Abstract
The existence of genetic variation in offspring size in plants and animals is puzzling because offspring size is often strongly associated with fitness and expected to be under stabilizing selection. An explanation for variation in seed size is conflict between parents and between parents and offspring. However, for this hypothesis to be true, it must be shown that the offspring genotype can affect its own size. The existence of paternal effects would support this hypothesis, but these have rarely been shown. Using a diallel cross among four natural accessions of Arabidopsis thaliana we show that maternal, paternal and positional effects jointly influence seed size, number and the frequency of seed abortion. We found that seed abortion (%) depends on the combination of maternal and paternal genotypes, suggesting the existence of mate choice or epistatic incompatibility among accessions of A. thaliana. In addition, since paternal genotype explains approximately 10 per cent of the variation in seed size, we propose that A. thaliana's offspring must influence the amount of resources allocated to themselves. Identification of paternal effects in Arabidopsis should facilitate dissection of the genetic mechanisms involved in paternal effects.
Keywords: seed number, seed size, trade-off, natural variation, selective seed abortion, intraspecific incompatibility
1. Introduction
Large variation in seed size is observed among species (Halpern 2005), between conspecific populations (Vaughton & Ramsey 1998), within populations (Simons & Johnston 2000), among individual plants (Schaal 1980; Obeso 1993) and within fruit (Thompson 1984). Larger seeds often have higher probability of germination (Jacobson 1998; Westoby et al. 2002) and seedlings of larger seeds tend to have greater survival and improved performance under a wide range of environmental conditions (Krannitz et al. 1991; Westoby et al. 1996; Manning et al. 2009). Thus, the existence of such variation is puzzling because seed size is expected to have a strong effect on fitness and be under strong stabilizing selection (Silvertown 1989; Manzaneda et al. 2009).
Some of the intraspecific variation in seed size can be attributed to environmental effects that provide more or less resources to the maternal plant to invest on seeds (Roach & Wulff 1987; Manning et al. 2009). In addition, because plants are modular, this variation can also be owing to positional or developmental effects that alter how much of the total resources available for the mother's reproduction is provided to each fruit and seed (resource allocation) according to their position and/or developmental timing (Schaal 1980; Simons & Johnston 2000; Marr & Marshall 2006; Boyd et al. 2007). Nevertheless, if seed size is under strong selection, little additive genetic variation (heritability) is expected (Mousseau & Roff 1987). However, contrary to theoretical expectations, there is considerable heritability of seed size in a range of plant species, particularly in grain crops (Silvertown 1989; Sadras 2007). A commonly suggested explanation for the maintenance of additive genetic variation in seed size is that the optimum seed size may differ between parents, and between parent and offspring (Banuelos & Obeso 2003; de Jong & Scott 2007). If this scenario is correct, it may explain the evolution of imprinting in genes that affect seed size (Haig & Westoby 1991; de Jong & Scott 2007). Alternatively, seed heritability may be maintained by different environments favouring seeds of different sizes (Levene 1953).
For each offspring, the larger the seed, the higher their fitness potential. However, because the total resources available to a mother for investment in reproduction is usually limited, a trade-off between the size of seeds and the number of seeds that can be produced by a maternal plant is expected (Harper et al. 1970; Henery & Westoby 2001; Sadras 2007). Thus, if the relationship between seed size and fitness increases with diminishing returns, the optimum seed size for mothers (which maximizes the total number of viable seeds produced) is smaller than for the offspring (Smith & Fretwell 1974; Sadras 2007). By contrast, the optimal seed size for the paternal parent, will depend on whether the species usually selfs or outcrosses (de Jong et al. 2005). According to kin-selection theory, in a completely selfing plant, mother and father are equally related to all seeds, and have the same inclusive fitness. Thus, the optimal size for both mother and father is expected to be the same. However, with an increased rate of outcrossing, the father's inclusive fitness will benefit from making their own seeds larger and more fit, even if it reduces the overall number of seeds produced by the mother. Similarly, with high outcrossing, each offspring is less likely to be related to the other offspring in the same plant. Thus, if the genotype of the offspring determines its own size, it would be advantageous to draw more resources from the mother at the detriment of its siblings (‘sibling rivalry’). However, for any of these theories to explain variation in seed size, it is important to demonstrate that the offspring genotype can affect its own size. The best way to demonstrate that the offspring controls its own size is to show that there are paternal effects, but their existence remains controversial (de Jong & Scott 2007).
The diploid seed of angiosperms consists of three parts that differ in their genetic composition: embryo, endosperm and seed coat. At a given locus, the embryo carries one copy of the maternal allele and one copy of the paternal allele, the endosperm typically carries two copies of the maternal allele and one copy of the paternal allele; and the seed coat is usually composed of diploid maternal tissue. Thus, offspring traits can be owing to the genotype of the embryo, of the endosperm, or the combination of the two. In addition, successful seed development requires genetic compatibility/cooperation between these three distinct tissues.
The triploid nature of the endosperm has been suggested to be an evolutionary strategy by the maternal genotype to control seed size (Haig & Westoby 1991). In agreement with this hypothesis is the fact that many studies have found maternal effects on seed size (Roach & Wulff 1987; Mousseau & Fox 1998; Galloway et al. 2009; Holland et al. 2009), and only rarely have paternal effects been observed (e.g. Marshall & Whittaker 1989; Andersson 1990). On the other hand, crosses between Arabidopsis thaliana and maize with different ploidies support the idea that there are parent-of origin effects on seed size (Birchler 1993; Scott et al. 1998). In A. thaliana, larger seeds are produced when the paternal genome is in excess, while an excess of maternal genotype causes reduction or abortion of seeds (Scott et al. 1998). From a molecular perspective, imprinting in genes expressed in the endosperm have been identified in both A. thaliana and maize (Kinoshita et al. 2004; Gutierrez-Marcos et al. 2006; Gehring et al. 2009). However, so far, only PHERES 1, from A. thaliana has been identified to be preferentially paternally expressed (Kohler et al. 2005). These results suggest that paternal effects on seed size can exist in A. thaliana, but natural variation among paternal genotype in seed size has not yet been demonstrated. In addition, molecular characterization of such effect remains sparse.
