Abstract
Huge areas of diverse tropical forest are lost or degraded every year with dramatic consequences for biodiversity. Deforestation and fragmentation, over-exploitation, invasive species and climate change are the main drivers of tropical forest biodiversity loss. Most studies investigating these threats have focused on changes in species richness or species diversity. However, if we are to understand the absolute and long-term effects of anthropogenic impacts on tropical forests, we should also consider the interactions between species, how those species are organized in networks, and the function that those species perform. I discuss our current knowledge of network structure and ecosystem functioning, highlighting empirical examples of their response to anthropogenic impacts. I consider the future prospects for tropical forest biodiversity, focusing on biodiversity and ecosystem functioning in secondary forest. Finally, I propose directions for future research to help us better understand the effects of anthropogenic impacts on tropical forest biodiversity.
Keywords: anthropogenic impacts, biodiversity, deforestation, ecosystem functioning, interactions, network structure
1. Introduction
Tropical forests are one of the most diverse habitats on Earth (Whitmore 1998). Over the past century tropical forests have been suffering from exceptional rates of change as they are degraded or destroyed by human activities. Approximately half of the tropical forest that was present at the beginning of the twentieth century has already disappeared, with peak deforestation in the 1980s and 1990s (Wright 2005). Evidence for a continuing decline in tropical forest area is unclear (Grainger 2008). This may be owing to difficulties in tracking the long term global trend in tropical forest area and forest regeneration (Grainger 2008). Estimates of a decline in deforestation may also result from the fact that even severely degraded forest is not included in the estimates of deforestation (Mayaux et al. 2005).
Estimates of tropical forest cover, for example from remote sensing, inform us about changes in forest extent, but crucially do not tell us how this relates to the biological diversity within. Biological diversity (hereafter biodiversity) refers to the ‘variability among living organisms from all sources including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part; this includes diversity within species, between species and of ecosystems’ (Convention on Biological Diversity 1992). Species–area curves can be used to predict the loss of species resulting from the loss of a particular area of forest (May et al. 1995), but their deceptive simplicity conceals a number of assumptions and complications that limit their usefulness (Lewis 2006).
In this paper, I focus on changes in tropical forest biodiversity over the past 100 years. During this period an increase in global population size and a concurrent increase in the exploitation of tropical forests have resulted in a vast reduction in biodiversity. Most studies have focused on changes in species richness and diversity, but it is becoming apparent that these metrics may not adequately represent anthropogenic impacts on ecological communities (Lewis 2009). Firstly, while overall species richness may not change following a particular disturbance the species identities may change, with implications for the persistence of ecological communities. Secondly, if we are to understand the absolute effect of anthropogenic impacts on tropical forests, we need to consider the interactions between species, how these species are organized (network structure) and the functions that these species perform (ecosystem functions). Ecosystem functions are biogeochemical activities, including primary production, decomposition and any other process that involves energy flow or nutrient cycling (Naeem 2009). A network structure and ecosystem functioning approach will supplement information on species richness and diversity by providing a more complete picture on how species coexist and influence one another, and in turn how anthropogenic impacts might perturb the organization and functioning of forests.
I begin by outlining the primary anthropogenic impacts on tropical forests and their effects at the species level. Then I discuss the importance of considering network structure and ecosystem functioning and describe the relatively small number of studies of anthropogenic impacts that have taken these approaches. Many of these studies focus on insects, because they are tractable study organisms, they make up more than half of all species and have a wide variety of functional roles in tropical forests. Next I consider the prospects for tropical forest biodiversity, focusing on biodiversity and ecosystem functioning in secondary forest. To conclude I suggest areas for future experimental and theoretical research, in order that we might better understand the tropical forest biodiversity crisis caused by anthropogenic impacts.
2. Anthropogenic influences on tropical forest biodiversity
The primary contemporary drivers of tropical forest biodiversity loss include direct effects of human activities such as habitat destruction and fragmentation (land-use change), invasive species and over-exploitation, as well as indirect effects of human activities such as climate change (Millennium Ecosystem Assessment 2005). The relative impacts of these threats vary among the world's major tropical forest regions (Corlett & Primack 2008). Primary drivers may also induce secondary effects, for example, altered disturbance dynamics (Barlow & Peres 2004). In this section, I discuss four major anthropogenic drivers of tropical biodiversity loss, focusing on their effects at the species level.
(a). Deforestation and fragmentation
Land-use change is thought to have the greatest impact on biodiversity in tropical forests (Sala et al. 2000). Forest clearance destroys the habitat and generally causes a decline in forest species abundance and diversity, particularly for species that are restricted in range. Diverse taxa show different and often variable responses (Lawton et al. 1998; Barlow et al. 2007). Following deforestation, the new habitat will determine biodiversity. For example, secondary forest regenerating after the natural forest has been cleared may never reach the same species richness and composition as primary forest (Chazdon 2008). However, the response is again taxon specific; for example, most primary forest leaf litter ant species in Ghana continued to survive in the agricultural landscape that replaced their original habitat (Belshaw & Bolton 1993). Apart from destroying habitat, forest clearance can fragment the remaining forest, leaving areas of forest that are too small for some species to persist, or too far apart for other species to move between (Fahrig 2003), resulting in a long process of decay in residual diversity from the remaining habitat (Krauss et al. 2010). Edge effects on fragments also affect species richness and composition (Ewers & Didham 2006).
(b). Over-exploitation
Over-exploitation of a particular species or group of species can result in that species, or group of species, being driven to local or even global extinction. It differs from the other drivers of biodiversity loss discussed here in specifically targeting individual species. The most well-known examples of over-exploitation of tropical forest species involve large mammals for bush-meat (Milner-Gulland et al. 2003), and tropical hardwoods for timber (Asner et al. 2005). A less well-known example is that of Chamaedorea palms (xaté) in Central America, whose leaves are harvested for the floricultural industry (Bridgewater et al. 2006).
