Abstract
Ecologists have long been fascinated by the flora and fauna of extreme environments. Physiological studies have revealed the extent to which lifestyle is constrained by low temperature but there is as yet no consensus on why the diversity of polar assemblages is so much lower than many tropical assemblages. The evolution of marine faunas at high latitudes has been influenced strongly by oceanic cooling during the Cenozoic and the associated onset of continental glaciations. Glaciation eradicated many shallow-water habitats, especially in the Southern Hemisphere, and the cooling has led to widespread extinction in some groups. While environmental conditions at glacial maxima would have been very different from those existing today, fossil evidence indicates that some lineages extend back well into the Cenozoic. Oscillations of the ice-sheet on Milankovitch frequencies will have periodically eradicated and exposed continental shelf habitat, and a full understanding of evolutionary dynamics at high latitude requires better knowledge of the links between the faunas of the shelf, slope and deep-sea. Molecular techniques to produce phylogenies, coupled with further palaeontological work to root these phylogenies in time, will be essential to further progress.
Keywords: biogeography, diversity, ice-sheet, latitude, Milankovitch, temperature
1. Introduction
It is certain that the fauna of any such region is qualitatively poorer than that of warm temperate and tropical areas of comparable effective precipitation. It is probably considered to be intuitively obvious that this should be so, but on analysis the obviousness tends to disappear. If we can have one or two species of a large family adapted to the rigors of Arctic existence, why can we not have more?
Ecologists have long been fascinated by the flora and fauna of extreme environments. What adaptations allow mammals to survive in the harshest of deserts, plants to colonize the highest mountaintops, or fish to live in sea water colder than the freezing point of their body fluids? Half a century ago the eminent ecologist Evelyn Hutchinson, in a justly famous essay (Hutchinson 1959), encapsulated the problem in the three succinct sentences quoted above. Although concerned primarily with the Arctic terrestrial environment, Hutchinson's remarks point to two important general themes in evolution: the nature of adaptation, and the relationship between evolutionary processes and diversity. In this paper we explore aspects of both these themes, using as our example the marine faunas of the two polar regions.
(a). What is an extreme environment?
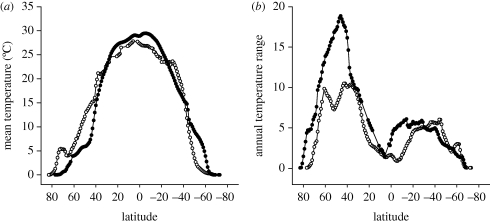
An extreme environment is one where the physical features of the habitat are towards the end of the range of natural variability. The polar oceans are thus extreme, in that they exhibit temperatures that are close to the freezing point, either seasonally or, at very high latitudes, year-round (figure 1).
Figure 1.
Polar regions as extreme marine environments. Mean and seasonal range of sea surface temperature along longitude 170°W in the Pacific Ocean (open circles) and longitude 30°W in the Atlantic Ocean (filled circles). Data were calculated for the period 1986–2001. (a) Mean temperature. (b) Mean annual temperature range. The AVHRR Pathfinder v. 5.0 SST data were obtained from the Physical Oceanography Distributed Active Archive Center (PO.DAAC) at the NASA Jet Propulsion Laboratory, Pasadena, California (http://podaac.jpl.nasa.gov).
An extreme environment is not necessarily a rare habitat. The deep-sea is extreme in that as well as being very cold (bottom temperatures are typically below 4°C: Gage & Tyler 1991) it is also at very high pressure. It is, however, also the largest single habitat on the face of the earth, and so while extreme in physical terms, it is also arguably the most typical environment on the planet.
Discussion of extreme environments in the ecological literature often involves the implicit assumption that such environments are tough or demanding places to live, although it is rare for this to be quantified in any way. There is also the danger of circularity in arguments relating biological diversity to perceived environmental harshness: there are few organisms living here because it is a tough environment, this must be a tough environment because so few organisms live here.
(b). Physiological performance and temperature
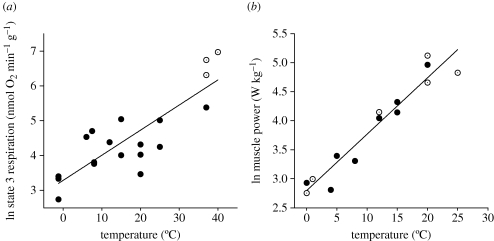
There are, however, some clearly established physiological constraints on the performance organisms can achieve in particular environments. Comparative studies of organisms that have adapted to different thermal environments over evolutionary time have shown clearly that the maximum rate of generation of ATP by mitochondria and the rate of power generation by muscle are both strongly temperature-dependent (figure 2a,b). These relationships indicate that, all other things being equal, lifestyles requiring the highest metabolic power, or the most intense muscle activity, are possible only with a warm body. Living with a warm body also carries with it a cost in terms of basal metabolism, in that it costs more energy to maintain a warm body than it does a cool one (Clarke & Fraser 2004). Taken together these three broad macrophysiological patterns indicate that in seeking to unravel the evolution of high latitude marine faunas we must recognize that a switch from a warm temperate to a polar environment may well have made certain high energy lifestyles untenable.
Figure 2.
Temperature and physiology, comparing species adapted to live at different environmental temperatures. (a) Relationship between mitochondrial power generation, measured as state 3 respiration rates (nmol O2 min−1 g liver fresh mass−1), and temperature. Data plotted separately for ectotherms (filled circles) and endotherms (open circles). (b) Fish muscle mass-specific power output as a function of habitat temperature. Open circles are data from measurement of muscle performance in fast-starts, closed circles are data from work-loop studies of isolated fibres. All data are for glycolytic (anaerobic) fibres; original data from Wakeling & Johnston (1998). Redrawn from Clarke & Pörtner (in press).
2. The marine fauna of the polar regions
(a). The global context
The marine environment is difficult to sample. As a result, knowledge of marine diversity has long lagged behind that of terrestrial systems, and we currently lack complete global syntheses of marine diversity for most groups. The intensity of the latitudinal cline in the sea can be seen by comparing the Southern Ocean fauna with the global diversity for several well-studied groups (table 1). Each of these groups has less than 1 per cent of its global diversity in the Southern Ocean, though the Antarctic contains around 12 per cent of the world's continental shelves (Walsh 1988).
Table 1.
A comparison of the Southern Ocean fauna with estimates of global species richness for the largest benthic groups. Global richness data come from Groombridge & Jenkins (2002), Bouchet & Rocroi (2005), De Grave et al. (2009); Southern Ocean (SO) richness data come from the Register of Antarctic Marine species (RAMS: www.scarmarbin.be/rams). Note that the shelled gastropod global figure includes terrestrial forms, but the vast majority of this group are marine, and the teleost figure includes deep-sea and pelagic species.
| group | global richness | SO richness |
|---|---|---|
| decapods | 15 000 | 13 |
| bivalves | 10 000 | 136 |
| shelled gastropods | 80 000 | 446 |
| teleost fish | 25 000 | 398 |
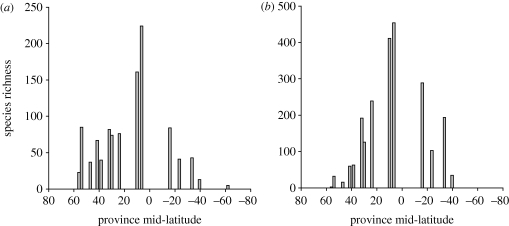
In the decapod groups Caridea (shrimps and prawns) and Brachyura (true crabs) shallow-water diversity along the coasts of North and South American peaks in the tropics, and declines towards both poles (figure 3). Diversity is not, however, symmetrical about the equator, for caridean richness declines more rapidly southwards than northwards, and the reverse pattern is found in brachyurans.
Figure 3.
