Abstract
Temporal variation in species abundances occurs in all ecological communities. Here, we explore the role that this temporal turnover plays in maintaining assemblage diversity. We investigate a three-decade time series of estuarine fishes and show that the abundances of the individual species fluctuate asynchronously around their mean levels. We then use a time-series modelling approach to examine the consequences of different patterns of turnover, by asking how the correlation between the abundance of a species in a given year and its abundance in the previous year influences the structure of the overall assemblage. Classical diversity measures that ignore species identities reveal that the observed assemblage structure will persist under all but the most extreme conditions. However, metrics that track species identities indicate a narrower set of turnover scenarios under which the predicted assemblage resembles the natural one. Our study suggests that species diversity metrics are insensitive to change and that measures that track species ranks may provide better early warning that an assemblage is being perturbed. It also highlights the need to incorporate temporal turnover in investigations of assemblage structure and function.
Keywords: biodiversity, turnover, estuarine, fish, abundance, rank
1. Introduction
Growing concern about the state of the planet and the impacts that we humans are having on it (Butchart et al. 2010) has led to renewed interest in methods used to track changes in biodiversity, which is generally done in one of two ways (Magurran et al. in press). The first approach is to combine information on the status of individual species to provide an overview of change, as, for example, the Living Planet Index (Loh et al. 2005) does. These types of indicators are popular with politicians and policy-makers and are increasingly widely used, but can have the disadvantage that conclusions are made on the basis of a non-random selection of species. Alternatively, one can look at the assemblage as a whole and monitor changes in biodiversity in relation to baseline changes—that is in comparison with the extent of temporal shifts in species identities and species abundances that would occur in an unperturbed assemblage.
Assemblage-based assessments track these shifts in an ecologically coherent group of organisms, and as such provide a link between biodiversity and ecosystem function (Cardinale et al. 2007, 2009; Loreau 2010). Temporal variation—turnover—in species abundances (Srivastava & Vellend 2005; Loreau 2010) can help maintain function if there is asynchrony in species abundances such that species that decline are replaced with those that have similar functional roles. Two closely related hypotheses—the ‘portfolio effect’ (Doak et al. 1998; Tilman et al. 1998) and the ‘insurance hypothesis’ (Yachi & Loreau 1999)—argue that ecosystem properties are more stable in diverse assemblages because there will be more species to compensate for any changes that do occur.
Although an assemblage-wide assessment of biodiversity is appealing, there are a number of difficulties. First, apart from a few well-known examples, such as the Park Grass experiment (Lawes & Gilbert 1880; Lawes et al. 1882) and the Continuous Plankton Recorder (Richardson et al. 2006), there are relatively few long-term datasets in which biodiversity data have been collected using consistent methods over a number of decades (Wolfe et al. 1987; Magurran et al. in press)—but this is now changing (e.g. Ernest et al. 2008). Second, although it is well known that all ecological communities experience temporal turnover (Darwin 1859; MacArthur & Wilson 1967), we still have limited information on how assemblage biodiversity changes through time (but see Grossman et al. 1982; Sale & Douglas 1984; Ross et al. 1985; Warwick et al. 2002; Thibault et al. 2004; Pavoine et al. 2009 for some exceptions). This arises not just as a consequence of data shortage but also because models of species abundance have focused on spatial rather than temporal patterns (Magurran 2007). As a result, it can be difficult to know whether reported changes in biodiversity are greater than those that might have happened by chance.
In general, it seems that mature assemblages—that is, those not undergoing any directional change such as succession—tend to show high persistence in the sense that species richness is maintained through time (Ross et al. 1985). This constancy can be underlain by considerable temporal variation in species rank and species abundance (Grossman et al. 1982, 1990; Ross et al. 1985), and thus is consistent with the idea that temporal asynchrony in species abundances can help stabilize community properties (Loreau 2010). However, Cottingham et al. (2001) found that the relationship between species richness and the regulation of aggregate assemblage variables (such as biomass and productivity) was more complex than anticipated. Moreover, it appears that species abundances do not always vary asynchronously. Valone & Barber (2008), for instance, discovered that the abundances of most pairs of species in an analysis of a range of terrestrial assemblages tended to covary positively—and attributed this to correlated responses to climatic fluctuations. Houlahan et al. (2007) found that the abundances of species in natural communities tended to covary positively and argued that the absence of compensatory dynamics is because abiotic factors, including climate, are likely to be more important than competition. On the other hand, Leary & Petchey (2009) showed that experimental communities of protists that contained species with different responses to environmental changes exhibited lower temporal variation in biomass. It is clear that our knowledge of the natural pattern of change in ecological assemblages is incomplete, and to address this, we need to examine data that have been collected using consistent methods, at regular intervals and ideally over decades so that we can be sure that natural variation is accounted for. Two factors warrant investigation: (i) do species abundances in natural assemblages vary asynchronously and (ii) which patterns of asynchrony (i.e. turnover in species abundances) produce assemblages where the structure is consistent with that found in nature and where assemblage properties are maintained through time.