(c). Invasive species
Invasive species are non-native species that have established outside their natural range, while introduced species have been established outside their natural range by human action. Both invasive and introduced species can cause extinctions, alter abiotic environments, become pests, or introduce diseases (Bradshaw et al. 2009), particularly targeting species with a lower reproductive potential or those that are naive to competitors or predators (Purvis et al. 2000). For example, the introduction of the brown snake on the island of Guam is thought to have caused the extinction of 12 of the 18 native birds (Wiles et al. 2003). Much of the evidence for the detrimental effects of invasive species is based on correlations between invasive species dominance and native species decline in degraded habitats (Didham et al. 2005). In these cases, invasive species could be driving the native species loss or could simply be taking advantage of habitat modification or another ecosystem change that is itself driving the native species loss (MacDougall & Turkington 2005). Invasive species may cause biotic homogenization, where species assemblages become dominated by a small number of widespread species that thrive in human-altered environments (McKinney & Lockwood 1999). Tropical forest communities that have been substantially altered by invasive or introduced species occur predominantly on heavily disturbed islands (Ghazoul & Sheil 2010). Intact continental rainforest may be more resistant to invasion because of the high species and functional group richness, high competitive exclusion rates and high pest loads (Denslow & DeWalt 2008). Invasive species can, however, dominate disturbed or open tropical forest areas, impacting their recovery (Ghazoul & Sheil 2010).
(d). Climate change
Climate change is expected to rival land-use change as the most important impact on tropical forest biodiversity. Many studies have shown that climate change causes range shifts to higher latitudes and elevations, as species expand into areas that become climatically suitable and contract from areas that become too warm (Wilson et al. 2007). Additionally, climate warming affects the phenology of species leading to potential mismatches between interacting species, for example, between pollinators and plants (Stenseth & Mysterud 2002). Climate change will also indirectly affect species by reducing the amount and availability of habitat, and by eliminating species that are essential to the species in question. Climate change is likely to have a particularly large impact on tropical ectotherms, even taking into account behavioural thermoregulation, because they are relatively sensitive to temperature change and are living very close to their optimal temperature (Deutsch et al. 2008; Huey et al. 2009; Kearney et al. 2009). The decline in amphibian populations in Neotropical montane tropical forests, notably golden toads (Bufo periglenes), has been linked to changing climate (Pounds 2001). Also, in central Panama, a change in climate in the form of a 25 year drying trend combined with increasingly severe dry seasons has led to a decline in the abundance of plant species with affinities for moist microhabitats (Condit et al. 1996). It is, however, difficult to make a causal link between climate change and changes in species richness because of the many other variables involved.
3. Anthropogenic impacts on network structure
The focus so far has been on changes at the species level, but species level metrics may not reveal all the changes occurring following a particular disturbance, because species identities can change without affecting species richness or diversity. In this section, I discuss how species are organized in networks, and the vulnerability of these networks to anthropogenic impacts. I highlight selected empirical studies that either take a network approach or consider cascading trophic effects.
(a). Networks of interacting species
Species are linked in networks to other species, with which they interact in a variety of ways, for example as predators or prey. Ecological network theory is dominated by diagrams of nodes and links, where the nodes represent species and each link represents the presence of an interaction or the mean interaction strength between two species. These mean values do not represent the observed ‘real world’ variability and we do not know the consequences of individual level variability to detected patterns of species interactions. Theoretically, the loss of one species has been shown to have widespread knock on effects on many other species (Montoya et al. 2006). This may lead to secondary or co-extinctions (Koh et al. 2004), but may also, in the short-term, benefit other species, if their competitors or predators are lost. Species interact both directly and indirectly, and the indirect interactions can be highly unpredictable (Yodzis 2000, Montoya et al. 2005). Consequently the loss of one species can result in the increase, decrease or extinction of apparently unconnected species.
Ecologists have found a number of network patterns that in theoretical studies affect the fragility of ecosystems to species loss. The lack of a theory quantifying the importance of these patterns holds us back from assessing their implications for biodiversity and its loss. The consequences of species loss depend on the connectance of the species within the network. Connectance studies have focused on unweighted trophic links, which may mask the role of skewed interaction strengths. Generally, however, in theoretical studies networks have been found to be robust, in terms of the secondary extinctions invoked, to random loss of species but not to loss of highly connected species (Sole & Montoya 2001; Dunne et al. 2002). Human activities are, however, causing secondary extinctions at greater levels than expected from random species losses (Dunne et al. 2002). Networks are less robust to the loss of highly connected species because the more trophic links that a species has to other species in a food web, the more potential it has to affect community structure. This, however, does not mean that the removal of a highly connected species will necessarily result in high species loss, nor that the removal of species with few trophic links will always result in few or no secondary extinctions.
The overall structure of networks may also affect their susceptibility to drivers of biodiversity loss. Theoretical studies have shown that a nested structure (where species with few links have a sub-set of the links of other species) makes the community more robust to both random extinctions (Memmott et al. 2004; Burgos et al. 2007) and habitat loss (Fortuna & Bascompte 2006). A modular pattern (with densely connected, non-overlapping subsets of species) may also increase network stability, retaining the impacts of a perturbation within a single module and minimizing impacts on other modules (Krause et al. 2003; Teng & McCann 2004). Nested groups and modules have been found in both mutualistic and antagonistic networks (Fortuna et al. 2010). Models with these network patterns have a well-known response to the deletion of species, but approaches considering explicit dynamics comparing those patterns using mutualistic and antagonistic networks are lacking (Bastolla et al. 2009).
Structural asymmetry may also be a key determinant of ecological stability, affecting the capacity of a system to recover from perturbations. Rooney et al. (2006) show that, in general, asymmetry in the amount of energy flowing through different channels, and in the pattern of predator attacks (interaction strengths), promotes network stability, by preventing dramatic overshoots following large perturbations while also permitting rapid recovery. The anthropogenic drivers of biodiversity loss directly erode this asymmetry by disproportionately reducing the abundance of top predators, and effectively homogenizing the energy channels, thus endangering the stability of natural ecosystems. In addition to eroding asymmetry, human-induced species loss is also likely to alter the number and configuration of stabilizing weak interactions (Berlow 1999). Despite our increasing knowledge of the patterns that influence the fragility of networks, there is still much to learn to increase our ability to predict how one species' extinction can cause multiple species losses.
A complete understanding of ecological networks may not be possible until ecologists begin to include all ecosystem components including detritus (Moore et al. 2004). Most ecological network studies focus entirely on living animals and plants, or more specifically on a subset of interactions, for example between a guild of hosts and their parasitoids. Quantitative food webs or networks are a useful tool for describing these subsets of interactions (Berlow et al. 2004; van Veen et al. 2006). They describe the species present in a network, and their abundances, and also show the frequency of interactions between them (although crucially do not take account of the variability of these interactions). Since they show a snapshot in time of a community, quantitative networks show only the potential for interactions and ecosystem functions, but they provide a framework for detecting the effects of human activities. The use of these quantitative networks is, however, limited by the intensive and time-consuming sampling required to document the interactions, particularly for antagonistic networks.
