Abstract
Across vertebrates, the observation that lower-pitched vocalizations are typically associated with larger and/or higher quality males has lead to the widespread belief that inter- and intra-sexual selection will produce male calls with low fundamental frequencies (F0). Here we investigated the response of oestrous red deer hinds to playback of re-synthesized male roars characterized by either higher than average or lower than average F0. We found that hinds prefer higher rather than lower ‘pitched’ roars, providing, to our knowledge, the first evidence of such a bias in nonhuman mammals. Our findings can be interpreted in relation to previous observations that the minimum F0 of roars is positively correlated with male reproductive success in free-ranging red deer stags, and that across Cervids the F0 of male mating calls shows extreme variability. Females showing preferences for higher-pitched roars might derive genetic benefits through more competitive male offspring. Our results emphasize the need for further investigations of female preferences in mammals in order to better understand the extreme variation of F0 values observed in male sexual calls.
Keywords: red deer, vocal communication, fundamental frequency, female mating preferences
1. Introduction
Recent theoretical and technical advances in mammalian bioacoustics, centred around the application of the source-filter theory of voice production to voiced signals (Fant 1960; Fitch 2002), have enabled investigators to understand the acoustic structure of mammal vocal signals according to their modes of production, and to predict the covariation of specific acoustic features with physiological or morphological attributes of callers (Fitch 1997; Riede & Fitch 1999; Reby & McComb 2003a; Harris et al. 2006; Sanvito et al. 2007; Vannoni & McElligott 2008; Charlton et al. 2009). The source filter theory states that vocal signals result from a two-stage production process, with the glottal wave generated in the larynx (the source), being subsequently filtered in the supra-laryngeal vocal tract (the filter; Fant 1960; Titze 1994). This theory predicts that independent indexical information can be contained in both the glottal wave (mainly characterized by its fundamental frequency, hereafter ‘F0’), and the spectral envelope of the radiated vocalization (mainly characterized by the vocal tract resonances or ‘formant’ frequencies).
Several recent publications applying the source-filter framework to the study of mammal signals have shown that vocal tract resonances, which reflect the length and shape of the vocal tract, are reliable acoustic cues to body size (Fitch 1997; Riede & Fitch 1999; Reby & McComb 2003a; Harris et al. 2006; Sanvito et al. 2007; Vannoni & McElligott 2008; Charlton et al. 2009) and identity (Rendall 2003; Kidjo et al. 2008). By contrast, despite its dependence on the length, mass and tension of vocal folds (Titze 1994), in mammals the fundamental frequency of vocalizations does not consistently provide reliable information on body size (Lass & Brown 1978; Reby & McComb 2003a; Rendall et al. 2005; but see Pfefferle & Fisher 2006). Nevertheless, F0 remains a highly distinctive and variable component of mammal calls and its covariation with caller attributes suggests that it might nonetheless provide important information for receivers in sexual contexts (e.g. on hormone profiles: Dabbs & Mallinger 1999; Evans et al. 2008; maturity: Reby & McComb 2003a; dominance: Vannoni & McElligott 2008).
Roaring in red deer has proved to be a productive model for studying the role of mammal vocal signals in sexual contexts (Clutton-Brock & Albon 1979; McComb 1987, 1991). A combination of observational and experimental studies has shown that vocal tract resonances or formants are reliable indices of body size (Reby & McComb 2003a), and that both red deer stags (Reby et al. 2005) and hinds (Charlton et al. 2007a,b, 2008a,b) perceive and use size-related formant information during the breeding season. Previous work investigating the effect of F0 variation in male roars on the behaviour of female red deer did not reveal differential responses to pitch variants (McComb 1991; Charlton et al. 2008b). Furthermore, correlational data from the Rum population (Reby & McComb 2003a) indicates that the relationship between F0 and reproductive success is not negative as might be predicted. Instead, the data reveal a positive correlation between minimum F0 and reproductive success (Reby & McComb 2003a), pointing to the need for further investigation.