In this paper, we explore temporal changes in an exceptionally well-documented assemblage of estuarine fishes and compare these patterns with those generated by a simulated fish assemblage with the same set of species, in which we manipulate the degree of within-species autocorrelation in abundance across years. We initially focus on the empirical dataset and begin by asking whether the abundances of the individual species change asynchronously, as predicted by the insurance and portfolio hypotheses discussed above. Next, we relate this pattern to assemblage structure with the expectation that this structure is preserved through time (i.e. that there will be no trend in a range of diversity metrics). Then, using the simulated data, we examine the conditions under which the aggregated abundances of the individual species produce an assemblage that resembles the natural one. We do this because while asynchrony in species abundances may be necessary to assure continuity of assemblage properties, year-to-year changes in the abundance of species will be constrained by their ecology, with the result that only a limited range of turnover scenarios are likely to be present in natural systems. For this analysis, we assume that the abundances of species in the assemblage vary independently, as each oscillates around its historical mean. Thus, we are asking how the correlation between the abundance of a species in a given year and its abundance in the previous year influences the structure of the overall assemblage. We assess assemblage responses to these varying levels of autocorrelation by using diversity measures that evaluate assemblage structure in terms of the relative abundances of species, but ignore species identities, and with metrics that track individual species identities. As Whittaker (1960) recognized, measures that take account of species composition can uncover differences between assemblages that indices that examine species richness or species relative abundance might not pick up (see also Grossman et al. 1982). We, therefore, expect the metrics that track species identities to reveal a smaller set of turnover scenarios in which the predicted assemblage resembles the real one.
2. Methods
(a). Data collection
The Bristol Channel estuarine fish assemblage has been sampled monthly for 30 years (Henderson 2007; Henderson & Bird 2010). To date, greater than 80 species and greater than 100 000 individuals have been recorded.
Fish samples were collected from the cooling water filter screens at Hinkley Point B power station, situated on the southern bank of the Bristol Channel in Somerset, England. The water intakes are in front of a rocky promontory within Bridgwater Bay, and to the east are the 40 km2 Stert mudflats. Depending upon the tide, the fish were sampled from water varying in depth from about 8 to 18 m. For a full description of the intake configuration and sampling methodology, see Henderson & Holmes (1991) and Henderson & Seaby (1994). The filter screens have a solid square mesh of 10 mm. Methodology has not changed over the three decades of the study.
Quantitative sampling commenced in 1980 when 24 h surveys of the diurnal pattern of capture were undertaken in October and November. From these surveys, it was concluded that samples collected during daylight were representative of the 24 h catch (Henderson & Holmes 1990) and monthly quantitative sampling commenced in January 1981. The total volume of water sampled per month, which has not varied over the entire period, is 4.27 × 105 m3. To standardize for tidal influence, all sampling dates were chosen for tides halfway between springs and neaps, with sampling commencing at high water (normally about 12.00 h). Fish were collected hourly from two filter screens for a 6 h period, identified to species and the number of individuals recorded.
The power station intakes at Hinkley Point are an effective sampler because of their position at the edge of a large inter-tidal mudflat in an estuary with extremely powerful tides resulting in suspended solid levels of up to 3 g l−1 and little light below 50 cm depth. The fishes, pelagic or benthic, are moved towards the intake in the tidal stream, often as they retreat from the inter-tidal zone where they feed, and it is likely that they are unable to see or otherwise detect the intake until they are too close to make an escape. Light is clearly important for avoidance because at power station intakes situated in clear water captures are higher at night. The efficiency of the sampling method is discussed in Henderson & Holmes (1991).
(b). Data analysis
We ask whether species abundances vary asynchronously by examining pairwise correlations, through time, for both the core assemblage (i.e. species that are present in majority of years; Magurran & Henderson 2003) and the entire assemblage (all species recorded). In both cases, we compare the empirical median correlation coefficient with the values generated by a randomization test (repeated 1000 times) in which the abundances of each species are randomly shuffled among years.
To examine the link between the patterns of autocorrelation and assemblage structure, we employ the following time-series model (Crawley 2007):
where Yt is the abundance of each species at time t, Yt−1 the abundance of species in the previous year, α the correlation structure (within species, between years) and Zt the noise in year t (set by a random number generator with mean = 0 and s.d. = empirical value).
Starting values are set at 0 and the output rescaled by adding the empirical mean (for each species) to obtain the predicted values. The model is run for values of α between −1 (strong anti-correlations) and +1 (random walk) in steps of 0.05. In each case, the autocorrelation value is equal for all species, while noise is independent for each species. Moreover, there is no among-species correlation included in the model, and species dynamics are completely independent. This analysis is repeated 1000 times and for both the core and the entire assemblage. We run the model for 50 years and use the last 30 years in our analyses. While we appreciate that strong negative correlations in the abundance of these estuarine fishes—many of which are relatively long lived—are biologically unlikely, we include these in our analysis to provide an insight into the consequences of these extreme values for temporal trends in assemblage diversity.
We assess the structure of the resulting assemblages by using three measures that place different weights on the relative abundance of species. These measures, which are well-known diversity statistics (Magurran 2004) and numerically related to one another (Hill 1973), are the exponential form of the Shannon index (N1 in Hill's terminology), the reciprocal form of the Simpson index (N2) and the Berger–Parker index (N∞). The exponential form of the Shannon index emphasizes the species richness component of diversity, the reciprocal form of the Simpson index emphasizes dominance, whereas the Berger–Parker index is a pure measure of dominance (specifically the relative abundance of the most abundant species). Together they provide an informative overview of the structure of the assemblage.
