influence the location of the animal in the environment and also directly influence temperature and the requirements for water (Nicholson 2009). Activity levels also influence the amounts and balance of nutrients needed (Raubenheimer & Simpson 1997), as does temperature. In endotherms, this is principally due to thermoregulation (e.g. Simpson & Raubenheimer 1997), while in ectotherms, it is due to the influence of temperature on growth, life history and nutritional physiology (Clements et al. 2009). Physiologically, feeding influences thermal and water status through its impact on heat and water exchange, and also through the production of metabolic water (Bozinovic & Gallardo 2006). In some animals, there exists a trade-off between storage capacity for water and energy (fat) (e.g. Mira & Raubenheimer 2002). Temperature also affects the interplay between nutrient intake, growth rate and efficiency of post-ingestive utilization (Miller et al. 2010). For sessile animals unable to move between microhabitats, behaviour can still play a role in driving trade-offs between temperature, aerobic respiration and water conservation via processes such as shell gaping in bivalves or alterations in posture in animals such as gastropods and anemones (Bayne et al. 1976).
Thermoregulation and nutrition are strongly mediated by behaviour, whereby regulation occurs with respect to an internal target state subject to the costs and benefits of the regulatory behaviour. In the case of thermoregulation, organisms have a particular target body temperature (or range of body temperatures) that optimizes performance: mobile organisms defend this target in the face of environmental variation by choosing different combinations of air temperature, humidity, wind speed and radiation, which constrain where and when the animal can be active. Activity is also potentially constrained by water loss rates via the hydration state. Similarly, feeding behaviour in the GF is driven by the organism striving to defend a target nutritional state against a nutritionally heterogeneous environment, through the quantities and combinations of food types consumed.
(b). Linkages between dynamic energy budget theory and biophysical ecology
The principles of BE provide a way to predict how different physical habitats, under different weather conditions, constrain thermal homeostasis. BE thus can be used as a behavioural ‘front-end’ to drive the body temperatures and hence rates in the DEB model as constrained by (and linked to) environmental gradients and habitat configurations. This integration of principles of BE with DEB theory is theoretically straightforward (Kooijman 2010), but is yet to be done in practice. All published applications linking DEB theory to environmental gradients to date have been in aquatic environments where the body temperature of focal species (ectotherms) could be assumed identical to water temperature. Applying DEB theory in the more thermally complex terrestrial or intertidal environments necessitates a detailed biophysical approach to accurately predict body temperature and water loss.
Key traits linking DEB theory and BE are size, shape and mass. Heat and mass exchange are strongly tied to these morphological characteristics, especially with regard to radiative, convective and evaporative heat transport. Biophysical models take such factors into account, and are thus able to predict the body temperatures of organisms in field conditions often with high fidelity. Importantly, however, these models generally do not permit the organism to grow or change its physiological responses to temperature over time. Instead, static thermal ‘snapshots’ are taken and compared against comparably static models of scope for growth. Conversely, DEB methods have very seldom used inputs from biophysical models as drivers of factors such as body temperature.
A truly mechanistic approach—one that involves geographical predictions of performance (including species interactions; Pincebourde & Casas 2006; Petes et al. 2008; Pincebourde et al. 2008) and survival using detailed physiological responses of organisms—is crucial if we are going to predict the effects of climate change (e.g. Chown & Gaston 2008; Helmuth 2009). Importantly, both BE and DEB approaches are capable of producing dynamic outputs needed for such an endeavour. However, both must be based on mechanism and not proxies for factors such as size. For example, the relationship between body size (length) and surface area subject to heat exchange may be very different from that for food uptake. An effective approach will account for body size for both thermodynamics and metabolic processes, and more importantly, will allow exploration of the linkages and feedbacks between these processes through the effect of size on factors such as thermal inertia, food intake requirements and behaviour. For example, in general, larger animals (with a smaller surface area to volume ratio) have a larger thermal inertia and thus heat more slowly than smaller animals. Larger organisms also live higher in the boundary layer (i.e. velocity gradient above the substrate), which exposes them to stronger convective regimes as well as greater forces from wind and waves.
We have illustrated these principles using mussels as an example, tying a biophysical model of heat exchange in the intertidal environment together with a DEB model parametrized for Mytilus spp., one of the most common genera of mussels worldwide (see electronic supplementary material for detailed methods and parameters). Using the biophysical model (described in the electronic supplementary material), we constructed a two-dimensional representation of the climatic niche of Mytilus with regard to air temperature and solar radiation, using assimilation rate (a process that occurs whether or not the mussels are submerged) as a performance measure (figure 3). As illustrated in the figure, the climatic niche as defined by our biophysical model also depends on wind speed and body size, with increases in both of these variables reducing the dependence of mussel temperature on solar radiation. When compared with combinations of air temperature and radiation that occur in a natural habitat of Mytilus (grey background points in figure 3), it can be seen that increases in body size and wind speed produce body temperatures closer to the physiologically optimal values during aerial exposure at low tide. As a corollary, vulnerability to high body temperature can be seen to depend on the size of the mussel at the time of the heat stress event. Depending on the date of settlement and the growth trajectory, mussels may be below the size threshold that would prevent heat stress occurring (electronic supplementary material, figure S1). Ecological forecasting of the potential for heat stress in organisms must therefore include the biophysics of heat transfer (Gilman et al. 2006) as well as the dynamics of growth (Hilbish & Koehn 1985).
Figure 3.
