Abstract
Seasonally driven cycles of incidence have been consistently observed for a range of directly transmitted pathogens. Though frequently observed, the mechanism of seasonality for directly transmitted human pathogens is rarely well understood. Despite significant annual variation in magnitude, measles outbreaks in Niger consistently begin in the dry season and decline at the onset of the seasonal rains. We estimate the seasonal fluctuation in measles transmission rates for the 38 districts and urban centres of Niger, from 11 years of weekly incidence reports. We show that transmission rates are consistently in anti-phase to the rainfall patterns across the country. The strength of the seasonal forcing of transmission is not correlated with the latitudinal rainfall gradient, as would be expected if transmission rates were determined purely by environmental conditions. Rather, seasonal forcing is correlated with the population size, with larger seasonal fluctuation in more populous, urban areas. This pattern is consistent with seasonal variation in human density and contact rates due to agricultural cycles. The stronger seasonality in large cities drives deep inter-epidemic troughs and results in frequent local extinction of measles, which contrasts starkly to the conventional observation that large cities, by virtue of their size, act as reservoirs of measles.
Keywords: seasonality, Niger, critical community size, TSIR model, epidemic metapopulation
1. Introduction
Measles is the exemplar for the study of the nonlinear dynamics of infectious disease (Bolker & Grenfell 1993; Grenfell et al. 1994; Earn et al. 2000; Keeling & Grenfell 2002; Conlan & Grenfell 2007; Ferrari et al. 2008). Seasonal variation in transmission rates, lifelong immunity following infection, the replenishment of susceptible individuals through births and demographic stochasticity leading to local extinction of measles are major determinants of its multi-annual dynamics (Bolker & Grenfell 1993; Earn et al. 2000; Rohani et al. 2002) and long-term persistence (Keeling & Grenfell 1997; Conlan & Grenfell 2007). Even very weak seasonal fluctuations in transmission rates interact with the epidemic clockwork to generate stable cycles of incidence (Aron & Schwartz 1984; Bauch & Earn 2003; Dushoff et al. 2004). The birth rate governs the accumulation of susceptible individuals in the troughs between outbreaks, and changes in birth rates change the dynamics from over-compensating multi-annual cycles to deterministic chaos (Earn et al. 2000).
While many pathogens exhibit behaviour characteristic of seasonally forced cycles, the explicit mechanism of seasonal forcing has rarely been identified (Altizer et al. 2006; Grassly & Fraser 2006). Seasonality in water-borne (e.g. cholera) and vector-borne (e.g. malaria) pathogens is well understood; however, teasing out the cause of seasonality for directly transmitted pathogens has proven harder. Undoubtedly the best-documented example of an explicit mechanism for seasonal transmission of a directly transmitted pathogen is the school-term forcing of measles transmission in England and Wales in the pre-vaccination era (Fine & Clarkson 1982; Schenzle 1984; Finkenstadt & Grenfell 2000). Transmission rates increased during school terms when children were aggregated, and declined during school holidays. Other studies of immunizing childhood infections, such as pertussis and chicken pox, have shown similar trends of increased transmission during school terms (London & Yorke 1973; Gomes et al. 1999; Deguen et al. 2000). Elsewhere, there is considerable debate as to the mechanistic basis of seasonality for directly transmitted pathogens (e.g. influenza: Lipsitch & Viboud 2009; meningitis: Broutin et al. 2007), ranging from seasonal fluctuations in susceptibility to environmental drivers or abundance of non-human hosts (Altizer et al. 2006; Grassly & Fraser 2006).
Measles dynamics in Niger are strongly seasonal, with outbreaks beginning at the start of the dry season and peaking at the onset of the rainy season (Ferrari et al. 2008). In the capital city, Niamey, this strong seasonality, combined with high birth rates, results in outbreaks that are highly variable in magnitude from year to year, and perhaps chaotic (Ferrari et al. 2008). Elsewhere in the country, while the magnitude of outbreaks is variable, the seasonal timing is highly consistent. Ferrari et al. (2008) proposed that the seasonal timing of measles outbreaks in Niger results from rural–urban migration within the country in response to agricultural cycles. At the onset of the rainy season, people tend to return from urban to rural areas to pursue agriculture, resulting in a drop in urban population densities and a concomitant decline in measles transmission rates. Guyer & McBean (1981) suggested that same mechanism may explain the seasonal measles outbreaks in Yaounde, Cameroon. From this hypothesis, we would predict seasonality to scale with human population density and urbanization.
