Abstract
Cooperatively breeding American crows (Corvus brachyrhynchos) suffer a severe disease-mediated survival cost from inbreeding, but the proximate mechanisms linking inbreeding to disease are unknown. Here, we examine indices of nestling body condition and innate immunocompetence in relationship to inbreeding and disease mortality. Using an estimate of microsatellite heterozygosity that predicts inbreeding in this population, we show that inbred crows were in relatively poor condition as nestlings, and that body condition index measured in the first 2–33 days after hatching, in addition to inbreeding index, predicted disease probability in the first 34 months of life. Inbred nestlings also mounted a weaker response along one axis of innate immunity: the proportion of bacteria killed in a microbiocidal assay increased as heterozygosity index increased. Relatively poor body condition and low innate immunocompetence are two mechanisms that might predispose inbred crows to ultimate disease mortality. A better understanding of condition-mediated inbreeding depression can guide efforts to minimize disease costs of inbreeding in small populations.
Keywords: American crows, body condition, disease, immunocompetence, inbreeding, inbreeding depression
1. Introduction
Inbred individuals might be less resistant to disease if they are unable to recognize as wide a breadth of pathogens and parasites as relatively outbred individuals, or if disease-causing agents are part of an environment that selects against individuals expressing deleterious recessive alleles (Coltman et al. 1999). Empirical evidence for disease costs of inbreeding has been documented in captive settings (e.g. Spielman et al. 2004; Hawley et al. 2005; Ross-Gillespie et al. 2007; Charpentier et al. 2008; Ilmonen et al. 2008) as well as in an array of free-living taxa, including California sea lions (Zalophus californianus; Acevedo-Whitehouse et al. 2003), Mediterranean striped dolphins (Stenella coeruleoalba; Valsecchi et al. 2004), Galapagos hawks (Buteo galapagoensis; Whiteman et al. 2006), Soay sheep (Ovis aries; Coltman et al. 1999), harbour seals (Phoca vitulina; Rijks et al. 2008) and American crows (Corvus brachyrhynchos; Townsend et al. 2009a). In some studies, however, the relationship between inbreeding and disease is unclear (e.g. Giese & Hedrick 2003) or controversial (Caro & Laurenson 1994), and in general, the disease costs of inbreeding are not well understood in wild populations (Keller & Waller 2002). In particular, scant information is available regarding the proximate factors that might predispose inbred individuals to disease in wild populations. A more complete picture of the causes and consequences of disease-mediated inbreeding depression in wild populations would contribute to our understanding of the evolution and maintenance of behaviours such as sex-biased dispersal (Charlesworth & Charlesworth 1987; Szulkin & Sheldon 2008), gregariousness (Jog & Watve 2005) and avoidance of kin matings (Koenig & Haydock 2004). Detailed knowledge about the factors that influence disease-mediated inbreeding depression, and ways in which the impacts of disease can be mitigated, is also critically important for the conservation of small, inbred populations (Keller & Waller 2002).
Factors that might potentially predispose inbred individuals to disease include differences in behavioural resistance (Calleri et al. 2006; Luong & Polak 2007) or immune response (Reid et al. 2007), which might be owing, in part, to differences in condition or nutrition (Moller et al. 1998; Blanco et al. 2001; Koski & Scott 2001) among individuals with different inbreeding coefficients. Previous field studies have explored links between inbreeding and components of the immune response (Reid et al. 2007), inbreeding and disease (e.g. Acevedo-Whitehouse et al. 2003; Charpentier et al. 2008; Townsend et al. 2009a), immune response, condition and parasite load (Moller & Haussy 2007; Parejo & Silva 2009), and population-level genetic diversity, immune response and ectoparasite abundance (Whiteman et al. 2006). To date, however, no studies have explicitly examined the links between inbreeding, immune response, condition and disease mortality of individuals in a wild, free-living population.
In this contribution, we begin the assessment of the mechanistic links between inbreeding and disease mortality in a large, open population of socially monogamous, cooperatively breeding American crows (C. brachyrhynchos) in Ithaca, NY. Previous work in this American crow population has shown that approximately 23 per cent of genetic parental dyads are either first-order kin (coefficient of relatedness (r ≈ 0.5)) or second-order kin (r ≈ 0.25; Townsend 2009; Townsend et al. 2009a,b). Inbred crows suffer from severe disease-mediated inbreeding depression: survival probability is lower for relatively inbred birds, and birds that die with signs of infectious disease during post-mortem examination have higher inbreeding indices than birds with other fates (Townsend et al. 2009a). Proximately, inbreeding in this population occurs because delayed dispersal and short dispersal distances in both sexes lead to interactions between related adults of the opposite sex (Townsend et al. 2009b). Inbreeding occurs through extrapair matings (i.e. matings outside of a socially monogamous pair bond) between mothers and their adult auxiliary sons within their family groups, as well as through within-pair matings between related (r ≈ 0.25) social pairs (Townsend 2009; Townsend et al. 2009a,b). Although ultimate factors that promote inbreeding in this population are currently unclear (Townsend et al. 2009a), the brood-level offspring production costs of incestuous mother–son matings might be balanced, to some extent, by the direct benefits provided by extrapair sires within cooperative groups (Townsend et al. 2010).