Mussel climatic niche space measured as surface area-specific food assimilation rate (J h−1 V2/3) for Mytilus edulis physiological parameters (electronic supplementary material, table S1). The assimilation rate is plotted in relation to air temperature and solar radiation for three different wind speeds and two different body sizes. A biophysical model (see electronic supplementary material) was used to calculate body temperature to infer assimilation rate. The grey cloud of points in the background represents hourly observed combinations of air temperature and solar radiation at the study site, Bodega CA, USA. (a,b) wind speed 0.1 m s−1; (c,d) wind speed 1.0 m s−1; (e,f) wind speed 9.0 m s−1.
The merger of BE methods with DEB approaches is a potentially powerful way to incorporate mechanism across a range of organizational scales. To date, however, despite the mechanistic nature of BE approaches, such ‘macroecological’ methods (e.g. Kerr et al. 2007) have tended to rely on simple correlates of fitness or measurements of physiological indicators of stress. For example, Wethey & Woodin (2008) used hindcasts of water temperature and historical records of species distribution patterns to explore the drivers of geographical ranges in intertidal barnacles and polychaetes. For barnacles, shifts (at a rate of 15–55 km per decade) were well correlated with winter water temperature maxima, a factor known to affect reproduction. For polychaetes, results were more ambiguous, and suggested that either cold winters or cool summers could explain the patterns observed. Nevertheless, evidence suggests that species range boundaries and population dynamics can often be set by far more subtle effects on physiological rate processes (Sanford 2002; Beukema et al. 2009). A true predictive framework thus mandates an equally mechanistic exploration of energy budgets in organisms and the subsequent effects on individual fitness and species interactions.
(c). Linkages between dynamic energy budget theory and the geometric framework
The GF is a tool for interpreting the observed relationships between food consumption, nutrient allocation and fitness components in multivariate nutritional space, and has been used to predict and understand feeding behaviour and post-ingestive allocation not only in individuals but also in groups (Simpson et al. 2006) and societies (Dussutour & Simpson 2009). The potential exists to map performance landscapes from individuals to population growth rates (Simpson et al. 2004), and this is where the DEB theory can provide a powerful modelling tool (e.g. Klanjscek et al. 2006). DEB models could be used as a computational engine for GF theory, e.g. to implement dynamic fitness-component landscapes in multivariate nutrient space with respect to growth rate, reproductive output and longevity, together with ancillary information about consequences of other organisms in the environment, such as toxic build-up of excreted waste. In relation to the latter point about inter-individual interactions, an important caveat to the translation from individual to population growth rate is that the transition need not be smooth or linear, owing to local interactions that yield sudden transitions in response (Simpson et al. 2010). Thus, the nutritional responses of a single forager ant cannot predict the nutritional regulation and allocation decisions made at the colony level (Dussutour & Simpson 2009), nor would the behaviour of a single protein-deprived Mormon cricket or locust in the absence of inter-individual interactions predict mass migration driven by cannibalism (Simpson et al. 2006).
The real power of the GF is in the interpretation of scenarios where foods are nutritionally unbalanced relative to the organism's needs, and vary in nutritional quality through space and time. The standard one-reserve DEB model can handle this scenario only very simplistically, whereby nutritionally imbalanced foods are reflected in different assimilation efficiencies of ingested food (Kooijman 2010, p. 107). This nutritionally implicit approach cannot tackle questions about the effects of different food components on fitness in different ecological contexts, nor the effect of those components on behavioural and physiological homeostatic responses (Raubenheimer et al. 2009). For example, herbivores and omnivores have been shown to have separate regulatory systems controlling the intake of protein and non-protein energy, but when the environment constrains animals to an imbalanced diet, protein intake dominates and leads to substantial changes in total energy content with consequent impact on levels of fat stored (Simpson & Raubenheimer 2005; Sørensen et al. 2008; Felton et al. 2009). This protein leverage effect has been proposed to explain development of obesity on a modern Western diet (Simpson & Raubenheimer 2005). Under a univariate DEB model, this ‘damming up’ of excess nutrient cannot be modelled without violating the strong homeostasis assumption of DEB theory.