(b). Empirical examples
(i). Habitat destruction and fragmentation
A recent study has shown for the first time that the conversion of forest to agriculture has a detrimental effect on both species diversity and network interactions. Tylianakis et al. (2007) studied food webs of Hymenoptera and their parasitoids along a land-use gradient from forest through coffee agroforests and pasture to rice fields. They found little change in species richness of Hymenoptera along the land-use gradient, although there was a marked change in food-web structure. Crucially, the frequency of the interactions in which they were involved differed greatly, resulting in a decline in interaction evenness in the highly modified pasture and rice fields (figure 1). In more modified habitats there was a higher ratio of parasitoid to host species, increased parasitism, and the most abundant parasitoid species was more specialized, all of which have implications for the ecosystem services of pollination and biological control provided by the bees and wasps in this system.
Figure 1.
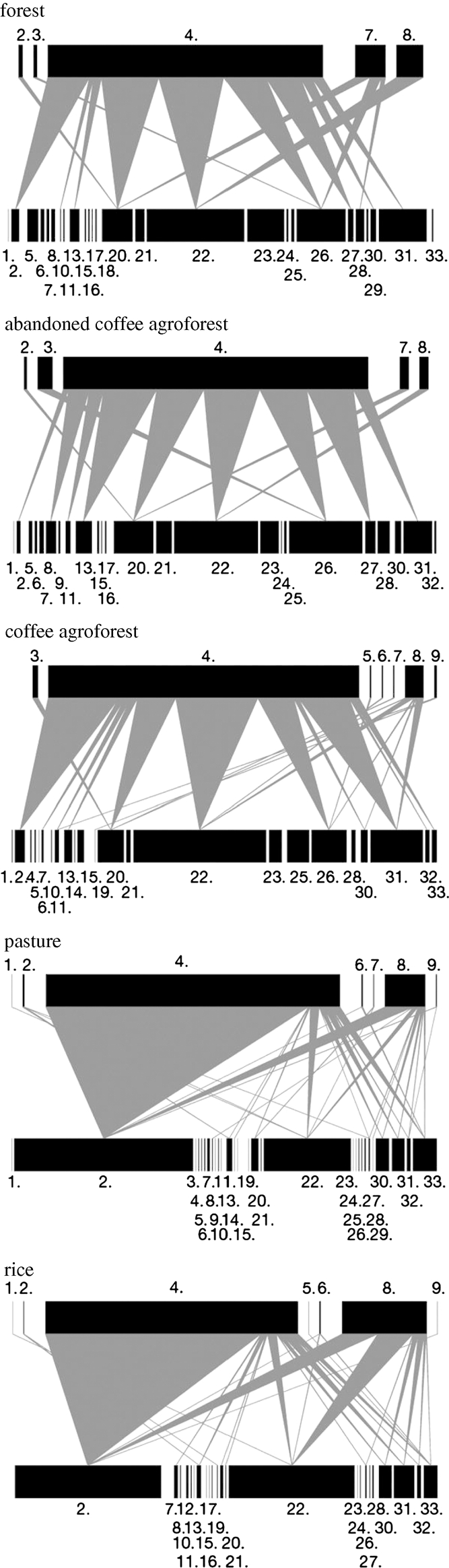
Quantitative host–parasitoid food webs along a land-use gradient from forest (top) through to rice fields (bottom). For each web, lower bars represent host bee and wasp abundances, and upper bars represent parasitoid abundances (drawn at different scales). The width of the links between upper and lower bars represents the frequency of each interaction. Host and parasitoid order are consistent across webs, and the numbers above and below the bars refer to species and are also consistent across webs. The evenness of interactions, in particular of the interactions shown by the most abundant parasitoid (species 4), declines significantly from the natural/semi-natural forest and agroforest habitats to the highly modified pasture and rice fields. Adapted with permission from Macmillan Publishers Ltd: Nature 445, 202–205, © 2007.
Fragmentation is likely to have profound effects on ecological interactions at the network level. For example, the loss of bird species in forest fragments resulted in deficient seed dispersal and reduced seedling recruitment of a bird dispersed rainforest tree (Cordeiro & Howe 2003). Generally, fragmentation reduces population densities of top predators (Holyoak 2000), and loss of top predators is likely to lead to strong trophic cascades (Adler 2008). Terborgh et al. (2001) studied forested islands in Lago Guri in Venezuela following their creation by the flooding of a tropical forest to construct a hydroelectric dam. While this fragmentation is qualitatively different to forest fragmentation resulting from conversion of forest to agricultural land, the resulting forest fragments created a natural experiment for studying the consequences of fragmentation. Terborgh et al. (2001) showed that trophic cascades resulted from the loss of top predators on these islands. An absence of large predators led to increased densities of seed predators and herbivores, and a correlated reduction in the densities of tree seedlings and saplings.
(ii). Over-exploitation
Over-hunting can cause cascading effects through tropical forest systems. The over-exploitation of large mammals has consequences for the structure, dynamics and species composition of tropical forest plant communities, through affecting their interactions with seed predators, seed dispersers, herbivores and browsers (Wright et al. 2007). The regional decline of mammals can also severely disrupt the diversity and abundance of dung beetle communities through the composition and availability of dung (Nichols et al. 2009). I am not aware of any studies investigating the effects of the natural over-exploitation of a single species or group of species on network structure. However, a manipulative experiment investigating the removal of a tropical forest shrub demonstrates the potential effects. While this study does not involve a traditionally exploited species, it serves as model species, showing how the removal of a particular species or group of species from a tropical forest may have cascading effects with profound implications for network structure. Morris et al. (2004) removed one species of shrub and its two associated leaf miners from replicate 4500 m2 plots in tropical forest in Belize, to investigate the propagation of indirect effects through the tri-trophic food web. They demonstrated pervasive effects on species abundance and parasitism a year after the initial manipulation.
