Abstract
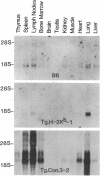
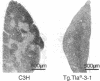
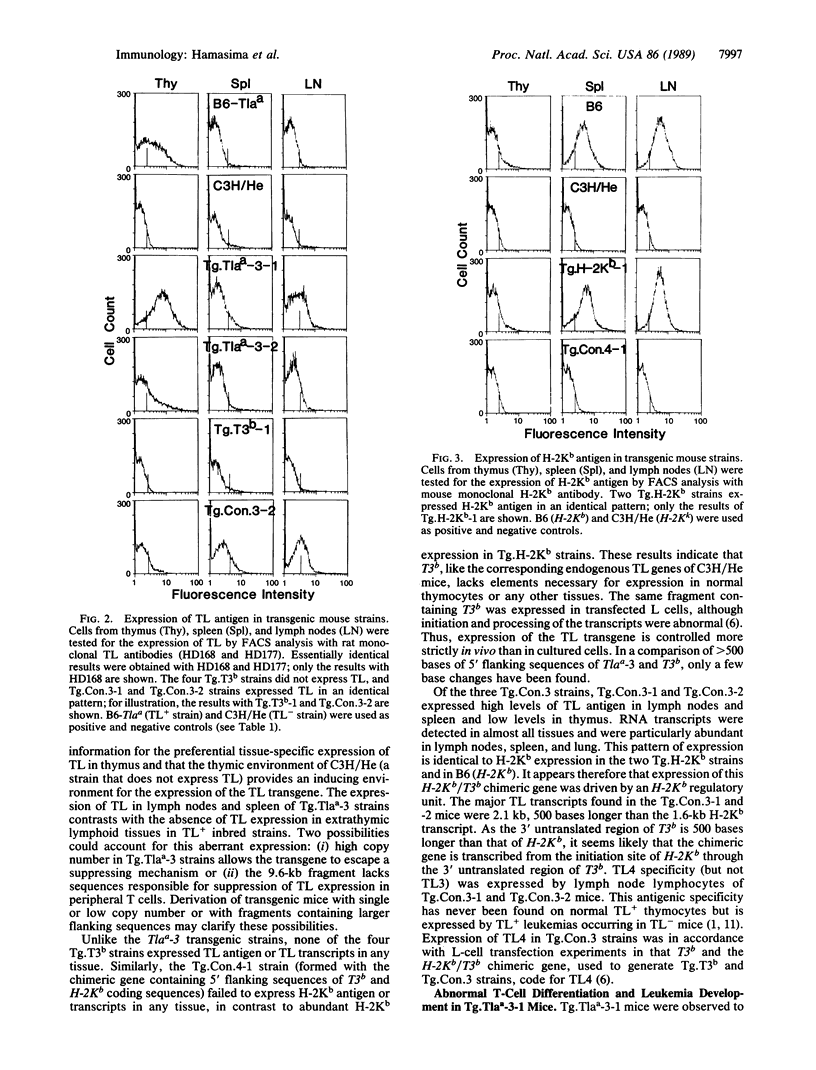
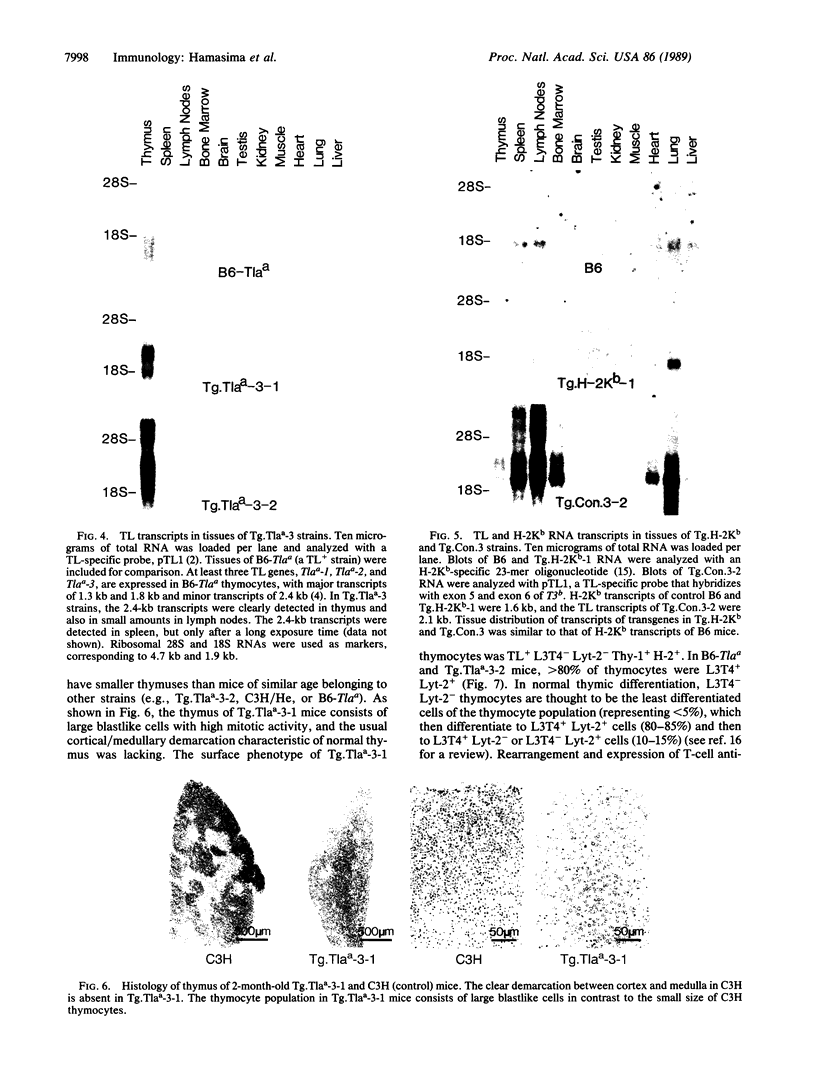
To investigate the genetic regulation of TL expression, 12 transgenic mouse strains on a C3H (TL-nonexpressing) background have been derived: two Tg.Tlaa-3 strains with Tlaa-3 isolated from A-strain TL+ thymocytes, four Tg.T3b strains with T3b from a TL+ leukemia arising in a C57BL/6 (TL-) mouse, three Tg.Con.3 strains with an H-2Kb/T3b chimeric gene (construct 3,5'flanking region and exon 1 of H-2Kb and exons 2-6 of T3b), one Tg.Con.4 strain with a T3b/H-2Kb chimeric gene (construct 4, 5' flanking region and exon 1 of T3b and exons 2-8 of H-2Kb), and two Tg.H-2Kb strains with H-2Kb. Expression of the transgenes was determined by the presence of TL or H-2Kb products or transcripts. Both Tg.Tlaa-3 strains expressed high levels of TL antigen in thymus, indicating that (i) the 9.6-kilobase Tlaa-3 DNA fragment contains sufficient information for correct tissue-specific expression in thymocytes and (ii) TL- thymocytes of C3H provide conditions for the transcriptional activation of Tlaa-3. In contrast, neither the four Tg.T3b strains nor the Tg.Con.4 strain expressed transgenes, indicating that (i) T3b lacks elements necessary for TL expression in normal thymocytes and (ii) the corresponding endogenous TL genes of C3H mice also lack these elements. The pattern of TL expression in two of the three Tg.Con.3 strains was similar to that of H-2Kb expression, indicating that transcription of this H-2Kb/T3b chimeric gene was driven by the regulatory sequences of H-2Kb. The thymuses of mice derived from the Tg.Tlaa-3-1 strain were smaller than C3H thymuses, and the surface phenotype of Tg.Tlaa-3-1 thymocytes resembled thymocyte precursors (TL+L3T4-Lyt-2-Thy-1+H-2+). These mice developed a high incidence of lymphomas with the same thymocyte precursor phenotype. The study of TL transgenic strains should prove useful in defining the role of TL in normal and abnormal T-cell differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. T., Obata Y., Stockert E., Old L. J. Thymus-leukemia (TL) antigens of the mouse. Analysis of TL mRNA and TL cDNA TL+ and TL- strains. J Exp Med. 1985 Oct 1;162(4):1134–1148. doi: 10.1084/jem.162.