Abstract
The seasonal reproductive cycle of photoperiodic rodents is conceptualized as a series of discrete melatonin-dependent neuroendocrine transitions. Least understood is the springtime restoration of responsiveness to winter-like melatonin signals (breaking of refractoriness) that enables animals to once again respond appropriately to winter photoperiods the following year. This has been posited to require many weeks of long days based on studies employing static photoperiods instead of the annual pattern of continually changing photoperiods under which these mechanisms evolved. Maintaining Siberian hamsters under simulated natural photoperiods, we demonstrate that winter refractoriness is broken within six weeks after the spring equinox. We then test whether a history of natural photoperiod exposure can eliminate the requirement for long-day melatonin signalling. Hamsters pinealectomized at the spring equinox and challenged 10 weeks later with winter melatonin infusions exhibited gonadal regression, indicating that refractoriness was broken. A photostimulatory effect on body weight is first observed in the last four weeks of winter. Thus, the seasonal transition to the summer photosensitive phenotype is triggered prior to the equinox without exposure to long days and is thereafter melatonin-independent. Distinctions between photoperiodic and circannual seasonal organization erode with the incorporation in the laboratory of ecologically relevant day length conditions.
Keywords: season, simulated natural photoperiod, melatonin
1. Introduction
Day length proximately controls seasonal rhythms of physiology and behaviour in animal taxa. In mammals, day length information is transduced into a neuroendocrine signal by the pineal gland; the critical information is coded by the duration of nocturnal melatonin secretion, which is proportional to the length of the night (Goldman 2001) and decoded by the melatonin-binding sites in the central nervous system (Badura & Goldman 1992; Freeman & Zucker 2001). In photoperiodic species, melatonin drives seasonal rhythms (Prendergast et al. 2009): seasonality is abrogated in both pinealectomized animals and pineal-intact individuals maintained in unchanging photoperiods. In circannual mammals by contrast, patterns of melatonin secretion entrain endogenous seasonal rhythms that persist in a range of static photoperiods in the absence of melatonin (Zucker 2001; Lincoln 2006). The physiological mechanisms that distinguish circannual from photoperiodic species are unspecified.
The study of mammalian photoperiodism has proceeded with a focus on long and short day lengths (hereafter denoted LD or SD, respectively) that drive key seasonal transitions, particularly in reproduction (Elliott 1976). Day lengths are respectively defined as long or short relative to a species-specific critical day length (13 h in Siberian hamsters; Hoffmann 1982). In hamsters, SDs inhibit gonadotrophin secretion and thereby prevent gonadal growth, or induce gonadal regression (Yellon & Goldman 1984). After several months of SD, however, the gonads undergo recrudescence (enlarge) despite continued SD (Hoffmann 1973). This loss of responsiveness to SD (i.e. refractoriness) occurs universally and thus is a defining feature of mammalian photoperiodism (Gorman et al. 2001). Responsiveness to SD lengths must be restored (i.e. refractoriness must be broken in the terminology of photoperiodism research) before animals can again respond to SDs in the next year. This requires several months of exposure to photoperiods longer than the critical day length during the ensuing spring and summer (Stetson et al. 1977). Until recently, it was believed that 12–15 weeks of LDs and their associated short duration melatonin signals were necessary to accomplish this restoration of reproductive responsiveness to day length (Reiter 1969; Stetson et al. 1977). Kauffman et al. (2003), however, demonstrated that continuous presence of melatonin is not required to break refractoriness: photorefractory Siberian hamsters pinealectomized after just six weeks of LD exposure underwent gonadal regression normally nine weeks later when challenged with melatonin infusions, indicating that photorefractoriness was broken. Notably, the nine weeks without melatonin are necessary for the process that breaks refractoriness to run to completion; hamsters pinealectomized and infused immediately after the six weeks of LD treatment remain refractory.
In photoperiodic hamsters, therefore, a full seasonal cycle requires two exogenously driven events—gonadal regression and breaking refractoriness—that bookend an endogenously controlled process, the development of refractoriness. Although rarely tested, this stage-wise analysis of seasonality is assumed to account for seasonal rhythms under natural conditions. This model is based on studies of rodents housed in static SD or LD and transferred abruptly between photoperiods in a single day, which does not simulate the incremental small daily changes in day length that occur in nature. The few studies that have examined seasonality in simulated natural photoperiods (SNP) reveal that change in day length may be a more salient indicator of time of year than absolute day length (Gorman & Zucker 1995, 1998; Butler et al. 2007a,b; Butler & Zucker 2009).
When animals become refractory is under the control of an interval timer whose duration is sensitive to incremental changes in day length. This plastic interval timer ensures that the onset of reproductive competence in spring is synchronized among hamsters that differ in age, pubertal status and the timing of autumn gonadal regression (Gorman 2001; Park et al. 2006; Butler et al. 2007a). Underlying plastic interval timing mechanisms have also been proposed to explain circannual rhythms (Goldman et al. 2004; Paul et al. 2008). If similar mechanisms underlie both photoperiodic and circannual rhythms, then in photoperiodic species, natural photoperiods may play a role in entraining endogenous clock-like mechanisms that are normally masked in artificial laboratory conditions. If so, then a progression of incremental changes in day length may be able to drive seasonal transitions without exposure to putative LD or SD.
In this study, we test the idea that a history of exposure to naturally progressing day lengths changes the photic cues required to break refractoriness. We first chart the natural history of the breaking of refractoriness under SNPs and then test the hypothesis that a natural photoperiodic history eliminates the requirement for melatonin signals after the spring equinox to break refractoriness. These experiments shed light on the salient cues that drive the breaking of refractoriness in natural populations.