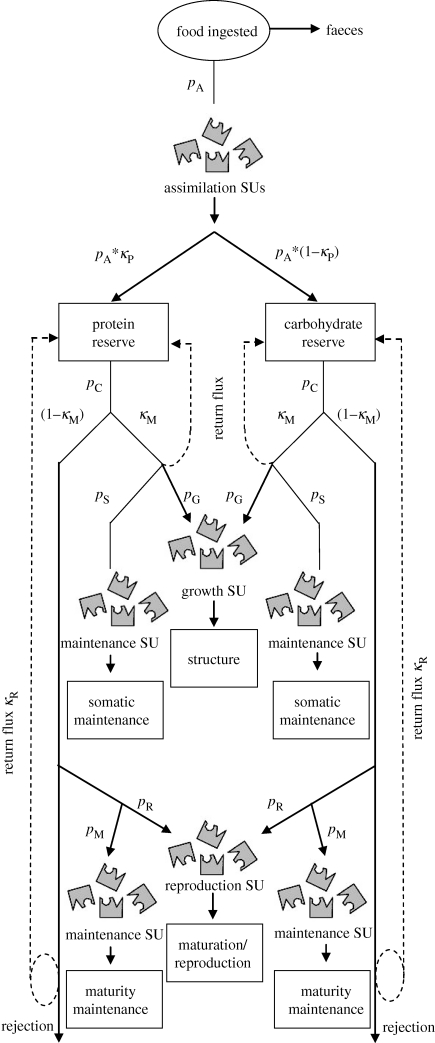
In standard DEB multi-reserve models, uptake of each nutrient is independent. To integrate DEB theory with GF in the context of multivariate nutritional space, a special kind of multivariate DEB model is required with one reserve for each nutritional component. In other words, a nutritionally explicit DEB model is required (sensu Raubenheimer et al. 2009; Simpson et al. 2010). This is achieved in general by specifying rules for SUs that transform food into separate nutrient reserve pools, and then regulate the assignment of mobilized reserves from each pool into maintenance, structure, maturity maintenance and reproductive output (figure 4). These rules can include a parameter dictating the return flux to the reserve pools of materials rejected by the SUs, controlling the extent to which nutrients ingested in excess are stored. Such a DEB model was first developed by Kuijper et al. (2004a) in the context of protein and carbohydrate (or non-protein) consumption. Kuijper et al.'s model was for adults with determinate growth, and hence did not include a growth SU. Including growth is more complex because the κ reserve mobilized from each reserve pool is not equally divided between maintenance and growth, and the growth rate is only defined implicitly as it both determines and depends on reserve mobilization rate (B. Kooijman 2009, personal communication). For example, in the context of a protein and carbohydrate reserve pool, carbohydrates and protein reserves are substitutable for maintenance with a strong preference for carbohydrate. For growth, overhead costs can be paid by carbohydrate or protein, but building blocks can only be made from protein reserves. Thus, protein and carbohydrate reserves are partly complementary and partly substitutable (see also Raubenheimer & Simpson (1994) and Simpson et al. (2004) for a discussion of this matter in relation to GF). The dynamics of the SU for growth dictate that growth rate is fast for the right mix of protein and carbohydrate, slow if one of them dominates and zero if protein is absent.
Figure 4.
A multiple-reserve DEB model including maintenance, growth, maturation, maturity maintenance and reproduction. Synthesizing units (SUs) control assimilation and allocation to growth, maintenance and reproduction. pA, lumped assimilated material; κP, protein fraction of assimilate; pC, mobilization rate; κM, fraction to growth/maintenance; pS, somatic maintenance; pG, growth allocation; pM, maturity maintenance; pR, reproduction allocation; κR, fraction returned to reserves.
We have applied the simpler scenario used by Kuijper et al. (2004a) to illustrate how DEB and GF can be integrated to model nutritional targets (see electronic supplementary material for detailed methods and parameters). We applied the DEB model to calculate egg production in a copepod as a function of ingested carbohydrate and protein, but extend Kuijper's approach by using the GF to integrate cost functions relating to longevity and stored nutrient excesses.
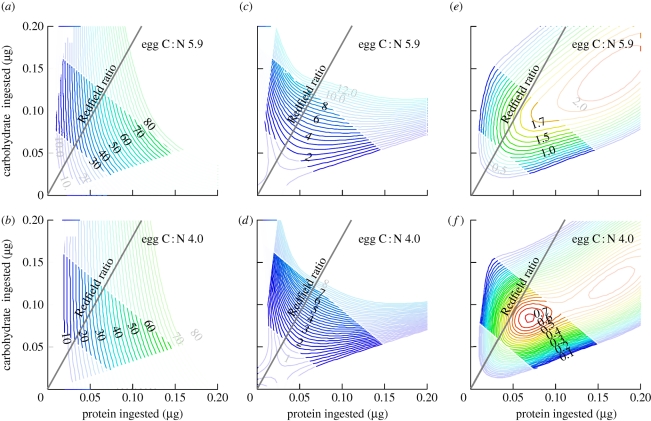
The results of our simulations are presented in figure 5. Tilman classified resource-dependent growth isoclines into eight categories (Tilman 1982, fig. 2), and we discuss our results in this context. From figure 5a, it can be seen that egg production rate increases with protein and carbohydrate consumed in the manner expected for Tilman's ‘hemi-essential’ case. Specifically, reproduction can occur in the absence of carbohydrate resource but not in the absence of protein resource. The reproduction isoclines bow towards the origin, indicating that less of each resource is required when both are consumed together. This interactive effect is diminished when the nitrogen content of eggs is altered from an observed C : N ratio of 5.9 to a value of 4.0 (figure 5b).
Figure 5.
Nutritional niche space calculated for the copepod Acartia tonsa with a DEB model incorporating separate reserves for protein and non-protein assimilates and represented using the geometric framework. (a,b) Egg production, (c,d) lifetime egg production (egg production rate × longevity) without and with (e,f) costs imposed for storage of excess nutrients are presented for eggs with two different C : N ratios. The Redfield ratio is represented as a nutritional rail, with interpolated regions of the state space emphasized relative to the extrapolated regions. See text for further details.
When longevity is linked to diet in the manner observed for many taxa (Lee et al. 2008; Maklakov et al. 2008), the resource-dependent growth isoclines predicted lifetime reproductive output to shift to the ‘interactive-essential’ category in the Tilman classification (figure 5c,d). Protein can no longer substitute for carbohydrate because excessive protein consumption very strongly shortens the lifespan. Altering the elemental composition of the reserves/eggs had minimal impact on the resource isoclines in this context (figure 5d).