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T., Obata Y., Stockert E., Takahashi T., Old L. J. Tla-region genes and their products. Immunol Res. 1987;6(1-2):30–45. doi: 10.1007/BF02918102. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Fisher D. A., Hunt S. W., 3rd, Hood L. Structure of a gene encoding a murine thymus leukemia antigen, and organization of Tla genes in the BALB/c mouse. J Exp Med. 1985 Aug 1;162(2):528–545. doi: 10.1084/jem.162.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyada C. G., Wallace R. B. Liver-specific expression of a Qa-encoded class I gene is associated with DNA hypomethylation. Mol Cell Biol. 1986 Jan;6(1):315–317. doi: 10.1128/mcb.6.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Chen Y. T., Stockert E., Old L. J. Structural analysis of TL genes of the mouse. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5475–5479. doi: 10.1073/pnas.82.16.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Stockert E., Chen Y. T., Takahashi T., Old L. J. Influence of 5' flanking sequences on TL and H-2 expression in transfected L cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3541–3545. doi: 10.1073/pnas.85.10.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- Pontarotti P. A., Mashimo H., Zeff R. A., Fisher D. A., Hood L., Mellor A., Flavell R. A., Nathenson S. G. Conservation and diversity in the class I genes of the major histocompatibility complex: sequence analysis of a Tlab gene and comparison with a Tlac gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1782–1786. doi: 10.1073/pnas.83.6.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scollay R., Wilson A., D'Amico A., Kelly K., Egerton M., Pearse M., Wu L., Shortman K. Developmental status and reconstitution potential of subpopulations of murine thymocytes. Immunol Rev. 1988 Aug;104:81–120. doi: 10.1111/j.1600-065x.1988.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Karjalainen K., Pelkonen J., Borgulya P., Rammensee H. G. The T-cell receptor for antigen in T-cell development and repertoire selection. Immunol Rev. 1988 Jan;101:21–37. doi: 10.1111/j.1600-065x.1988.tb00731.x. [DOI] [PubMed] [Google Scholar]