These diversity measures, however, ignore species identities. To track change in composition, we use two metrics, mean rank shift (MRS) and Bray–Curtis dissimilarity. MRS (Collins et al. 2008) is a measure of relative change in species rank abundance. The Bray–Curtis dissimilarity is a widely used measure of ecological distance (Southwood & Henderson 2000; Magurran 2004; Jost et al. in press) that assesses compositional similarity taking account of species abundances. Whereas the Hill numbers provide a value of diversity for each year of the series, MRS and Bray–Curtis dissimilarity make comparisons between consecutive pairs of years in the time series.
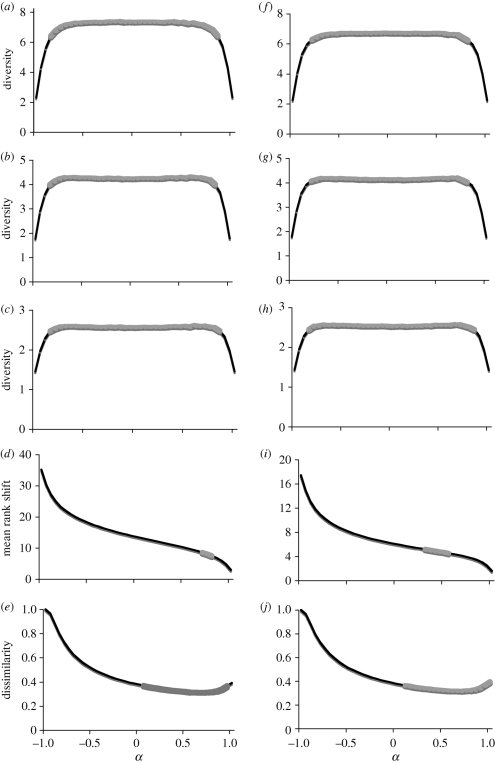
To compare the natural and simulated patterns, we first calculate the 95 per cent confidence limits for a given metric using the annual values in the empirical time series. We then take the mean predicted value for each level of autocorrelation and average these across the last 30 years of the simulated series (as above). These predicted values are then plotted in relation to the 95 per cent confidence limits of the empirical values (figure 4) as a simple method of examining the agreement between the observed and expected diversity metrics.
Figure 4.
Relationship between community characteristics (diversity (a–c, f–h) and community composition (d,e,i,j)) and correlation structure (α) for the entire community (a–e) and the core community (f,j). Graphs plot predicted values (mean of 30y). Points that lie within the 95% confidence limit of the empirical value are indicated by the thicker grey line. (a,f) N1, exp Shannon index, (b,g) N2, 1/Simpson index, (c,h) N∞, Berger–Parker index, (d,i) MRS and (e,j) Bray–Curtis index.
All analyses use the statistical programming language R (R Development Core Team 2008). Hill's diversity numbers and Bray–Curtis dissimilarity were calculated using the vegan (Oksanen 2010) package.
3. Results
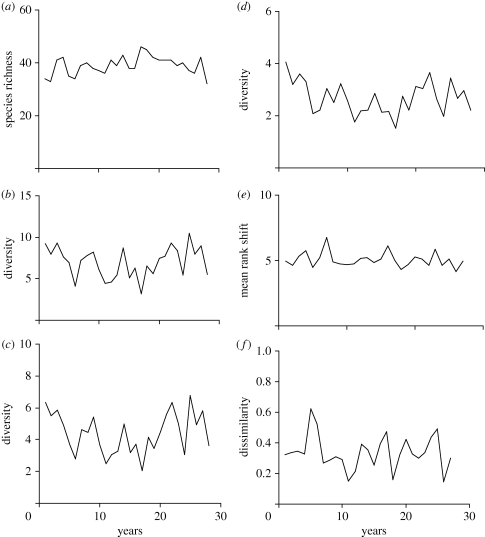
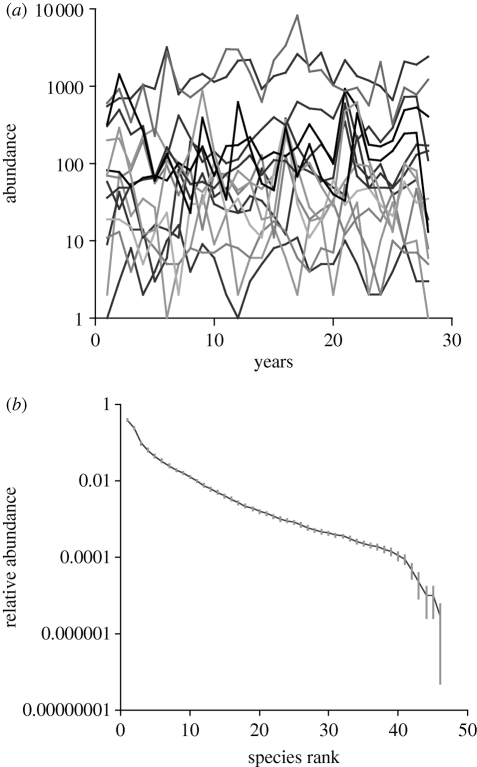
The structure of this estuarine assemblage has remained essentially unchanged through the study period (figure 1). This is true for the core species, as well as for the assemblage as a whole. Although some of our metrics show year-to-year variation, in no case is there a significant linear trend in the time series. This constancy at the assemblage level is maintained despite substantial changes in abundance of individual species, as figure 2a clearly reveals. The conservation of the assemblage's structure (which is also apparent in figure 2b) is not a product of temporal cross-correlations (either positive or negative) in species abundances. Pairwise correlations computed among species (through years) for the core assemblage (Magurran & Henderson 2003), and the entire assemblage, do not appear to differ from chance in a biologically meaningful way. The median correlation coefficient (Pearson's r) for the empirical dataset (core species) is −0.019. The 2.5 and 97.5 per cent quantiles (based on the randomization test) are −0.048 and −0.018, respectively. Equivalent values for the entire assemblage are −0.057 (quantiles −0.060 and −0.053).