2. Material and methods
(a). Terminology
Photoperiodism studies employ technical language that differs with respect to the model species under study. Terms used in the present account are defined as follows. Refractoriness refers to the neuroendocrine state in which SD or long melatonin signals no longer induce or maintain the winter phenotype. Long and short photoperiods (i.e. day lengths) are defined operationally by their stimulatory or inhibitory effects on seasonal traits, respectively. ‘SDs’ are also used specifically to refer to the winter solstice photoperiod in the methods and results sections. Regression and recrudescence refer, respectively, to the involution of the testes initiated by SDs or long melatonin signals, and the growth of the testes in spring due to the development of photorefractoriness.
(b). General
Nine cohorts of male Siberian hamsters (Phodopus sungorus) from a separate experiment were used for the present study (Butler et al. 2007a). Litters were born every two weeks over a 16 week interval beginning four weeks before the summer solstice (21 June) in a SNP corresponding to 53° N (annual range of 7.6–16.9 h of daylight, photophase midpoint 1100 h PST, 200–400 lux at cage level). The lighting schedule was generated and controlled by a latitudinal timer (EC71ST SunTracker, Paragon Electric Company, Two Rivers, WI). There was no illumination during the dark phase except as noted below in Experiment 2. Males were weaned at 23–24 days of age, and housed three per cage with both littermates and non-littermates of the same age in clear polypropylene cages (18 × 28 × 12 cm), furnished with Harlan Tek-Fresh bedding (Harlan Teklad, Madison, WI). Tap water and Lab Diet 5015 Mouse Diet (Brentwood, MO) were available ad libitum. All procedures were approved by the Animal Care and Use Committee of the University of California at Berkeley and complied with the NIH Guide for the Care and Use of Laboratory Animals.
Beginning on the day of weaning, and at weekly intervals thereafter, body mass and coat colour were assessed for all animals. Estimated testis size (ETV; width2 × length), a measure that correlates with testis mass (Butler et al. 2007a), was measured externally every two or three weeks in hamsters under light isoflurane anaesthesia. Regressed testes were defined by ETV less than 350.
(c). Primary responsiveness to SDs
Hamsters were categorized as photoresponsive or non-photoresponsive on the basis of their phenotype (gonad size, body mass, pelage colour and balano-preputial separation) during decreasing day lengths of late summer and autumn after their birth (Butler et al. 2007a). Only photoresponsive hamsters were retained for inclusion in the main body of the present study, although as described below, non-responsiveness was subsequently induced in some of these animals by long day lengths of the following spring.
(d). Secondary non-responsiveness induced by LDs
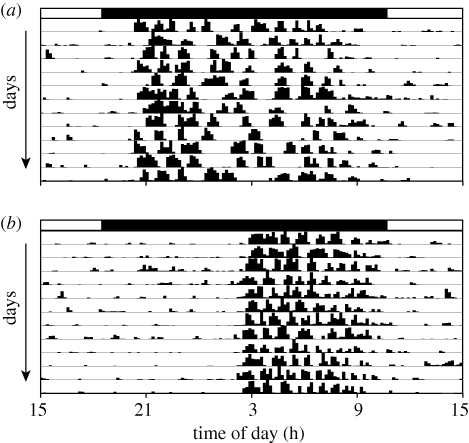
In Siberian hamsters, exposure to very long day lengths induces some individuals to maintain a summer-typical circadian entrainment pattern when transferred to SDs (figure 1; Gorman & Zucker 1997). Melatonin secretion durations remain compressed and consequently, the winter reproductive phenotype is not expressed. Environmentally induced non-responsiveness is also observed in SNP, albeit in fewer numbers than when hamsters are housed in static short photoperiods (Butler et al. 2007a). To identify circadian-based non-responsiveness, locomotor activity rhythms were recorded in SD. Circadian non-responders (cNR) were hamsters with activity onsets occurring later than 4 h after onset of darkness and with activity durations less than 10.5 h after stable entrainment to the photoperiod (Prendergast & Freeman 1999). Otherwise, they were considered circadian responders (cR). We adopted a longer activity duration threshold than used by Prendergast & Freeman (1999) because of the longer scotophase in the present experiment.
Figure 1.
Representative locomotor activity records for a circadian responder [(a) min. ETV = 97] and nonresponder [(b) min. ETV = 580] from Eq + 6. Hatch marks along each horizontal line represent the amount of activity; each horizontal line represents 24 h, and consecutive days are plotted from top to bottom. The dark phase (18.30 to 10.44 PST) is denoted by the black bar above each actogram.
(e). Activity measures
Locomotor activity of singly housed hamsters was monitored with passive infrared motion detectors (Quest PIR, Electronics Line USA, Boulder, CO) mounted on the cage lids (described in Butler et al. 2007a). Counts per 10 min bin were collected with Dataquest III (Mini-Mitter, Sun River, OR) and analysed with ClockLab (Actimetrics, Wilmette, IL). Activity onset and offset were calculated as described previously (Gorman 2001).
(f). Experiment 1: naturalistic termination of refractoriness by spring photoperiods
To identify when refractoriness is broken in the yearly cycle, hamsters born into SNP between 24 May and 2 August were studied the following spring. At five weeks after the winter solstice (SD-control, n = 21), and zero, three and six weeks after the spring equinox (Eq + 0, n = 29; Eq + 3, n = 36; Eq + 6, n = 33), hamsters were transferred to the winter solstice photoperiod (7 h 46 min light per day, SD). The simulated dates at transfer were 28 January, 22 March, 11 April and 2 May, and the day lengths at transfer were 9.01, 12.28, 13.49 and 15.12, respectively. The onset of the dark phase remained constant at all transfers. ETV was measured every three weeks for at least 15 weeks after transfer to the static SD; all hamsters had recrudesced testes with ETV > 500 at the spring equinox. To identify cR and cNR hamsters, locomotor activity was recorded for two weeks per animal over an eight week interval beginning at least 60 days after transfer to SD.