In the scenarios considered so far, ‘more is better’ with respect to both nitrogenous and non-nitrogenous resources. There is no target per se, but rather the organism would be expected to strive for as much as possible of each resource. This is frequently not the case; as already discussed, when an organism consumes a diet unbalanced with respect to its requirements, the non-limiting components of the diet can ‘dam up’ within the organism unless they are excreted to the environment. In our analysis, the imposition of a cost to storing nutrient excesses resulted in resource isoclines that form a target intake, as frequently observed in diet-selection studies (Simpson et al. 2004). This pattern falls under Tilman's ‘inhibition’ category, whereby excessive consumption ultimately results in reduced fitness, a scenario he regarded as rare (Tilman 1982).
Our brief example illustrates the potential for linking DEB theory to the concepts of the GF in a manner that enriches both approaches with respect to modelling niches. Parameterizing a multi-reserve DEB model for an organism provides a means to make a priori predictions of the dietary intake target purely from the perspective of the organism's demands for maintenance and building blocks. This provides an important null basis from which to interpret empirically observed intake targets. We would usually expect observed dietary targets to deviate from this null expectation, in part because of internally imposed costs such as lifespan or detrimental physiological or ecological impacts of nutrient acquisition and excessive reserve storage discussed above. The incorporation of physiological impacts would result in a model of what could be called the ‘fundamental nutritional niche’ of an organism. Ancillary DEB theory constructs may potentially accommodate such important additions. Additionally, GF-derived behavioural modules may be applied to DEB models to add behavioural realism to the feeding response. Observed dietary targets and rules of compromise also reflect the imprint of past and present ecology. For example, the spatial and temporal distribution of resources in relation to each other and in relation to temperature and humidity gradients may impose constraints on consumption, whereas nutritionally optimal food sources may be associated with higher predation risk or competitive interference (Simpson et al. 2010). The approach we have described for mechanistically modelling nutritional niches provides a means to quantify the relative merits of different feeding strategies in response to biotic and abiotic contingencies.
7. Developing a functional trait-based research agenda around the niche concept
Chase & Leibold (2003) have provided a vision for an ecological research agenda centred on the niche concept. Their approach generalizes the consumer-resource models of Tilman (figure 1a) to include other niche dimensions such as additional abiotic stresses and predators. However, it has been criticized for omitting a spatial environmental context and for not centralizing evolutionary processes (Hubbell 2004; McGill et al. 2006). These criticisms arise in part from the absence of a clearly elaborated link between the population-level responses depicted in the fitness-resource relationships and ZNGI plots (figure 1a), and the underling functional traits together with their relationship to the environment.
The approach we have described provides a more direct linkage with environmental gradients, and therefore greater potential to explain patterns such as body size clines and species distribution limits. With the revolution of GIS and remote-sensing technologies, we can depict such gradients with a greater accuracy and realism than ever before. These gradients may include standard climatic variables such as rainfall, temperature and soil type, as well as more subtle variables such as plant chemistry. The BE and GF approaches provide the mechanistic link between these environmental gradients, individual traits and performance currencies, allowing landscape-level questions such as species distribution limits and aggregation and migratory behaviour to be tackled on the basis of functional traits (Kearney & Porter 2009; Simpson et al. 2010). Moreover, mechanistic models naturally identify which traits and environmental gradients to measure. This reduces flexibility in the choice of environmental variables in comparison to correlative species distribution models. The benefit is greater explanatory and predictive power. Such an approach provides the capacity to ask questions such as ‘how would the direct effects of climate be expected to influence body size clines in endotherms?’ (Porter & Kearney 2009), ‘how do present or future environmental gradients alter the thermoregulatory priorities of ectotherms?’ (Kearney & Porter 2009) and ‘how does risk of heat stress under climate change vary with latitude?’ (Pörtner 2002; Gilman et al. 2006; Deutsch et al. 2008).
From an evolutionary perspective, functional trait-based models of the niche are much more amenable than are correlative models for the simple fact that traits and the fitness consequences of changing them are considered explicitly. Allowing model parameters to become ‘mutable’ in simulations, subject to heritabilities and selection strengths, permits inference on likely evolutionary trajectories (Simpson et al. 2010). For instance, the effect of evolutionary change on the potential geographical distribution of a mosquito was simulated under climate change by linking a quantitative genetics model to environmentally imposed selection on egg desiccation resistance (Kearney et al. 2009).
There are likely to be many obstacles in the path from traits to fundamental niches and ultimately to realized niches. The fundamental niche is a population-level phenomenon, but the approach we describe relates to the measurements of the traits of individuals. In some contexts, such as the modelling of range constraints, the capacity to infer a region as outside the fundamental niche based on individual traits can be highly informative (Kearney et al. 2008). However, to mechanistically underpin ZNGI diagrams with functional traits in the manner we have described, we must incorporate population dynamics models that include behavioural interactions between individuals. While such linkages are now being explored (Klanjscek et al. 2006; Buckley 2008), there is still a long way to go. The approach to modelling niches that we advocate here at the very least provides stronger scaffolding around the bridge between traits, environment and performance. This may well permit a more environmentally and evolutionarily explicit means to apply Chase and Leibold's research agenda. We are excited that functional trait-based approaches to understanding species' niches are becoming a key research agenda in ecology (Nisbet et al. 2000; Brown et al. 2004); perhaps Schoener's ‘mechanistic utopia’ is in sight?
Acknowledgements
We thank Bas Kooijman, Tânia Sousa, Jean-Christophe Poggiale, Tiago Domingos and two anonymous reviewers for discussion and comments on the manuscript. B.H. was supported by NASA grant NNG04GE43G and NOAA grant NA04NOS4780264 and S.J.S. was supported by an Australian Research Council Federation and Laureate Fellowships.