A better understanding of the genetic control of seed size would help evaluate the different evolutionary theories for the maintenance of heritable variation in seed size. From an applied perspective, identification of the contribution of maternal, paternal and developmental factors to seed size and number can be very useful in developing strategies to improve grain yield (Egli 1998; Spillane et al. 2002). Arabidopsis thaliana is an ideal species in which to investigate the relative contribution of these factors because it is one of the primary models of plant genetics and evolutionary ecology. In addition, extensive variation in seed size (up to 3.5-fold) has been observed among natural accessions of A. thaliana (Krannitz et al. 1991; Alonso-Blanco et al. 1999; Stokes et al. 2007; Ungru et al. 2008). While a number of ‘loss of function’ laboratory mutants have been identified to affect the maternal genotype contribution to seed size (Garcia et al. 2003, 2005; Jofuku et al. 2005), very little is known about the genetic basis of the observed natural variation (but see Jofuku et al. 2005). More importantly, evidence of natural variation in seed size can be subsequently followed up with gene identification, given the extensive gene mapping arsenal that is available in A. thaliana (Lukowitz et al. 2000; Jander et al. 2002).
The goal of this study is to investigate whether there is differential allocation of resources to A. thaliana seeds, according to maternal and paternal genotypes and positional effects. To reach this goal we used a set of diallel crosses among four natural accessions and examined the variation in fruit size, seed number and average seed size. We show that paternal effects clearly contribute to natural variation in seeds size, indicating that the offspring's genotype can influence the amount of resources allocated to them.
2. Material and methods
(a). Plant material
Seeds for the four accessions of A. thaliana used in this study were originally obtained from the Arabidopsis Information Management System (www.arabidopsis.org): Columbia (Col-0, CS6673), Landsberg erecta (Ler-0, CS20), Catania (Ct-1, CS6674), and Wilna (Wil-2, CS6889). These four accessions were chosen because they have similar flowering times and because they are part of the set of accessions used to produce the Multiparent Advanced Generation InterCross mapping lines (Kover et al. 2009), allowing future mapping of these crosses if useful. Each accession was grown and selfed for one generation to bulk seeds and remove any possible environmental maternal effects. Since A. thaliana mainly selfs, each of these accessions are essentially inbred lines.
(b). Reciprocal crosses
To perform reciprocal crosses between all four accessions, we planted three seeds of each accession into each of 10 pots (6.5 cm in diameter) containing John Innes no. 1 compost. All seeds were cold-treated for 3 days at 4°C to promote synchronous germination. Pots were then placed in a growth chamber at 21°C with a 14 : 10 light : dark regime, and watered as needed with tap water.
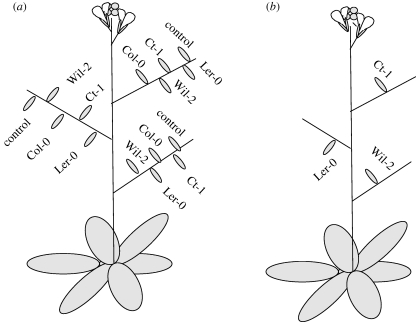
One plant in each pot was selected as a maternal plant. In each branch of maternal plants, four flower buds were emasculated prior to pollen maturation and hand pollinated with pollen from each of the three accessions. To reduce cross failure owing to pollen quality issues, pollen from at least three donor flowers of the same accession were used in each cross. The fourth bud was pollinated with pollen from a different flower from the same maternal plant (genetically equivalent to a selfed-fertilized cross), and a fifth bud was left undisturbed to produce naturally selfed-fertilized seeds (unmanipulated fruits). Thus, the size of seeds sired by different paternal genotype can be compared when growing side by side in the same branch of the same maternal plant. To ensure that the effect of maternal and paternal ecotypes on the allocation of resources to seeds was not obscured by the possible positional effects, we used a different order of paternal genotype across the buds in each branch (see figure 1a). Also, to remove developmental effects that may change resource allocation as the plant ages, all crosses in the same branch were carried out at the same time. The identity of the paternal accession used in each pollinated bud was recorded by colouring the pedicel of each bud with a waterproof pen (each paternal genotype was coded by a different colour). After elongation of the pedicels, small labels made of marking tape were used in addition to the pen markings to ensure the correct identification of the paternal genotype of each developing fruit. Typically, this protocol was repeated in three side branches of each maternal plant, so that each cross type was replicated 30 times. Besides the emasculated buds, a minimal number of buds were removed from the maternal plant so that observed seed sizes were in the context of a full and typical seed set for each maternal genotype.
Figure 1.
Schematic representation of cross scheme used. (a) Shows the scheme used in the first experiment, (b) the scheme used in the second experiment (see text for more details).
Two to three weeks after crossing, fruits were collected individually into glassine envelopes when they were deemed mature (as indicated by fruits opening upon light touch). During the process of fruit collection, failed crosses (which showed no elongation) and aborted fruits (fruits with less than 4 mm length) were recorded. Overall, 42 plants were used as maternal parents: 10 Col-0 plants, 10 Wil-2 plants, 10 Ct-1 plants and 12 Ler-0 plants. The two extra Ler-0 plants were needed because some plants only had two usable side branches. From these plants, a total of 580 fruits were collected.
In the first experiment (outlined above) we detected a significant effect of bud position but no effect of branch position (see results below). To ensure our results were robust to the experimental design we performed a second experiment. In this second experiment, maternal plants were grown as above, but only one cross was performed in each branch (so all crosses were performed on bud position 1), and a different paternal genotype was used in each branch (see figure 1b). Because most Ler-0 plants only have three usable branches for crosses, we only used three accessions in this experiment: Ct-1, Ler-0 and Wil-2.
(c). Measurement of reproductive traits
Each fruit was dissected individually under a dissecting microscope, where the fruit length and the number of viable and aborted seeds (smaller, darker and shrivelled seeds) were recorded. Since fruit size and seed number are highly correlated (r2 = 0.60, F1,380 = 570, p < 0.0001), we only present here the results for viable seed number.
Average seed area of a given fruit was obtained using the image of 10 random seeds acquired with a Leica DFC 320 R2 microscope with a digital camera and the program Leica IM50 Image Manager. Seed area was extracted using the measurement function of the program ImageJ v. 1.38x (NIH, Bethesda, MD). Seed area was used as a high throughput measurement of seed size, and seed size and seed weight has been previously shown to be highly correlated (r2 = 0.73 in Alonso-Blanco et al. 1999); and r2 = 0.6 in F. Goedecke and P. X. Kover 2006, unpublished results). Average seed area was only estimated for fruits in branches where all five crosses were successful to avoid any possible effect of reduced seed set on resource allocation (n = 214 fruits).