(g). Experiment 2: melatonin dependence of breaking of refractoriness
Hamsters born into SNP between 16 August and 13 September were studied the following spring to test whether endogenous melatonin signals after the spring equinox are necessary to break refractoriness. Two control groups were individually housed and challenged with SD either five weeks after the winter solstice (SD-control, n = 11) or at the spring equinox (Eq-Control; n = 10). A final group (n = 25) was pinealectomized on the spring equinox. Due to more rapid than expected increases in the timer-generated photoperiod by approximately 20 s d−1, the actual simulated day length at the equinox was 12 h 28 min, which qualifies as a SD (Hoffmann 1982; Duncan et al. 1985). In pinealectomized hamsters, refractoriness was assessed by administering melatonin infusions of a duration that simulates endogenous melatonin secretion in short winter day lengths (Kauffman et al. 2003). Ten weeks after pinealectomy, hamsters were fitted with subcutaneous polyethylene catheters while lightly anaesthetized with isoflurane vapours as previously described (Prendergast et al. 1996). Hamsters were infused with 100 ng melatonin (n = 15) or vehicle (n = 10) per night in a 10 h infusion beginning 1 h after lights-off using a variable flow rate pump (0.017 ml h−1, Razel Scientific, Stamford, CT; melatonin, M-5250, Sigma, St Louis, MO, in sterile 0.01% ethanolic saline). Pump syringes were filled with fresh melatonin solution twice weekly during the light phase so that any inadvertent melatonin delivered while filling the syringes would not affect the nocturnal infusion duration. During infusions, hamsters were maintained in SD, but with dim nocturnal illumination in the room (less than 0.01 lux at cage level, provided by two blue LEDs; Forever-Glo Nite Lite, American Tack and Hardware, Monsey, NY). ETV was measured at zero, six and nine weeks of the infusion period; all hamsters had recrudesced testes of ETV more than 500 at the equinox.
(h). Pinealectomy
Hamsters were anaesthetized with a ketamine–xylazine–acepromazine cocktail (21 mg ml−1, 2.4 mg ml−1, and 0.3 mg ml−1, respectively, injected i.p. at 0.34 ml per 100 g body weight) and pinealectomized as previously described (Kauffman et al. 2003). Buprenorphine was administered post-operatively (0.1ml of 0.015 mg ml−1 solution, injected s.c., Hospira, Lake Forest, IL). Pinealectomy was verified by radioimmunoassay of plasma melatonin according to the manufacturer's instructions (Buhlmann Melatonin Direct RIA, 01-RK-MDI, Alpco Diagnostics, Salem, NH). On the second night after the last infusion, blood was drawn from the retro-orbital sinus of isoflurane-anaesthetized intact and pinealectomized hamsters under dim red light (Bright Lab Jr. Safelight, Delta1/CPM Inc., Dallas, TX; wavelength limit of 610 nm) between 0345 h and 0435 h PST (lights off at the hours of 19.10). Blood was collected with heparinized Caraway tubes (#02–668–25, Fisher Scientific, Pittsburgh, PA) into 1.5 ml microfuge tubes containing 30 U heparin (1000 USP units per ml, Baxter, Deerfield, IL) on ice, spun at 5°C for 20 min at 1400 × g, and the plasma collected and stored at −80°C.
(i). Statistics
Differences in the pattern of testicular regression over time were assessed with repeated measures ANOVA. Cohort (birthday in spring/summer: Butler et al. 2007a) and group differences were analyzed with one-way ANOVA or t-test. Because cohort did not affect statistical measures, this variable was not considered further. Linear regressions tested for the relation between locomotor activity duration and minimum ETV, the former a surrogate for melatonin secretion duration (Elliott & Tamarkin 1994). Differences in the proportion of hamsters exhibiting gonadal regression were analysed for significance with the χ2-test for independence or post hoc Fisher's exact test (Statview 5.0, SAS Institute, Inc., Cary, NC). Means and standard errors are reported unless noted otherwise. The significance level was set at 0.05.
3. Results
(a). Experiment 1: termination of refractoriness in SNP
All SD-control hamsters had small testes upon transfer to SD (ETV = 203 ± 37). Their testes underwent recrudescence over the next several months, indicating neuroendocrine refractoriness to SDs. Testicular recrudescence was essentially complete by the spring equinox; all hamsters in Eq + 0, Eq + 3 and Eq + 6 groups had ETV > 500 at the time of transfer to SD. Increasing day lengths induced circadian non-responsiveness (cNR; figure 1), and the proportion of cNR hamsters differed by group (17%, 8% and 39% for Eq + 0, Eq + 3 and Eq + 6 groups, respectively, omnibus χ2: p < 0.01). In the Eq + 6 group, duration of locomotor activity and minimum ETV in SD were negatively correlated (R = −0.5, n = 33, p < 0.01). In contrast, activity duration and minimum ETV were not correlated in either the Eq + 0 or Eq + 3 groups (Eq + 0: R = 0.2, n = 29, p = 0.3; Eq + 3: R = −0.1, n = 36, p = 0.6). Considering all hamsters together without regard to their circadian entrainment status, the per cent displaying testicular regression in SD differed by group (0%, 17% and 39% for Eq + 0, Eq + 3 and Eq + 6 groups, respectively, omnibus χ2: p < 0.001), as did the temporal pattern of ETV (not shown). Given the cNR confound, however, the response to SD was separately considered among cR and cNR hamsters.