Latitudinal variation in the species richness of two groups of decapods. Data are pooled by faunal province, and the species richness of each province is plotted as a function of the mid-latitude of that province. Data replotted, with permission, from Boschi (2000). (a) Carideans (shrimps and prawns). (b) Brachyurans ( true crabs).
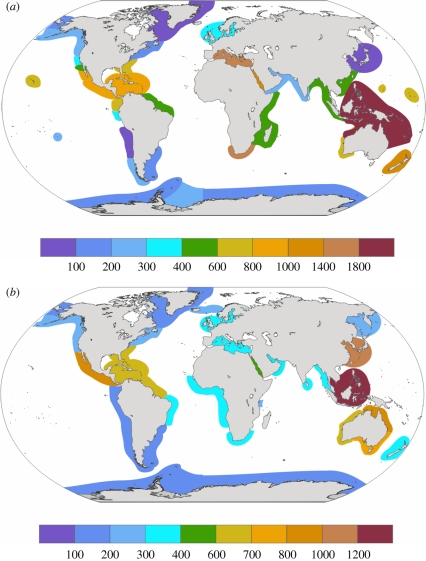
Molluscs are among the best-known marine taxa globally, and they exhibit two centres of richness, one in the Indo-West Pacific and the other on the Pacific and Atlantic coasts of central America (figure 4). In gastropods, other tropical or subtropical areas have much lower richness, often comparable with temperate areas, and the lowest values are found in the polar regions. Marine gastropods are a highly diverse group, with many species-rich clades. The species in these clades are often tiny, and frequently rare (for example Turridae, with more than 4000 species: Tucker 2004). A similar global pattern is shown by bivalves (Crame 2000a,b; Linse et al. 2006; Krug et al. 2009a), although not all bivalve gradients are either regular or symmetrical about the equator. For gastropod and bivalve molluscs together the fauna of New Caledonia has been estimated to contain more than 5000 species and that of the Philippines as many as 10 000–12 000 (Bouchet 2008).
Figure 4.
Global species richness in gastropod and bivalve molluscs. (a) Gastropods. (b) Bivalve molluscs. Reproduced, with permission, from Linse et al. (2006), where original data sources listed.
A comparison of the species abundance distributions for two well-sampled locations indicates that the marked difference in species richness between a tropical reef and a polar seabed is caused predominantly by the large number of rare species in tropical assemblages (Bouchet et al. 2002, 2009; table 2). Tropical/polar contrasts in richness for gastropod and bivalve molluscs are thus one of the most striking macroecological patterns in the sea. Whether this striking difference in the species/abundance distribution has any consequences for ecosystem functioning or evolutionary processes is currently unclear.
Table 2.
A comparison of gastropod species richness at two benthic sites, one polar (South Orkney Islands, Southern Ocean: Picken 1980) and one tropical (Koumac, New Caledonia: Bouchet et al. 2002). n(ind.), number of individuals sampled; SR, species richness; n(1), number of species represented by a single individual only; n.d., no data available. Note that in New Caledonia, 401 molluscan species were sampled only as empty shells.
| location | group | n(ind.) | SR | n(1) |
|---|---|---|---|---|
| New Caledonia | molluscsa | 127 652 | 2738 | 542 |
| gastropoda | 81 558 | 2187 | n.d. | |
| shelled gastropods | 76 343 | 1929 | n.d. | |
| Signy | shelled gastropods | 138 650 | 31 | 2 |
aExcluding cephalopods.
While the patterns for these well-known groups are often generalized to the marine fauna as a whole, it is important to recognize that we currently lack the biogeographic syntheses for many groups to establish whether these show similar patterns. The example of pycnogonids, which are remarkably diverse in the Southern Ocean (Clarke & Johnston 2003; Munilla & Membrives 2009), should warn against a simplistic generalization from well-studied groups such as crustaceans or molluscs to the fauna as a whole.
Although the Southern Ocean has been studied for well over a century, we still have only a working knowledge of the marine fauna (Clarke et al. 2007). Arntz et al. (1997) and Clarke & Johnston (2003) documented a total of more than 4000 benthic species from the Southern Ocean, and this total has continued to rise with continuing exploration and taxonomic work (Clarke 2008; Griffiths et al. in press). Knowledge of the Arctic marine fauna is also increasing: Sirenko & Piepenburg (1994) reported over 4000 macrobenthic taxa from the Arctic, and Sirenko (2001) increased this to about 4800 species, including about 400 from the deep Eurasian basins of the central Arctic Ocean.
These totals challenge the widely held assumption that the benthic fauna of the Arctic basin is less species-rich than that of the Southern Ocean (Piepenburg 2005). However these bare totals mix inventories from different habitats, and also from different depths, and we currently lack a rigorous comparison of the richness of the two polar marine faunas (Piepenburg 2005). We badly need a taxonomically controlled comparison of comparable habitats and regions in the two areas. Overall, however, we must conclude that the evidence for a difference in marine diversity between the two polar regions is currently equivocal.
(b). The Southern Ocean marine fauna
The continental shelf around Antarctica is unusually deep as a result of scouring from ice shelves at previous glacial maxima and depression by the enormous mass of continental ice. The average depth is approximately 450 m; trenches and basins scoured by ice or subglacial water flow can be considerably deeper (more than 1000 m), although the outer edge of the shelf is typically somewhat shallower. In contrast to all other continental shelves (including those of the Arctic), there is essentially no riverine input; mudflats are rare and estuaries almost non-existent. The low level of terrestrial input comes via glacial processes or, in a few places, wind. The unusual depth and topography of the Antarctic continental shelf mean that many habitats typical of shelves elsewhere are missing from Antarctica. These include most of the shallow-water habitats which traditionally support rich and diverse communities.
The modern benthic fauna of the continental shelves around Antarctica is typically an epifauna of sessile filter and particle feeders associated with coarse-grained glacial substrates (Arntz et al. 1994, 1997; Gutt 2000). Associated with these sessile forms is an errant fauna of ophiuroids, asteroids, echinoids, pycnogonids, isopods, amphipods, nemerteans and gastropods. Where soft sediments occur, then polychaetes are particularly important. One of the most important features of the Antarctic benthic fauna is the lack of durophagous (skeleton-breaking) predators, which are so characteristic of shallow waters elsewhere. Crabs, lobsters and sharks are essentially absent, and there is only a very limited diversity of teleosts and skates (Aronson & Blake 2001). The benthic fish fauna of Antarctica is also remarkable in its taxonomic balance: many teleost groups are almost completely absent and the fauna is dominated by striking radiations in two groups: the notothenioids, principally on the continental shelf, and the lipariids in the deeper waters of the continental slope (Eastman 1993; Eastman & Clarke 1998).
(c). The Arctic benthic fauna
The Arctic benthic fauna is dominated by crustaceans, molluscs and polychaetes (Sirenko & Piepenburg 1994), although in places echinoderms are also important. Key differences from the Southern Ocean are the importance of disturbance by biological agents, especially bottom-feeding whales and walrus, and the significant input of organic material from terrestrial sources.
While the most recent data suggest that the overall richness of the macrobenthic fauna of the Arctic and Antarctic continental shelves are more comparable than had previously been thought, one important difference that remains valid is the marked contrast in endemism. The Arctic is characterized by widespread boreal-Arctic taxa, with a high faunal similarity to the North Atlantic (Zenkevitch 1963, Knox & Lowry 1977; Dunton 1992; Piepenburg 2005). In contrast, the Southern Ocean fauna exhibits a high degree of endemism (White 1984; Dayton 1990; Arntz et al. 1997). This hemispheric asymmetry points clearly to a difference in the recent evolutionary history of the two regions (Crame 2004).