Here, we examine the relationships among inbreeding, innate immunocompetence, body condition and disease mortality in this American crow population. To generate indices of body condition, we used a mass by size residual (Schulte-Hostedde et al. 2005), hypothesizing that inbreeding would be linked to a general decline in body condition, which in turn would affect the probability of disease mortality (Beldomenico et al. 2008). We tested the specific predictions that: (i) inbred nestlings would have relatively low body condition indices; (ii) birds that ultimately died of infectious disease within the duration of this study (i.e. within the first 34 months of life) would have been in poor condition as nestlings relative to birds with other fates; and (iii) these condition indices would predict the probability of disease mortality. Furthermore, based on both theoretical expectations (Obrien & Evermann 1988; Coltman et al. 1999) and empirical observations in other taxa (Reid et al. 2007), we hypothesized that inbreeding would depress immune response, predicting that inbred individuals would have lower innate immunity scores than relatively outbred individuals. We ran two assays to characterize individual innate immune response: (i) a bacterial killing assay, which is a general assay of constitutive innate immunity that reflects the ability of the whole blood to stop a potential pathogen (Millet et al. 2007), and (ii) an assay for natural antibody (NAb)-mediated complement activation (Matson et al. 2005). NAbs and the complement play a critical role in early, efficient immunogenicity and preventing the spread of infection (Ochsenbein & Zinkernagel 2000), and complement deficiencies have been linked to disease in a wide array of taxa (Matson et al. 2005). Because of the potential costs associated with the production and maintenance of immune response (Moller et al. 1998; but see Klasing 1998), we also examined the relationship between immune response and condition or size. We did not, however, predict the direction of the relationship, because some studies suggest a positive correlation between condition and immune response (Moller et al. 1998; Blanco et al. 2001), whereas others suggest a trade-off between growth and immune response (Brommer 2004).
2. Material and methods
(a). Field sampling and histopathology analyses
From 2004 to 2009, we collected genetic samples from 375 nestlings belonging to 117 broods associated with 44 American crow family groups in a long-term suburban study population in Ithaca, NY (McGowan 2001; Clark et al. 2006). Crows in this population are socially monogamous, and family groups usually contain auxiliaries of either sex, most of which help to provision the incubating females, nestlings and fledgelings. Criteria and methods for classifying family groups, auxiliaries, male breeders and female breeders are described in Townsend et al. (2009b). Hatch date was estimated by the shifting behaviour of female breeders when their eggs begin to hatch, and we refined nestling age estimates at the time of banding. On days 1–33 after hatching, nestlings were individually marked with temporary bands, weighed and measured in tarsus, bill width and depth, exposed culmen and diameter of skull (measured from the back of the head to the proximal end of the exposed culmen). We collected blood (approx. 150 µl) from the brachial vein of live nestlings, and tissue samples from carcasses of dead nestlings found in and under these nests. Nestlings that survived past day 20 after hatching were remeasured and marked with unique combinations of metal bands, colour bands and patagial tags.
Marked focal nestlings (n = 299) from the cohorts of 2004–2008 were systematically monitored for fate at least once per month, following Townsend et al. (2009a). Post-mortem examinations were performed on the dead crows discovered between November 2006 and July 2008 that tested negative for West Nile virus (WNV), as described in Miller et al. (2010) and Townsend et al. (2009a). Of the 299 focal birds, 100 were still alive, 21 died with signs of infectious disease (poxviral dermatitis, n = 14; WNV, n = 3; bacterial infections, n = 2; fungal pneumonia, n = 1; enteritis; n = 1), 54 met with traumatic deaths and 124 died or disappeared of unknown causes by July 2008 (described in detail in Townsend et al. 2009a). Birds were only characterized as ‘diseased’ when observed infections were the likely cause of death.
(b). Molecular methods
DNA was extracted from blood samples using Perfect gDNA Blood Mini kits (Eppendorf, Westbury, NY, USA) and from feather tips using DNeasy tissue kits (Qiagen, Valencia, CA, USA). We sexed all individuals at diagnostic sex-linked alleles (Fridolfsson & Ellegren 1999). Following Townsend et al. (2009b), we genotyped each nestling at 10 microsatellite loci, and calculated two microsatellite-based individual heterozygosity indices that are frequently reported in the literature: (i) multi-locus heterozygosity (MLH), which is the frequency of heterozygous loci for each individual, and (ii) internal relatedness (IR), which accounts for background allele frequencies when estimating parental similarity from an offspring's genotype (Amos et al. 2001) using IRMacroN4 (http://www.zoo.cam.ac.uk/zoostaff/amos/#ComputerPrograms). Although simulations suggest that IR might reflect inbreeding somewhat more closely than MLH (Balloux et al. 2004), we emphasize MLH in this paper because it is more amenable to cross study comparisons. To determine if heterozygosity–fitness correlations were driven by heterozygosity at any single locus (‘local effects’; Hansson & Westerberg 2008), we sequentially dropped each marker from the marker set, calculated both individual heterozygosity indices in the reduced, nine-locus marker sets and ran post hoc tests using indices derived from the reduced marker set (Hawley et al. 2005).
If microsatellite and genome-wide heterozygosity are correlated in a given system, then heterozygosity estimated from one set of microsatellites should be positively correlated with heterozygosity from an independent set of microsatellites from the same individual (‘heterozygosity–heterozygosity correlations’ or HHCs; Balloux et al. 2004). In a previous study, using only IR, we showed that the strength of the HHCs was close to zero for relatively outbred birds and increased with the degree of inbreeding, suggesting that IR did correlate with genome-wide heterozygosity, and was therefore a valid index of inbreeding in this population (Townsend et al. 2009a). In this study, we extend HHC validation to MLH, categorizing offspring first by parental relatedness, then by disease fate. Methods used to categorize offspring by parental relatedness (e.g. offspring that were produced incestuously, offspring produced through second-order kin matings and relatively outbred offspring) are given in Townsend et al. (2009a). For fate categorization, we grouped focal offspring as ‘alive without signs of disease’ or ‘diseased’. The diseased grouping included the 21 individuals that suffered apparent disease mortality (described above) and four additional offspring from the 2009 cohort with detectable poxviral dermatitis lesions as of January 2010. We then conducted HHC simulations for offspring in each category by: (i) randomly splitting the 10 loci into two sets of five independent loci; (ii) calculating two MLH values—one from each set of five loci—for each individual; (iii) regressing the two MLH values for all individuals within each group against one another and calculating the r2 values of the regressions; and (iv) repeating this procedure 50 times. We used analysis of variance to compare the mean r2 values among the groups of offspring.