Figure 1.
Change in the Hinkley fish community through time: overall community view. (a) Species richness per year of time series (Hill's N0 index); (b) exponential form of the Shannon index (Hill's N1 index), annual values; (c) reciprocal of Simpson diversity index, annual values; (d) Berger–Parker index (Hill's N∞ index), annual values; (e) MRS index, sequential pairs of years; (f) Bray–Cutis dissimilarity values between sequential pairs of years. The different metrics exhibit year-to-year variation but in no case is there a significant linear trend: (a) y = 0.090x + 37.61, R2 = 0.045; (b) y = 0.006x + 6.90, R2 = 0.0007; (c) y = 0.0031x + 4.35, R2 = 0.004; (d) y = 0.015x + 2.91, R2 = 0.039; (e) y = −0.012x + 5.22, R2 = 0.03; (f) y = −0.0014x + 0.356, R2 = 0.009.
Figure 2.
(a) Temporal variation in the abundance of the 15 species that are present in every year of the time series; (b) rank abundance plot where values for first-ranked species disregarding species identity, second-ranked species, third-ranked species, etc. have been averaged across the time series. Grey bars show the 95% confidence limits around these mean values.
There is thus no strong signal of cross-correlation (either positive or negative) in species abundances through time. This outcome, which confirms that changes in the abundances of the species in the assemblage are effectively asynchronous, is consistent with a recent investigation of the dynamics of the dominant species in a range of vertebrate and invertebrate communities (Mutshinda et al. 2009). (Mutshinda et al. included a sub-set of our dataset in their analysis and reached a very similar conclusion.) This is not to say that no species pairs are correlated through time. For example, the abundances of two co-generic taxa, the pout, Trisopterous luscus, and poor cod, T. minutus, both rise and fall in concert, but show only modest temporal correlation (r = 0.31, n = 28) because they periodically switch their relative abundances. There are also instances of correlations in the abundances of closely related species in other communities (Sugihara et al. 2003).
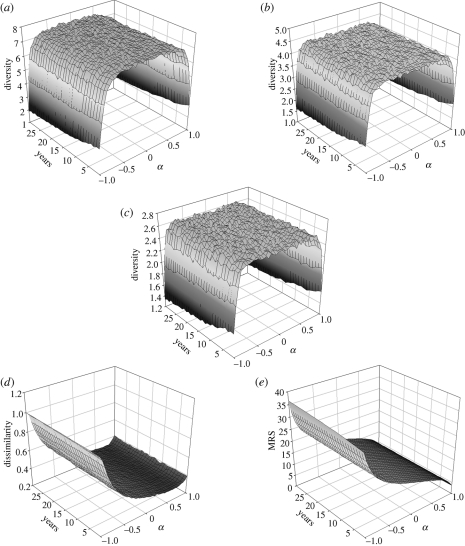
Our model predicts temporal patterns of species abundances that are consistent with the observed values. When α is around 0 (i.e. where the abundance of a species in one year is uncorrelated with its abundance in the previous year, resembling white noise) the results are similar to the empirical pattern. When α approaches −1 (strong anti-correlations) or α approaches 1 (a classic random walk) predicted abundances begin to diverge from the observed values. This result is particularly striking when we examine the 30 year trends of the Hill diversity numbers. In each case, and across a broad range of autocorrelation values, the same level of diversity is maintained through time (figure 3a–c). Moreover, when −0.8 < α < 0.8, predicted mean diversity falls within the confidence limits of the empirical values (figure 4a–c, f–h). This is true for both the core assemblage and the entire assemblage. In other words, the structure of the assemblage is retained as long as the autocorrelation in abundances between successive years is not too strong.
Figure 3.
Predicted values of (a) N1, exp Shannon index, (b) N2, 1/Simpson index, (c) N∞, Berger–Parker index, (d) Bray–Curtis dissimilarity and (e) MRS over 30 years in relation to varying values of α.
In contrast, metrics that take species identities into account reveal that the empirical assemblage is replicated over a narrower range of conditions. While both Bray–Curtis dissimilarity and MRS remain constant through time for a given value of α (figure 3d,e), the relationship between the extent of autocorrelation and the predicted value of the metric is an asymmetric one. As figure 4d,e and i,j indicates, only positive values of α lead to predictions that lie within the empirical confidence limits. This is particularly evident for MRS. Here, the predicted core assemblage resembles the empirical one when α ≈ 0.5 (figure 4i). The still narrower band of matching MRS values for the entire assemblage (figure 4d) is due to the occasional species which shift between being present at a low rank and being absent (Magurran & Henderson 2003).
4. Discussion
Our results show that abundances of species in an estuarine assemblage of fishes change asynchronously through time. We compared this pattern with a model that varied the level of autocorrelation in species abundances and used three informative diversity measures to describe different aspects of the species abundance distribution. Our model was able to replicate empirical patterns of diversity, not just in terms of the predicted level of diversity but also its constancy through time, for each of these three measures. Furthermore, this result was repeated for a wide range of autocorrelation values. In other words, we found that there are many different ways in which species abundances can change asynchronously and still produce an assemblage that is indistinguishable—based on diversity indices—from the natural one.