An important consequence of seasonality is that incidence can fall to very low levels in the troughs between outbreaks. Endemic persistence of measles transmission has classically been seen only for conurbations above a ‘critical community size (CCS)’ of 300 000–500 000 inhabitants (Bartlett 1957; Keeling & Grenfell 1997; Bjørnstad et al. 2002; Grenfell et al. 2002; Conlan & Grenfell 2007). These large cities then serve as local reservoirs that can seed outbreaks in smaller municipalities following local extinction, giving rise to travelling waves of outbreaks along a population gradient (Grenfell et al. 2001). Ferrari et al. (2008) showed that the combination of high birth rates and strong seasonality in Niamey drives frequent local extinction of measles. Despite several large population centres across Niger, strong seasonality in measles transmission rates drives local instability of measles dynamics and impacts regional persistence.
Here, we present an analysis of 11 years of weekly measles case reporting across Niger and show that the magnitude of seasonal forcing is positively correlated with population size, which lends support to the hypothesis that seasonal and other transmission heterogeneities in West African measles are driven by changes in human density. Further, we show that this correlation between population size and seasonal forcing means that all departments of Niger are below the CCS required for persistence. Thus, long-term, regional persistence of measles in Niger depends on regional metapopulation dynamics that are likely to extend well beyond national boundaries.
2. Material and methods
We analysed weekly case reports of measles from the Ministry of Health of Niger from 1 January 1995 to 31 December 2005. The country of Niger is divided into 38 administrative units: 34 districts and 4 urban centres (Agadez, Maradi, Niamey and Zinder). For reporting purposes, the city of Agadez was separated from the district of Tchirozerine in 2001, and in 2002 the district of Abalak was split into Abalak and Tchin-Tabaraden. As the reported data span this time frame we aggregated the reported cases after the splits into the earlier demarcations for consistency, resulting in 35 districts and 3 urban centres (Niamey, Maradi and Zinder).
The majority of the population in Niger is clustered in two major regions surrounding the capital city Niamey in the southwest, and surrounding the large cities of Maradi and Zinder in the south-centre of the country. The northern and extreme eastern portions of the country are very sparsely populated. Thus, for our analyses we divide the country into three broad regions: the arrondissements of Tillabery and Dosso (including the city of Niamey); the arrondissements of Tahoua, Maradi (including the city of Maradi) and Zinder (including the city of Zinder); and the northern and western arrondissements of Agadez and Diffa (see coloured regions in figure 2d). The former two regions tend to have large outbreaks that are more correlated within than between regions (figure 1c; Bharti et al. in press). The latter is characterized by much more episodic measles outbreaks (figure 1c). We also considered an alternative regional grouping that divides the arrondissement of Tahoua into eastern and western departments (allocated to the Niamey and Maradi regions, respectively), and the results we present are robust to this alternative grouping (see the electronic supplementary material, figure S3).
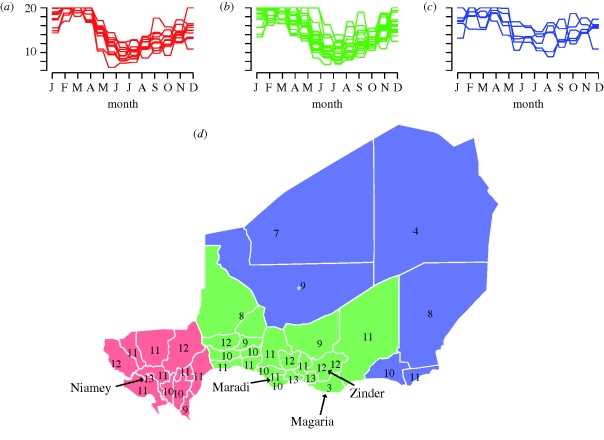
Figure 2.
(a) Estimated seasonal forcing of measles transmission rate (10× for display) for (a) the 11 districts and the city of Niamey corresponding to the red shaded area in (d), (b) the 18 districts and the cities of Maradi and Zinder shown in the green area in (d) and (c) the six districts shown in blue in (d). (d) Map of the departments and urban centres of Niger with the estimated range of the seasonal forcing (10× for display) shown for each.
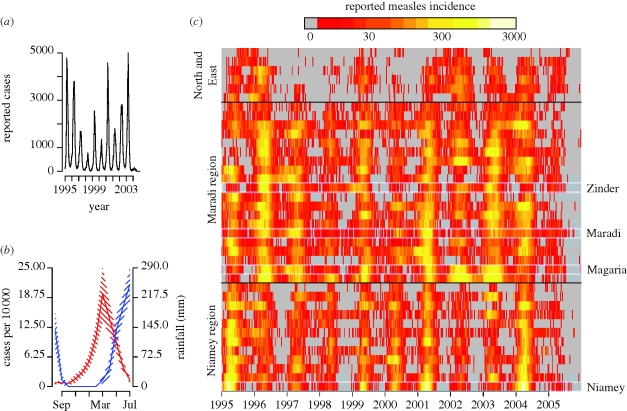
Figure 1.