(c). Innate immunity assays
Microbiocidal assays were carried out on nestlings sampled in 2009 following Millet et al. (2007). In brief, we diluted 0.75 µl whole blood from each nestling into sterile 1.5 ml capped tubes with 97.25 µl of prewarmed CO2-independent media (no. 18045; Gibco-Invitrogen, CA) plus 4 mM l-glutamine. We used Esherichia coli in this assay because vertebrates are likely to have constitutive components of their immune system that respond to it (Millet et al. 2007). The strain that we used (E. coli ATCC no. 8739; American Tissue and Cell Culture, VA, USA) was most susceptible to killing by a suite of other avian species (Millet et al. 2007). We diluted E. coli to a working culture in sterile phosphate-buffered saline. Ten 10 µl of the working culture (approx. 100 bacteria) were added to each diluted blood sample, vortexed and incubated for 30 min at 41°C. We vortexed the incubated samples, spread 50 µl aliquots onto agar plates, inverted them and incubated them at 37°C for 24 h. The number of bacteria in the inoculums was determined by adding the working culture to the media and l-glutamine mix, without blood and plating 10 µl. We also plated 10 µl of the media and l-glutamine mix, without blood or bacteria, as a negative control. The antimicrobial activity of blood was defined as the percentage of the inoculums killed, calculated as 1 − (viable bacteria after incubation/number inoculated).
The NAb-mediated complement assays were carried out on nestlings sampled in 2005 and 2007 following Matson et al. (2005). Starting with 50 µl of plasma from each individual, we carried out serial dilutions in 96-well round-bottomed assay plates, resulting in dilutions ranging from 1 to 1/1024 across the 12 columns, and added 25 µl of a 1 per cent rabbit blood cell suspension to each well. Plates were sealed with a plastic film, vortexed at low speed, incubated for 90 min at 37°C and assessed for agglutination. Plates were then kept at room temperature for an additional 70 min and scored for maximum haemolysis, which reflects the activity of the NAb-mediated complement (Matson et al. 2005). Haemolysis score was the column number of the last plasma dilution exhibiting haemolysis. Agglutination, which generally has a less clear endpoint than haemolysis (Matson et al. 2005), was not easy to observe or score in this study, and the results are not reported. All haemolysis assays were run in 2007. Plasma samples were stored at −20°C from the time of collection till the time of this assay.
(d). Statistical analyses
We calculated an index of body condition for each nestling as the residual from a regression of mass against size + (size × size), defining nestling size as the first principal component (PC1) on covariances of exposed culmen, skull, bill width and depth, and tarsus (Schulte-Hostedde et al. 2005). PC1 explained 94.8 per cent of the variation in these measurements. To explore the relationship between offspring body condition and inbreeding, we specified a body condition index of each nestling as the response in a linear mixed model (function lme in library nlme) in R v. 2.7.2, with heterozygosity index (IR or MLH), year, age (age + age), sex and all two-way interactions with inbreeding as fixed effects. Because some offspring were produced by the same breeders over multiple years, we included the identity of the family group as a random effect. Non-significant terms were removed from final models following the model-selection criteria of Hosmer & Lemeshow (2000).
The effect of nestling body condition on the probability of disease mortality of focal birds in the first 34 months of life was explored in a generalized linear model (R function glm), specifying death with signs of disease versus all other fates (alive, traumatic deaths and unknown fates) as the response (coded as 1/0) and nestling body condition index, heterozygosity index and their interaction as predictors (binomial distribution; parameter estimates β ± s.e. given in logit form). We examined the relationship between bactericidal activity and individual heterozygosity using a generalized linear mixed model fit by the Laplace approximation (function GLMER in R library lme4), with the proportion of bacteria killed as the response (weighted by the number of bacteria in the inoculums), heterozygosity index as a fixed effect and family group as a random effect (binomial distribution; β ± s.e. given in logit form). We examined the relationship between haemolysis and heterozygosity indices in a generalized linear model with haemolysis score as the response, and heterozygosity indices, collection year, nestling age, sex and all two-way interactions with heterozygosity as predictors (Poisson distribution; β ± s.e. given in log form).
The relationship between bacterial killing and body condition was examined in a linear mixed model with condition index as the response, proportion of bacteria killed and heterozygosity index as fixed effects, and family group as a random effect. The relationship between haemolysis score and condition was explored in a linear mixed model with condition index as the response, haemolysis score, year, age and sex as fixed effects, and family as a random effect. Relationships between size and both indices of innate immunity were examined in linear mixed effect models with nestling size (PC1) as the response, innate immunity index (haemolysis score or proportion of bacteria killed), year, age and sex as fixed effects, and family as a random effect.
3. Results
We collected complete morphometric measurements, genetic sexing data and complete genotypes at 10 loci from 375 offspring sampled between 2004 and 2009. There was a strong correlation between our two heterozygosity indices, IR and MLH (r2 = 0.97; β =− 1.2 ± 0.01; t(373) =− 114.2; p < 0.001; electronic supplementary material, figure S1). Because MLH is more amenable to cross-study comparisons than IR (and because the two indices yielded nearly identical results in all tests), statistical output is given for MLH only. Statistical output from tests using IR is available in the electronic supplementary material.
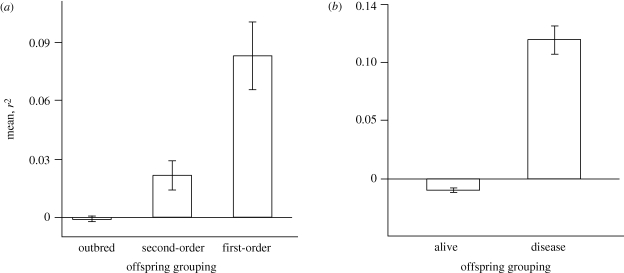
As expected if MLH reflects genome-wide heterozygosity (and inbreeding) in this population, there was significant variation in the strength of HHCs among offspring of different relatedness groupings (F2,147 = 15.8; p < 0.0001; figure 1a) and fate groupings (F1,98 = 106.2; p < 0.0001; figure 1b). The strength of the correlation was higher for offspring produced incestuously than for relatively outbred offspring (Tukey's HSD, α = 0.05), and was higher for diseased birds than for live birds without disease signs.
Figure 1.