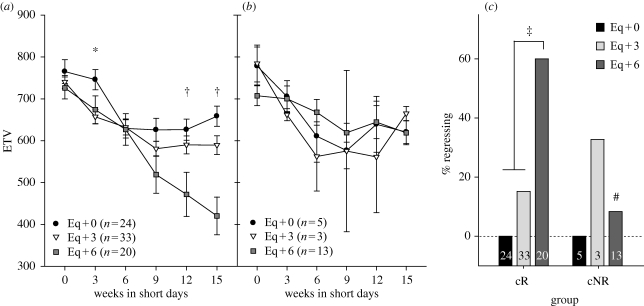
Among cR hamsters, refractoriness was broken in many more animals at six weeks after the equinox than prior to this time (figure 2). ETV decreased slightly over the first six to nine weeks of SD exposure in all groups, but only the Eq + 6 hamsters continued to undergo regression through week 15 (figure 2a). There were significant effects of the date of transfer, time in SDs, and transfer date × time on testis size (figure 2a; repeated measures ANOVA, p < 0.01). A greater proportion of cR hamsters in Eq + 6 underwent regression than in the Eq + 0 or Eq + 3 transfer groups (figure 2c; χ2, p < 0.001). Eq + 6 hamsters also had lower minimum ETVs compared with the other two groups (Tukey test, p < 0.05, not shown).
Figure 2.
(a) and (b) Time course of testicular regression in hamsters transferred at zero, three and six weeks after the spring equinox to short days (Eq + 0, Eq + 3 and Eq + 6, respectively). Data are divided between circadian responders (a), and nonresponders (b). One cNR hamster that died after week 9, with ETV > 750 is included in (c) as not showing gonadal regression. *p < 0.05, Tukey test between Eq + 0 and Eq + 3 groups; †p < 0.05, Tukey test between Eq + 6 and the other two groups. (c) Per cent of hamsters undergoing testicular regression among cR and cNR. Numbers within the bars denote n. ‡p < 0.01, pairwise χ2-test. #p < 0.01, χ2-test compared with cR Eq + 6.
Only two of 21 hamsters classified as cNR underwent gonadal regression. There were no significant effects of transfer date on the ETV pattern over time in SD (figure 2b), the proportion of hamsters undergoing regression (figure 2c), or minimum ETV (data not shown). There was a significant main effect of time in SD; as above, all three groups exhibited decreases in mean ETV from the point of transfer through six to nine weeks (p < 0.001). This decrease was owing to small ETV decreases in all hamsters and not owing to a small number of hamsters undergoing complete regression.
(b). Experiment 2: melatonin dependence of breaking of refractoriness
As in Experiment 1, all SD-controls had small testes that underwent recrudescence in SD. Eight of 10 Eq-Control hamsters transferred to SD at the spring equinox retained large testes; two exhibited testicular regression but no moult to the winter pelage. The incidence of gonadal regression did not differ significantly from that observed in Eq + 0 animals of Experiment 1 (p > 0.05, post hoc Fisher's Exact Test).
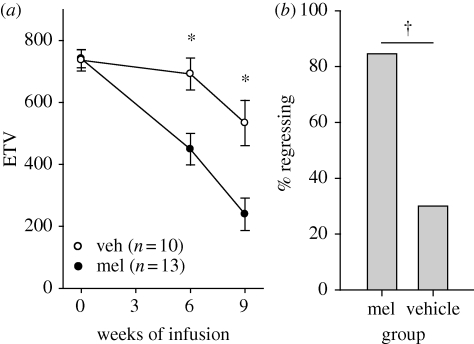
Pinealectomized hamsters responded to melatonin, but not vehicle infusions, with testicular regression (repeated measures ANOVA: infusion p < 0.01, time p < 0.001, infusion × time p < 0.01, figure 3a); i.e. day lengths shorter than 12.5 h broke refractoriness. ETV was significantly lower in melatonin-infused hamsters after six and nine weeks of treatment (t-test, p < 0.01; figure 3a). Eleven of 13 melatonin-infused, compared with 3/10 vehicle-infused hamsters, underwent gonadal regression (χ2: p < 0.01; figure 3b). The incidence of regression induced by melatonin-infusion was significantly greater than in Eq-Control hamsters (p < 0.001, post hoc Fisher's Exact Test). There were no differences between infusion groups in either body mass or pelage score (all tests: n.s.).
Figure 3.
(a) Testis size during nine weeks of infusion with either melatonin or vehicle. *p < 0.05 Tukey test. (b) Per cent of hamsters undergoing testicular regression during the infusions. †p < 0.05, χ2-test.
Pineal-intact hamsters all had elevated melatonin concentrations during the dark phase (mean ± s.d., 219 ± 134 pg ml−1, n = 13) that were much higher than those of pinealectomized hamsters (17.2 ± 15.0 pg ml−1, n = 18).
(c). Detecting photostimulatory conditions in SDs
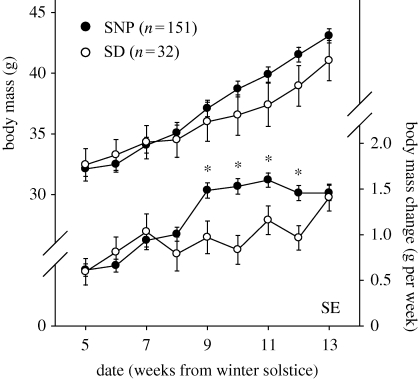
Because day lengths from the winter solstice to the spring equinox broke reproductive refractoriness, we performed a post hoc analysis of body mass in late winter day lengths to detect the onset of photostimulatory growth. Stimulatory effects of increasing day lengths on somatic and reproductive traits were observed in the month before the spring equinox. Compared with SD-Control hamsters in static photoperiods, increases in day length over the last eight weeks of simulated winter produced a divergence in body mass trajectory (figure 4: main effect of photoperiod, n.s., effects of time and time × photoperiod, p < 0.001, repeated measures ANOVA). The divergence reflects an increase in weight gain in SNP-housed hamsters, beyond that associated with refractoriness, beginning four weeks before the equinox, at a day length of 10 h 29 min that is increasing at 3.9 min day−1 (corresponding to 21 Feb) (effect of photoperiod, p < 0.05, effects of time and time × photoperiod, p < 0.001, repeated measures ANOVA). ETV data from Eq + 0, Eq + 3 and Eq + 6 also reveal photostimulation of the gonads beyond the size to which the testes of refractory hamsters recrudesce. Even when hamsters do not undergo regression (cR and cNR both), ETV decreases from approximately 750 to 650 after transfer from increasing day lengths to SDs, regardless of transfer date (figure 2).