(d). Statistical analysis
(i). Evaluation of the effect of hand pollination
To determine if hand pollination affected cross survival, we performed an ordinal logistic regression with female genotype, branch, and treatment (hand-pollinated or unmanipulated) as fixed effects, and plant nested within genotypes as a random effect. To remove heterogeneities owing to paternal genotype, this analysis only included crosses where mother and father were of the same accession (as in the naturally self-fertilized unmanipulated fruits). To examine whether hand-pollination per se had an effect on number of viable seeds and seed size, we used generalized linear mixed model where female genotype, branch, and treatment (hand- pollinated or unmanipulated) were included as fixed effects; and plant nested within maternal genotype was included as a random variable.
(ii). Survival of crosses and levels of seed abortion
Among hand-pollinated crosses, the effects of maternal and paternal genotypes on cross survival (yes or no), and on the proportion of seeds aborted, were tested using an ordinal logistic regression. Female and male genotypes, branch and bud position were included as fixed effects in the model. The identity of the particular plant used in each cross was also included in the model as a random variable nested within maternal genotype to control for possible maternal non-genetic effects.
(iii). Differential allocation of resources in hand-pollinated crosses
Among the successful crosses, we used a generalized linear mixed model to evaluate differences in viable seed number and seed size. Maternal and paternal genotypes were included as fixed effects in the model, as well as branch and bud position; while plants nested within maternal genotype was included as a random effect. Viable seed number was cubic root transformed prior to analysis to normalize the residuals. To further investigate the paternal effects observed on seed size, we performed post hoc contrasts using differences in least square means tests (LSMEANS/SLICE statement in the GLM procedure in SAS). To confirm the effect of paternal and maternal genotypes on the second experiment, we also used a general linear mixed model with maternal and paternal genotypes as fixed effects and plants nested within maternal genotype as a random effect.
(iv). Trade-off between seed size and number
To determine whether there is a resource trade-off at the fruit level between the number of viable seeds produced and seed size, we performed a linear regression of seed size on seed number including all fruits. This analysis assumes that total resources allocated to a given fruit are independent of fruit size. Thus, we repeated this analysis including fruit length as a covariate in case fruits of different size obtain different amounts of resources. In addition, we used a mixed model to test whether seed size was affected by the number of viable seeds across all hand-pollinated fruits,when mother genotype is included as a fixed variable. This analysis was carried out in case maternal genotype differ intrinsically in the amount of resources available, which could obscure a trade-off. Finally, we repeated the analysis on seed size, as described in the section above including the number of viable seeds as a covariate to determine whether the paternal effect on seed size was just owing to differences in the number of viable seeds produced.
All statistics were conducted in SAS (v. 9) and in each instance data are presented as the mean ± 1 s.e. While analyses involving viable seed number were performed on transformed data, the raw means and errors are presented for ease of interpretation.
3. Results
(a). Effect of hand-pollination treatment
Hand-pollination affected many aspects of resource allocation to fruits (table 1), with a higher proportion of fruits and seeds failing to develop in hand-pollinated crosses. While the difference in fruit success was only marginally significant (log-likelihood ratio (LLR) = 3.66, p = 0.056), the difference in the proportion of aborted seed was highly significant (LLR = 15.03, p < 0.001). In addition, hand-pollinated fruits tend to be significantly shorter (F1,118 = 29.55, p < 0.001), with fewer viable seeds in them (F1,28 = 5.35, p = 0.028). However, no effect of hand-pollination on seed size was observed (F1,28 = 0.46, p = 0.50). These results indicate that hand-pollinated outcrossed fruits and unmanipulated, selfed crosses are not equivalent. Thus, for the rest of the results in the paper, we focus exclusively on hand-pollinated crosses.
Table 1.
The effect of hand-pollination versus natural selfing on fruit traits.
| manipulation |
||
|---|---|---|
| trait | hand pollinated | unmanipulated |
| cross success (%) | 75 ± 4.3 | 87 ± 3.6 |
| seed abortion (%) | 3.6 ± 1.9 | 1.6 ± 0.9 |
| fruit length (mm) | 9.5 ± 0.35 | 12.4 ± 0.45 |
| viable seeds | 22.51 ± 1.94 | 41.82 ± 2.16 |
| seed area (mm2) | 0.142 ± 0.003 | 0.140 ± 0.003 |
(b). Effect of maternal and paternal genotypes in hand-pollinated crosses
(i). Fruit survival and levels of seed abortion
Although 23 per cent of the crosses failed, the likelihood that the cross succeeded seems independent of the paternal (LLR = 1.11, p = 0. 78) or maternal (LLR = 0.01, p = 1.0) genotype; as well as their interaction, (LLR = 7.65, p = 0.57). However, bud position had a significant effect on cross survival, with older, more proximal buds being more likely to set fruit than younger, more distal buds (LLR = 22.81, p < 0.0001). It is possible that, since all crosses were carried out simultaneously, some of the younger buds were not yet receptive to pollen. The branch used to perform the crosses did not significantly effect cross success (LLR = 3.86, p = 0.28).
Among the crosses that did succeed, there were a small number of aborted seeds (4% of all seeds that started developing were aborted). Likelihood ratio tests showed that male (LLR = 84.72, p < 0.001) and female (LLR = 27.35, p < 0.001) genotypes, as well as their interaction (LLR = 151.0 p < 0.001) had a significant effect on the proportion of seeds aborted. For example, in Col-0 mothers, the highest proportion of seed abortion occurs in crosses with Wil-2 as a father. However, in Ct-1 mothers, crosses with Wil-2 fathers had the lowest proportion of aborted seeds (table 2). Also, the lowest proportion of seed abortion tends to be when mothers are crossed with pollen from their own accession (table 2). These results suggest that seed abortion may be the result of epistatic incompatibilities within the embryo genotype and/or differential maternal allocation to embryos of different fathers. There was no effect of bud (LLR = 0.93, p = 0.81) or branch (LLR = 7.61, p = 0.06) on the proportion of aborted seeds.
Table 2.