Heterozygosity–heterozygosity correlations (HHCs), based on multilocus heterozygosity, for (a) offspring produced by parents with different degrees of probable relatedness (i.e. first-order kin, second-order kin and relatively outbred parental pairs), and (b) offspring with different fates (i.e. birds that were alive without signs of disease at the end of the study, and birds (either dead or alive) with signs of disease by the end of the study). Means and standard errors are shown.
(a). Condition, inbreeding and survival
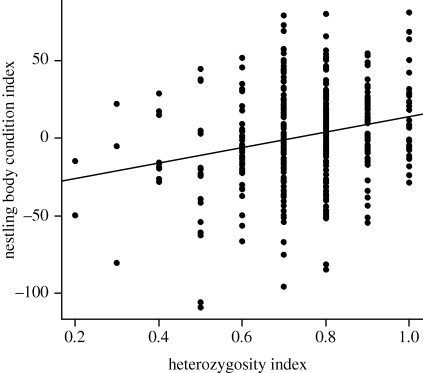
A least-squares regression suggested that condition increased with individual heterozygosity, although MLH explained only a small amount of the variation in condition (r2 = 0.05; figure 2 and electronic supplementary material, figure S2). A positive relationship between individual heterozygosity and body condition was supported by a linear mixed model, with body condition index as the response, MLH, year and sex as fixed effects, and family group as a random effect (β(MLH) = 38.5 ± 11.4, t(328) = 3.4, p = 0.0008; n = 375 offspring; electronic supplementary material, table S1). We ran the model first using the complete dataset (n = 375 offspring), including offspring measured from day 1 to 33 after hatching, and then limiting the dataset to the 339 offspring sampled within a shorter time frame (days 20–30 after hatching) to see if including nestlings at either age extreme affected the results. Results were congruent for the full and reduced datasets. Results from the reduced dataset are given in the electronic supplementary material, figure S3 and table S2. In this and all other analyses, results were consistent in post hoc tests using heterozygosity indices calculated from the sequentially reduced marker set, suggesting that no single locus was driving the patterns that we observed. Only results from the full marker set are reported.
Figure 2.
Correlation between nestling body condition index and heterozygosity index (MLH) (β ± s.e. = 50.12 ± 10.8; t373 = 4.6, p < 0.0001).
Among the 291 focal offspring that were monitored for fate and for which we had body condition indices, lifespan of the 21 birds that died with signs of infectious disease—and for which disease was the likely cause of death—ranged from 1 to 13 months (mean ± s.e. = 6.8 ± 0.9 months; details of the disease given in Townsend et al. 2009a). In a generalized linear model with disease mortality as a bivariate response, birds that died with signs of disease were in significantly worse condition as nestlings, and were less heterozygous than birds with other fates (table 1 and electronic supplementary material, table S3). The bivariate relationship between condition and fate is shown in the electronic supplementary material, figure S4.
Table 1.
Output from a generalized linear model showing the effects of body condition and individual heterozygosity (MLH) on the probability of dying with signs of disease (1 = diseased; 0 = other fates).
| β ± s.e. | z | p | |
|---|---|---|---|
| nestling body condition index | −0.01 ± 0.007 | −2.3 | 0.036 |
| individual heterozygosity | −5.37 ± 1.55 | −3.5 | 0.0005 |
(b). Innate immunity assays
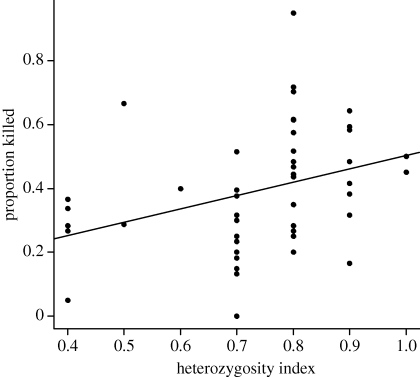
We collected bacterial killing data from 52 nestlings from the 2009 cohort. Least-squares regression analysis suggested that the proportion of bacteria killed by whole blood increased as individual heterozygosity increased (figure 3 and electronic supplementary material, figure S5). Likewise, a generalized linear mixed model with the proportion of bacteria killed as the response, MLH as a fixed effect and family as a random effect also suggested that more heterozygous birds killed more bacteria (β(MLH) ± s.e. = 1.74 ± 0.35; z = 5.0, p < 0.001). We collected rabbit red blood cell haemolysis data from 112 nestlings: 32 nestlings from the 2005 cohort and 80 from the 2007 cohort. Haemolysis score did not vary with MLH after accounting for year and nestling age in a generalized linear model with haemolysis score as the response (β(MLH) ± s.e. =− 7.4 ± 5.7; z108 =− 1.3, p = 0.20; electronic supplementary material, table S4).
Figure 3.
Correlation between individual heterozygosity index (MLH) and bactericidal activity against E. coli 8739 by diluted whole blood of crow nestlings (r2 = 0.12; β ± s.e. = 0.42 ± 0.16; t50 = 2.58, p = 0.013).
Innate immune response appeared to be independent of nestling body condition, but not size. Proportion of bacteria killed did not predict body condition index (linear mixed model with condition index as the response, heterozygosity index and proportion of bacteria killed as fixed effects and family group as a random effect; β (% killed) ±s.e. =− 28.0 ± 15.8, t34 =− 1.8, p = 0.09). Likewise, haemolysis score did not predict the body condition index (linear mixed model with condition index as the response, haemolysis score and year as fixed effects and family group as a random effect; β (haemolysis) ± s.e. = 3.3 ± 2.1, t87 = 1.6, p = 0.12). Proportion of bacteria killed had no effect on the nestling size (linear mixed effect model with size as the response, proportion of bacteria killed, age and sex as fixed effects, and family as a random effect; (β (% killed) ± s.e. =−1.9 ± 1.8; t32 =− 1.0, p = 0.31; electronic supplementary material, table S5a). However, nestlings with higher haemolysis scores were relatively large for their age (linear mixed effect model with size as the response and haemolysis score, age and sex as fixed effects; (β (haemolysis score) ± s.e. = 0.4 ± 0.2; t86 = 2.2, p = 0.03; electronic supplementary material, table S5b).