One important factor in interpreting the literature is that while previous playback studies were conducted during the breeding season, they did not exclusively target females in peak oestrus, when female preferences are most likely to emerge (Matsumoto-Oda 1999; Stumpf & Boesch 2005; for review see Charlton 2008). Consequently, in the current study investigating female preferences, we experimentally control the hormonal state of red deer hinds, ensuring (and verifying) that they are in peak oestrus at the time of the playback. More specifically, we use a two-speaker choice set-up to examine their behavioural responses to male roars characterized by either higher-than-average (160 Hz) or lower-than-average (70 Hz) F0.
2. Material and methods
(a). Experimental site and animals
This research took place at the Institut National de la Recherche Agronomique (INRA) Redon Experimental Farm, Clermont-Ferrand, France during the autumn breeding season of 2006. We used 26 adult female hinds aged 11–14 years as subjects for the playback experiments.
(b). Experimental protocol to control hind hormonal status
In order to synchronize oestrus during the experiment, a progestagen synchronization was used. This method has been successfully applied to induce oestrus in several domestic ruminant species such as sheep (Thimonier 1981) or goats (Freitas et al. 1997), as well as wild species such as red deer (Asher et al. 1992) and antelopes (Thompson & Monfort 1999). In the present study, intra-vaginal sponges impregnated with fluorogestone acetate (FGA) were used to provide a steady and continuous release of progestagen and inhibit the normal follicular growth and subsequent release of oestradiol (figure 1). These intra-vaginal sponges (2 × 45 mg, Intervet, Angers, France) were removed after 12 days and a 400 UI injection of Pregnant Mare Serum Gonadotrophin was given to each of the hinds. After removal of progestagen, the onset of oestrus and preovulatory luteinizing hormone (LH) surge in red deer are initiated by subsequent increase of plasma oestradiol concentrations (Asher et al. 1992; McLeod et al. 2001; Locatelli & Mermillod 2005). Previous studies (McLeod et al. 2001) have shown that the onset of oestrus is closely associated with a surge in LH, which occurs around 39.2 ± 4.1 h after progestagen device removal. In order to verify the efficiency of our synchronization method, in September 2007 five hinds from the Redon population were subjected to jugular venepuncture performed at 3 h intervals from 18 to 54 h after sponge withdrawal. Four of the five hinds exhibited a LH surge during the blood sample monitoring, with a mean time to LH surge of 42.75 ± 5.8 h after sponge removal, comparable with McLeod et al. (2001)'s observations. On this basis, the playback experiments were performed during the predicted peak oestrus period 35–48 h (m ± s.e. = 41.5 ± 3.9) after sponge removal.
Figure 1.
Diagrammatic representation of protocols used to induce oestrus and ovulation in red deer hinds. Exogenous progestagen are delivered by intravaginal sponges impregnated with fluorogestone acetate (FGA) to inhibit endogenous secretion of luteinizing hormone (LH), allowing regression of dominant follicle by atresia. Small follicles present on the ovary at the end of progestagen treatment are stimulated by administration of Pregnant Mare Serum Gonadotrophin (PMSG). At removal (R) of intravaginal sponges, growth of follicle is sustained by endogenous release of gonadotrophins. A massive release of oestradiol from the dominant oestrogenic follicle then triggers oestrus and the LH preovulatory surge.
(c). Hormone assays
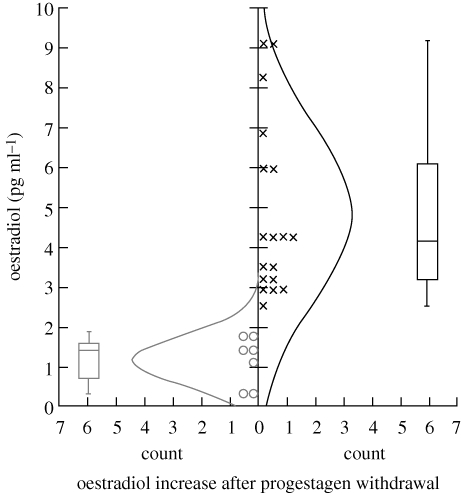
Blood samples were taken within 15 min after the playback experiments for determination of plasmatic oestradiol increase after sponge removal. The oestradiol plasma concentrations were estimated by ‘Laboratoire des Dosages Hormonaux, UMR-INRA PRC’ using the 125I E2 Diasorin RIA kit (Sorin Diagnostic, Antony, France) as described by Ben Saïd et al. (2007). Plasmatic oestradiol levels from hinds under FGA implant or during presumptive oestrus were compared by analysis of variance. Hinds presenting significantly higher oestradiol concentrations after sponge removal than the mean + (2 * s.e.) oestradiol concentration observed at the end of progestagen treatment were considered to be in oestrus (figure 2). Using this criterion, seven hinds not considered to be in oestrus during the experimental period were excluded from the analysis (402, 415, 424, 227, 228, 2404, 2409).