The proportion of seeds aborted (% ± s.e.) in hand-pollinated crosses as a function of male and female genotype.
| female genotype |
|||||
|---|---|---|---|---|---|
| male genotype | Col-0 | Ler-0 | Ct-1 | Wil-2 | overall |
| Col-0 | 0 ± 0 | 1.9 ± 1.3 | 4.5 ± 2.4 | 17.1 ± 7.3 | 5.0 ± 1.7 |
| Ler-0 | 1.9 ± 1.9 | 0 ± 0 | 7.9 ± 3.7 | 26.8 ± 10.3 | 8.1 ± 2.6 |
| Ct-1 | 5.8 ± 4.1 | 7.2 ± 2.6 | 3.2 ± 1.9 | 19.9 ± 9.6 | 8.2 ± 2.3 |
| Wil-2 | 15.6 ± 8.3 | 5.6 ± 2.5 | 0.3 ± 0.2 | 12.4 ± 8.0 | 8.1 ± 2.8 |
| overall | 6.0 ± 2.6 | 3.8 ± 1.0 | 4.0 ± 1.0 | 18.9 ± 4.4 | 7.3 ± 1.2 |
(ii). Viable seeds produced
The number of viable seeds was influenced only by male genotype (F3,250 = 7.39, p < 0.001); with fruits sired by Ler-0 fathers producing significantly less seeds (table 3). The effect of maternal genotype (F3,22 = 1.78, p = 0.171) and the interaction between maternal and paternal genotypes (F9,250 = 0.75, p = 0.665) was not significant. The effects of a genotype were not consistent as the paternal or maternal parent (table 3). For example, Ler-0 produced the most seeds when it was the maternal genotype but the least seeds when it was the paternal genotype (table 3). The position of the bud had a significant effect on the number of seeds produced, with more seeds being produced in the more proximal crosses than the distal crosses (F3,250 = 23.26, p < 0.001), but which branch was used to perform the crosses had no effect on the number of seeds produced (F3,250 = 0.27, p = 0.844).
Table 3.
Average number of viable seeds produced per fruit (mean ± s.e.) in successful hand-pollinated crosses as a function of maternal and paternal genotypes. (Superscript letters indicate maternal paternal genotypes that produce significantly different number of seeds according to least square mean differences test.)
| female genotype |
|||||
|---|---|---|---|---|---|
| male genotype | Col-0 | Ler-0 | Ct-1 | Wil-2 | overall |
| Col-0 | 27.8 ± 3.9 | 29.8 ± 4.1 | 17.5 ± 3.0 | 23.4 ± 4.2 | 24.4 ± 1.9a |
| Ler-0 | 15.2 ± 3.0 | 25.0 ± 4.0 | 9.25 ± 1.7 | 11.0 ± 3.0 | 15.5 ± 1.7b |
| Ct-1 | 26.1 ± 3.6 | 22.3 ± 3.5 | 16.9 ± 3.6 | 18.0 ± 4.5 | 20.9 ± 1.9a |
| Wil-2 | 22.8 ± 4.3 | 23.4 ± 4.2 | 22.6 ± 4.3 | 20.5 ± 3.8 | 22.4 ± 2.1a |
| overall | 22.8 ± 1.9a,b | 25.2 ± 1.9a | 16.7 ± 1.7a,b | 18.3 ± 1.9b | 20.9 ± 1.0 |
(iii). Seed size
Both male (F3,169 = 8.9, p < 0.0001) and female (F3,22 = 15.7, p < 0.0001) genotypes influenced the size of seeds produced, but there was no interaction between male and female genotypes on seed size (F9,169 = 1.5, p = 0.15). The effect of a particular genotype on seed size was again not consistent as the maternal or paternal genotypes (table 4). For example, when Ler-0 was the paternal genotype it produced on average the largest seeds, whereas when it was the maternal genotype it produced the smallest seeds. Similarly, among the maternal genotype, Ct-1 produced the largest seeds, but among the paternal genotype, Ct-1 produced relatively small seeds. While maternal genotype explained far more of the variation in seed size (29.3%), the paternal genotype also explains a substantial proportion of the variation (10.4%). Post hoc contrasts indicate that paternal genotypes produce significantly different seed sizes in Ct-1 and Ler-0 mothers (F3,169 = 3.6, p = 0.02; F3,169 = 10.7, p < 0.0001, respectively), and marginally significantly on Col-0 mothers (F3,169 = 2.4, p = 0.06). Seed sizes in Wil-0 mothers do not differ significantly among paternal genotype (F3,169 = 0.8, p = 0.5). There was no significant effect of branch (F1,169 = 0.8, p = 0.60) or bud position (F1,169 = 1.3, p = 0.69) on the size of seeds produced.
Table 4.
Average seed area (mm2 ± s.e.), as a function of male and female genotype in successful hand-pollinated crosses. (Superscript letters indicate paternal genotypes that are significantly different from other within a given maternal genotype, according to differences in least square means test.)
| female genotype |
|||||
|---|---|---|---|---|---|
| male genotype | Col-0 | Ler-0 | Ct-1 | Wil-2 | overall |
| Col-0 | 0.147 ± 0.007a,b | 0.101 ± 0.006b | 0.168 ± 0.004a | 0.130 ± 0.018a | 0.135 ± 0.005 |
| Ler-0 | 0.162 ± 0.013a | 0.145 ± 0.008a | 0.181 ± 0.009a | 0.137 ± 0.016a | 0.158 ± 0.006 |
| Ct-1 | 0.152 ± 0.007a | 0.100 ± 0.005b | 0.144 ± 0.005b | 0.122 ± 0.024a | 0.130 ± 0.005 |
| Wil-2 | 0.136 ± 0.009b | 0.103 ± 0.006b | 0.147 ± 0.003b | 0.118 ± 0.007a | 0.126 ± 0.005 |
| overall | 0.150 ± 0.005 | 0.112 ± 0.004 | 0.159 ± 0.004 | 0.126 ± 0.008 | 0.136 ± 0.003 |
In the second experiment, performed to confirm that the paternal and maternal effects on seed size were robust to the experimental design, we observed qualitatively similar results (table 5). As before, Ler-0 mothers produced on average the smallest seeds, and the Ler-0 fathers produced on average the largest seeds. In addition, the statistical analysis confirmed a significant effect of the maternal (F2,6 = 7.9, p = 0.021) and paternal (F2,27 = 7.32, p = 0.003) genotypes on seed size. No significant interaction between maternal and paternal effect was observed (F4,27 = 0.77, p = 0.552). Positional effects were not evaluated since only one cross was performed on each branch.
