Abstract
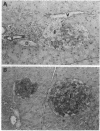
We have produced a panel of islet-specific T-cell clones from nonobese diabetic (NOD) mice. These clones proliferate and make interleukin 2 in an antigen-specific manner in response to NOD antigen-presenting cells and islet cells. Most of the clones respond to islet-cell antigen from different mouse strains but only in the presence of antigen-presenting cells bearing the class II major histocompatibility complex of the NOD mouse. In vivo, the clones mediate the destruction of islet, but not pituitary, grafts. Furthermore, pancreatic sections from a disease transfer experiment with one of the clones showed a pronounced cellular infiltration and degranulation of islets in nondiabetic (CBA x NOD)F1 recipients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., McDevitt H. O. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Ashwell J. D., Chen C., Schwartz R. H. High frequency and nonrandom distribution of alloreactivity in T cell clones selected for recognition of foreign antigen in association with self class II molecules. J Immunol. 1986 Jan;136(2):389–395. [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Linde S., Kofod H., Spector D., Delannoy M., Grant S., Hanahan D., Baekkeskov S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Chick W. L., Oie H. K., Sims H. L., King D. L., Weir G. C., Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K., Portas M., Bradley B., Wegmann D., Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988 Oct;37(10):1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- Herold K. C., Montag A. G., Fitch F. W. Treatment with anti-T-lymphocyte antibodies prevents induction of insulitis in mice given multiple doses of streptozocin. Diabetes. 1987 Jul;36(7):796–801. doi: 10.2337/diab.36.7.796. [DOI] [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Like A. A., Biron C. A., Weringer E. J., Byman K., Sroczynski E., Guberski D. L. Prevention of diabetes in BioBreeding/Worcester rats with monoclonal antibodies that recognize T lymphocytes or natural killer cells. J Exp Med. 1986 Oct 1;164(4):1145–1159. doi: 10.1084/jem.164.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Like A. A., Weringer E. J., Holdash A., McGill P., Atkinson D., Rossini A. A. Adoptive transfer of autoimmune diabetes mellitus in biobreeding/Worcester (BB/W) inbred and hybrid rats. J Immunol. 1985 Mar;134(3):1583–1587. [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Serreze D. V., Leiter E. H., Worthen S. M., Shultz L. D. NOD marrow stem cells adoptively transfer diabetes to resistant (NOD x NON)F1 mice. Diabetes. 1988 Feb;37(2):252–255. doi: 10.2337/diab.37.2.252. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Taylor-Edwards C., Banks B. A., Gregory A. K., Fathman C. G. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988 Apr 29;240(4852):659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hao L., Gill R. G., Lafferty K. J. Autoimmune diabetes in NOD mouse is L3T4 T-lymphocyte dependent. Diabetes. 1987 Apr;36(4):535–538. doi: 10.2337/diab.36.4.535. [DOI] [PubMed] [Google Scholar]
- Wicker L. S., Miller B. J., Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986 Aug;35(8):855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]