Figure 2.
Box-and-whisker diagram for plasma oestradiol concentrations in hinds at the time of presumed oestrus i.e. immediately after the playback experiment. Hinds showing a significant increase in oestradiol concentrations after sponge removal were considered to be in oestrus (cross), while those not showing this increase (open circle) were removed from the behavioural analyses.
(d). Preparation of playback stimuli
Roar bouts were selected from four adult red deer male exemplars (males 1–4, aged 5–8 years) unfamiliar to the current hinds and recorded at Redon in 1996 by D.R. using a Telinga pro-III-S/(DAT) microphone (without parabolic reflector) and a DAT Sony TCD-D7 recorder (amplitude resolution: 16 bits, sampling rate: 48 kHz) at distances ranging between 2 m and 10 m. In order to ensure that our playback calls adequately represented their class of stimuli (Wiley 2003), we selected roars characterized by varying harshness. The recordings were saved in audio interchange file format format (AIFF) as separate files.
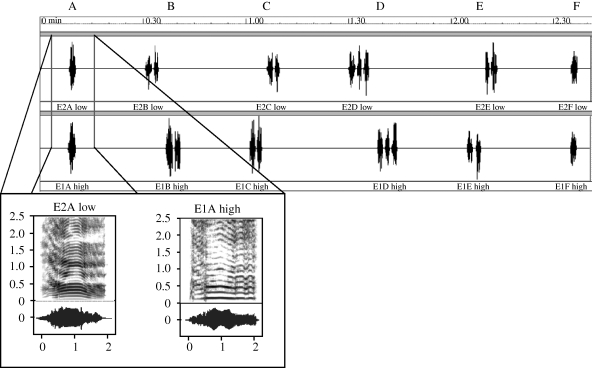
Playback trials were approximately 180 s long and consisted of the concurrent broadcast of two sequences of six roar bouts (A–F) from two different male exemplars (out of the four available males: 1, 2, 3, 4). Within each sequence bouts were arranged according to their number of roars (1–3) and their total roaring duration (with a mean residual difference of 0.1 ± 0.07 s), ensuring that bouts were matched across paired exemplar sequences. Bouts were also ordered so that the two males roared simultaneously at the beginning and at the end of the trial (bouts A and F) and consecutively (separated by 2 s) during the trial (bouts B–E). Roar duration and spacing were standardized so that the roaring rate was the same within each of the two bouts comprising a sequence and the pitch variant leading the consecutive bouts (B–E) was alternated within each playback and across playbacks to control for any preferences for bout leaders (McComb 1991; Charlton et al. 2007a,b). The composition and ordering of playback sequences are illustrated in figure 3.
Figure 3.
Diagram illustrating the structure of playback sequences. Sequences were designed to simulate a vocal exchange between a stag with low-pitched roars and a stag with high-pitched roars. The spectrograms represent a low pitch variant (70 Hz) and a high pitch variant (160 Hz).
Each male exemplar sequence was used for each experimental condition (high F0 = 160 Hz, low F0 = 70 Hz). The F0 of roars was rescaled multiplicatively using ‘Pitch Synchronous Overlap and Add’ in Praat (embedded in the ‘change gender…’ command). This works by determining the factor by which the signal's median pitch must be multiplied in order to achieve a predetermined target pitch median. The only arguments that were modified were: minimum pitch and maximum pitch (set to 30 and 250 Hz, respectively), the Formant shift ratio (set to 1.0) and the new pitch median, which was set to 70 Hz or 160 Hz. This created two pitch variants for each male exemplar with median F0's of 70 Hz and 160 Hz, reflecting the natural adult F0 range of red deer stags (Reby & McComb 2003a). Finally, in order to ensure that at any given stage of the sequence (A–F) bouts from each exemplar were played at comparable intensity, the mean intensity value of each bout of roars was rescaled independently from F0 using the ‘SCALE Intensity’ command in Praat. The resynthesis factors for relative intensity were calculated by dividing the intended value by the original measured value. The intended value was matched to the bout with the lower original value of the two exemplars, in order to avoid clipping in already 100 per cent normalized sound files. By rescaling the intensity of bouts rather than individual roars, the natural variability of intensity within bouts was preserved.