4. Discussion
Previously, we have shown that inbred birds in a population of American crows in Ithaca, NY, had a higher probability of disease mortality and a lower survival probability than relatively outbred birds (Townsend et al. 2009a). The proximate mechanisms linking inbreeding to disease, however, were unknown. Here, we show that inbred nestlings were in relatively poor condition: body mass residuals from a regression with body size increased with increasing heterozygosity index. Although the extent to which mass by size residuals reflect true physiological condition has been questioned (Green 2001; but see Schulte-Hostedde et al. 2005), crow condition indices appeared to have an important relationship with eventual disease mortality: nestling body condition indices were significantly lower for birds that died with signs of disease within the first 3 years of life than for birds with other fates. Inbreeding and condition appeared to have an additive effect on fate, because birds that died with signs of disease were both more inbred, and in worse condition as nestlings, than birds with other fates. Additionally, inbred nestlings appeared to mount a weaker response along some axes of innate immunity than relatively outbred birds: the proportion of bacteria killed by whole blood decreased with decreasing heterozygosity index. This bactericidal assay is likely to reflect a number of components of the innate immune system, both cell-mediated and humoral (described in Millet et al. 2007), and we do not know which component drove the observed patterns with inbreeding. However, the complement did not appear to be the component of innate immunity driving this pattern, because haemolysis scores, which reflect the activity of the complement (Matson et al. 2005), did not vary with individual heterozygosity.
(a). Inbreeding and microsatellite heterozygosity
Heterozygosity at a small panel of microsatellites is unlikely to correlate with genome-wide heterozygosity (and inbreeding) in all systems (Balloux et al. 2004; Slate et al. 2004; DeWoody & DeWoody 2005). However, this American crow population, although large and open, is characterized by a relatively high frequency and variance in the occurrence of inbreeding (Townsend et al. 2009a), the scenario under which microsatellite and genome-wide heterozygosity are expected to be most strongly correlated (Balloux et al. 2004; Slate et al. 2004). We found strong evidence to suggest that MLH of our 10 markers did reflect the degree of inbreeding in this population of crows: HHCs were significantly stronger for offspring that were produced incestuously than for offspring with less-related parents (see Townsend et al. 2009a for a similar analysis using IR). Furthermore, HHCs were stronger for offspring with signs of disease than for live offspring without signs of disease. These results are congruent with an analysis conducted with California sea lion data (Acevedo-Whitehouse et al. 2003), in which Balloux et al. (2004) found that HHCs were stronger among individuals with cancer than for those with other fates. The authors inferred that this group of diseased individuals contained highly inbred individuals (an inference that is strongly supported among diseased birds in our crow population; Townsend et al. 2009a), and concluded that microsatellite heterozygosity at a small panel of loci can correlate with inbreeding in philopatric populations containing highly inbred individuals.
One alternative explanation to inbreeding for the heterozygosity-fitness correlations which we observed is that these patterns were driven by local effects (i.e. a non-random association between a given microsatellite locus and a nearby fitness locus; Hansson & Westerberg 2008). However, post hoc tests, in which we sequentially dropped each locus from the marker set, yielded results congruent with the results using the full marker set, suggesting that no single microsatellite locus was driving the patterns that we observed.
(b). Nestling body condition and disease mortality
Poor body condition of crows when they were nestlings appeared to have long-term consequences, predisposing birds in this population to eventual death by disease. Our index of body condition was based on measurements taken when birds were only 1–33 days old, yet the mean age at which focal birds ultimately died with signs of disease was nearly seven months after hatching. Although a link between condition and disease is not unexpected (Beldomenico et al. 2008), field studies that document this link in wild populations are rare (e.g. Hakkarainen et al. 2007; Beldomenico et al. 2009). Effects of poor condition and infection can be synergistic, because individuals in poor condition might be less resistant to disease, and infection might further reduce individual condition (Beldomenico et al. 2008). Both condition and infection probability might be mediated, to some extent, by nutrition (Glick et al. 1981; Klasing 1998; Moller et al. 1998), and variation in diet might explain some of the individual variation in the relationship between condition and inbreeding that we observed: crow nestling size, blood protein and calcium have been linked to habitat and diet in this population (McGowan 2001; Heiss et al. 2009). It is likely that a number a factors in addition to inbreeding (genetic and extrinsic) affect individual body condition—and, therefore, disease mortality—in this population.
It is possible that the costs of inbreeding drop off quickly as the degree of inbreeding decreases, or that there is even an optimal, intermediate level of inbreeding in a given population (Bateson 1983). Previously, we showed that the relationship between microsatellite heterozygosity, disease and survival disappeared after incestuously produced crows were removed from the sample (Townsend et al. 2009a), a pattern that could have arisen because costs of inbreeding fell off rapidly as degree of inbreeding decreased. The same pattern would arise, however, if microsatellite heterozygosity did not correlate well with the degree of inbreeding among relatively outbred birds (which appeared to be the case in both the previous and current study), obscuring potential heterozygosity–fitness correlations among relatively outbred birds. With this marker set, we are therefore unable to determine how quickly the costs of inbreeding change with the degree of inbreeding in this population.
(c). Inbreeding and innate immunity
A weaker innate immune response might have been another proximate mechanism contributing to the higher probability of disease mortality among inbred birds. Although information on immunity and inbreeding in other wild bird populations is scant, a negative relationship between inbreeding and immune response has also been documented in an island population of song sparrows (Melospiza melodia), using an assay of cell-mediated immunity, which is one component of the avian acquired immune system (Reid et al. 2007). Likewise, among island populations of Galapagos hawks (Buteo galapagoensis), Whiteman et al. (2006) found that the NAb levels decreased with population-level genetic diversity, and individual ectoparasite abundance was negatively correlated with the level of NAbs.