Table 5.
The average seed area (mm2 ± s.e.) for experiment 2, as a function of male and female genotype in hand-pollinated crosses.
| female genotype |
||||
|---|---|---|---|---|
| male genotype | Ler-0 | Ct-1 | Wil-2 | overall |
| Ler-0 | 0.140 ± 0.011 | 0.164 ± 0.011 | 0.159 ± 0.009 | 0.153 ± 0.007 |
| Ct-1 | 0.113 ± 0.006 | 0.166 ± 0.005 | 0.145 ± 0.004 | 0.135 ± 0.008 |
| Wil-2 | 0.103 ± 0.004 | 0.145 ± 0.001 | 0.133 ± 0.006 | 0.121 ± 0.005 |
| overall | 0.119 ± 0.006 | 0.161 ± 0.006 | 0.143 ± 0.005 | 0.137 ± 0.006 |
(iv). Trade-off between seed size and number
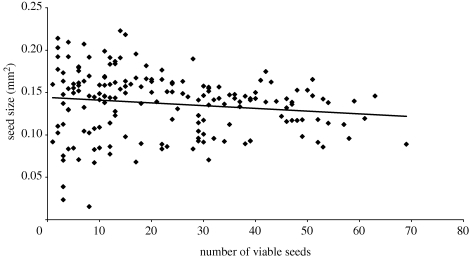
Across all fruits there is a negative relationship between the number of viable seeds produced in a fruit and the average seed size (figure 2), which is only marginally statistically significant (r2 = 0.02, F1,173 = 3.0, p = 0.085). Inclusion of fruit length in the model as a covariate results in no significant association between seed number and seed size in a given fruit (r2 = 0.003, F1,173 = 1.0, p = 0.514). The inclusion of maternal genotype in the model also does not alter this result: the effect of number of viable seeds on seed size remained non-significant (F1,147 = 0.01, p = 0.94). In addition, seed size was not influenced by the interaction between mother genotype and the number of viable seeds (F3,147 = 1.08, p = 0.36). Furthermore, when the number of viable seeds produced by fruit is included in the analysis of the seed size, we still obtain a significant effect of maternal (F3,22 = 13.36, p < 0.001) and paternal (F3,141 = 8.50, p < 0.001) genotypes; while the number of viable seeds (F1,141 = 0.02, p = 0.88), the branch used (F3,141 = 0.44, p = 0.73) and bud position (F1,141 = 1.34, p = 0.26) were not significant. This result indicates that the effect of paternal genotype on seed size is independent of fertilization success (i.e. of the number of seeds produced per fruit).
Figure 2.
The relationship between number of viable seed produced in a given fruit and the average seed size in the same fruit across all hand-pollinated crosses (y = − 0.003x + 0.1442; r2 = 0.0212).
4. Discussion
Here we show the existence of genetic variation among natural accessions of A. thaliana for reproductive allocation; with maternal, paternal and developmental effects jointly influencing seed size and number. As with previous experiments (Aarssen & Burton 1990; Platenkamp & Shaw 1993; Lemontey et al. 2000), we found that maternal genotype explains a larger proportion of the variance in seed size and number. However, paternal genotype also significantly affected resource allocation to seeds and explained a substantial amount of the variation (approx. 10% of the variation in seed size, and approx. 9% in seed number). The fact that there is significant genetic variation through maternal and paternal genotypes suggests that selection can still, in theory, increase seed size significantly. Although a response to selection would be more likely to come via selection on the maternal than paternal parent; the existence of paternal effects indicates that embryos must have some control over their own size. Thus, conflict over the ideal seed size between parents, and parents and offspring can explain the variation in seed number and size (de Jong & Scott 2007). However, the observed variation in seed number and size may also reflect past selection for these seed traits at their original collection sites, or stochastic factors (drift, bottlenecks) in the past history of the accessions.
We found that the same genotype has a different effect on the number of viable seeds and seed size when inherited maternally versus paternally. This may be the result of sex- specific expression (Hardenack et al. 1994; Ranz et al. 2003) or differential imprinting of an allele(s) when transmitted through the paternal genotype versus the maternal genotype (Dilkes & Comai 2004). In angiosperms silencing/suppression of paternal genes during seed development is more commonly observed (Kinoshita et al. 1999; Vielle-Calzada et al. 2000; Fitzgerald et al. 2008), leading to maternal effects. However, silencing/suppression of maternal genes have been observed (Kohler et al. 2005), supporting the idea that paternal effects can also exist.
In our experiment, paternal effects were particularly prominent in crosses involving Ler-0. Crosses with Ler-0 as a mother produce on average the smallest seeds of all four accessions, while crosses with Ler-0 as the paternal genotype have on average the largest seed size. Similar results were observed by Alonso-Blanco et al. (1999) in a reciprocal cross between Ler-0 and the accession Cvi-0. However, because only the two accessions were included in their study, the possibility of heterosis could not be distinguished from the occurrence of paternal effects. In our study, the effect of paternally inherited Ler-0 genes seems to be independent of the maternal genotype; i.e. within each maternal genotype, crosses with Ler-0 fathers always produces the largest seeds (table 4). This suggests that the genetic basis of this effect is stable across a wide genetic background, although the mechanism through which Ler-0 fathers produce larger seeds remains unknown. It is possible that the larger seed size is a direct consequence of the reduction in seed number since fruits with Ler-0 as fathers tend to produce less seeds (table 3). However, we argue that the larger size cannot be explained by a simple trade-off between size and number at the fruit level because we only observed a marginally significant trade-off, and the slope of the regression of seed size on seed number is very shallow (figure 2). In addition, in the mixed model for the effect of paternal and maternal genotypes on seed size, seed number was not significant.