Our model incorporates MacArthur's (1960) prediction that species typically return to their equilibrium level following a rise or decline in abundance. We are not making any assumption about whether the assemblage as a whole is in equilibrium here, but simply reflecting the fact that in the natural assemblage species tend to vary around their mean abundances. Because these changes in species abundances occur asynchronously, they are also consistent with the portfolio effect, as well as with the insurance hypothesis and its expectation that niche differences will cause species to respond to environmental variations in different ways and with different lags (Yachi & Loreau 1999; Loreau 2010). However, we have not formally explored the compensatory dynamics hypothesis (Tilman et al. 1998) which argues that ecosystem function is preserved as a result of negative covariance in species abundances and is in any case not well supported by empirical data (Houlahan et al. 2007). Although we have not shown that niche differences in this estuarine assemblage cause this asynchronicity, we have evidence that niche differences exist and underpin the relative abundances of species (Magurran & Henderson 2003; Henderson & Magurran 2010). Interestingly, the maintenance of structure through time is to a large extent due to the presence of the core species (figure 2b)—which are also the most common ones and contribute in excess of 98 per cent of the overall abundance in any given year (Magurran & Henderson 2003). It is increasingly recognized that common species make a significant contribution to assemblage structure and function (Gaston & Fuller 2008) and this is borne out by our work. On the other hand, the richness of the assemblage is maintained by the presence of the infrequent species; these predominantly rare species contribute a constant fraction of the overall richness of the assemblage through time (Magurran & Henderson 2003).
The conclusions we reach about the maintenance of assemblage structure using diversity measures contrast with those obtained with metrics that take account of species identities. In these latter cases, there is a more limited set of circumstances in which temporal turnover produces patterns that replicate those seen in nature. Thus, while the structure of the assemblage, in terms of the relative abundances of species, appears insensitive to variation in the rate of temporal turnover, there is a much smaller set of conditions that generate realistic year-by-year changes in species rankings and species composition.
An intriguing finding is that the metrics that track species composition suggest that the natural assemblage structure is generated by a positive autocorrelation of around 0.5. This value implies that the community metric has some predictability and the time series is dominated by longer wavelengths and has some memory or inertia to change. In fact, the reddening of population time series is a commonly observed feature. Kaitala et al. (2001) argue that reddened or pink population time series are a product of spatial structure and migration between populations. This must certainly be the case for the Hinkley data where there is a continual influx of migrants (Magurran & Henderson 2003). In addition to the role of metapopulation dynamics in reddening time series, we need also to consider the role of reddened environmental variables. Almost all physical time series influencing fishes at Hinkley, such as sea water temperature, solar radiation and North Atlantic Oscillation, have pink spectra. Finally, it is important to note that all longer-lived species have populations with memory. The number of offspring produced in any year is not just a product of events at reproduction but events over a number of previous years that have influenced the quality and abundance of the adults. This is the inertia or memory that can produce pink noise. It is therefore possible that an autocorrelation of around 0.5 is an emergent property of a community which has (i) some memory (the number of a species next year is linked to past reproductive success), (ii) metapopulation dynamics that tend to produce slower, longer-term changes, and (iii) a response to environmental variables with pink spectra.
A large number of metrics have been developed to assess the changes that occur in ecological communities as a result of human disturbance. A significant fraction of these have focused on changes in the species abundance distribution. For example, it has been suggested that an intact assemblage will have a lognormal pattern of species abundances, whereas an impacted one will be closer to a log-series distribution (May 1975). Unfortunately empirical data reveal a wide range of assemblage responses (Gray 1987; Tokeshi 1993; Dornelas et al. 2010). We suggest that because species abundance distributions are unlikely to change as long as the species richness of the assemblage is preserved, classical diversity measures may not be sensitive measures of disturbance. Like Collins et al. (2008) and MacNally (2007), we conclude that other indicators, such as those that track species ranks or changes in species composition, may provide better early warning that the assemblage is entering a transitional state, or has already been impacted.
Our results remind us that comparisons between the observed and expected distributions of relative abundances of species in an ecological assemblage will not necessarily be the best way of distinguishing between competing mechanistic models (McGill et al. 2007). We also note that we still have a very incomplete understanding of how ecological communities change through time. There have been numerous attempts (e.g. Motomura 1932; May 1975; Tokeshi 1996; Harte et al. 1999; Hubbell 2001; McGill 2003) to account for the characteristic shape of species abundance distributions. However, although some of the pioneers in the field (notably Fisher et al. 1943; Preston 1960) included temporal dynamics in their models, most attention has been directed to spatial patterns (but see Rosenzweig 1995; Lande et al. 2003; Magurran & Henderson 2003; Thibault et al. 2004 for some exceptions). These findings suggest that models that track temporal changes need to account for shifts in species composition as well as changes in species abundance. We anticipate that this new generation of models will become increasingly important in monitoring and managing changes in biodiversity.