(a) Weekly reported cases for all of Niger from 1995–2005. (b) The mean number of monthly cases in Niger over 1995–2005 (blue) compared with the mean monthly rainfall in Niamey (red). Shaded regions give ±2 s.e. (c) Logarithm of weekly reported measles incidence for all 35 districts and 3 urban centres over the time period 1995–2005. Each row of the matrix indicates one location. Weeks with 0 reported cases are shown in grey. Locations are grouped into the western arrondissements surrounding the city of Niamey, the southern arrondissements surrounding the city of Maradi, and the sparsely populated northern and eastern arrondissements (see map in figure 2).
We estimate the seasonal fluctuation in transmission rate for each reporting district by fitting the time series susceptible–infected–recovered (TSIR) model of Finkenstadt & Grenfell (2000) to the reported incidence aggregated into two week intervals, because 14 days is the approximate infectious generation time for measles (see the electronic supplementary material for more detail). Following Bjørnstad et al. (2002), we first reconstruct the unobserved time series of susceptible individuals as the residuals from the relationship between the cumulative reported incidence and the cumulative births. As some children will be vaccinated, and thus will not be susceptible to measles, we discounted the reported birth rate (51.73 births per 1000) by an assumed 70 per cent vaccination coverage (Ferrari et al. 2008). Vaccination coverage is likely to have increased over the time frame 1995–2005; WHO estimates that coverage with a single dose of measles vaccine ranged from 50 to 85 per cent over that timeframe (WHO 2009). In addition Niger conducted large-scale national campaigns in 2001 and 2005. Unfortunately, we do not have accurate regional reporting of vaccine coverage to permit a finer-scale accounting of vaccine coverage. However, while the changing vaccination coverage may bias estimates of the absolute transmission rate, estimates of the seasonal fluctuation in transmission rate are not strongly biased by assuming the mean vaccination coverage over that period (see the electronic supplementary material, appendix S2).
Measles incidence is likely to be under-reported. The TSIR model (below) accounts for this by estimating the reporting rate as the slope of the relationship between the cumulative reported incidence and the cumulative (vaccine-discounted) births. To account for variation in reporting rates, we estimated the time-varying reporting rate by fitting a smoothing spline with 2.5 d.f. (Bjørnstad et al. 2002). The TSIR model of Finkenstadt & Grenfell (2000) fits the time varying transmission rate as an unknown constant multiplied by a seasonally varying scaling factor. We modelled the seasonally varying component as a piecewise constant function with 13 (i.e. 4-week intervals) levels from January 1 to December 31. We estimated the parameters of the TSIR model from the reconstructed susceptible time series and the reported incidence as corrected by the reporting rate using least squares (Finkenstadt & Grenfell 2000). As the absolute value of the seasonal transmission rate is likely to be biased by the unknown vaccination rate, we present the seasonality as the variance of the seasonally varying scaling factor (see the electronic supplementary material, appendix S2).
To explore the effect of climatic factors and human aggregation on seasonality we fitted the estimated amplitude of seasonal forcing as a function of district rainfall and population size. Daily rainfall estimates were obtained from 2003 to 2007 from NOAA's Climate Prediction Center's CPC Morphing Technique (NOAA National Weather Service 2009). For analysis we used the average annual rainfall in each district over 2003–2007. As rainfall is 0 throughout the country during the dry season, this provides a measure of the magnitude of the difference between the dry and rainy seasons. District population sizes were taken from the official 1995 census report from Niger.
We explored the interaction between seasonality and the CCS for measles by simulating measles dynamics using the TSIR model with a two-week time step across a range of population sizes and amplitudes of seasonal forcing. We first simulated a generic case, with sinusoidal forcing βt=β1(1+β2 cos(2πt/26)), where β1 is the mean transmission rate and β2 controls the amplitude of seasonality (London & Yorke 1973). While the estimated seasonality for the Niger districts is not strictly a sinusoid (below), the pattern is crudely captured by a smooth wave. Thus, the sinusoid allows us to explore the general relationship of CCS and seasonal amplitude in terms of a single parameter, β2. In all simulations we scaled β1 relative to population size to give the same average RE (the expected number of secondary cases due to the first infectious case in a partially immune population) as was previously estimated for the capital city, Niamey (Ferrari et al. 2008). We further set the birth rate equal to the reported birth rate for Niger (50.73 births per 1000). We simulated time series for populations ranging from 100 000 to 1 000 000 and seasonal forcing β2 ranging from 0.1 to 0.8, which spans the range of seasonal amplitudes estimated for measles (pre-vaccine London ≈ 0.2 and contemporary Niamey ≈ 0.6; Ferrari et al. 2008). At each parameter combination we simulated 200 years of measles incidence with a small immigration rate of approximately 10 infected individuals per year. After a burn-in period of 150 years, we measured persistence as the proportion of years with two consecutive two-week intervals of 0 incidence.