3. The origin and age of polar marine faunas
During the latest Cretaceous and earliest Palaeogene the embryonic Arctic Ocean appears to have been fully marine but largely isolated from the rest of the world ocean (Marincovitch et al. 1985, 1990). By the late Palaeocene (56 Ma BP) there is evidence for an intermittent Arctic–Atlantic seaway and it is apparent that the Arctic Ocean was dominated by taxa of Atlantic affinities until the opening of the Bering Strait at approximately 3 Ma BP. At this time an asymmetric trans-Arctic interchange of taxa took place, with far more taxa moving from the Pacific to the Atlantic than in the reverse direction (Vermeij 1991). Modern cold-water molluscan clades originating in the North Pacific in the latest Eocene/earliest Oligocene include Buccinidae (whelks), Turritellinae, Fusitriton, the volutid Arctomelon, various protobranchs, the Clinocardiinae cockles, the tellinid Macoma and the anomalodesmatid clam Mya (Vermeij 2001).
The Arctic Ocean was largely ice-free with some connections to both the Atlantic and Pacific Ocean until the mid-Pliocene (about 3 Ma BP). At this point shallow-water temperatures started to cool; this cooling intensified at the start of the Pleistocene (1.8 Ma BP) and there were periodic alternations between colder glacial and warmer interglacial climates, producing the present environment characterized by a permanently ice-covered central Arctic Ocean fringed by shelf seas with seasonally varying sea-ice cover (Bleil & Thiede 1990). The cycles of glacial and interglacial conditions have had a dramatic effect on fauna of the shallow continental shelves of the Arctic basin, as variations in global sea-level have resulted in the shelves either being exposed, or covered by glacial ice. At the last glacial maximum (LGM) the continental shelves of northern Alaska (the Chukchi and Beaufort seas) and the East Siberian Sea remained largely unglaciated and almost entirely emergent. In contrast, the entire shelf of the Atlantic Arctic (from Greenland east into the Norwegian, Barents and Kara Seas) was covered with glacial ice that extended beyond the shelf-break and would have precluded the existence of a shallow-water fauna (Dunton 1992).
The current marine fauna has thus only occupied the entire Arctic continental shelf for at most 13 000 years, having colonized the shelf since the LGM. It is therefore often described as being relatively young, but it actually has a complex history (Dunton 1992). The shallow-water Arctic marine fauna would have been extirpated at each glacial maximum, and thus has had to recolonize the shelves repeatedly during interglacial periods when the seas re-flooded the shelves. The source of these colonists would have been refugia in unglaciated areas of the East Siberian and Beaufort Seas, the deeper bathyal waters of the Eurasian sector or the North Atlantic or North Pacific.
It has long been known that the benthic marine fauna of the Southern Ocean comprises a number of elements: a component the ancestry of which can be traced back to the Mesozoic, others which have clearly colonized the shelf from the deep sea or the continental slope, and some others that have dispersed into Antarctica along the Scotia arc (Hedgpeth 1970; Dell 1972; Knox & Lowry 1977; Lipps & Hickman 1982). Important evidence for the long history of the Antarctic marine benthic fauna comes from the gastropod family Struthiolariidae, which suggests that some elements of the modern benthic fauna have an ancestry at least as far back as the Upper Cretaceous, approximately 70 Ma BP (Zinsmeister 1982). The same is also true for the superfamily Buccinoidea (whelks: Kiel 2002; Stilwell et al. 2004; Beu 2009).
While the fauna was undoubtedly affected by the mass extinction event at the end of the Cretaceous, precisely to what extent is still uncertain. At least nine benthic molluscan taxa actually crossed the K/Pg boundary in Antarctica and of the 26 molluscan taxa in the beds directly above the boundary, 15 have been assigned to new species in surviving genera (Stilwell 2003). In the earliest Cenozoic there was a global expansion of a number of key benthic groups, prominent among which were the neogastropods, and it is interesting to note that bucciniform taxa underwent a pronounced radiation in Antarctica at this time (Oleinik & Zinsmeister 1996). Indeed, one of the new genera to appear in the Early Palaeocene, Probuccinum, still has a circum-Antarctic distribution at the present day (Stilwell et al. 2004). This was also a time of explosive radiation of many lineages of teleost fish (Aronson & Blake 2001).
4. The environmental context
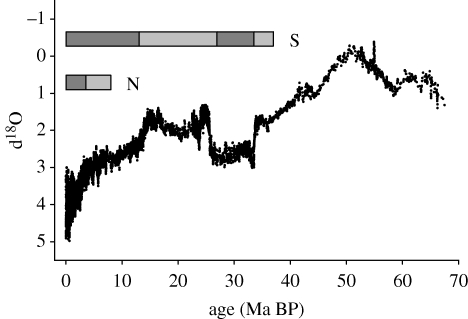
The overall thermal history of oceanic bottom temperatures during the Cenozoic is now well established from oxygen isotope analyses of foraminifera from deep-sea sediment cores (see Zachos et al. 2001). These data provide a broad indication of bottom temperatures in Antarctica throughout the Cenozoic, and indicate two periods of sharp change in isotopic signature (figure 5): at the end of the Eocene and in the middle of the Miocene. While changes in global ice volume will also have contributed to this signal, it seems probable that the early Oligocene marked the onset of continental glaciation, and the mid-Miocene the onset of rapid deep oceanic cooling (Lear et al. 2000).
Figure 5.
Cenozoic trends in global deep-sea temperatures determined from oxygen isotope composition of foraminifera. This trend gives a good indication of shallow-water temperatures at high southern latitudes. Data from Zachos et al. (2001). Also shown are approximate durations of Northern and Southern Hemisphere showing glaciations and the transition from a predominantly warm (glasshouse) world to one with significant high latitude glaciation (icehouse).
Bottom temperatures are driven principally by large-scale oceanography and glacial processes at high latitudes, and it has long been recognized that a key event in the evolution of Antarctic oceanography was the opening of the Drake Passage between South America and the Antarctic Peninsula. This allowed the onset of a circum-Antarctic current system and was almost certainly an important factor in the overall cooling of high latitudes (Barrett 1999).
Although the broad history of continental glaciation in Antarctica is reasonably well established (Barrett 1999), the details that are important to an understanding of the evolution of the Antarctic marine fauna are still frustratingly elusive. Full continental glaciation was probably initiated about 35 Ma BP, and the individual ice-sheets almost certainly fluctuated in size in response to orbital variations on Milankovitch frequencies (Barrett et al. 1987; Fielding et al. 2000; Powell et al. 2000; Naish et al. 2009; DeConto & Pollard 2003). The sharp cooling in the mid-Miocene marked a major growth in the continental ice-sheet, but quite what drove this is unknown.
The important question in terms of the evolutionary history of the Southern Ocean shallow-water marine fauna is to what degree fluctuations in sea level and the extent of the continental ice-sheet have driven changes in the depth and area of habitat on the continental shelves around Antarctica (Clarke & Crame 1989). Critical for understanding the impact of glacial maxima on the continental shelf fauna is to know whether the extension of the ice-sheet obliterated all habitat, or whether refugia existed. It seems probable that where substantial barriers to ice-flow existed, such as major islands, then these may have influenced ice flow and allowed for refugia on the seaward (lee) side. Possible examples of this are Alexander Island and Thurston Island. Furthermore, there is geophysical evidence that grounded ice did not cover the outer continental shelf in Prydz Bay (Domack et al. 1998), parts of the Ross Sea (Shipp et al. 1999) or George V Land (Beaman & Harris 2003). It is also possible that differences in flow rate of ice-streams meant that extension and retraction of the ice-sheet was not synchronous around Antarctica, and such diachronous flow may also have created a temporal mosaic refugia on the shelf.
While most attention has been directed at the LGM, and the most recent extension of the ice-sheet will have obliterated much of the record of previous extensions, cyclical extension and retraction of the ice-sheet had been proceeding for much of the later Cenozoic. Recent sediment cores from Cape Roberts have revealed at least 38 such cycles in the past 5 Myr (Naish et al. 2009) and a conservative estimate of the total number of such cycles would be more than 50.