Finally, we note that species complementarity—one of the explanations for increased productivity in diverse communities (Tilman 1997; Tilman et al. 1997, 2001; Hector et al. 1999)—has been shown to become increasingly important with time (Loreau et al. 2003; Cardinale et al. 2007; Fargione et al. 2007). The complementarity hypothesis argues that there are niche differences which mean that species exploit the available resources in different, and complementary, ways (Fargione et al. 2007). However, rapid changes in abundance could reduce complementarity and impair function. We agree with Schwartz et al. (2000) that the rate of temporal turnover in species abundances may play a critical role in ecosystem functioning and deserves more study. Temporal turnover may also be a variable that needs to be taken into account when assessing the capacity of natural communities to exhibit resilience in the face of environmental change (Thrush et al. 2009).
Acknowledgements
A.E.M. thanks the ERC for an advanced grant; she was also a member of a stimulating NCEAS working group entitled ‘Tools and fresh approaches for species abundance distributions’ led by Brian McGill. The long-term fieldwork would not have been possible without the aid of many researchers, including, in particular, Dr Richard Seaby and Mr Robin Somes. Finally, we thank Maria Dornelas and two anonymous referees whose comments greatly improved the paper.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Biological diversity in a changing world’.
References
- Butchart S. H. M., et al. 2010Global biodiversity: indicators of recent declines. Science 328, 1164–1168 (doi:10.1126/science.1187512) [DOI] [PubMed] [Google Scholar]
- Cardinale B. J., Wright J. P., Cadotte M. W., Carroll I. T., Hector A., Srivastava D. S., Loreau M., Weis J. J.2007Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl Acad. Sci. USA 104, 18 123–18 128 (doi:10.1073/pnas.0709069104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale B. J., et al. 2009Effects of biodiversity on the functioning of ecosystems: summary of 164 experimental manipulations of species richness. Ecology 90, 854 (doi:10.1890/08-1584.1) [Google Scholar]
- Collins S., et al. 2008Rank clocks and plant community dynamics. Ecology 89, 3534–3541 (doi:10.1890/07-1646.1) [DOI] [PubMed] [Google Scholar]
- Cottingham K. L., Brown B. L., Lennon J. T.2001Biodiversity may regulate the temporal variability of ecological systems. Ecol. Lett. 4, 72–85 (doi:10.1046/j.1461-0248.2001.00189.x) [Google Scholar]
- Crawley M. J.2007The R book. Chichester, UK: Wiley [Google Scholar]
- Darwin C.1859On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Doak D. F., Bigger D., Harding E. K., Marvier M. A., O'Malley R. E., Thomson D.1998The statistical inevitability of stability-diversity relationships in community ecology. Am. Nat. 151, 264–276 (doi:10.1086/286117) [DOI] [PubMed] [Google Scholar]
- Dornelas M., Soykan C., Ugland K. I.2010Biodiversity and disturbance. In Biological diversity: frontiers in measurement and assessment (eds Magurran A. E., McGill B. J.). Oxford, UK: Oxford University Press [Google Scholar]
- Ernest S. K. M., Brown J. H., Thibault K. M., White E. P., Goheen J. R.2008Zero sum, the niche and metacommunities: long-term dynamics of community assembly. Am. Nat. 172, E257–E269 [DOI] [PubMed] [Google Scholar]
- Fargione J., Tilman D., Dybzinski R., Lambers J. H. R., Clark C., Harpole W. S., Knops J. M. H., Reich P. B., Loreau M.2007From selection to complementarity: shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc. R. Soc. B 274, 871–876 (doi:10.1098/rspb.2006.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A., Corbet A. S., Williams C. B.1943The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 12, 42–58 [Google Scholar]
- Gaston K. J., Fuller R. A.2008Commonness, population depletion and conservation biology. Trends Ecol. Evol. 23, 14–19 (doi:10.1016/j.tree.2007.11.001) [DOI] [PubMed] [Google Scholar]
- Gray J. S.1987Species-abundance patterns. In Organization of communities—past and present (eds Gee J. H. R., Giller P. S.), pp. 53–67 Oxford, UK: Blackwell [Google Scholar]
- Grossman G. D., Moyle P. B., Whitaker J. O., Jr1982Stochasticity in structural and functional characteristics of an Indiana stream fish assemblage: a test of community theory. Am. Nat. 120, 423–454 [Google Scholar]
- Grossman G. D., Dowd J. F., Crawford M.1990Assemblage stability in stream fishes: a review. Environ. Manag. 14, 661–671 (doi:10.1007/BF02394716) [Google Scholar]
- Harte J., Kinzig A., Green J.1999Self-similarity in the distribution and abundance of species. Science 284, 334–336 (doi:10.1126/science.284.5412.334) [DOI] [PubMed] [Google Scholar]
- Hector A., et al. 1999Plant diversity and productivity in European grasslands. Science 286, 1123–1127 (doi:10.1126/science.286.5442.1123) [DOI] [PubMed] [Google Scholar]
- Henderson P. A.2007Discrete and continuous change in the fish community of the Bristol Channel in response to climate change. J. Mar. Biol. Assoc. UK 87, 589–598 (doi:10.1017/S0025315407052447) [Google Scholar]