We additionally conducted simulations using the estimated seasonality for the 38 districts and urban centres of Niger. These simulations were conducted as above, but using the estimated seasonality rather than generic sinusoidal forcing. For each location, we scaled the seasonal transmission rates by the population size (as above) to maintain the same average RE. As above, for each of the 38 seasonal patterns, we simulated 200 years of measles time series across a range of population size (10 000–700 000, the range of district population sizes) and recorded persistence in the last 50 years of the simulation.
3. Results
At the national scale, measles in Niger show regular annual outbreaks (figure 1a) that are strongly out of phase with the annual rainy season (figure 1b). Across the country, measles outbreaks tend to begin at the onset of the dry season and increase until the beginning of the rainy season (figure 1b,c). At the sub-national scale, measles dynamics in the 38 districts and urban centres are less regular. While the timing of measles outbreaks tends to be very regular, suggesting a common seasonal forcing, the magnitude of measles outbreaks varies greatly from year to year and across the country (figure 1c). Despite large variation in the amount of rain across the country (ranging from 1200 mm per year in the south to 50 mm per year in the North), the timing of measles outbreaks is highly correlated across the rainfall gradient (figure 1c).
The shape of the seasonal forcing of measles transmission is roughly consistent across the country (figure 2a–c; electronic supplementary material, figure S1). There is strong variation, however, in the amplitude of the seasonal fluctuation. The sparsely populated northern and eastern districts show consistently lower amplitude forcing than the southern and western districts. Within the southern and western districts, the highest seasonal amplitudes were associated with the densely populated urban centres of Niamey (in the west) and Maradi (in the east; figure 2d). Districts with high seasonal amplitude tended to cluster around the major urban centres in Niamey, Maradi and Zinder (figure 2d). The overall trend in seasonal amplitude follows the dominant north–south rainfall gradient and total annual rainfall is positively correlated with seasonal amplitude (p < 0.001, R2 = 0.37; figure 3a). However, this pattern is not consistent within each of the three regions of the country. Indeed, in the western districts surrounding Niamey the amplitude of seasonal forcing is negatively correlated with rainfall (p = 0.03, R2 = 0.3), and in the districts surrounding Maradi the relationship is not statistically significant (p = 0.2, R2 = 0.09; figure 3a). The relationship is also not statistically significant in the northern and eastern districts.
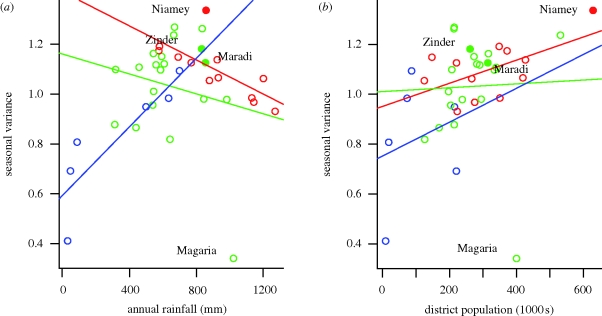
Figure 3.
(a) Estimated variance of seasonal forcing for the 38 departments and urban centres as function of mean annual rainfall during the period 2003–2006. Colours indicate the three regions (see map in figure 2). Solid lines are the regression fit for each region. Urban centres are shown as solid points. The department of Magaria, which is a strong outlier and was excluded from the analysis, is indicated. (b) Estimated variance of seasonal forcing for the 38 departments and urban centres as function of district population size (colours and lines are as in a).
The amplitude of seasonal forcing was strongly positively correlated with the population size in each department or urban centre across the entire country (p = 0.001, R2 = 0.27; figure 3b). This pattern also holds within the three regions of Niger; the linear relationship is significant in the western region containing Niamey (p = 0.05, R2 = 0.30) and the southern region containing Maradi and Zinder (excluding the department of Magaria, which is a marked outlier; p = 0.02, R2 = 0.24; figure 3b). To compare rainfall and population size, we fitted an ANCOVA with the three regions as categorical variables and population size and rainfall as covariates. Population size was a significant predictor of seasonality (p < 0.05) with rainfall in the model. Rainfall alone was not significant (p = 0.17), though there was a significant interaction of rainfall and region (p < 0.05), suggesting that the effect of rainfall on seasonality is not consistent across the country. These results hold for an alternative grouping of districts that assigns the departments of Tchin-Tabaraden, Illela, Tahoua and Bkonni to the Niamey region, and the department of Maine-Soroa to the Maradi region (see the electronic supplementary material, figure S3).
The Magaria outlier has an estimated range of seasonal forcing of 0.34, the lowest in the country, despite a relatively large population of 400 000 (figure 3a). This department is distinctive as it lies on the southern border with Nigeria. The border with Nigeria is very porous and there is frequent north–south movement of people between Niger and northern Nigeria (Raynaut 2001; Miles 2005; Bharti et al. in press), where measles is highly endemic and vaccination rates are low. The relatively low estimated seasonality in Magaria may reflect frequent reintroduction of measles following local fadeout (see figure 1c), which would manifest itself as though measles transmission rates were higher throughout the year.