Figure 4.
Body mass and weekly mass increases (mean ± s.e.) for hamsters in the increasing day lengths of the SNP or static winter solstice photoperiod (SD, 7 h 46 min). SE: spring equinox. *p < 0.05, Tukey test for body mass gain.
4. Discussion
Neuroendocrine responsiveness to SDs is restored in the majority of hamsters by the sixth week after the equinox. Pineal-intact hamsters challenged with SD three weeks earlier exhibited negligible testicular regression (Experiment 1). This corroborates an earlier study in a 40° N latitude SNP, in which incremental increases in day length through the seventh week after the equinox broke refractoriness (Gorman & Zucker 1995). The date at which refractoriness is broken in Experiment 1 points to the importance of the fourth to sixth weeks after the equinox. This interval's importance to breaking refractoriness may not be due to its increasing day lengths, however, but rather in allowing the process, triggered earlier, to complete. Experiment 2 shows that refractoriness is broken despite elimination of photoperiodic information conveyed by melatonin signals after the equinox. So although day lengths no longer than 15 h 12 min sufficed to break refractoriness (Experiment 1), refractoriness could also be broken after exposure to a maximum of 12 h 28 min of light per day (Experiment 2). The two experiments necessarily employed different methods to assess photoresponsiveness (SDs versus long-duration melatonin infusion), representing different assays of photoresponsiveness: saline controls for the infusion procedure in Experiment 2 indicate that the gonadal regression was melatonin-induced, reflecting bona fide restoration of neuroendocrine responsiveness. Together these experiments establish that refractoriness is not normally broken until approximately six weeks after the spring equinox, but that no post-equinox melatonin signals are required for this to transpire. How is this effected?
It is unlikely that the ultimate short duration melatonin signal (0 h) post-pinealectomy is interpreted as a LD and thereby contributes directly to breaking refractoriness. In several earlier studies, pinealectomy has never simulated LD treatment, or broken refractoriness, nor has LD equivalence been observed in other photoperiodic responses (Bittman & Zucker 1981; Carter & Goldman 1983; Kelly et al. 1994). By eliminating the neuroendocrine representation of day length, pinealectomy appears to prevent subsequent ambient day length from interfering with previously triggered but as yet uncompleted processes (Kauffman et al. 2003).
In the present context, gradual increases in day length from the winter solstice to the spring equinox initiate a transition to the photoresponsive state that continues in the absence of further increases in day length and is accomplished without exposure to LDs previously posited as essential (Stetson et al. 1977). With regard to the reproductive system, short but increasing day lengths are equivalent to LD lengths for restoration of photoresponsiveness.
A subset of hamsters, termed cNR, fails to produce long duration locomotor activity patterns and long-duration melatonin signals in SD (figure 1; Puchalski & Lynch 1986, 1991). These individuals do not undergo gonadal regression or other seasonal responses. Photoperiodic non-responsiveness due to loss of responsiveness to melatonin is common among seasonal mammals (Prendergast et al. 2001), but to date, non-responsiveness arising from atypical circadian entrainment appears to be unique to Siberian hamsters. The incidence of circadian non-responsiveness is influenced by photoperiodic history: day lengths longer than 15 h permanently induce summer-like circadian entrainment and reproductive phenotypes in a substantial fraction of hamsters subsequently housed in SD (Gorman & Zucker 1997; Goldman et al. 2000). Our results confirm that environmental induction of non-responsiveness is more prevalent with increasing day length (figure 2c).
(a). Delimiting the critical period for melatonin effects (what is ‘stimulatory’?)
The present studies suggest a re-evaluation of what constitutes a stimulatory day length under ecologically relevant conditions. Increases in day length per se appear to be a powerful stimulatory signal (Stetson et al. 1986; Gorman 1995; Gorman & Zucker 1995). Day lengths begin to increase after the winter solstice, but it is not known when day length and its rate of increase are first interpreted by hamsters as photostimulatory. Body mass provides a somatic measure of photostimulation: weight gain is elevated in SNP-housed hamsters compared with controls beginning four weeks before the spring equinox (21 Feb). This indicates that absolute day lengths as short as 10.5 h can be read as stimulatory when increasing daily by approximately 4 min d−1.
Photostimulation by otherwise typical short day lengths suggests that seasonal mechanisms that control body mass and reproduction may critically depend on how rate of change of photoperiod is coded. Different molecular signatures of LD and SD have been identified in several brain tissues associated with photoperiod processing (e.g. Lincoln et al. 2003; Hazlerigg et al. 2005; Dupré et al. 2008). Whether the molecular patterns manifest at markedly different day lengths also apply to the decoding of incrementally changing photoperiod and melatonin signals remains to be investigated. As different seasonally changing traits are mediated by distinct neural pathways, change in photoperiod may affect these traits differently (Maywood & Hastings 1995). Understanding where and how photoperiod history is encoded is an important step (Teubner & Freeman 2007; Teubner et al. 2008).
(b). Integrating photoperiodic and circannual timers
Our results point to similarities in circannual and photoperiodic seasonal mechanisms. In circannual mammals, photoperiod and associated melatonin signals, entrain the reproductive cycle to a period of 12 months; generation of the cycle is not, however, dependent on day length or melatonin (Lee & Zucker 1991; Barrell et al. 2000; Lincoln 2006; Hazlerigg & Loudon 2008). Light and melatonin may play a similar entraining role in Siberian hamsters, which is more apparent in naturally changing day lengths than in standard static photoperiods used in most laboratory studies. Our finding that the seasonal transition in photoresponsiveness occurs without exposure to melatonin signals after the spring equinox, and importantly, without any LD trigger, supports the idea that hamster seasonality involves an endogenous component that, as is the case for circannual rhythms, is entrained by the natural sequence of changing day lengths. Under static photoperiods, these endogenous processes are disrupted or masked.