(iii). Invasive species
The invasive yellow crazy ant on Christmas Island, in the Indian Ocean, has had pervasive effects on the rainforest ecosystem, facilitated by introduced honeydew-secreting scale insects (Abbott & Green 2007). Observational and experimental studies have demonstrated the effect of the ants on at least three trophic levels (O'Dowd et al. 2003). In invaded areas the red land crab, the dominant native omnivore, is eliminated, resulting in increased seedling recruitment and enhanced seedling species richness. Invasion, and in particular the formation of high-density ant supercolonies, also disrupts frugivory by endemic birds (Davis et al. 2010). Presence of the ants directly decreases handling time and indirectly influences bird abundances and behaviour through changes in resources and habitat structure, and may negatively impact on the ecological function of seed dispersal. Another example of introduced species affecting tropical forest communities comes from Hawaii. Henneman & Memmott (2001) demonstrated the pervasive effects of introduced biocontrol agents in a food web in the Alakai swamp forest on Kaua'i, where 83 per cent of parasitoids attacking native moths were the naturalized progeny of released biological control agents.
(iv). Climate change
I am not aware of any published studies investigating the effects of climate change on network structure in tropical forest, although I am aware of at least one study in progress.
4. Anthropogenic impacts on ecosystem functioning
(a). Ecosystem functioning
If a species is lost from an ecosystem it is not just the species itself that is lost, but its interactions, and the ecological functions that result from these interactions, for example, seed dispersal. These interactions can be critical to the survival or functioning of another species or the ecosystem itself. There are a huge number of studies focusing on the positive relationship between species diversity and ecosystem functioning, dominated by studies on productivity in temperate plants (Loreau et al. 2002). Until recently there was little focus on the relationship between network structure and ecosystem functioning, but there is an increasing realization that the loss of interactions can have pervasive effects on both ecosystem structure and functioning (Memmott et al. 2007), and that species diversity, network structure and ecosystem functioning are closely linked. However, that is not to say that high connectance automatically results in a high level of ecosystem functioning. Overall ecosystem functioning is dependent on the variety of interactions represented, and the presence of particular key functions, rather than on the total number of interactions or functions. More than one species may provide the same function in an ecosystem, providing ecological redundancy, for example, there may be many insects that pollinate a particular plant species (Walker 1992). This may buffer the effects of the loss of one species, but how many species can we lose before we start to affect ecosystem functioning (Purvis & Hector 2000)? Loss of tropical forest at extraordinarily high rates is likely to overcome this buffer, and result in the loss of ecosystem functioning, with dramatic effects not only on tropical forests, but on ecosystem services that benefit humans, such as pollination.
(b). Empirical examples
There are few studies that explicitly measure the effect of anthropogenic impacts on ecosystem functioning, and as far as I am aware, none for deforestation, invasive species or climate change. The studies mentioned under network structure may however, also be relevant here in terms of their implications for ecosystem functioning. Traill et al. (2010) recently reviewed how global warming will alter species interactions (and subsequently function), but found a paucity of information on ecosystem function.
(i). Fragmentation
Any species loss owing to habitat destruction may have a delayed impact on ecosystem functioning (Gonzalez et al. 2009). Many studies draw inferences about the loss of ecosystem functioning following fragmentation from species richness data; however, few have measured the functional process directly (Didham et al. 1996). Larsen et al. (2005) studied dung beetle communities on forested islands in Lago Guri in Venezuela. They found that habitat loss disrupted ecosystem functioning (in this case dung burial) by not only affecting species richness, but also species abundance and extinction order. Using both simulation models and empirical studies they demonstrated that the non-random response of communities to disturbance has unexpectedly large functional consequences. In particular, large-bodied beetle species tended to be both the most extinction-prone and the most functionally efficient, leading to rapid functional loss.
(ii). Over-exploitation
The cascading effects of over-hunting through tropical forest systems are likely to have impacts on ecosystem function, but I am not aware of any studies investigating this. A manipulative experiment on dung beetles by Slade et al. (2007) demonstrates how the removal of a particular group of species from a tropical forest may have cascading effects with profound implications for ecosystem functioning. While dung beetles are not a traditionally exploited species, over-hunting of mammals in tropical forests resulting in a reduction in dung could in fact indirectly lead to the local elimination of dependent dung beetles, with implications for the maintenance of key ecosystem processes including nutrient recycling and secondary seed dispersal (Andresen & Laurance 2007). Slade et al. (2007) experimentally manipulated dung beetle communities in primary forest in Malaysian Borneo, by controlling the access of different functional groups of beetles to patches of resource, thus mimicking the loss of nocturnal or diurnal, rolling or tunnelling beetles. The ecosystem functions of both dung removal and seed removal increased with dung beetle functional group richness, indicating that any loss of species occurring naturally would result in a lower ecosystem function if the loss of that species led to a reduced functional group composition.
5. Future prospects
(a). Prospects for tropical forest biodiversity
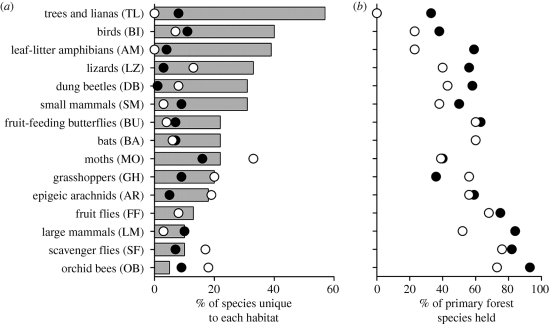
Tropical forest destruction is likely to continue in the future, causing an extinction crisis among tropical forest species (Bradshaw et al. 2009). Controversially, Wright & Muller-Landau (2006) suggested that tropical deforestation will decrease. They based their projections on slowing population growth rates and increasing urbanization, as well as an increase in natural forest regeneration. Their paper was widely challenged, particularly over the complex relationship between rural and urban population growth and deforestation, the potential irreversibility of the momentum set in motion by current patterns of deforestation and population growth, and the assumption that primary, secondary and degraded forests should all have the same ecological value (Brook et al. 2006; Gardner et al. 2007; Sloan 2007). Secondary forest tends to have a very different composition and structure to primary forest (Chazdon 2008). There is evidence that secondary forest contains less native biodiversity than primary forest (figure 2), but we do not know how many species depend on primary forest, and taxa appear to respond very differently (Barlow et al. 2007; Gardner et al. 2007).
Figure 2.
(a) The percentage of species unique to primary, secondary and plantation forests and (b) the percentage of species recorded in primary forest that were also recorded in secondary forest and plantations. Primary, secondary and plantation forests are represented by grey bars, black circles and white circles, respectively. Adapted with permission from the National Academy of Sciences: Proceedings of the National Academy of Sciences of the United States of America 104, 18 555–18 560, © 2007.