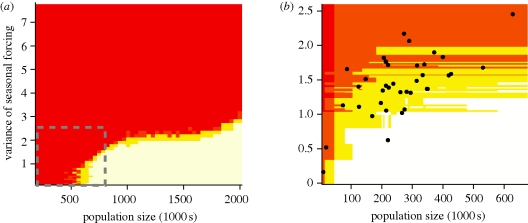
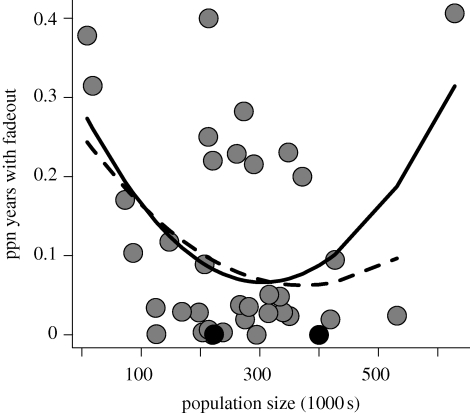
Bartlett (1957) first suggested that measles should be more persistent in large cities. For the simple generic model with sinusoidal forcing, an increase in the strength of seasonal forcing will increase the CCS for the long-term persistence of measles cycles (figure 4a). Above a critical degree of seasonal forcing, the threshold population size for persistence increases rapidly by several orders of magnitude (not shown). For the explicit seasonal patterns estimated for the Niger departments, the predicted pattern is qualitatively the same, though less smooth due to variation in the shape of the seasonal forcing (figure 4b). While many of the districts of Niger are large enough to support persistent measles cycles with weak seasonality, the correlation between population size and seasonal forcing means that local persistence is unlikely across the full range of district population sizes in Niger. Notably, while measles is expected to fade out locally at least once in 50 years in most of the Nigerien districts, the likelihood of local extinction is, on average, lower for districts of moderate size (figure 5). Indeed, the proportion of years with a local fade-out increases for large conurbations in the three largest districts (including Niamey, the largest). The quadratic relationship is significant both with and without Niamey in the analysis (p = 0.047 and p = 0.066, respectively). This stands in stark contrast to the classic pattern observed in Europe and North America, where the largest cities act as reservoirs for local measles persistence.
Figure 4.
Probability of measles fade-out as a function of population size and the amplitude of seasonal forcing. (a) The percentage of years (out 50) with two consecutive two-week intervals with 0 measles incidence for a model with sinusoidal seasonal forcing. The grey dashed box indicates the approximate parameter range for (b). (b) The same as (a) using estimated seasonal forcing for the 38 districts of Niger. Black dots indicate the population size and amplitude of seasonal forcing for the districts of Niger. (Red, more than 50%; orange, between 10% and 50%; yellow, between 0% and 10%; white, no fade-outs).
Figure 5.
The proportion of years with fade-outs for the 38 districts of Niger (grey dots). The two districts that had fadeouts every year are shown in black. Curves are the best-fit quadratic function with (solid) and without (dashed) Niamey included.
4. Discussion
Measles transmission in Niger is strongly seasonal across the entire country and the magnitude of seasonal forcing correlates with population size. The amplitude of seasonal forcing was broadly correlated rainfall. However, while the rainfall gradient is probably the ultimate determinant of human settlement and aggregation in Niger, population size is the most consistent predictor of measles seasonality across the country (figure 3a,b). Thus, the pattern of seasonality is consistent with the hypothesis that seasonal transmission is driven by anthropogenic rather than climatic factors. Previously, Guyer & McBean (1981) attributed the seasonality of measles in Cameroon to aggregation of people in cities during the dry season in response to agricultural cycles. It is probable that such seasonal aggregation is a contributing factor to the seasonal pattern in Niger; however, it is difficult to exclude the influence of seasonal variation in mobility (due to the saturation of roads in the rainy season) and reporting of measles cases. While variation in reporting rates may somewhat bias the observed seasonal amplitude, the consistent pattern of large magnitude variation in measles outbreaks across the country is consistent with strongly over-compensating cycles due to seasonal forcing (Ferrari et al. 2008) rather than observational errors due to reporting. Seasonal changes in density due to school terms have been classically implicated in driving transmission rates of childhood infections in Western Europe (Fine & Clarkson 1982; Gomes et al. 1999; Deguen et al. 2000; Finkenstadt & Grenfell 2000) and North America (London & Yorke 1973). In Niger, however, school terms are unlikely to be a major driver of measles seasonality as incidence is predominantly in children younger than school age (Ferrari et al. 2010).