Footnotes
One contribution of 14 to a Theme Issue ‘Developments in dynamic energy budget theory and its applications’.
References
- Bayne B. L., Bayne C. J., Carefoot T. C., Thompson R. J.1976The physiological ecology of Mytilus californianus conrad. Oecologia 22, 211–228 (doi:10.1007/BF00344793) [DOI] [PubMed] [Google Scholar]
- Behmer S. T., Joern A.2008Coexisting generalist herbivores occupy unique nutritional feeding niches. Proc. Natl Acad. Sci. USA 105, 1977–1982 (doi:10.1073/pnas.0711870105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukema J. J., Dekker R., Jansen J. M.2009Some like it cold: populations of the tellinid bivalve Macoma balthica (L.) suffer in various ways from a warming climate. Mar. Ecol. Prog. Ser. 384, 135–145 (doi:10.3354/meps07952) [Google Scholar]
- Bozinovic F., Gallardo P.2006The water economy of South American desert rodents: from integrative to molecular physiological ecology. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 142, 163–172 (doi:10.1016/j.cbpc.2005.08.004) [DOI] [PubMed] [Google Scholar]
- Brown J. H. Macroecology. Chicago, IL:: The University of Chicago Press; 1995. [Google Scholar]
- Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B.2004Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- Buckley L.2008Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am. Nat. 171, E1–E19 (doi:10.1086/523949) [DOI] [PubMed] [Google Scholar]
- Campbell G. S., Norman J. M.1998Environmental biophysics. New York, NY: Springer [Google Scholar]
- Chase J. M., Leibold M. A. Ecological niches: interspecific interactions. Chicago, IL:: The University of Chicago Press; 2003. [Google Scholar]
- Cheng K., Simpson S. J., Raubenheimer D.2008A geometry of regulatory scaling. Am. Nat. 172, 681–693 (doi:10.1086/591686) [DOI] [PubMed] [Google Scholar]
- Chown S. L., Gaston K. J.2008Macrophysiology for a changing world. Proc. R. Soc. B 275, 1469–1478 (doi:10.1098/rspb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements K. D., Raubenheimer D., Choat J. H.2009Nutritional ecology of marine herbivorous fishes: ten years on. Funct. Ecol. 23, 79–92 (doi:10.1111/j.1365-2435.2008.01524.x) [Google Scholar]
- Couzin I. D., Krause J.2003Self-organization and the collective behavior of vertebrates. Adv. Study Behav. 32, 1–75 (doi:10.1016/S0065-3454(03)01001-5) [Google Scholar]
- Davis A. J., Jenkinson L. S., Lawton J. H., Shorrocks B., Wood S.1998Making mistakes when predicting shifts in species range in response to global warming. Nature 391, 783–786 (doi:10.1038/35842) [DOI] [PubMed] [Google Scholar]
- de Laguna G. A.1962The role of teleonomy in evolution. Phil. Sci. 29, 117–131 (doi:10.1086/287855) [Google Scholar]
- Denny M. W.1988Biology and the mechanics of the wave-swept environment. Princeton, NJ: Princeton University Press [Google Scholar]
- Denny M. W., Hunt L. J. H., Harley C. D. G.2009On the prediction of extreme ecological events. Ecol. Monogr. 79, 397–421 (doi:10.1890/08-0579.1) [Google Scholar]
- Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., Martin P. R.2008Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussutour A., Simpson S. J.2009Communal nutrition in ants. Curr. Biol. 19, 740–744 (doi:10.1016/j.cub.2009.03.015) [DOI] [PubMed] [Google Scholar]
- Elith J., Leathwick J. R.2009Species distribution models: ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. System. 40, 677–697 (doi:10.1146/annurev.ecolsys.110308.120159) [Google Scholar]
- Elton C.1927Animal ecology. London, UK: Sidgwick & Jackson [Google Scholar]
- Felton A. M., Felton A., Raubenheimer D., Simpson S. J., Foley W. J., Wood J. T., Wallis I. R., Lindenmayar D. B.2009Protein content of diet dictates the daily energy intake of a free-ranging primate. Behav. Ecol. 20, 685–690 (doi:10.1093/beheco/arp021) [Google Scholar]
- Gates D. M.1980Biophysical ecology. New York, NY: Springer [Google Scholar]
- Gilman S. E., Wethey D. S., Helmuth B.2006Variation in the sensitivity of organismal body temperature to climate change over local and geographic scales. Proc. Natl Acad. Sci. USA 103, 9560–9565 (doi:10.1073/pnas.0510992103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. H.1971A multivariate statistical approach to the Hutchinsonian niche: bivalve molluscs of central Canada. Ecology 52, 543–556 (doi:10.2307/1934142) [DOI] [PubMed] [Google Scholar]
- Grinnell J.1917The niche-relationships of the California Thrasher. Auk 34, 427–433 [Google Scholar]
- Helmuth B.1998Intertidal mussel microclimates: predicting the body temperature of a sessile invertebrate. Ecol. Monogr. 68, 51–74 (doi:10.1890/0012-9615(1998)068[0051:IMMPTB]2.0.CO;2) [Google Scholar]
- Helmuth B.2009From cells to coastlines: how can we use physiology to forecast the impacts of climate change? J. Exp. Biol. 212, 753–760 (doi:10.1242/jeb.023861) [DOI] [PubMed] [Google Scholar]
- Hilbish T. J., Koehn R. K.1985The physiological basis of natural selection at the Lap locus. Evolution 39, 1302–1317 (doi:10.2307/2408787) [DOI] [PubMed] [Google Scholar]