(a). Benthic habitats at glacial maxima
Ecological conditions for shallow-water benthos would have been very different during glacial maxima from those we observe at present, and it has been proposed (Thatje et al. 2005) that glacial processes such as enhanced iceberg scour on the shelf and sediment gravity flows down the slope would have made survival of benthic communities impossible on either the shelf or slope. At glacial maxima, significant quantities of scoured material flowed down the continental slope to generate large sediment fans on the adjacent abyssal plain (Dowdeswell et al. 2008). At the same time, the development of multi-year ice close to shore, and a significant northward extension of the seasonal sea-ice zone would have effectively eliminated primary production close to shore, possibly apart from within polynias (Thatje et al. 2008).
Taken together these two scenarios might imply that no benthic fauna could have survived on the Antarctic continental shelf or slope at glacial maxima, as has been suggested by Thatje et al. (2005). However, it is probable that the fast ice-streams that generated the abyssal sediment fans were confined to specific channels (Ó Cofaigh et al. 2005), and hence the scouring of the continental slope confined to particular locations. Furthermore, there is also evidence of biological production though the LGM in both the Ross Sea (Brambati et al. 2002) and Amundsen Sea (Thatje et al. 2008).
While it is clear that there are no modern analogues for the environmental conditions pertaining at glacial maxima, the presence of lineages of benthic organisms that extend back to the Mesozoic indicate that the question is not whether shallow-water benthic organisms survived successive glacial advances, but how and where they did so.
5. Climate, milankovitch cycles and evolutionary dynamics in the polar seas
We can now use our developing knowledge of the tectonic, climatic and glacial evolution of the Antarctic and Southern Ocean to provide a context for the evolution of the marine fauna. In doing so we update previous reviews of the history of polar marine faunas (Lipps & Hickman 1982; Clarke & Crame 1989; Aronson & Blake 2001; Clarke et al. 2004; Crame 2004) and make some very general predictions about how key events in the past have shaped the modern fauna. While all such change is gradual, we can identify four particular processes that will have had particular impact on the marine fauna.
(a). The breakup of Gondwana
Although the fossil record is far from complete, there is growing evidence to suggest that distinctive, temperate, shallow marine faunas have characterized the southernmost high latitudes since at least the late Palaeozoic Era (Crame 2004). By the Late Cretaceous the Antarctic shallow marine fauna formed part of a cool-water Weddellian Province that could be traced around the southern Gondwana margins from Patagonia, through West Antarctica, to New Zealand and south-eastern Australia (Zinsmeister 1982). There are also connections with warmer water faunas from central Chile and South Africa (Kiel 2002). Although a rigorous analysis has yet to be undertaken, it would appear that as far back as the Campanian/Maastrichtian (approx. 80 Ma BP), both the Weddellian province to the south and the North Pacific province were less rich than the well-studied subtropical faunas from the US Gulf Coast (Sohl 1971; Kiel 2002), indicating that latitudinal clines in richness have a long history in the sea.
The fragmentation of Gondwana would have led to vicariance within the Weddellian province, and also the appearance of elements from the Tethyan fauna. A nice example is shown by the notothenioid fishes, which are the dominant group of fishes on the continental shelf of Antarctica today. The initial diversification took place along the coast of Gondwana, and the present distribution of the non-Antarctic lineages (Bovichtidae, Pseudaphritidae and Eleginopidae) in the coastal waters of South America, southern Australia and Tasmania result from vicariance associated with the fragmentation of Gondwana (Near 2004).
(b). The K/Pg mass extinction event
The shallow-water benthic marine fauna of Antarctica was undoubtedly affected by the mass extinction event that marked the end of the Cretaceous, but to precisely what extent is still uncertain. At least nine benthic molluscan taxa actually crossed the K/Pg boundary in Antarctica, including the prominent, large suspension feeding bivalve, Lahillia. Of the 26 molluscan taxa in the beds directly above the K/Pg boundary, 15 have been assigned to new species in surviving genera (Stilwell 2003).
Although this extinction event led to an overall reduction in marine diversity in the early Palaeocene in Antarctica, this was to some extent mitigated by the global expansion of a number of key benthic groups. Prominent among these were the neogastropods, and it is interesting to note that bucciniform taxa underwent a pronounced radiation in Antarctica at this time (Oleinik & Zinsmeister 1996).
(c). The onset of continental glaciation and associated cooling
The main impact of continental glaciation would have been to eradicate key habitats. In particular, the extension of continental ice to the shoreline and beyond would have eliminated rivers and estuaries, and also the associated deltaic environments and intertidal mudflats. The latter in particular are rich habitats for marine organisms, and it would seem probable that the loss of many fish groups from the Antarctic during the mid-Cenozoic would have been the direct result of this loss of habitat.
Once sea-ice had started to form this would have scoured the intertidal and subtidal habitats, eventually making these habitats untenable for the traditional epifaunal invertebrates of this habitat, such as macroalgae, cirripede barnacles, mussels and limpets. Today only a single species of limpet is found in the intertidal zone of the Antarctic continent (Nacella concinna) and this migrates to deeper water for the ice-bound winter season. The current intertidal fauna has essentially no epifaunal taxa, though a diverse cryptic intertidal fauna has recently been described (Waller et al. 2006).
At the same time as the development of continental glaciation, the Antarctic marine fauna was also subject to cooling (figure 5). The three aspects of this long-term temperature change that might affect the fauna are if the rate of change exceeds the capacity of a species to adapt, if the lower temperature precludes some lifestyles or ecologies, and if a particular threshold for activity or survival is crossed.
Although the Cenozoic marks a change from a temperate to a polar marine environment, the overall rate of change appears to have been generally slow and would not seem to have posed a particular physiological challenge to the fauna (Clarke 1993). All ectotherms living at lower temperatures will, however, have had reduced capacities to generate metabolic power, and lowered capacities for energy-intensive activities (Peck 2002; Peck et al. 2006). This may well have constrained the lifestyles possible in the new cooler environment, and it is possible that such constraints are behind the absence of fast-swimming predatory fish in the Southern Ocean.
Two groups do, however, appear to have been affected by threshold phenomena, namely teleost fish and decapods. Teleost fish are unusual in that the osmolarity of their body fluids is roughly half that of sea water. As a result the freezing point of these body fluids is well above that of polar sea water, and is thus liable to freeze. Freezing is prevented in polar fish by the presence of a suite of antifreeze molecules, which can either be proteins or glycoproteins. The fish fauna of Antarctica in the early Eocene was diverse, and typical of warmer waters elsewhere (Eastman 1993; Aronson & Blake 2001). The Eocene was a time of rapid diversification of the teleosts, and this radiation appears to have increased the diversity and richness of the Southern Ocean as much as elsewhere (Eastman 1993). The present fish fauna is far less diverse, and taxonomically highly restricted. On the continental shelf the fauna is dominated by a single clade, the notothenioids, and in the deeper waters of the continental slope there has also been a substantial radiation of liparids (snail-fishes) (Eastman 1993).
The Southern Ocean marine fauna is also unusual in the paucity of decapods; there are fewer than a dozen species of carideans (shrimps) and no true crabs (Brachyura) or lobsters, although there are a range of anomuran lithodids (stone-crabs) in the deeper waters of the continental slope (Thatje & Arntz 2004). Physiological work has pointed to a failure of magnesium regulation at low temperatures as one factor in the paucity of decapods in Antarctica (Frederich et al. 2001). But this explanation is unsatisfactory on its own, for it begs two further questions: why should this failure occur in decapods and not other marine invertebrates, and to return to Hutchinson's question, if some decapods can overcome this problem and adapt to the modern polar environment, why cannot others? Decapods were common in the warm water fauna of Gondwana in the late Mesozoic and early Cenozoic, and at least one lineage of lobsters was still present in the Pliocene (Feldmann & Quilty 1997); but the development of glaciation and the gradual cooling of the environment is associated with the loss of most of the decapod fauna.