(e). Playback design
Paired stimuli sequences were broadcast simultaneously from two separate speakers, mimicking a natural exchange between two male red deer, and therefore emulating a mate choice situation (Charlton et al. 2007b). Both the pitch variant and male exemplar played from each speaker were randomized across playback trials to control for any preferences for areas of the experimental site or particular exemplars. Each hind therefore received one of 16 different and unique sequence pairs differing in the following characteristics: the male exemplars that were used in the pair (male 1 versus 2 or male 3 versus 4), the arrangement of the bouts within the sequences (see figure 3), the pitch (70 Hz versus 160 Hz) at which each exemplar was resynthesized, and finally the speaker (left or right) from which each exemplar was played. This ensured that each of the first 16 hinds received a unique sequence corresponding to a unique combination of male (exemplar)/arrangement/location/condition and ordered in a latin square design. The next 10 hinds were subsequently played the first 10 of these 16 combinations.
(f). Experimental set-up
The playback stimuli were presented using two Anchor Audio Liberty 6000HIC loudspeakers hidden behind semi-opaque farm gates (2 m high by 4 m wide) covered with black matt plastic mesh, and connected by coaxial cable to an Apple Macintosh G3 computer. Roars were broadcast from the speakers at equal sound pressure levels (SPLs) (105 dB peak SPL at 1 m from the source, determined using a Radio Shack Sound Level Meter set for C-weighted fast response) and at a height of 1.5 m from the ground. Speakers were placed 16 m apart and 20 m away from the hind's position at playback onset. By not placing the speakers directly opposite each other we ensured that any aversion responses would not be confused with movements towards either speaker position. Two adjacent ‘proximity zones’ of approximately 100 m2 were clearly demarcated using white chalk around each speaker. Behavioural responses were video-recorded during the experimental period and for 2 min after the last roar was broadcast.
(g). Behavioural analyses
The video sequences were analysed using Gamebreaker 5.1 (SportsTec, Sydney; see the electronic supplementary material). We measured the number and cumulative duration of looks given while stationary towards either of the speaker positions and the amount of time spent within the ‘proximity zones’ in front of either speaker. Looking was defined as starting when the hind raised or turned her head to face the speaker, having previously faced away, before maintaining a fixed head position, and ended when the head moved away from this first fixed position. We also measured the total time spent by hinds in each of the proximity zones. Video analyses were carried out by B.D.C. and D.R. double-coded both duration measures for five hinds (26%). An overall 99.7 per cent agreement was achieved between B.D.C. and D.R. on both measures.
(h). Statistical analyses
Log transformations were used to normalize the data distribution so that parametric statistics could be used. To retain subjects with behavioural responses of zero in the dataset we added 1 to each data point before applying the log transformation. Paired t-tests were used to detect significant differences in behavioural response between the conditions. Significance levels were set at 0.05 and two-tailed probability values quoted.
3. Results
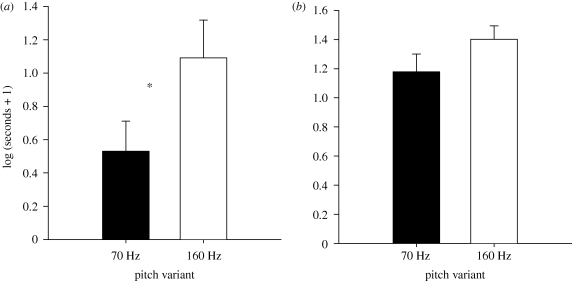
Out of the oestrous hinds (n = 19), 12 entered one or both of the proximity zones: five hinds entered the ‘proximity zone’ of the high pitch variant only, one hind entered the ‘proximity zone’ of the low pitch variant only, and six hinds entered both zones. On average, hinds spent significantly more time in the ‘proximity zone’ of the high pitch variant (56.61 ± 16.77 s) than in that of the low pitch variant (17.40 ± 8.37 s), (t18 = −2.198, p = 0.041); see figure 4. By contrast, there were no significant differences in look duration between high (34.84 ± 6.6 s) and low (27.26 ± 8.26 s) pitch variants (t18 = −1.149, p = 0.266), nor in the number of looks between high (9.74 ± 6.03 s) and low (7.26 ± 5.15 s) pitch variants (t18 = −1.283, p = 0.216).