- Holt R. D.2009Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl Acad. Sci. USA 106, 19 659–19 665 (doi:10.1073/pnas.0905137106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell S.2004Ecological niches: linking classical and contemporary approaches. Interspecific interactions. By Jonathan M. Chase and Mathew A. Leibold. Q. Rev. Biol. 79, 96–97 (doi:10.1086/421654) [Google Scholar]
- Hutchinson G. E.1957Concluding remarks. Cold Spring Harbour Symp. Quant. Biol. 22, 415–427 (doi:10.1101/SQB.1957.022.01.039) [Google Scholar]
- Jones S. J., Mieszkowska N., Wethey D. S.2009Linking thermal tolerances and biogeography: Mytilus edulis (L.) at its southern limit on the East Coast of the United States. Biol. Bull. 217, 73–85 [DOI] [PubMed] [Google Scholar]
- Kearney M.2006Habitat, environment and niche: what are we modelling? Oikos 115, 186–191 (doi:10.1111/j.2006.0030-1299.14908.x) [Google Scholar]
- Kearney M., Porter W. P.2006Ecologists have already started rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 481–482 (doi:10.1016/j.tree.2006.06.019) [DOI] [PubMed] [Google Scholar]
- Kearney M., Porter W. P.2009Mechanistic niche modelling: combining physiological and spatial data to predict species' ranges. Ecol. Lett. 12, 334–350 (doi:10.1111/j.1461-0248.2008.01277.x) [DOI] [PubMed] [Google Scholar]
- Kearney M., Phillips B. L., Tracy C. R., Christian K. A., Betts G., Porter W. P.2008Modelling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423–434 (doi:10.1111/j.0906-7590.2008.05457.x) [Google Scholar]
- Kearney M., Porter W. P., Williams C. K., Ritchie S., Hoffmann A. A.2009Integrating biophysical models and evolutionary theory to predict climatic impacts on species' ranges: the dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 23, 528–538 (doi:10.1111/j.1365-2435.2008.01538.x) [Google Scholar]
- Kerr J. T., Kharouba H. M., Currie D. J.2007The macroecological contribution to global change solutions. Science 316, 1581–1584 (doi:10.1126/science.1133267) [DOI] [PubMed] [Google Scholar]
- Kim K., Lasker H. R.1997Flow-mediated resource competition in the suspension feeding gorgonian Plexaura homomalla (Esper). Exp. Mar. Biol. Ecol. 215, 49–64 (doi:10.1016/S0022-0981(97)00015-4) [Google Scholar]
- Kingsolver J. G.1979Thermal and hydric aspects of environmental heterogeneity in the pitcher plant mosquito. Ecol. Monogr. 49, 357–376 (doi:10.2307/1942468) [Google Scholar]
- Klanjscek T., Caswell H., Neubert M. G., Nisbet R. M.2006Integrating dynamic energy budgets into matrix population models. Ecol. Model. 196, 407–420 (doi:10.1016/j.ecolmodel.2006.02.023) [Google Scholar]
- Kooijman S. A. L. M.2010Dynamic energy budget theory for metabolic organization. Cambridge, UK: Cambridge University Press [Google Scholar]
- Kuijper L. D. J., Anderson T. R., Kooijman S. A. L. M.2004aC and N gross growth efficiencies of copepod egg production studied using a dynamic energy budget model. J. Plankton Res. 26, 213–226 (doi:10.1093/plankt/fbh020) [Google Scholar]
- Kuijper L. D. J., Kooi B. W., Anderson T. R., Kooijman S. A. L. M.2004bStoichiometry and food-chain dynamics. Theor. Popul. Biol. 66, 323–339 (doi:10.1016/j.tpb.2004.06.011) [DOI] [PubMed] [Google Scholar]
- Lee K. P., Simpson S. J., Clissold F. J., Brooks R., Ballard J. W. O., Taylor P. W., Soran N., Raubenheimer D. J.2008Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA 105, 2498–2503 (doi:10.1073/pnas.0710787105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold M. A.1995The niche concept revisited: mechanistic models and community context. Ecology 76, 1371–1382 (doi:10.2307/1938141) [Google Scholar]
- MacArthur R. H.1972Geographical ecology: patterns in the distribution of species. New York, NY: Harper & Row [Google Scholar]
- Macarthur R. H., Levins R.1967The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385 (doi:10.1086/282505) [Google Scholar]
- Maguire B., Jr1973Niche response structure and the analytical potentials of its relationship to the habitat. Am. Nat. 107, 213–246 [Google Scholar]
- Maklakov A. A., Simpson S. J., Zajitschek F., Hall M. D., Dessmann J., Clissold F., Raubenheimer D. J., Bonduriansky R., Brooks R. C.2008Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1066 (doi:10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- McGill B. J., Enquist B. J., Weiher E., Westoby M.2006Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 (doi:10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- McIntosh R. P.1998The myth of the community as an organism. Perspect. Biol. Med. 41, 426–438 [Google Scholar]
- Miller G. A., Clissold F. J., Mayntz D., Simpson S. J.2010Speed over efficiency: locusts select body temperatures that favour growth rate over efficient nutrient utilization. Proc. R. Soc. B 276, 3581–3589 (doi:10.1098/rspb.2009.1030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira A., Raubenheimer D.2002Divergent nutrition-related adaptations in two cockroach populations inhabiting different environments. Physiol. Entomol. 27, 330–339 (doi:10.1046/j.1365-3032.2002.00306.x) [Google Scholar]