One ecologically significant consequence of the cooling was the associated substantial reduction or complete loss of durophagous (skeleton-crushing) predators such as brachyuran crabs, lobsters and fish such as skates and rays from the continental shelf environment of Antarctica (Aronson & Blake 2001; Aronson et al. 2007, 2009). This appears to have induced a significant shift in the assemblage composition of the benthos (Aronson & Blake 2001; Aronson et al. 2009), to one that looks remarkably archaic in its make-up. The shift to glacial conditions with the associated reduction in sedimentation will also have been a factor, and distinguishing the relative importance of these is not straightforward (Gili et al. 2006).
(d). Milankovitch variability and the diversity pump
Perhaps the most profound environmental factor driving evolutionary dynamics in polar marine faunas has been the variation in size and extent of the continental ice-sheets, in response to Milankovitch climate variability (Clarke & Crame 1989, 1992). These variations would have affected the area of continental shelf habitat through coverage or exposure of habitat by ice, and sea-level change.
The change in area would have fragmented ranges, confining taxa to refugia on the shelf, or forcing a shift in distribution down the continental slope or even into the deep sea. As the ice-sheets waned, habitat area would once again be available on the shelf, and previously isolated populations would come into secondary contact.
The fragmentation of ranges might drive some species to extinction through mechanisms such as Allee effects or stochastic environmental changes acting on small populations. Alternatively, founder effects or genetic drift might result in a change in population genetic structure. The degree of isolation of populations confined to refugia would have depended on the nature of geneflow (Lester et al. 2007; Teske et al. 2007; Burrows et al. 2009), itself a feature of reproductive strategy (and notably the extent of dispersal through long-lived larval stages).
That a shift of populations into deeper water was a feature of previous glacial maxima is suggested by the generally wider bathymetric range of Antarctic benthic marine invertebrates compared with those elsewhere (Brey et al. 1996; Brandt et al. 2009). Such exchange would have been facilitated by two factors, namely the general similarity in temperature between the shelf, slope and deep-sea in Antarctica (Clarke et al. 2009) and the unusually deep nature of the Antarctic continental shelf which would have required a degree of pressure adaptation.
Once previously isolated populations were able to coalesce, some may have developed reproductive isolation and hence diverged into separate lineages, whereas others will have combined once more with geneflow homogenizing the population once more. Clarke & Crame (1989, 1992) likened this model of Milankovitch-driven range fragmentation and combination to the climate-driven diversity pump of Valentine (1967). Cycles of range contraction and expansion driven by Milankovitch climate variability have also been a major feature of the evolution of terrestrial biota, where they have been called ‘orbitally forced species' range dynamics’ (Dynesius & Jansson 2000; Jansson & Dynesius 2002).
(e). Summary: glaciation and the polar marine benthos
Are there any general conclusions we can make concerning glaciations and evolutionary processes in high latitude marine communities? The major unresolved issue at present would appear to be the question of to what extent marine benthos was able to survive in refugia on the continental shelf, or perhaps the adjacent slope, at glacial maxima. While geophysical evidence suggests a radically different disturbance regime at glacial maxima, the presence of lineages with a fossil record back to the Mesozoic points clearly to survival of assemblages in situ. An important area for future work here would be a rigorous bipolar comparison of the shelf fauna of Antarctica, the European Arctic shelf and the East Siberian Arctic shelf. The European Arctic shelf would appear to provide an example of a biota that has been assembled de novo following the LGM, whereas the East Siberian shelf provides an example of a fauna that contains a component that survived the LGM in refugia.
6. Is there anything special about evolution at high latitudes?
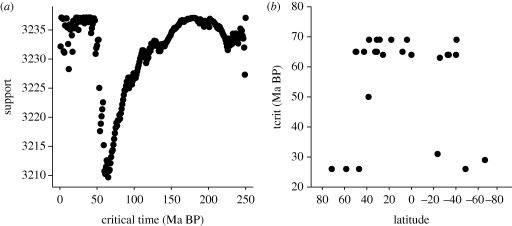
A recent important study by Krug et al. (2009b) has demonstrated two key features of the Cenozoic diversification. The first was unequivocal support for the K/Pg extinction event being the most significant factor affecting the speciation dynamics in marine bivalves in the past 250 Myr (figure 6a), confirming the apparent global homogeneity of the K/Pg extinction event (Raup & Jablonski 1993).
Figure 6.
Cenozoic trends in bivalve mollusc origination rate. (a) Support for potential inflection points in the backwards survivorship curves for genera of all extant bivalve molluscs. The optimization routine is such that the ordinate scale is the negative of the support, and hence the lower the value the greater the support. Note the clear indication of the K/Pg, mass extinction at 65 Ma BP. (b) Latitudinal differences in the timing of the inflection point (tcrit, Ma BP) for bivalve molluscs, estimated for different biogeographic provinces. By convention, southern latitudes are negative. Replotted from Krug et al. (2009b), with data supplied by the author.
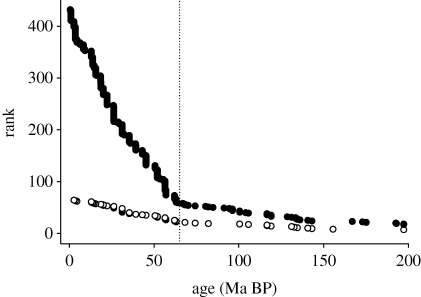
Krug et al. (2009b) repeated this analysis on a province by province basis, and this demonstrated a strong latitudinal pattern. For tropical and temperate faunas the major signal came from the K/Pg extinction event, but for the two polar regions the dominant signal came from an event at 25–30 Ma BP (figure 7). This does not mean that the polar regions were unaffected by the K/Pg extinction, for fossil evidence shows clearly that they were; it only means that an event around the Oligocene (perhaps the onset of widespread glaciation in the Southern Hemisphere) had an even greater impact.
Figure 7.
Age/frequency distributions of marine bivalve genera from tropical West Pacific (open circles) and the Arctic (filled circles). Replotted from Krug et al. (2009b), with data supplied by the author.
The second was that the recovery immediately following the K/Pg mass extinction was, however, asymmetric in that the rate of subsequent diversification during the Cenozoic was more rapid in the tropical regions than at high latitudes (Crame 2009; figure 6b). Partly this would have been because tropical diversification started from a higher standing diversity, but the study also points to a significant underlying difference in absolute rates. Per lineage origination rates were 0.020 genera per lineage per million years in the 50 Myr before the K/Pg event and 0.026 immediately afterwards (Krug et al. 2009b).
The underlying reason for the difference in origination rates between tropical and polar molluscan faunas is unclear, but it would appear to go back at least 65 Myr. While fossil data tend to indicate a faster rate of diversification in tropical seas, the marked longitudinal variation in tropical diversity indicates that diversification involves more than temperature. Briggs (2003), for example, has argued that tropical diversification is further increased by a positive feedback from ecological (sympatric) speciation once standing diversity reaches a particular threshold.
7. The wider evolutionary impact of high latitude glaciations
The fluctuations in the size and extent of the continental ice-sheets driven by Milankovitch orbital variations has influenced marine diversity in more than just the polar regions. In particular they have been a major driver of variation in global sea-level, which has in turn been a key factor in the diversification of tropical faunas. The area of shallow water in the Indo-West Pacific has fluctuated significantly as sea-level varied. This has resulted in a pattern of range fragmentation and recombination, exactly analogous to that experienced by the continental shelf marine fauna of Antarctica, and providing opportunities for allopatric speciation. By contrast, on linear coasts such as characterizes much of the Americas, there is the opportunity for species to move laterally and ranges tend not to fragment.