In general, there may be trade-offs among the many different components of the immune system (Adamo 2004; Forsman et al. 2008): individuals that mount a weak response along one axis of immunity might mount a stronger response along another. Therefore, for a complete assessment of immunity and inbreeding in crows, it will be necessary to examine multiple axes of the immune response (innate and acquired; cell-mediated and humoral) using a larger array of immunocompetence assays (Adamo 2004; Matson et al. 2005; Millet et al. 2007), within the same individual birds. The field of eco-immunology is an emerging one, and many assumptions underlying the interpretation of the immunological assays that we employed are untested in populations of wild birds (Ardia & Schat 2008). For example, the assumed relationship between innate immune response and disease should be empirically tested in crows, because immunity scores do not always reflect disease resistance (Adamo 2004). Preliminary analyses suggested that haemolysis scores (which did not vary with inbreeding) did not predict disease mortality in this population (A. Townsend 2010, unpublished data). We did not have data collected over a time frame sufficient to assess links between bactericidal score (which did vary with inbreeding) and fate in this study. The relationship between bactericidal score and inbreeding that we report here, although suggestive, should therefore be regarded as preliminary.
(d). Nestling body condition, size and innate immunity
The relationship between immune response and condition is complex. Individuals in good condition might be able to mount a stronger immune response than individuals in poor condition (Moller et al. 1998; Blanco et al. 2001), particularly if immune response is costly. However, if individuals in poor condition experience a higher infection probability or are more likely to be suffering from a current infection than individuals in good condition (Beldomenico et al. 2008, 2009), then they might invest more resources into immune defence and therefore exhibit a stronger response to experimental challenges. Costly allocation to immune response by individuals in poor condition might further impact condition or growth (e.g. Soler et al. 2003; Bonato et al. 2009). We found no evidence for a relationship between body condition and innate immunocompetence indices in these nestlings, although nestlings that were larger for their age appeared to mount a stronger NAb-mediated complement response than relatively small birds, suggesting that individuals with greater resources might be mounting a stronger response at least along some axes of immunity.
5. Conclusions
Although a link between disease and inbreeding is a theoretical expectation (Obrien & Evermann 1988; Coltman et al. 1999), evidence for this relationship in wild populations is scarce (reviewed in Townsend et al. 2009a). Empirical investigations of the potential mechanisms mediating the disease-inbreeding link in wild populations are rarer still. This study is the first, to our knowledge, to document the links among nestling body condition, innate immunity, inbreeding and disease mortality in a wild population of birds. Because of the potential synergistic interactions among immunity, condition and infections, and the correlative nature of this study, however, we have not distinguished between cause and effect among the links. For example, it is unclear whether poor condition of individuals as nestlings led to their relatively high probability of infection and disease-mediated mortality (i.e. within the first 13 months of their life), or if these individuals were already suffering from infections as nestlings, which led to their poor nestling body condition and contributed to eventual disease mortality. More information regarding the direction of causation among these links could be gained by repeated measures of condition, immune response and infection status over time (Beldomenico et al. 2009), and by experimental manipulation of condition (e.g. by manipulating resources). A better understanding of the condition-mediated links between inbreeding and disease can be used to guide conservation efforts to minimize disease costs of inbreeding in small populations.
Acknowledgements
All capture, handling, marking, observation and blood sampling of American crows was carried out under permit from the US Geological Survey Bird Banding Laboratory, NY State (no. 22263) and under protocols approved by the Binghamton University (nos 537-03 and 607-07) and Cornell University (no. 1988-0210) Institutional Animal Care and Use Committees.
We thank B. Cramer and L. Stenzler for their logistical support and advice with the immunocompetence assays, and I. Lovette, A. T. Schat, D. Robinson, J. Fitzpatrick, W. Koenig and J. Dickinson for discussion. Support for this work was provided by the National Science Foundation, the National Institute of Health, the Animal Behaviour Society, Cornell Sigma Xi Grant-in-Aid of Research, the Frank M. Chapman Memorial Fund, the Cooper Ornithological Society, the Wilson Ornithological Society, the Andrew W. Mellon Foundation, an Eloise Gerry Fellowship from Sigma Delta Epsilon/Graduate Women in Science, and the American Association of University Women.
References
- Acevedo-Whitehouse K., Gulland F., Greig D., Amos W.2003Disease susceptibility in California sea lions. Nature 422, 35 (doi:10.1038/422035a) [DOI] [PubMed] [Google Scholar]
- Adamo S. A.2004How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 68, 1443–1449 (doi:10.1016/j.anbehav.2004.05.005) [Google Scholar]
- Amos W., Wilmer J. W., Fullard K., Burg T. M., Croxall J. P., Bloch D., Coulson T.2001The influence of parental relatedness on reproductive success. Proc. R. Soc. Lond. B 268, 2021–2027 (doi:10.1098/rspb.2001.1751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardia D. R., Schat K. A.2008Ecoimmunology. In Avian immunology (eds Davison T. F., Kaspers B., Schat K. A.), pp. 421–441 Amsterdam/Boston/London: Elsevier/Academic Press [Google Scholar]