Figure 4.
Error bar charts showing means ± s.e. of hind behavioural responses to the pitch variants (*p < 0.05; p-values are from t-tests; n = 18); (a) time in mate choice zones and (b) looking duration.
4. Discussion
We found that oestrous hinds given a relative choice between the two male pitch variants spent more time in close proximity to speakers broadcasting high-pitched male roars. Given that previous work on red deer failed to detect a differential response by females to playback stimuli representing different male pitch variants (McComb 1991; Charlton et al. 2008b), the current research emphasizes that experimental investigation of female choice in mammals should be conducted during the female's peak conception times (for review see Charlton 2008). Differences in the behaviour of oestrous versus non-oestrous females in mate choice situations have previously been reported in fallow deer, with oestrous females displaying a strong preference that was absent in non-oestrous ones (McComb & Clutton-Brock 1994).
To our knowledge, the results presented here constitute the first evidence of a female preference based on the fundamental frequency of a male call in a non-human mammal—but crucially, in the opposite direction to that normally assumed. While the mean fundamental frequency of male red deer roars is not correlated to any known aspect of male quality, including body weight, the minimum fundamental frequency is positively correlated with the caller's reproductive success, (the mean and maximum also have non-significant trends in this direction, Reby & McComb 2003a). A larger sample would be needed to further investigate the potential for F0 characteristics to provide information on the caller's reproductive success, and indeed the basis for this relationship (physiological or behavioural). Nonetheless, females preferring males with overall higher F0 might obtain indirect genetic benefits through more competitive (as indicated by the positive relationship between minimum F0 and reproductive success) or attractive offspring, in turn exerting positive sexual selection pressures on the F0 of male red deer roars.
Our findings are also consistent with the observation that despite red deer being a strongly sexually size-dimorphic species (males are 1.5 times heavier, Clutton-Brock et al. 1982) call fundamental frequency is comparable in males and females (Vankova et al. 1997; Reby & McComb 2003a). Similarly, in North American Elk or Wapiti (Cervus e. nelsoni), the very strong sexual dimorphism in body size does not translate into vocal fold length dimorphism (Riede & Titze 2008), nor is it associated with F0 dimorphism (Feighny et al. 2006). This is in stark contrast to Fallow deer (Dama dama) and Corsican deer (another subspecies of red deer, C. elaphus corsicanus), in which a comparably pronounced sexual dimorphism in body size is reflected in an extreme sexual dimorphism in F0 (Reby & McComb 2003b; Kidjo et al. 2008). Moreover, across cervid species there is a counterintuitive positive relationship between body size and male F0: species with larger males have the highest F0, and vice-versa. Taken together these observations suggest that very different, and possibly opposite selection pressures may be operating on F0 phenotypes within closely related species of polygynous deer.
Investigating the role of F0 in mammal sexual vocal communication could also contribute to our understanding of the evolution and role of F0 in human speech. Recent human studies have shown that the F0 of male speech influences male dominance attributions and may be important as a cue to the speaker's testosterone levels (Dabbs & Mallinger 1999; Evans et al. 2008; Puts 2007). Moreover, human females find males with lower F0 more masculine and more attractive (Collins 2000; Feinberg et al. 2005; Puts 2005), and in hunter–gatherer societies males with low F0 have higher reproductive success (Apicella et al. 2006) and may be preferred as mating partners (Apicella & Feinberg 2009).