The generation of cold, highly oxygenated bottom water at high latitudes and its subsequent transport into lower latitudes is critical to the existence of life in the deep sea (Gage & Tyler 1991). At periods in the past when neither pole was glaciated, large areas of the deep sea have been anoxic and devoid of life. Glaciation at high latitudes has thus been a key factor in generating diversity in the largest marine habitat on Earth, the deep sea.
Acknowledgements
We thank Zack Krug for generously providing data to allow the plotting of figures 6 and 7. We also thank our colleagues for many stimulating discussions over the past years on the topic of the evolution of the polar marine fauna, especially David Barnes, Angelika Brandt, David Jablonski, Katrin Linse, Lloyd Peck, Kaustov Roy, Andrew Smith, Sven Thatje and Paul Tyler. We thank William Austin and an anonymous referee for constructive comments that greatly helped clarify a number of points in the paper.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Biological diversity in a changing world’.
References
- Arntz W. E., Brey T., Gallardo V. A.1994Antarctic zoobenthos. Adv. Mar. Biol. 32, 241–304 [Google Scholar]
- Arntz W. E., Gutt J., Klages M.1997Antarctic marine biodiversity: an overview. In Antarctic communities: species, structure and survival (eds Battaglia B., Valencia J., Walton D. W. H.), pp. 3–14 Cambridge, UK: Cambridge University Press [Google Scholar]
- Aronson R. B., Blake D. B.2001Global climate change and the origin of modern benthic communities in Antarctica. Am. Zool. 41, 27–39 (doi:10.1668/0003-1569(2001)041[0027:GCCATO]2.0.CO;2) [Google Scholar]
- Aronson R. B., Thatje S., Clarke A., Peck L. S., Blake D. B., Wilga C. D., Seibel B. A.2007Climate change and invasibility of the Antarctic benthos. Annu. Rev. Ecol. Syst. 38, 129–154 (doi:10.1146/annurev.ecolsys.38.091206.095525) [Google Scholar]
- Aronson R. B., Moody R. M., Ivany L. C., Blake D. B., Werner J. E., Glass A.2009Climate change and trophic response of the Antarctic bottom fauna. PLoS ONE 4, e4385 (doi:10.1371/journal.pone.0004385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P. J.1999Antarctic climate history over the last 100 million years. Terra Antarctica 3, 53–72 [Google Scholar]
- Barrett P. J., Elston D. P., Harwood D. M., McKelvey B. C., Webb N.1987Mid-Cenozoic record of glaciation and sea-level change on the margin of the Victoria Land basin, Antarctica. Geology 15, 634–637 (doi:10.1130/0091-7613(1987)15<634:MROGAS>2.0.CO;2) [Google Scholar]
- Beaman R. J., Harris P. T.2003Seafloor morphology and acoustic facies of the George V Land shelf. Deep-Sea Res. Part II 50, 1343–1355 (doi:10.1016/S0967-0645(03)00071-7) [Google Scholar]
- Beu A. G.2009Before the ice: biogeography of Antarctic Paleogene molluscan faunas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 284, 191–226 (doi:10.1016/j.palaeo.2009.09.025) [Google Scholar]
- Bleil U., Thiede J.1990Geologic history of the polar oceans: Arctic versus Antarctic. 823 pp Dordrecht, The Netherlands: Kluwer [Google Scholar]
- Boschi E. E.2000Species of decapod crustaceans and their distribution in the American marine zoogeographic provinces. Rev. Invest. Desarrollo Pesquero 13, 1–136 [Google Scholar]
- Bouchet P.2008The mighty numbers of Philippine marine mollusks. In Philippine marine mollusks, vol. I (Gastropoda—Part I) (ed. G. T. Poppe), pp. 8–16 Hackenheim, Germany: Conch Books [Google Scholar]
- Bouchet P., Rocroi P.2005Classification and nomenclator of gastropod families. Malacologia 47, 1–397 [Google Scholar]
- Bouchet P., Lozouet P., Maestrati P., Heros V.2002Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 75, 421–436 (doi:10.1046/j.1095-8312.2002.00052.x) [Google Scholar]
- Bouchet P., Lozouet P., Sysoev A.2009An inordinate fondness for turrids. Deep-Sea Res. II 56, 1724–1731 (doi:10.1016/j.dsr2.2009.05.033) [Google Scholar]
- Brambati A., Melis R., Quaia T., Salvi G.2002Late Quaternary climate change in the Ross Sea, Antarctica. Antarctica at a close of a millennium. Bull. R. Soc. New Zealand 35, 359–364 [Google Scholar]
- Brandt A., Linse K., Schüller M.2009Bathymetric distribution patterns of Southern Ocean macrofaunal taxa: Bivalvia, Gastropoda, Isopoda and Polychaeta. Deep-Sea Res. Part I 56, 2013–2025 (doi:10.1016/j.dsr.2009.06.007) [Google Scholar]
- Brey T., Dahm C., Gorny M., Klages M., Stiller M., Arntz W. E.1996Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarctic Sci. 8, 3–6 (doi:10.1017/S0954102096000028) [Google Scholar]
- Briggs J. C.2003Marine centres of origin as evolutionary engines. J. Biogeogr. 30, 1–18 (doi:10.1046/j.1365-2699.2003.00810.x) [Google Scholar]
- Burrows M. T., Harvey R., Robb L., Poloczanska E. S., Mieszkowska N., Moore P., Leaper R., Hawkins S. J., Benedetti-Cecchi L.2009Spatial scales of variance in abundance of intertidal species: effects of region, dispersal mode, and trophic level. Ecology 90, 1242–1254 (doi:10.1890/08-0206.1) [DOI] [PubMed] [Google Scholar]
- Clarke A.1993Temperature and extinction in the sea: a physiologist's view. Paleobiology 19, 499–518 [Google Scholar]
- Clarke A.2008Antarctic marine benthic diversity: patterns and processes. J. Exp. Mar. Biol. Ecol. 366, 48–55 (doi:10.1016/j.jembe.2008.07.008) [Google Scholar]
- Clarke A., Crame J. A.1989The origin of the Southern Ocean marine fauna. In Origins and evolution of the Antarctic biota., vol. 47 (ed. Crame J. A.), pp. 253–268 London, UK: Special Publications of Geological Society [Google Scholar]
- Clarke A., Crame J. A.1992The Southern Ocean benthic fauna and climate change: a historical perspective. Phil. Trans. R. Soc. Lond. B 338, 299–309 (doi:10.1098/rstb.1992.0150) [Google Scholar]
- Clarke A., Fraser K. P. P.2004Why does metabolism scale with temperature? Funct. Ecol. 18, 243–251 (doi:10.1111/j.0269-8463.2004.00841.x) [Google Scholar]
- Clarke A., Johnston N. M.2003Antarctic marine benthic diversity. Oceanogr. Mar. Biol. Annu. Rev. 41, 47–114 [Google Scholar]
- Clarke A., Pörtner H. O.In press Temperature, metabolic power and the evolution of endothermy. Biol. Rev. [DOI] [PubMed] [Google Scholar]
- Clarke A., Aronson R. B., Crame J. A., Gili J.-M., Blake D. B.2004Evolution and diversity of the benthic fauna of the Southern Ocean continental shelf. Antarctic Sci. 16, 559–568 (doi:10.1017/S0954102004002329) [Google Scholar]
- Clarke A., Griffiths H. J., Linse K., Barnes D. K. A., Crame J. A.2007How well do we know the Antarctic marine fauna? A preliminary study of macroecological and biogeographical patterns in Southern Ocean gastropod and bivalve molluscs. Divers. Distributions 13, 620–632 (doi:10.1111/j.1472-4642.2007.00380.x) [Google Scholar]