- Henderson P. A., Bird D. J.2010Fish and macro-crustacean communities and their dynamics in the Severn Estuary. Mar. Pollut. Bull. 61, 100–114 (doi:10.1016/j.marpolbul.2009.12.017) [DOI] [PubMed] [Google Scholar]
- Henderson P. A., Holmes R. H. A.1990Population stability over a ten-year period in the short-lived fish Liparis liparis (L.). J. Fish Biol. 37, 605–616 (doi:10.1111/j.1095-8649.1990.tb05893.x) [Google Scholar]
- Henderson P. A., Holmes R. H. A.1991On the population dynamics of dab, sole and flounder within Bridgwater Bay in the lower Severn Estuary, England. Neth. J. Sea Res. 27, 337–344 (doi:10.1016/0077-7579(91)90036-Z) [Google Scholar]
- Henderson P. A., Magurran A. E.2010Linking species abundance distributions in numerical abundance and biomass through simple assumptions about community structure. Proc. R. Soc. B 277, 1561–1570 (doi:10.1098/rspb.2009.2189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. A., Seaby R. M. H.1994On the factors influencing juvenile flatfish abundance in the lower Severn Estuary. Neth. J. Sea Res. 32, 321–330 (doi:10.1016/0077-7579(94)90009-4) [Google Scholar]
- Hill M. O.1973Diversity and evenness: a unifying notation and its consequences. Ecology 54, 427–431 (doi:10.2307/1934352) [Google Scholar]
- Houlahan J. E., et al. 2007Compensatory dynamics are rare in natural ecological communities. Proc. Natl Acad. Sci. USA 104, 3273 (doi:10.1073/pnas.0603798104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell S. P.2001The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- Jost L., Chao A., Chazdon R. L.In press Compositional similarity and beta diversity. In Biological diversity: frontiers in measurement and assessment (eds Magurran A. E., McGill B. J.). Oxford, UK: Oxford University Press [Google Scholar]
- Kaitala V., Alaja S., Ranta E.2001Temporal self-similarity created by spatial individual-based population dynamics. Oikos 94, 273–278 (doi:10.1034/j.1600-0706.2001.940207.x) [Google Scholar]
- Lande R., Engen S., Saether E.2003Stochastic population dynamics in ecology and conservation. Oxford, UK: Oxford University Press [Google Scholar]
- Lawes J., Gilbert J.1880Agricultural, botanical and chemical results of experiments on the mixed herbage of permanent grassland, conducted for many years in succession on the same land. I. Phil. Trans. R. Soc. Lond. B 171, 289–416 (doi:10.1098/rstl.1880.0010) [Google Scholar]
- Lawes J., Gilbert J., Masters M.1882Agricultural, botanical and chemical results of experiments on the mixed herbage of permanent meadow, conducted for more than twenty years in succession on the same land. II. The botanical results. Phil. Trans. R. Soc. Lond. B 173, 1181–1413 (doi:10.1098/rstl.1882.0029) [Google Scholar]
- Leary D. J., Petchey O. L.2009Testing a biological mechanism of the insurance hypothesis in experimental aquatic communities. J. Anim. Ecol. 78, 1143–1151 (doi:10.1111/j.1365-2656.2009.01586.x) [DOI] [PubMed] [Google Scholar]
- Loh J., Green R. E., Ricketts T., Lamoreux J., Jenkins M., Kapos V., Randers J.2005The Living Planet Index: using species population time series to track trends in biodiversity. Phil. Trans. R. Soc. B 360, 289–295 (doi:10.1098/rstb.2004.1584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M.2010Linking biodiversity and ecosystems: towards a unifying ecological theory. Phil. Trans. R. Soc. B 365, 49–60 (doi:10.1098/rstb.2009.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M., Mouquet N., Gonzalez A.2003Biodiversity as spatial insurance in heterogenous landscapes. Proc. Natl Acad. Sci. USA 100, 12 765–12 770 (doi:10.1073/pnas.2235465100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R.1960On the relative abundance of species. Am. Nat. 94, 25–36 [Google Scholar]
- MacArthur R. H., Wilson E. O.1967The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- MacNally R.2007Use of the abundance spectrum and relative-abundance distributions to analyze assemblage change in massively altered landscapes. Am. Nat. 170, 319–330 (doi:10.1086/519859) [DOI] [PubMed] [Google Scholar]
- Magurran A. E.2004Measuring biological diversity. Oxford, UK: Blackwell Science [Google Scholar]
- Magurran A. E.2007Species abundance distributions over time. Ecol. Lett. 10, 347–354 (doi:10.1111/j.1461-0248.2007.01024.x) [DOI] [PubMed] [Google Scholar]
- Magurran A. E., Henderson P. A.2003Explaining the excess of rare species in natural species abundance distributions. Nature 422, 714–716 (doi:10.1038/nature01547) [DOI] [PubMed] [Google Scholar]
- Magurran A. E., Baillie S. R., Buckland S. T., Dick J. M., Scott E. M., Smith R. I., Somerfield P. J., Watt A. D.In press Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol. Evol. (doi:10.1016/j.tree.2010.06.016) [DOI] [PubMed] [Google Scholar]
- May R. M.1975Patterns of species abundance and diversity. In Ecology and evolution of communities (eds Cody M. L., Diamond J. M.), pp. 81–120 Cambridge, MA: Harvard University Press [Google Scholar]
- McGill B. J.2003A test of the unified neutral theory of biodiversity. Nature 422, 881–885 (doi:10.1038/nature01583) [DOI] [PubMed] [Google Scholar]
- McGill B. J., et al. 2007Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10, 995–1015 (doi:10.1111/j.1461-0248.2007.01094.x) [DOI] [PubMed] [Google Scholar]