As with a previous study (Stokes et al. 2007), we found that seed number and cross viability is affected by the hand-pollination treatment, confirming that care should be taken when comparing resource allocation in hand-pollinated and naturally selfed-crosses. The cross manipulation may have affected fertilization success by limiting pollen delivery, delivering pollen when the stigma was not receptive or the manipulation itself may have induced some fertilization failure. Although previous studies have suggested that emasculated flowers tend to produce larger seeds owing to the smaller number of seeds produced (Barth et al. 2003; Meyer et al. 2004), we did not find such an effect. Hand-pollinated fruits produced significantly less seeds, but no significant difference in seed size was observed. Stokes et al. (2007) also found no significant differences in seed size between naturally and hand-pollinated crosses. This result further indicates that the trade-off between seed size and seed number does not operate very strongly at the local fruit level, and that the reduction in seed number in one particular fruit does not necessarily result in larger seeds. Thus, an explanation for the difference in seed size among different paternal genotype must be searched for in the father genetic information.
Our study also revealed a strong genetic basis for the frequency of seed abortion, which seems to be strongly dependent on the combination of maternal and paternal genotypes. This can be owing to ‘mate choice’, post-zygotic endosperm incompatibility/imbalance, or pre-embryonic epistatic incompatibility. Mate choice refers to the possibility that mothers control seed development and may selectively abort seeds depending on the embryo or the father genotype (Marshall & Ellstrand 1988; Willson 1994; Korbecka et al. 2002). Endosperm imbalance or incompatibility has been observed in crosses between species, and is thought to be one of the main barriers to hybridization in angiosperms (Kinoshita 2007); it tends to disrupt the proper development of the endosperm causing seed abortion. Epistatic incompatibility is also common among different populations or subspecies of plants and animals, and is believed to be owing to interaction between genes that have evolved independently (e.g. Zeh & Zeh 1996; Fishman & Willis 2009). Incompatibility among A. thaliana accessions has been previously shown post-germination, as the result of autoimmune activity (Bomblies et al. 2007), thus it is possible that an analogous mechanism of epistatic incompatibility operates during seed development. However, further research will need to be carried out to investigate the mechanisms underlying this observation.
In summary, our study presents strong evidence for the presence of paternal effect on Ler-0, with the direction of the effect being compatible with a conflict between fathers and mothers for the ideal seed size. Such a pattern is expected in outcrossing species, but not in selfers (de Jong & Scott 2007). While it is hard to explain the maintenance of this effect in nearly inbred lines of A. thaliana, it is possible that the genetic factor underlying this effect is a new mutation that is neutral, since Ler-0 is mainly a laboratory strain. Alternatively, it is also possible that this paternal effect is ancestral to the evolution of selfing in A. thalianas (which might be very recent, see Bomblies & Weigel 2007). The closest relative of A. thaliana, A. lyrata is a self-incompatible outcrosser (Mable et al. 2005). Such a genetic effect could have become fixed during evolution by chance, or through selection as maternal and paternal contribution to seed size will be co-selected in a selfing species. Finally, it is also possible that such a genetic factor may have been favoured during the speciation of A. thaliana as a mechanism to promote post-zygotic hybridization barriers through endosperm incompatibility as suggested by Nyshiyama & Yabuno (1978). Independent of the mechanisms, further investigation of the genetic basis of this effect may provide valuable insights into the control of seed size and reveal possible avenues of manipulation of seed size.
Acknowledgements
We thank J. Wolf for helpful discussion and statistical advice and C. Klingenberg for use of imaging equipment and software. J.H. was funded by NERC and a Royal Society Fellowship, P.X.K. was funded by NERC and BBSRC.
References
- Aarssen L. W., Burton S. M.1990Maternal effects at four levels in Senecio vulgaris (Asteraceae) grown on a soil nutrient gradient. Am. J. Bot. 77, 1231–1240 (doi:10.2307/2444634) [Google Scholar]
- Alonso-Blanco C., Vries H. B.-D., Hanhart C. J., Koornneef M.1999Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 96, 4710–4717 (doi:10.1073/pnas.96.8.4710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S.1990Paternal effects on seed size in a population of Crepis tectorum (Asteraceae). Oikos 59, 3–8 (doi:10.2307/3545114) [Google Scholar]
- Banuelos M. J., Obeso J. R.2003Maternal provisioning, sibling rivalry and seed mass variability in the dioecious shrub Rhamnus alpinus. Evol. Ecol. 17, 19–31 (doi:10.1023/A:1022430302689) [Google Scholar]
- Barth S., Busimi A. K., Friedrich Utz H., Melchinger A. E.2003Heterosis for biomass yield and related traits in five hybrids of Arabidopsis thaliana L. Heynh. Heredity 91, 36–42 (doi:10.1038/sj.hdy.6800276) [DOI] [PubMed] [Google Scholar]
- Birchler J.1993Dosage analysis of maize endosperm development. Annu. Rev. Genet. 27, 181–204 (doi:10.1146/annurev.ge.27.120193.001145) [DOI] [PubMed] [Google Scholar]
- Bomblies K., Weigel D.2007Arabidopsis: a model genus for speciation. Curr. Opin. Genet. Dev. 17, 500–504 (doi:10.1016/j.gde.2007.09.006) [DOI] [PubMed] [Google Scholar]
- Bomblies K., Lempe J., Epple P., Warthmann N., Lanz C., Dangl J. L., Weigel D.2007Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biol. 5, e236 (doi:10.1371/journal.pbio.0050236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd E. W., Dorn L. A., Weinig C., Schmitt J.2007Maternal effects and germination timing mediate the expression of winter and spring annual life histories in Arabidopsis thaliana. Int. J. Plant Sci. 168, 205–214 (doi:10.1086/509587) [Google Scholar]