Wright & Muller-Landau (2006) predict that most forest will be secondary forest in the future, but will this be a suitable habitat for primary forest species? Degraded and secondary forests may have the potential to attain a structure and species composition similar to primary forests in the long term. However, since they are unlikely to be sufficiently connected to sources of primary forest species or to be protected from further disturbance, this potential is unlikely to be reached (Gardner et al. 2007). We currently have little insight into whether ecosystem functioning is restored. Understanding how secondary forest functioning differs from primary forest functioning is a major concern if an increasing proportion of the world's tropical forests will be secondary forest in the future. Slade (2007) experimentally investigated the herbivores of a Dipterocarp tree seedling and their predators, over a gradient of logging disturbance in Malaysian Borneo. She found that predation was higher in more disturbed sites, while herbivory did not differ, suggesting that ecosystem functions might be affected differently, and that generalizations will be difficult. We need to know the critical threshold at which primary forest becomes so degraded that it no longer performs the ecosystem functions and services that would be expected, and, conversely, the point at which regenerated tropical forest becomes equivalent to primary forest in terms of ecosystem functions and services. Even if the species themselves can recover, the restoration of interactions and functions may not follow (Memmott et al. 2007).
(b). Prospects for science
If we are to understand the contemporary threat of anthropogenic impacts on tropical forests, we need to understand exactly how ecological networks respond to perturbations. We need to conduct studies that tell us exactly how species diversity, network structure and ecosystem functioning are connected, and their relative importance. Then we can determine the elements of a tropical forest network that are crucial for functioning and most pertinent to measure. We need general insights rather than a case-by-case understanding of particular systems. Given that it is unlikely that we will stop destroying tropical forests completely, Gardner et al. (2009) discuss how we might combine social and ecological factors to enhance biodiversity conservation. Here, I focus on how we can increase our scientific understanding so we can make recommendations for the development of rational policies to mitigate the effects of human activities on tropical forests.
(i). Large-scale experiments
In order to fully understand and make generalizations about the effects of drivers of biodiversity loss there is a crucial need for more experimental investigations. These experiments must take place at large spatial and temporal scales given that most of the processes that we are concerned about are likely to be occurring at such scales. We need to understand how network structure and functioning change as drivers of biodiversity loss fluctuate in strength, as there is likely to be substantial variability in both magnitude and direction of effects (Tylianakis et al. 2008). Also, we need to investigate the synergistic effects of multiple drivers of biodiversity loss, particularly those involving climate change, which is having a pervasive effect (Brook et al. 2008). A recent microcosm experiment revealed that habitat fragmentation and overharvesting combined with environmental warming resulted in rotifer populations declining up to 50 times more rapidly when all three threats were combined than when acting alone (Mora et al. 2007). There is increasing concern that multiple drivers of ecological change will interact synergistically to accelerate biodiversity loss, but the prevalence and magnitude of these synergies remain uncertain (Brook et al. 2008). A meta-analysis revealed synergistic effects in a third of experiments and also found that unexpected effects were more common than additive effects (Darling & Cote 2008).
The variability in the effects of single global environmental change drivers along with the higher-order effects among multiple drivers acting simultaneously means that predicting future responses to tropical forest loss and extrapolating results across entire networks will be extremely challenging. The Biological Dynamics of Forest Fragments Project in Brazil, the world's largest scale and longest running habitat fragmentation experiment, has provided unique insights into the ecological decay of forest fragments (Laurance et al. 2002). It has paved the way for new similarly ambitious experiments investigating multiple interacting drivers.
(ii). Predicting the effects of anthropogenic impacts
We are missing vital basic information about how species are organized, and consequently cannot expect to be able to predict how perturbations will affect tropical forest biodiversity. Our focus should be on developing and testing theories about patterns of biodiversity and their mechanisms, and consequently on making the leap to understanding biodiversity from multiple interaction types and trophic levels. We are lacking specific and universal predictions about how perturbations propagate through networks causing species extinctions and potentially ecosystem collapse. Do patterns of extinctions derived from species level interaction networks capture the observed patterns, taking into account the dynamics of species interactions, observed individual variability and a limited sampling effort? Neutral theory has been useful in understanding the coexistence of species in tropical forests (Hubbell 2001). Would neutral theory help us to understand ecological networks and how they are affected by anthropogenic impacts? Gilbert et al. (2006) found that neutral theory accurately predicted the pace of local extinctions in forest fragments, but consistently underestimated changes in species composition because it does not recognize underlying differences among species.
We need to improve the leap from diversity and structure to ecosystem function. For example, if we know the species diversity and network structure for a particular tropical forest, can we predict the level of ecosystem functioning? And if we know the level of ecosystem functioning at the plant trophic level can we predict levels of ecosystem functioning at higher trophic levels? A crucial unanswered question for the conservation of tropical forest biodiversity and for global biodiversity in general, is ‘Do critical thresholds exist at which the loss of species diversity, or the loss of particular species, disrupts ecosystem functions and services, and how can these thresholds be predicted?’ (Sutherland et al. 2009).
(iii). Combining experiments and theory
Currently empiricists and theoreticians tend to work independently, but combining their approaches by conducting in situ tropical forest experiments to measure the effects of different quantifiable levels of single and interacting drivers of biodiversity loss, while modelling the real world variability in responses, could make the field more predictive. If we are modelling and experimenting on the same system we will be able to gain a deeper insight into how diversity, network structure and ecosystem function are affected by biodiversity loss, whether these three are inextricably linked, at what point ecosystem function is disrupted, and whether structure and ecosystem function can be restored. If enough studies can be conducted along these lines then we may be able to make general predictions about the critical thresholds that disrupt tropical forest ecosystem functioning.
6. Conclusion
In this paper, I have described the impacts of the main anthropogenic threats to tropical forests. It is clear that habitat destruction and fragmentation, over-exploitation, invasive species and climate change have the potential to create havoc in tropical forests. If we are to truly understand the impact of changes in biodiversity loss, we must look beyond species richness and diversity. It is evident that we do not fully understand the effects at the network and ecosystem functioning level and that much work is yet to be done. By combining well-designed large-scale experiments with improved theory that can predict how and when the loss of species results in the loss of structure and functioning, we can reach a greater scientific understanding that can be applied to mitigate the impact of human activities on tropical forests.
Acknowledgements
R.M. is funded by a Royal Society University Research Fellowship. I am grateful to Anne Magurran for the invitation to participate in the Royal Society Discussion Meeting and to Charles Godfray, Owen Lewis and two anonymous reviewers for helpful and insightful suggestions on earlier drafts of this manuscript.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Biological diversity in a changing world’.