This account is supported by reports of annual rhythms in body mass and reproduction in a small minority of Siberian hamsters held in an unvarying 9 h light: 15 h dark cycle for up to 2 years (Anchordoquy & Lynch 2000), and in two other photoperiodic hamster species, where melatonin-independent seasonal timing suggestive of a circannual process is unmasked by pinealectomy (Masson-Pévet et al. 1987; Butler et al. 2008). The interval timer in Siberian hamsters that determines the onset of refractoriness also exhibits properties of an endogenous annual rhythm. It is modulated by photoperiod such that reproductive effort is synchronized among hamsters with diverse developmental trajectories and photoperiodic histories (Gorman 2001, 2003; Park et al. 2006).
The evolutionary relation between photoperiodic and circannual timing mechanisms remains poorly understood (Lincoln et al. 2005; Hazlerigg & Loudon 2008). The present study raises the possibility that circannual mechanisms might underlie the classic model of mammalian photoperiodism. The converse also has been proposed: mechanisms of photoperiodism—interval timing and switching between sensitive and refractory states—may form a basis for circannual processes (Goldman et al. 2004; Paul et al. 2008). Establishing whether one mechanism has emerged from the other, or even whether they are truly distinct, will require a better understanding of each and how they map onto phylogeny. Mechanistically, the regulation of thyroid hormone T3 availability by differential expression of types 2 and 3 deiodinases may be a point of convergence of circannual and photoperiodic mechanisms. In both sheep and hamsters, photoperiod regulates deiodinase expression in the mediobasal hypothalamus (Watanabe et al. 2004; Hanon et al. 2008). Indeed, in both species, changes in thyroid hormone regulation in the brain are important for seasonal transitions: in sheep, thyroidectomy prevents ewes from returning to their summer anoestrus (Moenter et al. 1991), in hamsters, thyroidectomy advances the onset of refractoriness (Prendergast et al. 2002).
The categorical distinction between photoperiodic and circannual timing—made on the basis of experiments with static photoperiods that never prevail in nature—blurs with the incorporation of natural progressions in day lengths. The present data redefine the day lengths sufficient to break refractoriness; melatonin signals reflective of day lengths around the equinox, and far from the long day lengths previously thought necessary, suffice to initiate this neuroendocrine transition. These results emphasize the importance of photoperiodic history in the actions of melatonin.
Acknowledgements
We thank Jeff Elliott for conducting the melatonin assay and Chris Tuthill, Kim Pelz, Justin Trumbull, and Sean Dunn for technical assistance. This research was supported by NIH Grants MH-61 171 and HD-36 460. MPB was a Howard Hughes Medical Institute Predoctoral Fellow, Robert Katz Fellow, and a Wang Family Fellow.
References
- Anchordoquy H. C., Lynch G. R.2000Evidence of an annual rhythm in a small proportion of Siberian hamsters exposed to chronic short days. J. Biol. Rhythms 15, 122–125 (doi:10.1177/074873040001500206) [DOI] [PubMed] [Google Scholar]
- Badura L. L., Goldman B. D.1992Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res. 598, 98–106 (doi:10.1016/0006-8993(92)90172-6) [DOI] [PubMed] [Google Scholar]
- Barrell G. K., Thrun L. A., Brown M. E., Viguie C., Karsch F. J.2000Importance of photoperiodic signal quality to entrainment of the circannual reproductive rhythm of the ewe. Biol. Reprod. 63, 769–774 (doi:10.1095/biolreprod63.3.769) [DOI] [PubMed] [Google Scholar]
- Bittman E. L., Zucker I.1981Photoperiodic termination of hamster refractoriness: participation of the pineal gland. Biol. Reprod. 24, 568–572 (doi:10.1095/biolreprod24.3.568) [DOI] [PubMed] [Google Scholar]
- Butler M. P., Zucker I.2009Seasonal pelage changes are synchronized by simulated natural photoperiods in Siberian hamsters (Phodopus sungorus). J. Exp. Zool. A 311, 475–482 (doi:10.1002/jez.544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. P., Turner K. W., Park J. H., Butler J. P., Trumbull J. J., Dunn S. P., Villa P., Zucker I.2007aSimulated natural day lengths synchronize seasonal rhythms of asynchronously born male Siberian hamsters. Am. J. Physiol. 293, R402–R412 (doi:10.1152/ajpregu.00146.2007) [DOI] [PubMed] [Google Scholar]
- Butler M. P., Trumbull J. J., Turner K. W., Zucker I.2007bTiming of puberty and synchronization of seasonal rhythms by simulated natural photoperiods in female Siberian hamsters. Am. J. Physiol. 293, R413–R420 (doi:10.1152/ajpregu.00216.2007) [DOI] [PubMed] [Google Scholar]
- Butler M. P., Turner K. W., Zucker I.2008A melatonin-independent seasonal timer induces neuroendocrine refractoriness to short day lengths. J. Biol. Rhythms 23, 242–251 (doi:10.1177/0748730408317135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. S., Goldman B. D.1983Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology 113, 1261–1267 (doi:10.1210/endo-113-4-1261) [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Goldman B. D., Di Pinto M. N., Stetson M. H.1985Testicular function and pelage color have different critical daylengths in the Djungarian hamster, Phodopus sungorus sungorus. Endocrinology 116, 424–430 (doi:10.1210/endo-116-1-424) [DOI] [PubMed] [Google Scholar]