We suggest that a comparative research effort is now needed to investigate the basis and function of F0 variation in a wider range of mammalian species. More specifically, future studies could investigate the covariation of male F0 and vocal fold dimensions with caller attributes such as body size, hormonal status, social rank and reproductive success. Resynthesis and playback experiments could then be used to assess female preferences for F0 variants, and the results of these investigations examined in relation to phylogenetic relationships and variations in sexual dimorphisms (including F0 and vocal fold anatomy), reproductive systems and ecology. With well-established experimental procedures for revealing female preference at the time of oestrus and the exceptional range of F0 variation observed within very closely related subspecies and species, polygynous deer constitute a particularly interesting group for investigating the evolution of the values and function of F0 in mammal sexual signals.
Acknowledgements
This work follows the Association for the study of Animal Behaviour/Animal Behaviour Society guidelines for the use of animals in research, and was carried out under authorization A37801 of the French Ministry of Agriculture.
We thank the Institut National de la Recherche Agronomique for allowing us to conduct our experiment at Redon experimental red deer farm. We warmly thank André Guittard (dédé), Benjamin Larret, Yoan Thomas and Marcel Verdier for their invaluable help. We also thank Elisabetta Vannoni for her assistance during the experiments. This research was supported by a Nuffield research grant to D.R.
References
- Apicella C. L., Feinberg D. R.2009Voice pitch alters mate-choice-relevant perception in hunter–gatherers. Proc. R. Soc. B 276, 1077–1082 (doi:10.1098/rspb.2008.1542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella C. L., Feinberg D. R., Marlowe F. W.2006Voice pitch predicts reproductive success in male hunter–gatherers. Biol. Lett. 3, 682–684 (doi:10.1098/rsbl.2007.0410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G. W., Fisher M. W., Jabbour H. N., Smith J. F., Mulley R. C., Morrow C. J., Veldhuizen F. A., Langridge M.1992Relationship between the onset of oestrus, the preovulatory surge in luteinizing hormone and ovulation following oestrous synchronization and superovulation of farmed red deer (Cervus elaphus). J. Reprod. Fertil. 96, 261–273 [DOI] [PubMed] [Google Scholar]
- Ben Saïd S., Lomet D., Chesneau D., Lardic L., Canepa S., Guillaume D., Briant C., Fabre-Nys C., Caraty A.2007Differential estradiol requirement for the induction of estrus behavior and the luteinizing hormone surge in two breeds of sheep. Biol. Reprod. 76, 673–680 (doi:10.1095/biolreprod.106.057406) [DOI] [PubMed] [Google Scholar]
- Charlton B. D.2008Female mate choice in nonhuman mammals. In Animal behavior: new research (eds Weber E. A., Krause L. H.), pp. 35–56 New York, NY: Nova Science Publishers Inc [Google Scholar]
- Charlton B. D., Reby D., McComb K.2007aFemale perception of size-related formants in red deer, Cervus elaphus. Anim. Behav. 74, 707–714 (doi:10.1016/j.anbehav.2006.09.021) [Google Scholar]
- Charlton B. D., Reby D., McComb K.2007bFemale red deer prefer the roars of larger males. Biol. Lett. 3, 382–385 (doi:10.1098/rsbl.2007.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton B. D., McComb K., Reby D.2008aFree-ranging red deer hinds show greater attentiveness to roars with formant frequencies typical of young males. Ethology 114, 1023–1031 (doi:10.1111/j.1439-0310.2008.01539.x) [Google Scholar]
- Charlton B. D., Reby D., McComb K.2008bEffect of combined source (F0) and filter (formant) variation on red deer hind responses to male roars. J. Acoust. Soc. Am. 123, 2936–2943 (doi:10.1121/1.2896758) [DOI] [PubMed] [Google Scholar]