- de Jong T., Scott R.2007Parental conflict does not necessarily lead to the evolution of imprinting. Trends Plant Sci. 12, 439–443 (doi:10.1016/j-tplants.2007.07.003) [DOI] [PubMed] [Google Scholar]
- de Jong T., Van Dijk H., Klinkhamer P.2005Hamilton's rule, imprinting and parent-offspring conflict over seed mass in partially selfing plants. J. Evol. Biol. 18, 676–682 (doi:10.1111/j.1420-9101.2004.00856.x) [DOI] [PubMed] [Google Scholar]
- Dilkes B. P., Comai L.2004A differential dosage hypothesis for parental effects in seed development. Plant Cell 16, 3174–3180 (doi:10.1105/tpc.104.161230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D.1998Seed biology and the yield of grain crops. Oxfordshire, UK: CAB International [Google Scholar]
- Fishman L., Willis J. H.2009Evidence for Dobzhansky–Muller incompatibilities contributing to the sterility of hybrids between Mimulus guttatus and M. nasutus. Evolution 55, 1932–1942 [DOI] [PubMed] [Google Scholar]
- Fitzgerald J., Luo M., Chaudhury A., Berger F.2008DNA methylation causes predominant maternal controls of plant embryo growth. PLoS ONE 3, e2298 (doi:10.1371/journal.pone.0002298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway L. D., Etterson J. R., McGlothlin J. W.2009Contribution of direct and maternal genetic effects to life-history evolution. New Phytol. 183, 826–838 (doi:10.1111/j.1469-8137.2009.02939.x) [DOI] [PubMed] [Google Scholar]
- Garcia D., Saingery V., Chambrier P., Mayer U., Jurgens G., Berger F.2003Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiol. 131, 1661–1670 (doi:10.1104/pp.102.018762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D., Fitzgerald J. N., Berger F.2005Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis. Plant Cell 17, 52–60 (doi:10.1105/tpc.104.027136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M., Bubb K. L., Henikoff S.2009Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science 324, 1447–1451 (doi:10.1126/science.1171609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Marcos J. F., Costa L. M., Pra M. D., Scholten S., Kranz E., Perez P., Dickinson H. G.2006Epigenetic asymmetry of imprinted genes in plant gametes. Nat. Genet. 38, 876–878 (doi:10.1038/ng1828) [DOI] [PubMed] [Google Scholar]
- Haig D., Westoby M.1991Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Phil. Trans. R. Soc. Lond. B 333, 1–13 (doi:10.1098/rstb.1991.0057) [Google Scholar]
- Halpern S. L.2005Sources and consequences of seed size variation in Lupinus perennis (Fabaceae): adaptive and non-adaptive hypotheses. Am. J. Bot. 92, 205–213 (doi:10.3732/ajb.92.2.205) [DOI] [PubMed] [Google Scholar]
- Hardenack S., Ye D., Saedler H., Grant S.1994Comparison of MADS box gene expression in developing male and female flowers of the dioecious plant White Campion. Plant Cell 6, 1775–1787 (doi:10.1105/tpc.6.12.1775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. L., Lovell P. H., Moore K. G.1970The shapes and sizes of seeds. Annu. Rev. Ecol. Syst. 1, 327–356 (doi:10.1146/annurev.es.01.110170.001551) [Google Scholar]
- Henery M., Westoby M.2001Seed mass and seed nutrient content as predictors of seed output variation between species. Oikos 92, 479–490 (doi:10.1034/j.1600-0706.2001.920309.x) [Google Scholar]
- Holland J. N., Chamberlain, Scott A., Waguespack, Aline M., Kinyo Anthony S.2009Effects of pollen load and donor diversity on seed and fruit mass in the columnar cactus, Pachycereus schottii (Cactaceae). Int. J. Plant Sci. 170, 467–475 (doi:10.1086/597266) [Google Scholar]
- Jacobson D. J.1998Persistent, systemic, asymptomatic infections of Albugo. Can. J. Bot. Revue Canadienne De Botanique 76, 739–750 (doi:10.1139/cjb-76-5-739) [Google Scholar]
- Jander G., Norris S., Rounsley S., Bush D., Levin I.2002Arabidopsis map-based cloning in the post-genome era. Plant Physiol. 129, 440–450 (doi:10.1104/pp.003533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku J., Omidyar P., Gee Z., Okamuro J.2005Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc. Natl Acad. Sci. USA 102, 3117–3122 (doi:10.1073/pnas.0409893102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T.2007Reproductive barrier and genomic imprinting in the endosperm of flowering plants. Genes Genet. Syst. 82, 177–186 (doi:10.1266/ggs.82.177) [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Yadegari R., Harada J. J., Goldberg R. B., Fischer R. L.1999Imprinting of the MEDEA polycomb gene in the Arabidopsis endosperm. Plant Cell 11, 1945–1952 (doi:10.1105/tpc.11.10.1945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Miura A., Choi Y., Kinoshita Y., Cao X., Jacobsen S. E., Fischer R. L., Kakutani T.2004One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science. 303, 521–523 (doi:10.1126/science.1089835) [DOI] [PubMed] [Google Scholar]
- Kohler C., Page D. R., Gagliardini V., Grossniklaus U.2005The Arabidopsis thaliana MEDEA polycomb group protein controls expression of PHERES1 by parental imprinting. Nat. Genet. 37, 28–30 [DOI] [PubMed] [Google Scholar]
- Korbecka G., Klinkhamer P. G. L., Vrieling K.2002Selective embryo abortion hypothesis revisited: a molecular approach. Plant Biol. 4, 298–310 (doi:10.1055/s-2002-32331) [Google Scholar]
- Kover P. X., Valdar W., Trakalo J., Scarcelli N., Ehrenreich I., Purugganan M., Durrant C., Mott R.2009A multiparent advanced generation intercross to fine-map quantitative traits in Arabidopsis thaliana. PLoS Genet. 5, e1000551 (doi:10.1371/journal.pgen.1000551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krannitz P. G., Aarssen L. W., Dow J. M.1991The effect of genetically based differences in seed size on seedling survival in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 78, 446–450 (doi:10.2307/2444967) [Google Scholar]
- Lemontey C., Mousset-Declas C., Munier-Jolain N., Boutin P.2000Maternal genotype influences pea seed size by controlling both mitotic activity during early embryogenesis and final endoreduplication level/cotyledon cell size in mature seed. J. Exp. Bot. 51, 167–175 (doi:10.1093/jexbot/51.343.167) [DOI] [PubMed] [Google Scholar]