Seasonal forcing of measles transmission can have strong consequences for the predictability and stability of both local (Bolker & Grenfell 1993; Keeling & Grenfell 1997; Earn et al. 2000; Rohani et al. 2002; Conlan & Grenfell 2007) and regional dynamics (Bolker & Grenfell 1995; Grenfell et al. 1995). In Niamey, very large seasonal fluctuations in measles transmission rates coupled with high birth rates drive large outbreaks followed by stochastic extinction of measles, which must be followed by reintroduction of infection from elsewhere. Our analysis of the reported incidence from 1995–2005 shows that the dynamics are characterized by seasonal outbreaks followed by frequent local extinctions. Thus maintenance of endemic measles in Niger requires rescue from local extinction either by internal migration of cases within Niger or transnational/international imports. The characteristic stability of metapopulations arises from the asynchrony in local extinction and the possibility for recolonization from elsewhere in the metapopulation (Hanski 1998). The strong synchrony of the seasonal transmission rates across Niger means that epidemic troughs tend to occur at the same time (figure 1c), reducing regional persistence due to synchronous extinction risk (Grenfell et al. 1995; Keeling et al. 2004).
Pre-vaccination England and Wales are perhaps the best-studied seasonally forced epidemic metapopulation (Grenfell & Bolker 1998; Grenfell et al. 2001; Xia et al. 2004). In that setting, seasonal forcing due to school terms was roughly constant across the metapopulation and endemic measles in the largest cities gave rise to hierarchical waves of measles spread outward from cities larger than approximately 300 000 people to smaller municipalities that tended to experience local fade-outs in the inter-epidemic troughs (Grenfell & Bolker 1998; Grenfell et al. 2001; Xia et al. 2004). The CCS for measles has been classically assumed to be in the order of 250 000 to 500 000, based on both theoretical and empirical investigation (Keeling & Grenfell 1997). Conlan & Grenfell (2007) showed that the CCS may be lower for high-birth-rate settings. These previous studies, however, assumed relatively low seasonality, consistent with the school term forcing of pre-vaccination England and Wales. Here we show, both for a generic sinusoidal model of seasonality and for the explicit seasonal pattern observed in Niger, that the CCS scales non-linearly with the amplitude of seasonal forcing, such that at the observed limits of measles seasonality (the urban districts of Niger) the CCS is likely to be several millions.
In Niger, seasonal forcing correlates with population size, meaning that measles is less persistent in the largest cities than it is in cities of moderate size. As such, the large cities of Niger do not play the classic role as reservoirs for measles infection during the seasonal troughs. This may, in part, explain why the dynamics in Niger do not exhibit the hierarchical wave-like spread that was seen in England and Wales (Grenfell et al. 2001).
The combination of highly synchronous seasonal forcing (though of varying magnitude) and dynamic instability of measles in large cities conspires to maintain relatively weak spatial correlation of measles dynamics across Niger. Since no single region in Niger appears to be able to act as a long-term reservoir for measles, based on the empirical observation (figure 1) or the theoretical prediction (figure 5), the connectivity between regions—and perhaps beyond the national borders—(Yameogo et al. 2005; Bharti et al. in press), is likely to be important to the large-scale persistence of measles in this seasonally forced metapopulation. This phenomenon may present challenges to public health planning as the magnitude of outbreaks is locally variable and there may be many outbreaks across the country separated by large distances, which can increase the costs of response. Large-scale pulsed vaccination strategies, such as the supplemental immunization activities currently conducted in conjunction with the Measles Initiative, may be a useful strategy to coordinate regional dynamics with the effect of reducing regional variation in the magnitude of measles outbreaks (Choisy et al. 2006). Further, these dynamics suggest that the maintenance of measles within Niger is a function of metapopulation dynamics at a scale beyond national boundaries (Bharti et al. in press), as is seen, for example, in the dynamics of meningitis (Bharti et al. in preparation). Thus, to the extent that it is logistically possible, control strategies aimed at measles elimination should consider the coordination of vaccination activities at the regional scale.
Acknowledgements
We would like to acknowledge the support of the Niger Ministry of Health and Médicins Sans Frontières for access to the Nigerien surveillance data. M.F., B.G. and O.B. were funded by RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health and a grant from the Bill and Melinda Gates Foundation. B.G. was additionally funded by the National Institutes of Health (R01 GM083 983-01).