References
- Abbott K. L., Green P. T.2007Collapse of an ant-scale mutualism in a rainforest on Christmas Island. Oikos 116, 1238–1246 (doi:10.1111/j.0030-1299.2007.15629.x) [Google Scholar]
- Adler G. H.2008Resource limitations of insular animals: causes and consequences. In Tropical forest community ecology (eds Carson W. P., Schnitzer S. A.). Oxford, UK: Wiley-Blackwell [Google Scholar]
- Andresen E., Laurance S. G. W.2007Possible indirect effects of mammal hunting on dung beetle assemblages in Panama. Biotropica 39, 141–146 (doi:10.1111/j.1744-7429.2006.00239.x) [Google Scholar]
- Asner G. P., Knapp D. E., Broadbent E. N., Oliveira P. J. C., Keller M., Silva J. N.2005Selective logging in the Brazilian Amazon. Science 310, 480–482 (doi:10.1126/science.1118051) [DOI] [PubMed] [Google Scholar]
- Barlow J., Peres C. A.2004Ecological responses to El Nino-induced surface fires in central Brazilian Amazonia: management implications for flammable tropical forests. Phil. Trans. R. Soc. Lond. B 359, 367–380 (doi:10.1098/rstb.2003.1423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J., et al. 2007Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl Acad. Sci. USA 104, 18 555–18 560 (doi:10.1073/pnas.0703333104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastolla U., Fortuna M. A., Pascual-Garcia A., Ferrera A., Luque B., Bascompte J.2009The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 458, 1018–1091 (doi:10.1038/nature07950) [DOI] [PubMed] [Google Scholar]
- Belshaw R., Bolton B.1993The effect of forest disturbance on the leaf litter ant fauna in Ghana. Biodiv. Conserv. 2, 656–666 (doi:10.1007/BF00051965) [Google Scholar]
- Berlow E. L.1999Strong effects of weak interactions in ecological communities. Nature 398, 330–334 (doi:10.1038/18672) [Google Scholar]
- Berlow E. L., et al. 2004Interaction strengths in food webs: issues and opportunities. J. Anim. Ecol. 73, 585–598 (doi:10.1111/j.0021-8790.2004.00833.x) [Google Scholar]
- Bradshaw C. J. A., Sodhi N. S., Brook B. W.2009Tropical turmoil: a biodiversity tragedy in progress. Front. Ecol. Environ. 7, 79–87 (doi:10.1890/070193) [Google Scholar]
- Bridgewater S. G. M., Pickles P., Garwood N. C., Penn M., Bateman R. M., Morgan H. P., Wicks N., Bol N.2006Chamaedorea (Xate) in the Greater Maya Mountains and the Chiquibul Forest Reserve, Belize: An economic assessment of a non-timber forest producy. Econ. Bot. 60, 265–283 (doi:10.1663/0013-0001(2006)60[265:CXITGM]2.0.CO;2) [Google Scholar]
- Brook B. W., Bradshaw C. J. A., Koh L. P., Sodhi N. S.2006Momentum drives the crash: mass extinction in the tropics. Biotropica 38, 302–305 (doi:10.1111/j.1744-7429.2006.00141.x) [Google Scholar]
- Brook B. W., Sodhi N. S., Bradshaw C. J. A.2008Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (doi:10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- Burgos E., Ceva H., Perazzo R. P. J., Devoto M., Medan D., Zimmermann M., Delbue A. M.2007Why nestedness in mutualistic networks? J. Theoret. Biol. 249, 307–313 (doi:10.1016/j.jtbi.2007.07.030) [DOI] [PubMed] [Google Scholar]
- Chazdon R. L.2008Chance and determinism in tropical forest succession. In Tropical forest community ecology (eds Carson W. P., Schnitzer S. A.). Oxford, UK: Wiley-Blackwell [Google Scholar]
- Condit R., Hubbell S. P., Foster R. B.1996Changes in tree species abundance in a Neotropical forest: impact of climate change. J. Trop. Ecol. 12, 231–256 (doi:10.1017/S0266467400009433) [Google Scholar]
- Convention on Biological Diversity. 1992. See http://www.cbd.int/convention/convention.shtml .
- Cordeiro N. J., Howe H. F.2003Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc. Natl Acad. Sci. USA 100, 14 052–14 056 (doi:10.1073/pnas.2331023100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett R. T., Primack R. B.2008Tropical rainforest conservation: a global perspective. In Tropical forest community ecology (eds Carson W. P., Schnitzer S. A.). Oxford, UK: Wiley-Blackwell [Google Scholar]
- Darling E. S., Cote I. M.2008Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286 (doi:10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- Davis N. E., O'dowd D. J., Mac Nally R., Green P. T.2010Invasive ants disrupt frugivory by endemic island birds. Biol. Lett. 6, 85–88 (doi:10.1098/rsbl.2009.0655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow J. S., Dewalt S. J.2008Exotic plant invasions in tropical forests: patterns and hypotheses. In Tropical forest community ecology (eds Carson W. P., Schnitzer S. A.). Oxford, UK: Wiley-Blackwell [Google Scholar]
- Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., Martin P. R.2008Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672 (doi:10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didham R. K., Ghazoul J., Stork N. E., Davis A. J.1996Insects in fragmented forests: a functional approach. Trends Ecol. Evol. 11, 255–260 (doi:10.1016/0169-5347(96)20047-3) [DOI] [PubMed] [Google Scholar]
- Didham R. K., Tylianakis J. M., Hutchison M. A., Ewers R. M., Gemmell N. J.2005Are invasive species the drivers of ecological change? Trends Ecol. Evol. 20, 470–474 (doi:10.1016/j.tree.2005.07.006) [DOI] [PubMed] [Google Scholar]
- Dunne J. A., Williams R. J., Martinez N. D.2002Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett. 5, 558–567 (doi:10.1046/j.1461-0248.2002.00354.x) [Google Scholar]
- Ewers R. M., Didham R. K.2006Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 81, 117–142 (doi:10.1017/S1464793105006949) [DOI] [PubMed] [Google Scholar]
- Fahrig L.2003Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 34, 487–515 (doi:10.1146/annurev.ecolsys.34.011802.132419) [Google Scholar]
- Fortuna M. A., Bascompte J.2006Habitat loss and the structure of plant–animal mutualistic networks. Ecol. Lett. 9, 281–286 (doi:10.1111/j.1461-0248.2005.00868.x) [DOI] [PubMed] [Google Scholar]