- Levene H.1953Genetic equilibrium when more than one ecological niche is available. Am. Nat. 87, 331–333 (doi:10.1086/281792) [Google Scholar]
- Lukowitz W., Gillmor C., Scheible W.2000Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol. 123, 795–805 (doi:10.1104/pp.123.3.795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mable B. K., Robertson A. V., Dart S., Berardo C. D., Witham L.2005Breakdown of self-incompatibility in the perennial Arabidopsis lyrata (Brassicaceae) and its genetic consequences. Evolution 59, 1437–1448 [PubMed] [Google Scholar]
- Manning P., Houston K., Evans T.2009Shifts in seed size across experimental nitrogen enrichment and plant density gradients. Basic Appl. Ecol. 10, 300–308 (doi:10.1016/j.baae.2008.08.004) [Google Scholar]
- Manzaneda A., Rey P., Alcantara J.2009Conflicting selection on diaspore traits limits the evolutionary potential of seed dispersal by ants. J. Evol. Biol. 22, 1407–1417 (doi:10.1111/j.1420-9101.2009.01752.x) [DOI] [PubMed] [Google Scholar]
- Marr D. L., Marshall M. L.2006The role of fungal pathogens in flower size and seed mass variation in three species of Hydrophyllum (Hydrophyllaceae). Am. J. Bot. 93, 389–398 (doi:10.3732/ajb.93.3.389) [DOI] [PubMed] [Google Scholar]
- Marshall D. L., Ellstrand N. C.1988Effective mate choice in wild radish: evidence for selective seed abortion and its mechanism. Am. Nat. 131, 739–756 (doi:10.1086/284816) [Google Scholar]
- Marshall D. L., Whittaker K. L.1989Effects of pollen donor identity on offspring quality in wild radish, Raphanus sativus. Am. J. Bot. 76, 1081–1088 (doi:10.2307/2444530) [Google Scholar]
- Meyer R. C., Torjek O., Becher M., Altmann T.2004Heterosis of biomass production in Arabidopsis. Establishment during early development. Plant Physiol. 134, 1813–1823 (doi:10.1104/pp.103.033001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau T. A., Fox C. W.1998The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- Mousseau T. A., Roff D. A.1987Natural selection and the heritability of fitness components. Heredity 59, 181–197 (doi:10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- Nyshiyama I., Yabuno T.1978Casual relationships between the polar nuclei in double fertilization and interspecific cross incompatibility in Avena. Cytologia 43, 453–466 [Google Scholar]
- Obeso J. R.1993Seed mass variation in the perennial herb Aspodelus albus: sources of variation and position effect. Oecologia 93, 571–575 (doi:10.1007/BF00328967) [DOI] [PubMed] [Google Scholar]
- Platenkamp G. A. J., Shaw R. G.1993Environmental and genetic maternal effects on seed characters in Nemophila menziesii. Evolution 47, 540–555 (doi:10.2307/2410070) [DOI] [PubMed] [Google Scholar]
- Ranz J. M., Castillo-Davis C. I., Meiklejohn C. D., Hartl D. L.2003Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science 300, 1742–1745 (doi:10.1126/science.1085881) [DOI] [PubMed] [Google Scholar]
- Roach D. A., Wulff R. D.1987Maternal effects in plants. Annu Rev. Ecol. Syst. 18, 209–235 [Google Scholar]
- Sadras V. O.2007Evolutionary aspects of the trade-off between seed size and number in crops. Field Crops Res. 100, 125–138 (doi:10.1016/j.fcr.2006.07.004) [Google Scholar]
- Schaal B. A.1980Reproductive capacity and seed size in Lupinus texensis. Am. J. Bot. 67, 703–709 (doi:10.2307/2442663) [Google Scholar]
- Scott R. J., Spielman M., Bailey J., Dickinson H. G.1998Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125, 3329–3341 [DOI] [PubMed] [Google Scholar]
- Silvertown J.1989The paradox of seed size and adaptation. Trends Ecol. Evol. 4, 24–26 (doi:10.1016/0169-5347(89)90013-X) [DOI] [PubMed] [Google Scholar]
- Simons A. M., Johnston M. O.2000Variation in seed traits of Lobelia inflata (Campanulaceae): sources and fitness consequences. Am. J. Bot. 87, 124–132 (doi:10.2307/2656690) [PubMed] [Google Scholar]
- Smith C. C., Fretwell S. D.1974The optimal balance between size and number of offspring. Am. Nat. 108, 499–506 (doi:10.1086/282929) [Google Scholar]
- Spillane C., Grossniklaus U., Vielle-Calzada J.2002Parent-of-origin effects and seed development: genetics and epigenetics. In Transgenic plants and crops (eds Khachatourians G., McHughen A., Scorza R., Nip W., Hui Y.), pp. 109–136 London, UK: Taylor & Francis [Google Scholar]
- Stokes D., Morgan C., O'Neill C., Bancroft I.2007Evaluating the utility of Arabidopsis thaliana as a model for understanding heterosis in hybrid crops. Euphytica 156, 157–171 (doi:10.1007/s10681-007-9362-1) [Google Scholar]
- Thompson J. N.1984Variation among individual seed masses in Lomatium grayi (Umbelliferae) under controlled conditions: magnitude and partitioning of the variance. Ecology 65, 626–631 (doi:10.2307/1941425) [Google Scholar]
- Ungru A., Nowack M. K., Reymond M., Shirzadi R., Kumar M., Biewers S., Grini P. E., Schnittger A.2008Natural variation in the degree of autonomous endosperm formation reveals independence and constraints of embryo growth during seed development in Arabidopsis thaliana. Genetics 179, 829–841 (doi:10.1534/genetics.107.084889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughton G., Ramsey M.1998Sources and consequences of seed mass variation in Banksia marginata (Proteaceae). J. Ecol. 86, 563–573 (doi:10.1046/j.1365-2745.1998.00279.x) [Google Scholar]
- Vielle-Calzada J.-P., Baskar R., Grossniklaus U.2000Delayed activation of the paternal genome during seed development. Nature 404, 91–94 (doi:10.1038/35003595) [DOI] [PubMed] [Google Scholar]
- Westoby M., Leishman M., Lord J.1996Comparative ecology of seed size and dispersal. Phil. Trans R. Soc. Lond. B 351, 1309–1318 (doi:10.1098/rstb.1996.0114) [Google Scholar]
- Westoby M., Falster D. S., Moles A. T., Vesk P., Wright I.2002Plant ecological strategies: some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 33, 125–159 (doi:10.1146/annurev.ecolsys.33.010802.150452) [Google Scholar]
- Willson M. F.1994Sexual selection in plants: perspective and overview. Am. Nat. 144, S13–S39 (doi:10.1086/285651) [Google Scholar]
- Zeh J. A., Zeh D. W.1996The evolution of polyandry. I: intragenomic conflict and genetic incompatibility. Proc. R. Soc. Lond. B 263, 1711–1717 (doi:10.1098/rspb.1996.0250) [Google Scholar]