- Dupré S. M., et al. 2008Identification of melatonin-regulated genes in the ovine pituitary pars tuberalis, a target site for seasonal hormone control. Endocrinology 149, 5527–5539 (doi:10.1210/en.2008-0834) [DOI] [PubMed] [Google Scholar]
- Elliott J. A.1976Circadian rhythms and photoperiodic time measurement in mammals. Fed. Proc. 35, 2339–2346 [PubMed] [Google Scholar]
- Elliott J. A., Tamarkin L.1994Complex circadian regulation of pineal melatonin and wheel running in Syrian hamsters. J. Comp. Physiol. [A] 174, 469–484 (doi:10.1007/BF00191713) [DOI] [PubMed] [Google Scholar]
- Freeman D. A., Zucker I.2001Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc. Natl Acad. Sci. USA 98, 6447–6452 (doi:10.1073/pnas.111140398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B. D.2001Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms 16, 283–301 (doi:10.1177/074873001129001980) [DOI] [PubMed] [Google Scholar]
- Goldman S. L., Dhandapani K., Goldman B. D.2000Genetic and environmental influences on short-day responsiveness in Siberian hamsters (Phodopus sungorus). J. Biol. Rhythms 15, 417–428 (doi:10.1177/074873000129001503) [DOI] [PubMed] [Google Scholar]
- Goldman B. D., Gwinner E., Karsch F. J., Saunders D., Zucker I., Ball G.2004Circannual rhythms and photoperiodism. In Chronobiology: biological timekeeping (eds Dunlap J. C., Loros J. J., DeCoursey P. J.), pp. 107–144 Sunderland, MA: Sinauer Associates [Google Scholar]
- Gorman M. R.1995Seasonal adaptations of Siberian hamsters. I. Accelerated gonadal and somatic development in increasing versus static long day lengths. Biol. Reprod. 53, 110–115 (doi:10.1095/biolreprod53.1.110) [DOI] [PubMed] [Google Scholar]
- Gorman M. R.2001A plastic interval timer synchronizes pubertal development of summer- and fall-born hamsters. Am. J. Physiol. 281, R1613–R1623 [DOI] [PubMed] [Google Scholar]
- Gorman M. R.2003Melatonin implants disrupt developmental synchrony regulated by flexible interval timers. J. Neuroendocrinol. 15, 1084–1094 (doi:10.1046/j.1365-2826.2003.01104.x) [DOI] [PubMed] [Google Scholar]
- Gorman M. R., Zucker I.1995Seasonal adaptations of Siberian hamsters. II. Pattern of change in day length controls annual testicular and body weight rhythms. Biol. Reprod. 53, 116–125 (doi:10.1095/biolreprod53.1.116) [DOI] [PubMed] [Google Scholar]
- Gorman M. R., Zucker I.1997Environmental induction of photononresponsiveness in the Siberian hamster. Phodopus sungorus. Am. J. Physiol. 272, R887–R895 [DOI] [PubMed] [Google Scholar]
- Gorman M. R., Zucker I.1998Mammalian seasonal rhythms: New perspectives gained from the use of simulated natural photoperiods. In Biological clocks, mechanisms and applications (ed. Touitou Y.), pp. 195–204 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- Gorman M. R., Goldman B. D., Zucker I.2001Mammalian Photoperiodism. In Circadian clocks, handbook of behavioral neurobiology, volume 12 (eds Takahashi J. S., Turek F. W., Moore R. Y.), pp. 481–508 New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]
- Hanon E. A., Lincoln G. A., Fustin J. M., Dardente H., Masson-Pevet M., Morgan P. J., Hazlerigg D. G.2008Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 18, 1147–1152 (doi:10.1016/j.cub.2008.06.076) [DOI] [PubMed] [Google Scholar]
- Hazlerigg D., Loudon A.2008New insights into ancient seasonal life timers. Curr. Biol. 18, R795–R804 (doi:10.1016/j.cub.2008.07.040) [DOI] [PubMed] [Google Scholar]
- Hazlerigg D. G., Ebling F. J., Johnston J. D.2005Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr. Biol. 15, R449–R450 (doi:10.1016/j.cub.2005.06.010) [DOI] [PubMed] [Google Scholar]
- Hoffmann K.1973Influence of photoperiod and melatonin on testis size, body weight, and pelage color in Djungarian hamster (Phodopus sungorus). J. Comp. Physiol. 85, 267–282 (doi:10.1007/BF00694233) [Google Scholar]
- Hoffmann K.1982The critical photoperiod in the Djungarian hamster Phodopus sungorus. In Vertebrate circadian systems (eds Aschoff J., Daan S., Groos G.), pp. 297–304 Heidelberg, Germany: Springer-Verlag [Google Scholar]
- Kauffman A. S., Freeman D. A., Zucker I.2003Termination of neuroendocrine refractoriness to melatonin in Siberian hamsters (Phodopus sungorus). J. Neuroendocrinol. 15, 191–196 (doi:10.1046/j.1365-2826.2003.00966.x) [DOI] [PubMed] [Google Scholar]
- Kelly K. K., Goldman B. D., Zucker I.1994Gonadal growth and hormone concentrations in photoregressed Siberian hamsters: pinealectomy versus photostimulation. Biol. Reprod. 51, 1046–1050 (doi:10.1095/biolreprod51.5.1046) [DOI] [PubMed] [Google Scholar]
- Lee T. M., Zucker I.1991Suprachiasmatic nucleus and photic entrainment of circannual rhythms in ground squirrels. J. Biol. Rhythms 6, 315–330 (doi:10.1177/074873049100600403) [DOI] [PubMed] [Google Scholar]
- Lincoln G. A.2006Melatonin entrainment of circannual rhythms. Chronobiol. Int. 23, 301–306 (doi:10.1080/07420520500464452) [DOI] [PubMed] [Google Scholar]