- Motomura I.1932On the statistical treatment of communities. Zool. Mag. Tokyo 44, 379–383 [In Japanese.] [Google Scholar]
- Mutshinda C. M., O'Hara R. B., Woiwod I. P.2009What drives community dynamics? Proc. R. Soc. B 276, 2923–2929 (doi:10.1098/rspb.2009.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J., et al. 2010Vegan: R package, version 1.17-3. See http://CRAN.R-project.org/package=vegan
- Pavoine S., Love M., Bonsall M. B.2009Hierarchical partitioning of evolutionary and ecological patterns in the organization of phylogenetically-structured species assemblages: application to rockfish (genus: Sebastes) in the Southern California Bight. Ecol. Lett. 12, 898–908 (doi:10.1111/j.1461-0248.2009.01344.x) [DOI] [PubMed] [Google Scholar]
- Preston F. W.1960Time and space and the variation of species. Ecology 41, 612–627 [Google Scholar]
- R Development Core team 2008R: A language and environment for statistical computing. In R foundation for statistical computing (ed. R Development Core Team). Austria: Vienna; See http://www.R-project.org [Google Scholar]
- Richardson A. J., Walne A. W., John A. W. G., Jonas T. D., Lindley J. A., Sims D. W., Stevens D., Witt M.2006Using continuous plankton recorder data. Prog. Oceanogr. 68, 27–74 (doi:10.1016/j.pocean.2005.09.011) [Google Scholar]
- Rosenzweig M. L.1995Species diversity in space and time. Cambridge, UK: Cambridge University Press [Google Scholar]
- Ross S. T., Matthews W. J., Echelle A. A.1985Persistence of stream fish assemblages: effects of environmental change. Am. Nat. 126, 24–40 [Google Scholar]
- Sale P. F., Douglas W. A.1984Temporal variability in the community structure of fish on coral patch reefs and the relation of community structure to reef structure. Ecology 65, 409–422 (doi:10.2307/1941404) [Google Scholar]
- Schwartz M. W., Brigham C. A., Hoeksema J. D., Lyons K. G., Mills M. H., van Mantgem P. J.2000Linking biodiversity to ecosystem function: implications for conservation biology. Oecologia 122, 297–305 (doi:10.1007/s004420050035) [DOI] [PubMed] [Google Scholar]
- Southwood R., Henderson P. A.2000Ecological methods. Oxford, UK: Blackwell Science [Google Scholar]
- Srivastava D. S., Vellend M.2005Biodiversity-ecosystem function research: is it relevant to conservation? Annu. Rev. Ecol. Evol. Syst. 36, 267–294 [Google Scholar]
- Sugihara G., Bersier L., Southwood T. R. E., Pimm S. L., May R. M.2003Predicted correspondence between species abundances and dendrograms of niche similarities. Proc. Natl Acad. Sci. USA 100, 5246–5251 (doi:10.1073/pnas.0831096100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault K., White E., Ernest S. K. M.2004Temporal dynamics in the structure and composition of a desert rodent community. Ecology 85, 2649–2655 (doi:10.1890/04-0321) [Google Scholar]
- Thrush S. F., Hewitt J. E., Dayton P. K., Coco G., Lohrer A. M., Norkko A., Norkko J., Chiantore M.2009Forecasting the limits of resilience: integrating empirical research with theory. Proc. R. Soc. B 276, 3209–3217 (doi:10.1098/rspb.2009.0661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D.1997Distinguishing between the effects of species diversity and species composition. Oikos 80, 185 (doi:10.2307/3546532) [Google Scholar]
- Tilman D., Knops J., Wedin D., Reich P. B., Ritchie M., Siemann E.1997The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302 (doi:10.1126/science.277.5330.1300) [Google Scholar]
- Tilman D., Lehman C. L., Bristow C. E.1998Diversity-stability relationships: statistical inevitability or ecological consequence. Am. Nat. 151, 277–282 [DOI] [PubMed] [Google Scholar]
- Tilman D., Reich P. B., Knops J., Wedin D., Mielke T., Lehman C.2001Diversity and productivity in a long-term grassland experiment. Science 294, 843–845 (doi:10.1126/science.1060391) [DOI] [PubMed] [Google Scholar]
- Tokeshi M.1993Species abundance patterns and community structure. Adv. Ecol. Res. 24, 112–186 [Google Scholar]
- Tokeshi M.1996Power fraction: a new explanation for species abundance patterns in species-rich assemblages. Oikos 75, 543–550 (doi:10.2307/3545898) [Google Scholar]
- Valone T. J., Barber N. A.2008An empirical evaluation of the insurance hypothesis in diversity-stability models. Ecology 89, 522–531 (doi:10.1890/07-0153.1) [DOI] [PubMed] [Google Scholar]
- Warwick R. M., et al. 2002Inter-annual changes in the biodiversity and community structure of the macrobenthos in Tees Bay and the Tees estuary UK, associated with local and regional environmental events. Mar. Ecol. Prog. Ser. 234, 1–13 (doi:10.3354/meps234001) [Google Scholar]
- Whittaker R. H.1960Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 30, 279–338 (doi:10.2307/1943563) [Google Scholar]
- Wolfe D. A., Champ M. A., Flemer D. A., Mearns A. J.1987Long-term biological data sets: their role in research, monitoring, and management of estuarine and coastal marine systems. Estuaries 10, 181–193 (doi:10.2307/1351847) [Google Scholar]
- Yachi S., Loreau M.1999Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468 (doi:10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]