- Fortuna M. A., Stouffer D. B., Olesen J. M., Jordano P., Mouillot D., Krasnov B. R., Poulin R., Bascompte J.2010Nestedness versus modularity in ecological networks: two sides of the same coin? J. Anim. Ecol. 79, 811–817 (doi:10.1111/j.1365-2656.2010.01688.x) [DOI] [PubMed] [Google Scholar]
- Gardner T. A., Barlow J., Parry L. W., Peres C. A.2007Predicting the uncertain future of tropical forest species in a data vacuum. Biotropica 39, 25–30 (doi:10.1111/j.1744-7429.2006.00228.x) [Google Scholar]
- Gardner T. A., Barlow J., Chazdon R., Ewers R. M., Harvey C. A., Peres C. A., Sodhi N. S.2009Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 12, 561–582 (doi:10.1111/j.1461-0248.2009.01294.x) [DOI] [PubMed] [Google Scholar]
- Ghazoul J., Sheil D.2010Tropical rain forest ecology, diversity, and conservation. Oxford, UK: Oxford University Press [Google Scholar]
- Gilbert B., Laurance W. F., Leigh E. G., Nascimento H. E. M.2006Can neutral theory predict the responses of Amazonian tree communities to forest fragmentation? Am. Nat. 168, 304–317 (doi:10.1086/506969) [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Mouquet N., Loreau M.2009Biodiversity as spatial insurance: the effects of habitat fragmentation and dispersal on ecosystem functioning. In Biodiversity, ecosystem functioning, and human wellbeing (eds Naeem S., Bunker D. E., Hector A., Loreau M., Perrings C.). Oxford, UK: Oxford University Press [Google Scholar]
- Grainger A.2008Difficulties in tracking the long-term global trend in tropical forest area. Proc. Natl Acad. Sci. USA 105, 818–823 (doi:10.1073/pnas.0703015105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman M. L., Memmott J.2001Infiltration of a Hawaiian community by introduced biological control agents. Science 293, 1314–1316 (doi:10.1126/science.1060788) [DOI] [PubMed] [Google Scholar]
- Holyoak M.2000Habitat subdivision causes changes in food web structure. Ecol. Lett. 3, 509–515 (doi:10.1046/j.1461-0248.2000.00180.x) [Google Scholar]
- Hubbell S. P.2001A unified neutral theory of biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- Huey R. B., Deutsch C. A., Tewksbury J. J., Vitt L. J., Hertz P. E., Perez H. J. A., Garland T.2009Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948 (doi:10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M., Shine R., Porter W. P.2009The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840 (doi:10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh L. P., Dunn R. R., Sodhi N. S., Colwell R. K., Proctor H. C., Smith V. S.2004Species coextinctions and the biodiversity crisis. Science 305, 1632–1634 (doi:10.1126/science.1101101) [DOI] [PubMed] [Google Scholar]
- Krause A., Frank K., Mason D., Ulanowickz R., Taylor W.2003Compartments revealed in food-web structure. Nature 426, 282–285 (doi:10.1038/nature02115) [DOI] [PubMed] [Google Scholar]
- Krauss J., et al. 2010Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol. Lett. 13, 597–605 (doi:10.1111/j.1461-0248.2010.01457.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen T. H., Williams N. M., Kremen C.2005Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol. Lett. 8, 538–547 (doi:10.1111/j.1461-0248.2005.00749.x) [DOI] [PubMed] [Google Scholar]
- Laurance W. F., et al. 2002Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv. Biol. 16, 605–618 (doi:10.1046/j.1523-1739.2002.01025.x) [Google Scholar]
- Lawton J. H., et al. 1998Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature 391, 72–76 (doi:10.1038/34166) [Google Scholar]
- Lewis O. T.2006Climate change, species–area curves and the extinction crisis. Phil. Trans. R. Soc. B 361, 163–171 (doi:10.1098/rstb.2005.1712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis O. T.2009Biodiversity change and ecosystem function in tropical forests. Basic Appl. Ecol. 10, 97–102 (doi:10.1016/j.baae.2008.08.010) [Google Scholar]
- Loreau M., Naeem S., Inchausti P.2002Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford, UK: Oxford University Press [Google Scholar]
- Macdougall A. S., Turkington R.2005Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86, 42–55 (doi:10.1890/04-0669) [Google Scholar]
- May R. M., Lawton J. H., Stork N. E.1995Assessing extinction rates. In Extinction rates (eds Lawton J. H., May R. M.), pp. 1–24 Oxford, UK: Oxford University Press [Google Scholar]
- Mayaux P., Holmgren P., Achard F., Eva H., Stibig H., Branthomme A.2005Tropical forest cover change in the 1990s and options for future monitoring. Phil. Trans. R. Soc. B 360, 373–384 (doi:10.1098/rstb.2004.1590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckinney M. L., Lockwood J. L.1999Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (doi:10.1016/S0169-5347(99)01679-1) [DOI] [PubMed] [Google Scholar]
- Memmott J., Waser N. M., Price M. V.2004Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 (doi:10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott J., Gibson R., Gigante Carvalheiro L., Henson K., Huttel Heleno R., Lopezaraiza Mikel M., Pearce S.2007The conservation of ecological interactions. In Proc. Royal Entomological Society's 23rd Symp. Insect Conservation Biology (eds Stewart A. J. A., New T. R., Lewis O. T.), ch. 10, pp. 226–244. Wallingford, UK: CABI Publishing [Google Scholar]
- Millennium Ecosystem Assessment 2005Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute [Google Scholar]
- Milner-Gulland E. J., Bennett E. L.& the SCB 2002 Annual Meeting Wild Meat Group 2003Wild meat: the bigger picture. Trends Ecol. Evol. 18, 351–357 (doi:10.1016/S0169-5347(03)00123-X) [Google Scholar]
- Montoya J. M., Emmerson M. C., Sole R. V., Woodward G.2005Perturbations and indirect effects in complex food webs. In Dynamic food webs: multispecies assemblages, ecosystem development, and environmental change (eds De Ruiter P., Wolters V., Moore J. C.). New York, NY: Academic Press [Google Scholar]