- Balloux F., Amos W., Coulson T.2004Does heterozygosity estimate inbreeding in real populations? Mol. Ecol. 13, 3021–3031 (doi:10.1111/j.1365-294X.2004.02318.x) [DOI] [PubMed] [Google Scholar]
- Bateson P.1983Optimal outbreeding. In Mate choice (ed. Bateson P.), pp. 257–277 Cambridge, UK: Cambridge University Press [Google Scholar]
- Beldomenico P. M., Telfer S., Gebert S., Lukomski L., Bennett M., Begon M.2008Poor condition and infection: a vicious circle in natural populations. Proc. R. Soc. B 275, 1753–1759 (doi:10.1098/rspb.2008.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldomenico P. M., Telfer S., Lukomski L., Gebert S., Bennett M., Begon M.2009Host condition and individual risk of cowpox virus infection in natural animal populations: cause or effect? Epidemiol. Infect. 137, 1295–1301 (doi:10.1017/S0950268808001866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G., De la Puente J., Corroto M., Baz T., Colas J.2001Condition-dependent immune defence in the magpie: how important is ectoparasitism? Biol. J. Linn. Soc. 72, 279–286 (doi:10.1111/j.1095-8312.2001.tb01317.x) [Google Scholar]
- Bonato M., Evans M. R., Hasselquist D., Cloete S. W. P., Cherry M. I.2009Growth rate and hatching date in ostrich chicks reflect humoral but not cell-mediated immune function. Behav. Ecol. Sociobiol. 64, 183–191 (doi:10.1007/s00265-009-0835-1) [Google Scholar]
- Brommer J. E.2004Immunocompetence and its costs during development: an experimental study in blue tit nestlings. Proc. R. Soc. Lond. B 271, S110–S113 (doi:10.1098/rsbl.2003.0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleri D. V., Reid E. M., Rosengaus R. B., Vargo E. L., Traniello J. F. A.2006Inbreeding and disease resistance in a social insect: effects of heterozygosity on immunocompetence in the termite Zootermopsis angusticollis. Proc. R. Soc. B 273, 2633–2640 (doi:10.1098/rspb.2006.3622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro T. M., Laurenson M. K.1994Ecological and genetic factors in conservation: a cautionary tale. Science 263, 485–486 (doi:10.1126/science.8290956) [DOI] [PubMed] [Google Scholar]
- Charlesworth D., Charlesworth B.1987Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 237–268 (doi:10.1146/annurev.es.18.110187.001321) [Google Scholar]
- Charpentier M. J. E., Williams C. V., Drea C. M.2008Inbreeding depression in ring-tailed lemurs (Lemur catta): genetic diversity predicts parasitism, immunocompetence, and survivorship. Conserv. Genet. 9, 1605–1615 (doi:10.1007/s10592-007-9499-4) [Google Scholar]
- Clark A. B., Robinson D. A., McGowan K. J.2006Effects of West Nile virus mortality on social structure of an American crow (Corvus brachyrhynchos) population in New York state. Ornith. Monogr. 60, 65–78 (doi:10.1642/0078-6594(2006)60[65:EOWNVM]2.0.CO;2) [Google Scholar]
- Coltman D. W., Pilkington J. G., Smith J. A., Pemberton J. M.1999Parasite-mediated selection against inbred Soay sheep in a free-living, island population. Evolution 53, 1259–1267 (doi:10.2307/2640828) [DOI] [PubMed] [Google Scholar]
- DeWoody Y. D., DeWoody J. A.2005On the estimation of genome-wide heterozygosity using molecular markers. J. Hered. 96, 85–88 (doi:10.1093/jhered/esi017) [DOI] [PubMed] [Google Scholar]
- Forsman A. M., Vogel L. A., Sakaluk S. K., Grindstaff J. L., Thompson C. F.2008Immune-challenged house wren broods differ in the relative strengths of their responses among different axes of the immune system. J. Evol. Biol. 21, 873–878 (doi:10.1111/j.1420-9101.2008.01503.x) [DOI] [PubMed] [Google Scholar]
- Fridolfsson A. K., Ellegren H.1999A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121 (doi:10.2307/3677252) [Google Scholar]
- Giese A. R., Hedrick P. W.2003Genetic variation and resistance to a bacterial infection in the endangered Gila topminnow. Anim. Conserv. 6, 369–377 (doi:10.1017/S1367943003003445) [Google Scholar]
- Glick B., Day E. J., Thompson D.1981Calorie-protein deficiencies and the immune response of the chicken. I. Humoral immunity. Poult. Sci. 60, 2494–2500 [DOI] [PubMed] [Google Scholar]
- Green A. J.2001Mass/length residuals: measures of body condition or generators of spurious results? Ecology 82, 1473–1483 (doi:10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2) [Google Scholar]
- Hakkarainen H., Huhta E., Koskela E., Mappes T., Soveri T., Suorsa P.2007Eimeria-parasites are associated with a lowered mother's and offspring's body condition in island and mainland populations of the bank vole. Parasitology 134, 23–31 (doi:10.1017/S0031182006001120) [DOI] [PubMed] [Google Scholar]
- Hansson B., Westerberg L.2008Heterozygosity-fitness correlations within inbreeding classes: local or genome-wide effects? Conserv. Genet. 9, 73–83 (doi:10.1007/s10592-007-9309-z) [Google Scholar]
- Hawley D. M., Sydenstricker K. V., Kollias G. V., Dhondt A. A.2005Genetic diversity predicts pathogen resistance and cell-mediated immunocompetence in house finches. Biol. Lett. 1, 326–329 (doi:10.1098/rsbl.2005.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss R. S., Clark A. B., McGowan K. J.2009Growth and nutritional state of American crow nestlings vary between urban and rural habitats. Ecol. Appl. 19, 829–839 (doi:10.1890/08-0140.1) [DOI] [PubMed] [Google Scholar]
- Hosmer D. W., Lemeshow S.2000Applied logistic regression. New York, NY: John Wiley & Sons, Inc [Google Scholar]
- Ilmonen P., Penn D. J., Damjanovich K., Clarke J., Lamborn D., Morrison L., Ghotbi L., Potts W. K.2008Experimental infection magnifies inbreeding depression in house mice. J. Evol. Biol. 21, 834–841 (doi:10.1111/j.1420-9101.2008.01510.x) [DOI] [PubMed] [Google Scholar]
- Jog M., Watve M.2005Role of parasites and commensals in shaping host behaviour. Curr. Sci. 89, 1184–1191 [Google Scholar]
- Keller L. F., Waller D. M.2002Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 (doi:10.1016/S0169-5347(02)02489-8) [Google Scholar]