- Charlton B. D., Zhihe Z., Snyder R. J.2009The information content of giant panda, Ailuropoda melanoleuca, bleats: acoustic cues to sex, age and size. Anim. Behav. 78, 893–898 (doi:10.1016/j.anbehav.2009.06.029) [Google Scholar]
- Clutton-Brock T. H., Albon S. D.1979The roaring of red deer and the evolution of honest advertising. Behaviour 69, 145–170 (doi:10.1163/156853979X00449) [Google Scholar]
- Clutton-Brock T. H., Albon S., Guinness F. E.1982Red deer: behavior and ecology of two sexes. Chicago, IL: University of Chicago Press [Google Scholar]
- Collins S. A.2000Men's voices and women's choices. Anim. Behav. 60, 773–780 [DOI] [PubMed] [Google Scholar]
- Dabbs J. M., Mallinger A.1999High testosterone levels predict low voice pitch among men. Pers. Individ. Dif. 27, 801–804 (doi:10.1016/S0191-8869(98)00272-4) [Google Scholar]
- Evans S., Neave N., Wakelin D., Hamilton C.2008The relationship between testosterone and vocal frequencies in human males. Physiol. Behav. 93, 783–788 (doi:10.1016/j.physbeh.2007.11.033) [DOI] [PubMed] [Google Scholar]
- Fant G.1960Acoustic theory of speech production. The Hague, The Netherlands: Mouton [Google Scholar]
- Feighny J. A., Williamson K. E., Clarke J. A.2006North American elk bugle vocalizations: male and female bugle call structure and context. J. Mammal. 87, 1072–1077 (doi:10.1644/06-MAMM-A-079R2.1) [Google Scholar]
- Feinberg D. R., Jones B. C., Little A. C., Burt D. M., Perrett D. I.2005Manipulations of fundamental and formant frequencies influence the attractiveness of human male voices. Anim. Behav. 69, 561–568 (doi:10.1016/j.anbehav.2004.06.012) [Google Scholar]
- Fitch W. T.1997Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 102, 1213–1222 (doi:10.1121/1.421048) [DOI] [PubMed] [Google Scholar]
- Fitch W. T.2002Comparative vocal production and the evolution of speech: reinterpreting the descent of the larynx. In The transition to language (ed. Wray A.), pp. 21–45 Oxford, UK: Oxford University Press [Google Scholar]
- Freitas V. J. F., Baril G., Saumande J.1997Estrus synchronization in dairy goats: use of fluorogestone acetate vaginal sponges or norgestomet ear implants. Anim. Reprod. Sci. 46, 237–244 (doi:10.1016/S0378-4320(96)01614-4) [DOI] [PubMed] [Google Scholar]
- Harris T. R., Fitch W. T., Goldstein L. M., Fashing P. J.2006Black and white colobus monkey (Colobus guereza) roars as a source of both honest and exaggerated information about body mass. Ethology 112, 911–920 (doi:10.1111/j.1439-0310.2006.01247.x) [Google Scholar]
- Kidjo N., Cargnelutti B., Charlton B. D., Wilson C., Reby D.2008Vocal behaviour in the endangered Corsican deer, description and phylogenetic implications. Bioacoustics 18, 159–181 [Google Scholar]
- Lass N. J., Brown W. S.1978Correlational study of speakers' heights, weights, body surface areas and speaking fundamental frequencies. J. Acoust. Soc. Am. 63, 1218–1220 (doi:10.1121/1.381808) [DOI] [PubMed] [Google Scholar]
- Locatelli Y., Mermillod P.2005Caractéristiques et maîtrise de la fonction de reproduction chez les cervidés. Productions Animales 18, 3–25 [Google Scholar]
- Matsumoto-Oda A.1999Female choice in the opportunistic mating of wild chimpanzees (Pan troglodytes schweinfurthii) at Mahale. Behav. Ecol. Sociobiol. 46, 258–266 (doi:10.1007/s002650050618) [Google Scholar]
- McComb K.1987Roaring by red deer stags advances the date of oestrus in hinds. Nature 330, 648–649 (doi:10.1038/330648a0) [DOI] [PubMed] [Google Scholar]
- McComb K. E.1991Female choice for high roaring rates in red deer, Cervus elaphus. Anim. Behav. 41, 79–88 (doi:10.1016/S0003-3472(05)80504-4) [Google Scholar]
- McComb K., Clutton-Brock T.1994Is mate choice copying or aggregation responsible for skewed distributions of females on leks? Proc. R. Soc. Lond. B 255, 13–19 (doi:10.1098/rspb.1994.0003) [DOI] [PubMed] [Google Scholar]