- Montoya J. M., Pimm S. L., Sole R. V.2006Ecological networks and their fragility. Nature 442, 259–264 (doi:10.1038/nature04927) [DOI] [PubMed] [Google Scholar]
- Moore J. C., et al. 2004Detritus, trophic dynamics and biodiversity. Ecol. Lett. 7, 584–600 (doi:10.1111/j.1461-0248.2004.00606.x) [Google Scholar]
- Mora C., Metzger R., Rollo A., Myers R. A.2007Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proc. R. Soc. B 274, 1023–1028 (doi:10.1098/rspb.2006.0338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. J., Lewis O. T., Godfray H. C. J.2004Experimental evidence for apparent competition in a tropical forest food web. Nature 428, 310–313 (doi:10.1038/nature02394) [DOI] [PubMed] [Google Scholar]
- Naeem S.2009Biodiversity, ecosystem functioning, and ecosystem services. In The Princeton guide to ecology (ed. Levin S. A.). Princeton, NJ: Princeton University Press [Google Scholar]
- Nichols E., Gardner T. A., Peres C. A., Spector S.& the Scarabaeinae Research Network 2009Co-declining mammals and dung beetles: an impending ecological cascade. Oikos 118, 481–487 (doi:10.1111/j.1600-0706.2009.17268.x) [Google Scholar]
- O'Dowd D. J., Green P. T., Lake P. S.2003Invasional ‘meltdown’ on an oceanic island. Ecol. Lett. 6, 812–817 (doi:10.1046/j.1461-0248.2003.00512.x) [Google Scholar]
- Pounds J. A.2001Climate and amphibian declines. Nature 410, 639–640 (doi:10.1038/35070683) [DOI] [PubMed] [Google Scholar]
- Purvis A., Hector A.2000Getting the measure of biodiversity. Nature 405, 212–219 (doi:10.1038/35012221) [DOI] [PubMed] [Google Scholar]
- Purvis A., Gittleman J. L., Cowlishaw G., Mace G. M.2000Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952 (doi:10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney N., McCann K., Gellner G., Moore J. C.2006Structural asymmetry and the stability of diverse food webs. Nature 442, 265–269 (doi:10.1038/nature04887) [DOI] [PubMed] [Google Scholar]
- Sala O. E., et al. 2000Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- Slade E. M.2007The effects of tropical forest management on biodiversity and ecosystem functioning. DPhil thesis, Department of Zoology, University of Oxford [Google Scholar]
- Slade E. M., Mann D. J., Villanueva J. F., Lewis O. T.2007Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. J. Anim. Ecol. 76, 1094–1104 (doi:10.1111/j.1365-2656.2007.01296.x) [DOI] [PubMed] [Google Scholar]
- Sloan S.2007Fewer people may not mean more forest for Latin American forest frontiers. Biotropica 39, 443–446 (doi:10.1111/j.1744-7429.2007.00288.x) [Google Scholar]
- Sole R. V., Montoya J. M.2001Complexity and fragility in ecological networks. Proc. R. Soc. Lond. B 268, 2039–2045 (doi:10.1098/rspb.2001.1767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N. C., Mysterud A.2002Climate, changing phenology, and other life history and traits: nonlinearity and match-mismatch to the environment. Proc. Natl Acad. Sci. USA 99, 13 379–13 381 (doi:10.1073/pnas.212519399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland W. J., et al. 2009One hundred questions of importance to the conservation of global biological diversity. Conserv. Biol. 23, 557–567 (doi:10.1111/j.1523-1739.2009.01212.x) [DOI] [PubMed] [Google Scholar]
- Teng J., McCann K. S.2004Dynamics of compartmented and reticulate food webs in relation to energetic flows. Am. Nat. 164, 85–100 (doi:10.1086/421723) [DOI] [PubMed] [Google Scholar]
- Terborgh J., et al. 2001Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (doi:10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- Traill L. W., Lim M., Sodhi N. S., Bradshaw C. J. A.2010Mechanisms driving change: altered species interactions and ecosystem function through global warming. J. Anim. Ecol. 79, 937–947 (doi:10.1111/j.1365-2656.2010.01695.x) [DOI] [PubMed] [Google Scholar]
- Tylianakis J. M., Tscharntke T., Lewis O. T.2007Habitat modification alters the structure of tropical host–parasitoid food webs. Nature 445, 202–205 (doi:10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- Tylianakis J. M., Didham R. K., Bascompte J., Wardle D. A.2008Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- Van Veen F. J. F., Morris R. J., Godfray H. C. J.2006Apparent competition, quantitative food webs, and the structure of phytophagous insect communities. Annu. Rev. Entomol. 51, 187–208 (doi:10.1146/annurev.ento.51.110104.151120) [DOI] [PubMed] [Google Scholar]
- Walker B. H.1992Biodiversity and ecological redundancy. Conserv. Biol. 6, 18–23 (doi:10.1046/j.1523-1739.1992.610018.x) [Google Scholar]
- Whitmore T. C.1998An introduction to tropical rain forests. Oxford, UK: Oxford University Press [Google Scholar]
- Wiles G. J., Bart J., Beck R. E., Aguon C. F.2003Impacts of the brown tree snake: patterns of decline and species persistence in Guam's avifauna. Conserv. Biol. 17, 1350–1360 (doi:10.1046/j.1523-1739.2003.01526.x) [Google Scholar]
- Wilson R. J., Davies Z. G., Thomas C. D.2007Insects and climate change: processes, patterns and implications for conservation. In Proc. Royal Entomological Society's 23rd Symp. Insect Conservation Biology (eds Stewart A. J. A., New T. R., Lewis O. T.), ch. 11, pp. 245–279. Wallingford, UK: CABI Publishing [Google Scholar]
- Wright S. J.2005Tropical forests in a changing environment. Trends Ecol. Evol. 20, 553–560 (doi:10.1016/j.tree.2005.07.009) [DOI] [PubMed] [Google Scholar]
- Wright S. J., Muller-Landau H. C.2006The future of tropical forest species. Biotropica 38, 287–301 (doi:10.1111/j.1744-7429.2006.00154.x) [Google Scholar]
- Wright S. J., Stoner K. E., Beckman N., Corlett R. T., Dirzo R., Muller-Landau H. C., Nunez-Iturri G., Peres C. A., Wang B. C.2007The plight of large animals in tropical forests and the consequences for plant regeneration. Biotropica 39, 289–291 (doi:10.1111/j.1744-7429.2007.00293.x) [Google Scholar]
- Yodzis P.2000Diffuse effects in food webs. Ecology 81, 261–266 (doi:10.1890/0012-9658(2000)081[0261:DEIFW]2.0.CO;2) [Google Scholar]