References
- Altizer S., Dobson A., Hosseini P., Hudson P., Pascual M., Rohani P.2006Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484 (doi:10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- Aron J. L., Schwartz I. B.1984Seasonality and period-doubling bifurctions in an epidemic model. J. Theor. Biol. 110, 665–679 (doi:10.1016/S0022-5193(84)80150-2) [DOI] [PubMed] [Google Scholar]
- Bartlett M. S.1957Measles periodicity and community size. J. R. Statist. Soc. Ser. 120, 48–70 (doi:10.2307/2342553) [Google Scholar]
- Bauch C. T., Earn D. J. D.2003Transients and attractors in epidemics. Proc. R. Soc. Lond. B 270, 1573–1578 (doi:10.1098/rspb.2003.2410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti N., Djibo A., Ferrari M. F., Grais R. F., Tatem A. J., McCabe C. A., Bjornstad N., Grenfell B. T.In press Measles hotspots and epidemiological connectivity. Epidemiol. Infect. (doi:10.1017/S0950268809991385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti N., Broutin H., Grais R. F., Djibo A., Grenfell B. T.In preparation Spatial dynamics of meningitis in Niger: observed patterns in comparison with measles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnstad O. N., Finkenstadt B. F., Grenfell B. T.2002Dynamics of measles epidemics: estimating scaling of transmission rates using a time series SIR model. Ecol. Monogr. 72, 169–184 (doi:10.1890/0012-9615(2002)072) [Google Scholar]
- Bolker B. M., Grenfell B. T.1993Chaos and biological complexity in measles dynamics. Proc. R. Soc. Lond. B 251, 75–81 (doi:10.1098/rspb.1993.0011) [DOI] [PubMed] [Google Scholar]
- Bolker B., Grenfell B.1995Space, Persistence and dynamics of measles epidemics. Phil. Trans. R. Soc. Lond. B 348, 309–320 (doi:10.1098/rstb.1995.0070) [DOI] [PubMed] [Google Scholar]
- Broutin H., Philippon S., de Magny G. C., Courel M. F., Sultan B., Guegan J. F.2007Comparative study of meningitis dynamics across nine African countries: a global perspective. Int. J. Health Geogr. 6, (doi:10.1186/1476-072X-6-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choisy M., Guegan J. F., Rohani P.2006Dynamics of infectious diseases and pulse vaccination: teasing apart the embedded resonance effects. Physica D-Nonlinear Phenomena 223, 26–35 (doi:10.1016/j.physd.2006.08.006) [Google Scholar]
- Conlan A. J., Grenfell B. T.2007Seasonality and the persistence and invasion of measles. Proc. R. Soc. B 274, 1133–1141 (doi:10.1098/rspb.2006.0030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguen S., Thomas G., Chau N. P.2000Estimation of the contact rate in a seasonal SEIR model: application to chickenpox incidence in France. Statist. Med. 19, 1207–1216 (doi:10.1002/(SICI)1097-0258(20000515)19:9<1207::AID-SIM423>3.0.CO;2-L) [DOI] [PubMed] [Google Scholar]
- Dushoff J., Plotkin J. B., Levin S. A., Earn D. J. D.2004Dynamical resonance can account for seasonality of influenza epidemics. Proc. Natl Acad. Sci. USA 101, 16 915–16 916 (doi:10.1073/pnas.0407293101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earn D. J. D., Rohani P., Bolker B. M., Grenfell B. T.2000A simple model for complex dynamical transitions in epidemics. Science 287, 667–670 (doi:10.1126/science.287.5453.667) [DOI] [PubMed] [Google Scholar]
- Ferrari M. J., Grais R. F., Bharti N., Conlan A. J. K., Bjornstad O. N., Wolfson L. J., Guerin P. J., Djibo A., Grenfell B. T.2008The dynamics of measles in sub-Saharan Africa. Nature 451, 679–684 (doi:10.1038/nature06509) [DOI] [PubMed] [Google Scholar]
- Ferrari M. J., Djibo A., Grais R. F., Grenfell B. T., Bjornstad O. N.2010Episodic outbreaks bias estimates of age-specific force of infection: a corrected method using measles as an example. Epidemiol. Infect. 138, 108–116 (doi:10.1017/S0950268809990173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E. M., Clarkson J. A.1982Measles in England and Wales. 1. An analysis of factors underlying seasonal patterns. Int. J. Epidemiol. 11, 5–14 (doi:10.1093/ije/11.1.5) [DOI] [PubMed] [Google Scholar]
- Finkenstadt B. F., Grenfell B. T.2000Time series modelling of childhood diseases: a dynamical systems approach. J. R. Statist. Soc. Ser. C-Appl. Statist. 49, 187–205 (doi:10.1111/1467-9876.00187) [Google Scholar]