- McLeod B. J., Meikle L. M., Fisher M. W., Shackell G. H., Heath D. A.2001Gonadotrophin-induced follicle development in red deer hinds during the breeding and non-breeding seasons. Reproduction 122, 111–119 (doi:10.1530/rep.0.1220111) [PubMed] [Google Scholar]
- Pfefferle D., Fischer J.2006Sounds and size: identification of acoustic variables that reflect body size in hamadryas baboons, Papio hamadryas. Anim. Behav. 72, 43–51 (doi:10.1016/j.anbehav.2005.08.021) [Google Scholar]
- Puts D. A.2005Mating context and menstrual phase affect women's preferences for male voice pitch. Evol. Hum. Behav. 26, 388–397 (doi:10.1016/j.evolhumbehav.2005.03.001) [Google Scholar]
- Puts D. A.2007Men's voices as dominance signals: vocal fundamental and formant frequencies influence dominance attributions among men. Evol. Hum. Behav. 28, 340–344 (doi:10.1016/j.evolhumbehav.2007.05.002) [Google Scholar]
- Reby D., McComb K.2003aAnatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim. Behav. 65, 519–530 (doi:10.1006/anbe.2003.2078) [Google Scholar]
- Reby D., McComb K.2003bVocal communication and reproduction in deer. Adv. Stud. Behav. 33, 231–264 (doi:10.1016/S0065-3454(03)33005-0) [Google Scholar]
- Reby D., McComb K., Cargnelutti B., Darwin C., Fitch W. T., Clutton-Brock T. H.2005Red deer stags use formants as assessment cues during intrasexual agonistic interactions. Proc. R. Soc. B 272, 941–947 (doi:10.1098/rspb.2004.2954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendall D.2003Acoustic correlates of caller identity and affect intensity in the vowel-like grunt vocalizations of baboons. J. Acoust. Soc. Am. 113, 3390–3402 (doi:10.1121/1.1568942) [DOI] [PubMed] [Google Scholar]
- Rendall D., Kollias S., Ney C.2005Pitch (F0) and formant profiles of human vowels and vowel-like baboon grunts: the role of vocalizer body size and voice-acoustic allometry. J. Acoust. Soc. Am. 117, 944–955 (doi:10.1121/1.1848011) [DOI] [PubMed] [Google Scholar]
- Riede T., Fitch W. T.1999Vocal tract length and acoustics of vocalization in the domestic dog (Canis familiaris). J. Exp. Biol. 202, 2859–2867 [DOI] [PubMed] [Google Scholar]
- Riede T., Titze I. R.2008Vocal fold elasticity of the Rocky Mountain elk (Cervus elaphus nelsoni): producing high fundamental frequency vocalization with a very long vocal fold. J. Exp. Biol. 211, 2144–2154 (doi:10.1242/jeb.017004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvito S., Galimberti F., Miller E. H.2007Vocal signalling in male southern elephant seals is honest but imprecise. Anim. Behav. 73, 287–299 (doi:10.1016/j.anbehav.2006.08.005) [Google Scholar]
- Stumpf R. M., Boesch C.2005Does promiscuous mating preclude female choice? Female sexual strategies in chimpanzees (Pan troglodytes verus) of the Tai National Park, Cote d'Ivoire. Behav. Ecol. Sociobiol. 57, 511–524 (doi:10.1007/s00265-004-0868-4) [Google Scholar]
- Thimonier J.1981Control of seasonal reproduction in sheep and goats by light and hormones. J. Reprod. Fertil. Suppl. 30, 33–45 [PubMed] [Google Scholar]
- Thompson K. V., Monfort S. L.1999Synchronization of oestrous cycles in sable antelope. Anim. Reprod. Sci. 57, 185–197 (doi:10.1016/S0378-4320(99)00061-5) [DOI] [PubMed] [Google Scholar]
- Titze I. R.1994Principles of voice production. Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- Vankova D., Bartos L., Malek J.1997The role of vocalization in the communication between red deer hinds and calves. Ethology 103, 795–808 [Google Scholar]
- Vannoni E., McElligott A. G.2008Low frequency groans indicate larger and more dominant fallow deer (Dama dama) males. PLoS ONE 3, e3113 (doi:10.1371/journal.pone.0003113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley R. H.2003Is there an ideal behavioural experiment? Anim. Behav. 66, 585–588 (doi:10.1006/anbe.2003.2231) [Google Scholar]