- Clarke A., Griffiths H. J., Barnes D. K. A., Meredith M. P., Grant S. M.2009Spatial variation in seabed temperature in the Southern Ocean: implications for benthic ecology and biogeography. J. Geophys. Res. Biogeosciences 114, G03003 (doi:10.1029/2008JG000886) [Google Scholar]
- Crame J. A.2000aEvolution of taxonomic diversity gradients in the marine realm: evidence from the composition of recent bivalve faunas. Paleobiology 26, 188–214 [Google Scholar]
- Crame J. A.2000bThe nature and origin of taxonomic diversity gradients in marine bivalves. In The evolutionary biology of the Bivalvia, vol. 177 (eds Harper E. M., Taylor J. D., Crame J. A.), pp. 247–257 London, UK: Special Publications of Geological Society [Google Scholar]
- Crame J. A.2004Pattern and process in marine biogeography: a view from the poles. In Modern biogeography: new directions in the geography of nature (eds Lomolino M. V., Heaney L.), pp. 271–291 Sunderland, MA: Sinauer [Google Scholar]
- Crame J. A.2009Time's stamp on modern biogeography. Science 323, 720–721 (doi:10.1126/science.1169410) [DOI] [PubMed] [Google Scholar]
- Dayton P. K.1990Polar benthos. In Polar oceanography, Part B: chemistry, biology and geology, vol. 2 (ed. Smith W. O.), pp. 631–685 San Diego, CA: Academic Press [Google Scholar]
- DeConto R. M., Pollard D.2003Rapid Cenozoic glaciation of Antarctic induced by declining atmospheric CO2. Nature 421, 245–249 (doi:10.1038/nature01290) [DOI] [PubMed] [Google Scholar]
- De Grave S., et al. 2009A classification of living and fossil genera of decapod crustaceans. Raffles Bull. Zool. Suppl. 21, 1–109 [Google Scholar]
- Dell R. K.1972Antarctic benthos. Adv. Mar. Biol. 10, 1–216 (doi:10.1016/S0065-2881(08)60416-2) [Google Scholar]
- Domack E., O'Brien P., Harris P., Taylor F., Quilty P. G., De Santis L., Raker B.1998Late Quaternary sediment facies in Prydz Bay, East Antarctica and their relationship to glacial advance onto the continental shelf. Antarctic Sci. 10, 236–246 (doi:10.1017/S0954102098000339) [Google Scholar]
- Dowdeswell J. A., Ó Cofaigh C., Noormets R., Larter R. D., Hillenbrand C.-D., Benetti S., Evans J., Pudsey C. J.2008A major trough-mouth fan on the continental margin of the Bellingshausen Sea, West Antarctica: the Belgica Fan. Mar. Geol. 252, 129–140 (doi:10.1016/j.margeo.2008.03.017) [Google Scholar]
- Dunton K.1992Arctic biogeography: the paradox of the marine benthic fauna and flora. Trends Ecol. Evol. 7, 183–189 (doi:10.1016/0169-5347(92)90070-R) [DOI] [PubMed] [Google Scholar]
- Dynesius M., Jansson R.2000Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA 97, 9115–9120 (doi:10.1073/pnas.97.16.9115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman J. T.1993Antarctic fish biology: evolution in a unique environment. San Diego, CA: Academic Press [Google Scholar]
- Eastman J. T., Clarke A.1998A comparison of adaptive radiations of Antarctic fish with those of nonantarctic fish. In Fishes of Antarctica: a biological overview (eds di Prisco G., Pisano E., Clarke A.), pp. 3–26 Berlin, Germany: Springer [Google Scholar]
- Feldmann R. M., Quilty P. G.1997First Pliocene decapod crustacean (Malacostraca: Palinuridae) from the Antarctic. Antarctic Sci. 9, 56–60 (doi:10.1017/S0954102097000084) [Google Scholar]
- Fielding C. R., Naish T. R., Woolfe K. J., Lavelle M. A.2000Facies analysis and sequence stratigraphy of CRP-2/2A, Victoria Land Basin, Antarctica. Terra Antartica 7, 323–338 [Google Scholar]
- Frederich M., Sartoris F. J., Pörtner H. O.2001Distribution patterns of decapod crustaceans in polar areas: a result of magnesium regulation? Polar Biol. 24, 719–723 (doi:10.1007/s003000100270) [Google Scholar]
- Gage J. D., Tyler P. A.1991Deep-sea biology: a natural history of organisms at the deep-sea floor. Cambridge, UK: Cambridge University Press [Google Scholar]
- Gili J.-M., Arntz W. E., Palanques A., Orejas C., Clarke A., Dayton P. K., Teixidó N., Rossi S., López-González P. J.2006A unique assemblage of epibenthic sessile suspension feeders with archaic features in the high-Antarctic. Deep-Sea Res. Part II 53, 1029–1052 (doi:10.1016/j.dsr2.2005.10.021) [Google Scholar]
- Griffiths H. J., Danis B., Clarke A.In press Quantifying Antarctic marine biodiversity: the SCAR-MarBIN data portal. Deep-Sea Res. Part II. [Google Scholar]
- Groombridge B., Jenkins M. D.2002World atlas of biodiversity, 340 pp Berkeley, CA: University of California Press [Google Scholar]
- Gutt J.2000Some ‘driving forces’ structuring communities of the sublittoral Antarctic macrobenthos. Antarctic Sci. 12, 297–313 (doi:10.1017/S0954102000000365) [Google Scholar]
- Hedgpeth J. W.1970Marine biogeography of the Antarctic regions. In Antarctic ecology, vol. 1 (ed. Holdgate M. W.), pp. 97–104 New York, NY: Academic Press [Google Scholar]
- Hutchinson G. E.1959Homage to Santa Rosalia, or why are there so many kinds of animals? Am. Nat. 93, 145–159 [Google Scholar]
- Jansson R., Dynesius M.2002The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Syst. 33, 741–777 (doi:10.1146/annurev.ecolsys.33.010802.150520) [Google Scholar]
- Kiel S.2002Notes on the biogeography of Campanian—Maastrichtian gastropods. Schriftenreihe des Erdwissenschaftlichen Kommision. Östereichische Akad. Wissensch. Wien 15, 109–127 [Google Scholar]
- Knox G. A., Lowry J. K.1977A comparison between the benthos of the Southern Ocean and the North Polar Ocean with special reference to the Amphipoda and the Polychaeta. In Polar oceans (ed. Dunbar M. J.), pp. 423–462 Calgary, Canada: Arctic Institute of North America [Google Scholar]
- Krug A. Z., Jablonski D., Valentine J. W.2009aGeneration of Earth's first-order biodiversity pattern. Astrobiology 9, 113–124 (doi:10.1089/ast.2008.0253) [DOI] [PubMed] [Google Scholar]
- Krug A. Z., Jablonski D., Valentine J. W.2009bSignature of the End-Cretaceous mass extinction in the modern biota. Science 323, 767–771 (doi:10.1126/science.1164905) [DOI] [PubMed] [Google Scholar]
- Lear C. H., Elderfield H., Wilson P. H.2000Cenozoic deep-sea temperatures and global ice volumes from Mg/Ca in benthic foraminiferal calcite. Science 287, 269–272 (doi:10.1126/science.287.5451.269) [DOI] [PubMed] [Google Scholar]
- Lester S. E., Ruttenberg B. I., Gaines S. D., Kinlan B. P.2007The relationship between dispersal ability and geographic range size. Ecol. Lett. 10, 745–758 (doi:10.1111/j.1461-0248.2007.01070.x) [DOI] [PubMed] [Google Scholar]
- Linse K., Griffiths H. J., Barnes D. K. A., Clarke A.2006Biodiversity and biogeography of Antarctic and Sub-Antarctic Mollusca. Deep-Sea Res. Part II 53, 985–1008 (doi:10.1016/j.dsr2.2006.05.003) [Google Scholar]