- Nicholson S. W.2009Water homeostasis in bees, with the emphasis on sociality. J. Exp. Biol. 212, 429–434 (doi:10.1242/jeb.022343) [DOI] [PubMed] [Google Scholar]
- Nisbet R. M., Muller E. B., Lika K., Kooijman S. A. L. M.2000From molecules to ecosystems through dynamic energy budget models. J. Anim. Ecol. 69, 913–926 (doi:10.1046/j.1365-2656.2000.00448.x) [Google Scholar]
- O'Connor R. J.2000Why ecology lags behind biology. Scientist 14, 35 [Google Scholar]
- Pearman P. B., Guisan A., Broennimann O., Randin C. F.2008Niche dynamics in space and time. Trends Ecol. Evol. 23, 149–158 (doi:10.1016/j.tree.2007.11.005) [DOI] [PubMed] [Google Scholar]
- Petes L. E., Mouchka M. E., Milston-Clements R. H., Momoda T. S., Menge B. A.2008Effects of environmental stress on intertidal mussels and their sea star predators. Oecologia 156, 671–680 (doi:10.1007/s00442-008-1018-x) [DOI] [PubMed] [Google Scholar]
- Pincebourde S., Casas J.2006Multitrophic biophysical budgets: thermal ecology of an intimate herbivore insect–plant interaction. Ecol. Monogr. 76, 175–194 (doi:10.1890/0012-9615(2006)076[0175:MBBTEO]2.0.CO;2) [Google Scholar]
- Pincebourde S., Sanford E., Helmuth B.2008Body temperature during low tide alters the feeding performance of a top intertidal predator. Limnol. Oceanogr. 53, 1562–1573 [Google Scholar]
- Poggiale J.-C., Baklouti M., Queguiner B., Kooijman S. A. L. M.2010How far details are important in ecosystem modelling: the case of multi-limiting nutrients in phytoplankton–zooplankton interactions. Phil. Trans. R. Soc. B 365, 3495–3507 (doi:10.1098/rstb.2010.0165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W. P., Gates D. M.1969Thermodynamic equilibria of animals with environment. Ecol. Monogr. 39, 227–244 (doi:10.2307/1948545) [Google Scholar]
- Porter W. P., Kearney M.2009Size, shape, and the thermal niche of endotherms. Proc. Natl Acad. Sci. USA 106, 19 666–19 672 (doi:10.1073/pnas.0907321106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W. P., Mitchell J. W., Beckman W. A., DeWitt C. B.1973Behavioral implications of mechanistic ecology—thermal and behavioral modeling of desert ectotherms and their microenvironment. Oecologia 13, 1–54 (doi:10.1007/BF00379617) [DOI] [PubMed] [Google Scholar]
- Porter W. P., Munger J. C., Stewart W. E., Budaraju S., Jaeger J.1994Endotherm energetics: from a scalable individual-based model to ecological applications. Austr. J. Zool. 42, 125–162 (doi:10.1071/ZO9940125) [Google Scholar]
- Pörtner H. O.2002Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. Part A: Physiol. 132, 739–761 (doi:10.1016/S1095-6433(02)00045-4) [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Gäde G.1994Hunger–thirst interactions in the locust, Locusta migratoria. J. Insect Physiol. 40, 631–639 (doi:10.1016/0022-1910(94)90151-1) [Google Scholar]
- Raubenheimer D., Gäde G.1996Separating food and water deprivation in locusts: effects on the patterns of feeding, locomotion and growth. Physiol. Entomol. 21, 76–84 (doi:10.1111/j.1365-3032.1996.tb00838.x) [Google Scholar]
- Raubenheimer D., Simpson S. J.1994The analysis of nutrient budgets. Funct. Ecol. 8, 783–791 (doi:10.2307/2390238) [Google Scholar]
- Raubenheimer D., Simpson S. J.1997Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 10, 151–179 (doi:10.1079/NRR19970009) [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Simpson S. J.2009Nutritional PharmEcology: doses, nutrients, toxins and medicines. Integr. Comp. Biol. 49, 329–337 (doi:10.1093/icb/icp050) [DOI] [PubMed] [Google Scholar]
- Raubenheimer D., Simpson S. J., Mayntz D.2009Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 23, 4–16 (doi:10.1111/j.1365-2435.2009.01522.x) [Google Scholar]
- Roff D. A.2002Life history evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- Rossberg A. G., Brännström Å., Dieckmann U.2009How trophic interaction strength depends on traits. Theor. Ecol. 3, 13–24 (doi:10.1007/s12080-009-0049-1) [Google Scholar]
- Roughgarden J., Porter W., Heckel D.1981Resource partitioning of space and its relationship to body temperature in Anolis lizard populations. Oecologia (Berl) 50, 256–264 (doi:10.1007/BF00348048) [DOI] [PubMed] [Google Scholar]
- Sanford E.2002Water temperature, predation, and the neglected role of physiological rate effects in rocky intertidal communities. Integr. Comp. Biol. 42, 881–891 (doi:10.1093/icb/42.4.881) [DOI] [PubMed] [Google Scholar]
- Schoener T. W.1986Mechanistic approaches to community ecology: a new reductionism? Am. Zool. 26, 81–106 (doi:10.1093/icb/26.1.81) [Google Scholar]
- Schoener T. W.1989The ecological niche. In Ecological concepts: the contribution of ecology to an understanding of the natural world (ed. Cherrett J. M.), pp. 79–114 Oxford, UK: Blackwell Scientific [Google Scholar]