- Lincoln G. A., Andersson H., Loudon A.2003Clock genes in calendar cells as the basis of annual timekeeping in mammals—a unifying hypothesis. J. Endocrinol. 179, 1–13 (doi:10.1677/joe.0.1790001) [DOI] [PubMed] [Google Scholar]
- Lincoln G. A., Johnston J. D., Andersson H., Wagner G., Hazlerigg D. G.2005Photorefractoriness in mammals: dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology 146, 3782–3790 (doi:10.1210/en.2005-0132) [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M., Pévet P., Vivien-Roels B.1987Pinealectomy and constant release of melatonin or 5-methoxytryptamine induce testicular atrophy in the European hamster (Cricetus cricetus, L.). J. Pineal. Res. 4, 79–88 (doi:10.1111/j.1600-079X.1987.tb00843.x) [DOI] [PubMed] [Google Scholar]
- Maywood E. S., Hastings M. H.1995Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology 136, 144–153 [DOI] [PubMed] [Google Scholar]
- Moenter S. M., Woodfill C. J., Karsch F. J.1991Role of the thyroid gland in seasonal reproduction: thyroidectomy blocks seasonal suppression of reproductive neuroendocrine activity in ewes. Endocrinology 128, 1337–1344 (doi:10.1210/endo-128-3-1337) [DOI] [PubMed] [Google Scholar]
- Park J. H., Kauffman A. S., Paul M. J., Butler M. P., Beery A. K., Costantini R. M., Zucker I.2006Interval timer control of puberty in photoinhibited Siberian hamsters. J. Biol. Rhythms 21, 373–383 (doi:10.1177/0748730406292315) [DOI] [PubMed] [Google Scholar]
- Paul M. J., Zucker I., Schwartz W. J.2008Tracking the seasons: the internal calendars of vertebrates. Phil. Trans. R. Soc. B 363, 341–361 (doi:10.1098/rstb.2007.2143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast B. J., Freeman D. A.1999Pineal-independent regulation of photo-nonresponsiveness in the Siberian hamster (Phodopus sungorus). J. Biol. Rhythms 14, 62–71 (doi:10.1177/074873099129000452) [DOI] [PubMed] [Google Scholar]
- Prendergast B. J., Kelly K. K., Zucker I., Gorman M. R.1996Enhanced reproductive responses to melatonin in juvenile Siberian hamsters. Am. J. Physiol. 271, R1041–R1046 [DOI] [PubMed] [Google Scholar]
- Prendergast B. J., Kriegsfeld L. J., Nelson R. J.2001Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q. Rev. Biol. 76, 293–325 (doi:10.1086/393989) [DOI] [PubMed] [Google Scholar]
- Prendergast B. J., Mosinger B., Jr, Kolattukudy P. E., Nelson R. J.2002Hypothalamic gene expression in reproductively photoresponsive and photorefractory Siberian hamsters. Proc. Natl Acad. Sci. USA 99, 16 291–16 296 (doi:10.1073/pnas.232490799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast B. J., Nelson R. J., Zucker I.2009Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In Hormones, brain, and behavior, 2nd edn (eds Pfaff D. W., Arnold A. P., Etgen A. M., Fahrbach S. E., Rubin R. T.), pp. 507–538 San Diego, CA: Academic Press [Google Scholar]
- Puchalski W., Lynch G. R.1986Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J. Comp. Physiol. [A] 159, 7–11 (doi:10.1007/BF00612490) [DOI] [PubMed] [Google Scholar]
- Puchalski W., Lynch G. R.1991Circadian characteristics of Djungarian hamsters: effects of photoperiodic pretreatment and artificial selection. Am. J. Physiol. 261, R670–R676 [DOI] [PubMed] [Google Scholar]
- Reiter R. J.1969Pineal function in long term blinded male and female golden hamsters. Gen. Comp. Endocrinol. 12, 460–468 (doi:10.1016/0016-6480(69)90162-2) [DOI] [PubMed] [Google Scholar]
- Stetson M. H., Watson-Whitmyre M., Matt K. S.1977Termination of photorefractoriness in golden hamsters-photoperiodic requirements. J. Exp. Zool. 202, 81–88 (doi:10.1002/jez.1402020110) [DOI] [PubMed] [Google Scholar]
- Stetson M. H., Elliott J. A., Goldman B. D.1986Maternal transfer of photoperiodic information influences the photoperiodic response of prepubertal Djungarian hamsters (Phodopus sungorus sungorus). Biol. Reprod. 34, 664–669 (doi:10.1095/biolreprod34.4.664) [DOI] [PubMed] [Google Scholar]
- Teubner B. J., Freeman D. A.2007Different neural melatonin-target tissues are critical for encoding and retrieving day length information in Siberian hamsters. J. Neuroendocrinol. 19, 102–108 (doi:10.1111/j.1365-2826.2006.01511.x) [DOI] [PubMed] [Google Scholar]
- Teubner B. J., Smith C. D., Freeman D. A.2008Multiple melatonin target tissues mediate termination of photorefractoriness by long day lengths in Siberian hamsters. J. Biol. Rhythms 23, 502–510 (doi:10.1177/0748730408325233) [DOI] [PubMed] [Google Scholar]
- Watanabe M., Yasuo S., Watanabe T., Yamamura T., Nakao N., Ebihara S., Yoshimura T.2004Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology 145, 1546–1549 (doi:10.1210/en.2003-1593) [DOI] [PubMed] [Google Scholar]
- Yellon S. M., Goldman B. D.1984Photoperiod control of reproductive development in the male Djungarian hamster (Phodopus sungorus). Endocrinology 114, 664–670 (doi:10.1210/endo-114-2-664) [DOI] [PubMed] [Google Scholar]
- Zucker I.2001Circannual Rhythms. In Circadian clocks, volume 12, Handbook of behavioral neurobiology (eds Takahashi J. S., Turek F. W., Moore R. Y.), pp. 509–528 New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]