- Lipps J. H., Hickman C. S.1982Origin, age and evolution of Antarctic and deep-sea faunas. In Environment of the deep sea (eds Ernst W. G., Morris J. G.), pp. 324–356 Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- Marincovich L., Brouwers E. M., Carter L. D.1985Early Tertiary marine fossils from northern Alaska: implications for Arctic Ocean palaeogeography and faunal evolution. Geology 13, 770–773 (doi:10.1130/0091-7613(1985)13<770:ETMFFN>2.0.CO;2) [Google Scholar]
- Marincovich L., Brouwers E. M., Hopkins D. M., McKenna M. C.1990Late Mesozoic and Cenozoic paleogeographic and paleoclimate history of the Arctic Ocean basin, based on shallow-water marine faunas and terrestrial vertebrates. In The Arctic Ocean region. The geology of North America. Volume I, vol. 1 (eds Grantz A., Johnson L., Sweeney J. F.), pp. 403–426 Boulder, CO: Geological Society of America [Google Scholar]
- Munilla T., Membrives A. S.2009Check-list of the pycnogonids from Antarctic and sub-Antarctic waters: zoogeographic implications. Antarctic Sci. 21, 99–111 (doi:10.1017/S095410200800151X) [Google Scholar]
- Naish T., et al. 2009Obliquity-paced Pliocene West Antarctic ice sheet oscillations. Nature 458, 322–328 (doi:10.1038/nature07867) [DOI] [PubMed] [Google Scholar]
- Near T. J.2004Estimating divergence times of notothenioid fishes using a fossil-calibrated molecular clock. Antarctic Sci. 16, 37–44 (doi:10.1017/S0954102004001798) [Google Scholar]
- Ó Cofaigh C. O., Larter R. D., Dowdeswell J. A., Hillenbrand C.-D., Pudsey C. J., Evans J., Morris P.2005Flow of the West Antarctic Ice Sheet on the continental margin of the Bellingshausen Sea at the Last Glacial Maximum. J. Geophys. Res. Solid Earth 110, B11103 (doi:10.1029/2005JB003619) [Google Scholar]
- Oleinik A. E., Zinsmeister W. J.1996Paleocene diversification of bucciniform gastropods on Seymour Island, Antarctica. J. Paleontol. 70, 923–934 [Google Scholar]
- Peck L. S.2002Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol. 25, 31–40 (doi:10.1007/s003000100308) [Google Scholar]
- Peck L. S., Convey P., Barnes D. K. A.2006Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol. Rev. 81, 75–109 (doi:10.1017/S1464793105006871) [DOI] [PubMed] [Google Scholar]
- Picken G. B.1980The nearshore prosobranch gastropod epifauna of Signy Island, South Orkney Islands, 148 pp. Aberdeen, UK: Department of Zoology, University of Aberdeen [Google Scholar]
- Piepenburg D.2005Recent research on Arctic benthos: common notions need to be revised. Polar Biol. 28, 733–755 (doi:10.1007/s00300-005-0013-5) [Google Scholar]
- Powell R. D., Krissek L. A., van der Meer J. J. M.2000Preliminary depositional environment analysis of CRP-2/2A, Victoria Land Basin, Antarctica: palaeoglaciological and palaeoclimatic inferences. Terra Antartica 7, 313–322 [Google Scholar]
- Raup D. M., Jablonski D.1993Geography of end-Cretaceous marine bivalve extinctions. Science 260, 971–973 (doi:10.1126/science.11537491) [DOI] [PubMed] [Google Scholar]
- Shipp S., Anderson J. B., Domack E. W.1999Seismic signature of the Late Pleistocene fluctuation of the West Antarctic Ice Sheet system in the Ross Sea: a new perspective, Part I. Geol. Soc. Am. Bull. 111, 1496–1516 [Google Scholar]
- Sirenko B. I.2001List of species of free-living invertebrates of Eurasian Arctic seas and adjacent deep waters. Crustaceana 75, 1285–1286 [Google Scholar]
- Sirenko B. I., Pieperburg D.1994Current knowledge of biodiversity and benthic zonation patterns of Eurasian Arctic shelf seas, with special reference to the Laptev Sea. Berich. Polarforsch. 144, 69–77 [Google Scholar]
- Sohl N. F.1971North American Cretaceous biotic provinces delineated by gastropods. In Proc. of the Second North American Paleontological Convention, 1969, Chicago, vol. 2, pp. 1610–1637 Lawrence, KS: Allen Press [Google Scholar]
- Stilwell J. D.2003Patterns of biodiversity and faunal rebound following the K-T boundary extinction event in Austral Palaeocene molluscan faunas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 195, 319–356 (doi:10.1016/S0031-0182(03)00364-X) [Google Scholar]
- Stilwell J. D., Zinsmeister W. J., Oleinik A. E.2004Early Paleocene mollusks of Antarctica: systematics, paleoecology and paleogeographic significance. Bull. Am. Paleontol. 367, 1–89 [Google Scholar]
- Teske P. R., Papadopoulos I., Zardi G. I., McQuaid C. D., Edkins M. T., Griffiths C. L., Barker N. P.2007Implications of life history for genetic structure and migration rates of southern African coastal invertebrates: planktonic, abbreviated and direct development. Marine Biol. 152, 697–711 (doi:10.1007/s00227-007-0724-y) [Google Scholar]
- Thatje S., Arntz W. E.2004Antarctic reptant decapods: more than a myth? Polar Biol. 27, 195–201 (doi:10.1007/s00300-003-0583-z) [Google Scholar]
- Thatje S., Hillenbrand C.-D., Larter R.2005On the origin of Antarctic marine benthic community structure. Trends Ecol. Evol. 20, 534–540 (doi:10.1016/j.tree.2005.07.010) [DOI] [PubMed] [Google Scholar]
- Thatje S., Hillenbrand C.-D., Mackensen A., Larter R.2008Life hung by a thread: endurance of Antarctic fauna in glacial periods. Ecology 89, 682–692 (doi:10.1890/07-0498.1) [DOI] [PubMed] [Google Scholar]
- Tucker J. K.2004Catalogue of recent and fossil turrids (Mollusca: Gastropoda). Zootaxa 682, 1–1295 [Google Scholar]
- Valentine J. W.1967Influence of climatic fluctuations on species diversity within the Tethyan provincial system. In Aspects of Tethyan biogeography, vol. 7 (eds Adams C. G., Ager D. V.), pp. 153–166 London, UK: Systematics Association Publication [Google Scholar]
- Vermeij G. J.1991Anatomy of an invasion: the trans-Arctic interchange. Paleobiology 17, 281–307 [Google Scholar]
- Vermeij G. J.2001Community assembly in the sea. Geologic history of the living shore biota. In Marine community ecology (ed. Bertness M. D.), pp. 39–60 Sunderland, MA: Sinauer [Google Scholar]
- Wakeling J. M., Johnston I. A.1998Muscle power output limits fast-start performance in fish. J. Exp. Biol. 201, 1505–1526 [DOI] [PubMed] [Google Scholar]
- Waller C. L., Barnes D. K. A., Convey P.2006Ecological contrasts across an Antarctic land-sea interface. Austral. Ecol. 31, 656–666 (doi:10.1111/j.1442-9993.2006.01618.x) [Google Scholar]
- Walsh J. J.1988On the nature of continental shelves. San Diego, CA: Academic Press [Google Scholar]
- White M. G.1984Marine benthos. In Antarctic ecology, vol. 2 (ed. Laws R. M.), pp. 421–461 London, UK: Academic Press [Google Scholar]
- Zachos J., Pagani M., Sloan L., Thomas E., Billups K.2001Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (doi:10.1126/science.1059412) [DOI] [PubMed] [Google Scholar]
- Zenkevitch L. A.1963Biology of the seas of the USSR [English translation from the Russian by S. Botcharskaya], 955pp. London: George Allen & Unwin [Google Scholar]
- Zinsmeister W. J.1982Late Cretaceous-early Tertiary molluscan biogeograohy of the southern circum-Pacific. J. Paleontol. 56, 84–102 [Google Scholar]