- Schoener T. W.2009Ecological niche. In The princeton guide to ecology (eds Levin S. A., Carpenter S. R., Godfray H. C., Kinzig A. P., Loreau M., Losos J. B., Walker B., Wilcove D. S.), pp. 3–13 Princeton, NJ: Princeton University Press [Google Scholar]
- Schwartz M. W., Iverson L. R., Prasad A. M., Matthews S. N., O'Connor R. J.2006Predicting extinctions as a result of climate change. Ecology 87, 1611–1615 (doi:10.1890/0012-9658(2006)87[1611:PEAARO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Seebacher F., Grigg G. C., Beard L. A.1999Crocodiles as dinosaurs: behavioural thermoregulation in very large ectotherms leads to high and stable body temperatures. J. Exp. Biol. 202, 77–86 [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Raubenheimer D.1997The geometric analysis of feeding and nutrition in the rat. Appetite 28, 201–213 (doi:10.1006/appe.1996.0077) [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Raubenheimer D.2005Obesity: the protein leverage hypothesis. Obes. Rev. 6, 133–142 (doi:10.1111/j.1467-789X.2005.00178.x) [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Sibly R. M., Pum Lee K., Behmer S. T., Raubenheimer D.2004Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 68, 1299–1311 (doi:10.1016/j.anbehav.2004.03.003) [Google Scholar]
- Simpson S. J., Sword G. A., Lorch P. D., Couzin I. D.2006Cannibal crickets on a forced march for protein and salt. Proc. Natl Acad. Sci. USA 103, 4152–4156 (doi:10.1073/pnas.0508915103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S. J., Raubenheimer D., Charleston M. A., Clissold F. J. & the ARC-NZ vegetation function network herbivory working group 2010Modelling nutritional interactions: from individuals to communities. Trends Ecol. Evol. 25, 53–60 (doi:10.1016/j.tree.2009.06.012) [DOI] [PubMed] [Google Scholar]
- Soberón J.2007Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 (doi:10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- Sørensen A., Mayntz D., Raubenheimer D., Simpson S. J.2008Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity 16, 566–571 (doi:10.1038/oby.2007.58) [DOI] [PubMed] [Google Scholar]
- Sousa T., Domingos T., Kooijman S. A. L. M.2008From empirical patterns to theory: a formal metabolic theory of life. Phil. Trans. R. Soc. B 363, 2453–2464 (doi:10.1098/rstb.2007.2230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotila J. R., Lommen P. W., Bakken G. S., Gates D. M.1973A mathematical model for body temperatures of large reptiles: implications for dinosaur ecology. Am. Nat. 107, 391–404 (doi:10.1086/282842) [Google Scholar]
- Stevenson R. D.1985Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am. Nat. 125, 102–177 (doi:10.1086/284330) [Google Scholar]
- Thomas C. D., et al. 2004Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- Thomas Y., Maurizé J., Pouvreau S., Bacher C., Gohin F., Struski C., Le Mao P.2006Modelling the growth of Mytilus edulis (L.) by coupling a dynamic energy budget model with satellite-derived environmental data. In Rapport d'avancement de contrat N° 05/2 210 106/F, France: See http://www.fao.org/fishery/gisfish/servlet/CDSServlet?status=ND0xMTM0LjQzNzMmNj1lbiYzMz1hcXVhX2RvYyYzNz1pbmZv [Google Scholar]
- Thompson N. S.1987The misappropriation of teleonomy. In Perspectives in ethology (eds Bateson P. P. G., Klopfer P. H.), pp. 259–274 New York, NY: Plenum [Google Scholar]
- Tilman D.1982Resource competition and community structure. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- Tracy C. R.1976A model of the dynamic exchanges of water and energy between a terrestrial amphibian and its environment. Ecol. Monogr. 46, 293–326 (doi:10.2307/1942256) [Google Scholar]
- Tracy C. R., Christian K. A.1986Ecological relations among space, time, and thermal niche axes. Ecology 67, 609–615 (doi:10.2307/1937684) [Google Scholar]
- van der Meer J.2006Metabolic theories in ecology. Trends Ecol. Evol. 21, 136–140 (doi:10.1016/j.tree.2005.11.004) [DOI] [PubMed] [Google Scholar]
- Violle C., Jiang L.2009Towards a trait-based quantification of species niche. J. Plant Ecol. 2, 87–93 (doi:10.1093/jpe/rtp007) [Google Scholar]
- Warbrick-Smith J., Raubenheimer D., Simpson S. J., Behmer S. T.2009Three hundred and fifty generations of extreme food specialisation: testing predictions of nutritional ecology. Entomologia Experimentalis et Applicata 132, 65–75 (doi:10.1111/j.1570-7458.2009.00870.x) [Google Scholar]
- Wethey D. S., Woodin S. A.2008Ecological hindcasting of biogeographic responses to climate change in the European intertidal zone. Hydrobiologia 606, 139–151 (doi:10.1007/s10750-008-9338-8) [Google Scholar]
- Widdows J., Johnson D.1988Physiological energetics of Mytilus edulis: scope for growth. Mar. Ecol. Progr. Ser. 46, 113–121 (doi:10.3354/meps046113) [Google Scholar]
- Wiens J. J., Graham C. H.2005Niche conservatism: integrating evolution, ecology and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539 (doi:10.1146/annurev.ecolsys.36.102803.095431) [Google Scholar]