- Klasing K. C.1998Nutritional modulation of resistance to infectious diseases. Poult. Sci. 77, 1119–1125 [DOI] [PubMed] [Google Scholar]
- Koenig W. D., Haydock J.2004Incest and incest avoidance. In Ecology and evolution of cooperative breeding in birds (eds Koenig W. D., Dickinson J. L.), pp. 142–156 Cambridge, UK: Cambridge University Press [Google Scholar]
- Koski K. G., Scott M. E.2001Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annu. Rev. Nutr. 21, 297–321 (doi:10.1146/annurev.nutr.21.1.297) [DOI] [PubMed] [Google Scholar]
- Luong L. T., Polak M.2007Costs of resistance in the Drosophila macrocheles system: a negative genetic correlation between ectoparasite resistance and reproduction. Evolution 61, 1391–1402 (doi:10.1111/j.1558-5646.2007.00116.x) [DOI] [PubMed] [Google Scholar]
- Matson K. D., Ricklefs R. E., Klasing K. C.2005A hemolysis-hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Dev. Comp. Immunol. 29, 275–286 (doi:10.1016/j.dci.2004.07.006) [DOI] [PubMed] [Google Scholar]
- McGowan K. J.2001Demographic and behavioral comparisons of suburban and rural American crows. In Avian ecology and conservation in an urbanizing world (eds Marzluff J. M., Bowman R., Donelly D.), pp. 365–381 Norwell, MA: Klumer Academic Press [Google Scholar]
- Miller A. D., Townsend A. K., McGowan K. J., Clark A. B., Glaser A. L., Patrican L. A., Dobson E., Buckles E. L.2010Non-West Nile virus-associated mortality in a population of American crows (Corvus brachyrhynchos): a gross and histopathologic study. J. Vet. Diagn. Invest. 22, 120–126 [DOI] [PubMed] [Google Scholar]
- Millet S., Bennett J., Lee K. A., Hau M., Klasing K. C.2007Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 31, 188–201 (doi:10.1016/j.dci.2006.05.013) [DOI] [PubMed] [Google Scholar]
- Moller A. P., Christe P., Erritzoe J., Meller A. P.1998Condition, disease and immune defence. Oikos 83, 301–306 (doi:10.2307/3546841) [Google Scholar]
- Moller A. P., Haussy C.2007Fitness consequences of variation in natural antibodies and complement in the barn swallow Hirundo rustica. Funct. Ecol. 21, 363–371 (doi:10.1111/j.1365-2435.2006.01215.x) [Google Scholar]
- Obrien S. J., Evermann J. F.1988Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol. Evol. 3, 254–259 (doi:10.1016/0169-5347(88)90058-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein A. F., Zinkernagel R. M.2000Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21, 624–630 (doi:10.1016/S0167-5699(00)01754-0) [DOI] [PubMed] [Google Scholar]
- Parejo D., Silva N.2009Immunity and fitness in a wild population of Eurasian kestrels Falco tinnunculus. Naturwissenschaften 96, 1193–1202 (doi:10.1007/s00114-009-0584-z) [DOI] [PubMed] [Google Scholar]
- Reid J. M., Arcese P., Keller L. F., Elliott K. H., Sampson L., Hasselquist D.2007Inbreeding effects on immune response in free-living song sparrows (Melospiza melodia). Proc. R. Soc. B 274, 697–706 (doi:10.1098/rspb.2006.0092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijks J. M., Hoffman J. I., Kuiken T., Osterhaus A., Amos W.2008Heterozygosity and lungworm burden in harbour seals (Phoca vitulina). Heredity 100, 587–593 (doi:10.1038/hdy.2008.18) [DOI] [PubMed] [Google Scholar]
- Ross-Gillespie A., O'Riain M. J., Keller L. F.2007Viral epizootic reveals inbreeding depression in a habitually inbreeding mammal. Evolution 61, 2268–2273 (doi:10.1111/j.1558-5646.2007.00177.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hostedde A. I., Zinner B., Millar J. S., Hickling G. J.2005Restitution of mass-size residuals: validating body condition indices. Ecology 86, 155–163 (doi:10.1890/04-0232) [Google Scholar]
- Slate J., David P., Dodds K. G., Veenvliet B. A., Glass B. C., Broad T. E., McEwan J. C.2004Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: theoretical expectations and empirical data. Heredity 93, 255–265 (doi:10.1038/sj.hdy.6800485) [DOI] [PubMed] [Google Scholar]
- Soler J. J., de Neve L., Perez-Contreras T., Soler M., Sorci G.2003Trade-off between immunocompetence and growth in magpies: an experimental study. Proc. R. Soc. Lond. B 270, 241–248 (doi:10.1098/rspb.2002.2217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman D., Brook B. W., Briscoe D. A., Frankham R.2004Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv. Genet. 5, 439–448 (doi:10.1023/B:COGE.0000041030.76598.cd) [Google Scholar]
- Szulkin M., Sheldon B. C.2008Dispersal as a means of inbreeding avoidance in a wild bird population. Proc. R. Soc. B 275, 703–711 (doi:10.1098/rspb.2007.0989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. K.2009Extrapair copulations predict extrapair fertilizations in the American crow. Condor 111, 387–392 (doi:10.1525/cond.2009.090010) [Google Scholar]
- Townsend A. K., Clark A. B., McGowan K. J., Buckles E. L., Miller A. D., Lovette I. J.2009aDisease-mediated inbreeding depression in a large, open population of cooperative crows. Proc. R. Soc. B 276, 2057–2064 (doi:10.1098/rspb.2008.1852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. K., Clark A. B., McGowan K. J., Lovette I. J.2009bReproductive partitioning and the assumptions of reproductive skew models in the cooperatively breeding American crow. Anim. Behav. 77, 503–512 (doi:10.1016/j.anbehav.2008.10.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. K., Clark A. B., McGowan K. J.2010Genetic costs and direct benefits of extrapair paternity for female American crows. Am. Nat. 175, E1–E9 (doi:10.1086/648553) [DOI] [PubMed] [Google Scholar]
- Valsecchi E., Amos W., Raga J. A., Podesta M., Sherwin W.2004The effects of inbreeding on mortality during a morbillivirus outbreak in the Mediterranean striped dolphin (Stenella coeruleoalba). Anim. Conserv. 7, 139–146 (doi:10.1017/S1367943004001325) [Google Scholar]
- Whiteman N. K., Matson K. D., Bollmer J. L., Parker P. G.2006Disease ecology in the Galapagos hawk (Buteo galapagoensis): host genetic diversity, parasite load and natural antibodies. Proc. R. Soc. B 273, 797–804 (doi:10.1098/rspb.2005.3396) [DOI] [PMC free article] [PubMed] [Google Scholar]