- Gomes M. C., Gomes J. J., Paulo A. C.1999Diphtheria, pertussis, and measles in Portugal before and after mass vaccination: a time series analysis. Eur. J. Epidemiol. 15, 791–798 (doi:10.1023/A:1007615513441) [DOI] [PubMed] [Google Scholar]
- Grassly N. C., Fraser C.2006Seasonal infectious disease epidemiology. Proc. R. Soc. B 273, 2541–2550 (doi:10.1098/rspb.2006.3604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell B. T., Bolker B. M.1998Cities and villages: infection hierarchies in a measles metapopulation. Ecol. Lett. 1, 63–70 (doi:10.1046/j.1461-0248.1998.00016.x) [Google Scholar]
- Grenfell B. T., Kleczkowski A., Ellner S. P., Bolker B. M.1994Measles as a case study in nonlinear forecasting and chaos. Phil. Trans. R. Soc. Lond. A 348, 515–530 (doi:10.1098/rsta.1994.0108) [Google Scholar]
- Grenfell B. T., Bolker B. M., Kleczkowski A.1995Seasonality and extinction in chaotic metapopulations. Proc. R. Soc. Lond. B 259, 97–103 (doi:10.1098/rspb.1995.0015) [Google Scholar]
- Grenfell B. T., Bjornstad O. N., Kappey J.2001Travelling waves and spatial hierarchies in measles epidemics. Nature 414, 716–723 (doi:10.1038/414716a) [DOI] [PubMed] [Google Scholar]
- Grenfell B. T., Bjornstad O. N., Finkenstadt B. F.2002Dynamics of measles epidemics: scaling noise, determinism, and predictability with the TSIR model. Ecol. Monogr. 72, 185–202 (doi:10.1890/0012-9615(2002)072[0185:DOMESN]2.0.CO;2) [Google Scholar]
- Guyer B., McBean A. M.1981The epidemiology and control of measles in Yaounde, Cameroun, 1968–1975. Int. J. Epidemiol. 10, 263–269 (doi:10.1093/ije/10.3.263) [DOI] [PubMed] [Google Scholar]
- Hanski I.1998Metapopulation dynamics. Nature (Lond.) 396, 41–49 (doi:10.1038/23876) [Google Scholar]
- Keeling M. J., Grenfell B. T.1997Disease extinction and community size: modeling the persistence of measles. Science 275, 65–67 (doi:10.1126/science.275.5296.65) [DOI] [PubMed] [Google Scholar]
- Keeling M. J., Grenfell B. T.2002Understanding the persistence of measles: reconciling theory, simulation and observation. Proc. R. Soc. Lond. B 269, 335–343 (doi:10.1098/rspb.2001.1898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling M., Bjornstad O. N., Grenfell B. T.2004Metapopulation dynamics of infectious diseases. In Ecology, genetics, and evolution of metapopulations (eds Hanski I., Gaggiotti O.), pp. 415–445 Burlington, MA: Elsevier Academic Press [Google Scholar]
- Lipsitch M., Viboud C. C.2009Influenza seasonality: lifting the fog. Proc. Natl Acad. Sci. USA 106, 3645–3646 (doi:10.1073/pnas.0900933106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- London W. P., Yorke J. A.1973Recurrent outbreaks of measles, chickenpox and mumps. I. Seasonal variation in contact rates. Am. J. Epidemiol. 98, 453–468 [DOI] [PubMed] [Google Scholar]
- Miles W. F. S.2005Development, not division: local versus external perceptions of the Niger–Nigeria boundary. J. Mod. Afr. Stud. 43, 297–320 (doi:10.1017/S0022278X05000844) [Google Scholar]
- NOAA National Weather Service. NOAA CPC Morphing Technique (‘CMORPH’). 2009 See http://www.cpc.ncep.noaa.gov/products/janowiak/cmorph_description.html. [Google Scholar]
- Raynaut C.2001Societies and nature in the Sahel: ecological diversity and social dynamics. Global Environ. Change-Human and Policy Dimensions 11, 9–18 (doi:10.1016/S0959-3780(00)00041-8) [Google Scholar]
- Rohani P., Keeling M. J., Grenfell B. T.2002The interplay between determinism and stochasticity in childhood diseases. Am. Nat. 159, 469–481 (doi:10.1086/339467) [DOI] [PubMed] [Google Scholar]
- Schenzle D.1984An age-structured model of pre- and post-vaccination measles transmission. IMA J. Math. Appl. Med. Biol. 1, 169–191 (doi:10.1093/imammb/1.2.169) [DOI] [PubMed] [Google Scholar]
- WHO 2009Reported estiamtes of MCV coverage. See http://www.who.int/immunization_monitoring/en/globalsummary/timeseries/tscoveragemcv.htm [Google Scholar]
- Xia Y. C., Bjornstad O. N., Grenfell B. T.2004Measles metapopulation dynamics: a gravity model for epidemiological coupling and dynamics. Am. Nat. 164, 267–281 (doi:10.1086/422341) [DOI] [PubMed] [Google Scholar]
- Yameogo K. R., et al. 2005Migration as a risk factor for measles after a mass vaccination campaign, Burkina Faso, 2002. Int. J. Epidemiol. 34, 556–564 (doi:10.1093/ije/dyi001) [DOI] [PubMed] [Google Scholar]