Abstract
Global climate change has the potential to substantially alter the production and community structure of marine fisheries and modify the ongoing impacts of fishing. Fish community composition is already changing in some tropical, temperate and polar ecosystems, where local combinations of warming trends and higher environmental variation anticipate the changes likely to occur more widely over coming decades. Using case studies from the Western Indian Ocean, the North Sea and the Bering Sea, we contextualize the direct and indirect effects of climate change on production and biodiversity and, in turn, on the social and economic aspects of marine fisheries. Climate warming is expected to lead to (i) yield and species losses in tropical reef fisheries, driven primarily by habitat loss; (ii) community turnover in temperate fisheries, owing to the arrival and increasing dominance of warm-water species as well as the reduced dominance and departure of cold-water species; and (iii) increased diversity and yield in Arctic fisheries, arising from invasions of southern species and increased primary production resulting from ice-free summer conditions. How societies deal with such changes will depend largely on their capacity to adapt—to plan and implement effective responses to change—a process heavily influenced by social, economic, political and cultural conditions.
Keywords: climate change, fish communities, social–ecological systems, biodiversity
1. Introduction
Achieving sustainable fisheries is among the most challenging large-scale management problems globally. Just as evidence for reductions in exploitation rates is emerging in some wealthier regions (Beddington et al. 2007; Worm et al. 2009), concerns are growing about the fisheries implications of global climate warming. Although climate-driven change is expected in every marine ecosystem, the science needed for regional-scale ecological understanding is immature and thus the magnitude and extent of effects remain largely unknown. Yet adaptation of fisheries and fisheries management to a changing environment is necessary when nearly 1.5 billion people rely on fish for more than 20 per cent of their protein (Badjeck et al. 2010) and global fisheries contribute $91.2 billion USD to global agricultural trade (2006 data; FAO 2008).
To date, warming within the world's oceans has been variable in magnitude though unequivocal in scope; all but two of the world's 64 large marine ecosystems (LMEs) experienced warming between 1982 and 2006 (Sherman et al. 2009), with the largest increases among shallow shelf and inland sea areas in the North Atlantic. Warming trends are expected to have widespread effects on catch diversity as the distribution of populations changes to reflect the spatial movement of thermal optima (figure 1a; Planque & Frédou 1999; Pörtner 2010). These changes are in addition to well-documented climate variability effects on fisheries from changes in temperature, winds and hydrological cycles (Brander 2010). Warming will probably alter the location of critical fish habitat (Brander 2010); competition and predation dynamics (Graham & Harrod 2009); ecosystem functional roles (Munday et al. 2008); food availability (Chase & Liebold 2002); and reproductive success (Edwards & Richardson 2004; Overland et al. 2010). Finally, warming trends will also alter ocean chemistry, with potentially negative effects such as ocean acidification and hypoxia (Pörtner 2010) that, given their limited effects to date, we do not consider here.
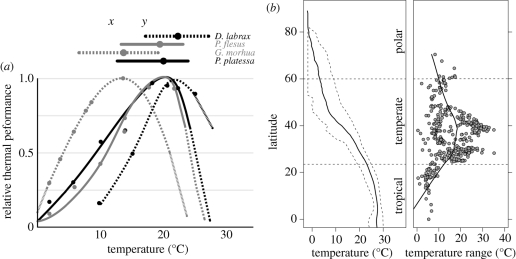
Figure 1.
(a) Laboratory-based thermal performance profiles for North Sea demersal fishes Pleuronectes platessa (black solid line), Pleuronectes flesus (grey solid line), Gadus morhua (grey dotted line) and Dicentrarchus labrax (black dotted line). Relative performance profiles include temperature performance breadths (>69% performance; top horizontal lines) and varying tolerance ranges (solid/dotted curves). Communities assembled at temperature x will have a different composition from communities at temperature y because of diversity in thermal performance, although performance breadth is likely to be narrower in situ. Data from Freitas et al. (2007). (b) Average Northern Hemisphere sea-surface temperatures (solid line, left panel) with 95% quantile range (of 365 days per 1° latitude–longitude grid cell; dashed lines, left panel) from the 2009 HadISST1 dataset (Rayner et al. 2003) alongside seasonal temperature ranges (max–min; grey circles, right panel) from National Oceanic and Atmospheric Administration buoy data (http://www.ndbc.noaa.gov), both by latitude. Buoy data includes a local average line (solid line, right panel) based on the default Loess.smooth function in R.
Beyond the direct effects of increasing temperature, warming also adds energy to the ocean-climate system, increasing the severity of acute disturbance events and generating higher environmental variability. As a result, both the frequency of regional climate anomalies (e.g. El Niño) and the magnitude of physical disturbance events (e.g. storms, coral bleaching) are expected to increase (Timmermann et al. 1999). More extreme seasonal shifts may lead to a match–mismatch between larval fish populations and their zooplankton prey (Stenseth et al. 2002), as well as unpredictable levels of seasonal upwelling and more variable recruitment (Usher et al. 2005; Brander 2009). Although warming trends and acute disturbances are expected to increase across many ecosystems, impacts will be system-dependent (table 1). Polar and tropical ecosystems will probably be more susceptible than temperate systems to climate change because of their low levels of seasonal temperature variation and their proximity to thermal extremes (figure 1b).
Table 1.
Major climate change phenomena currently impacting the diversity of marine fisheries. Primary drivers refer to increased warming through time (trend) and more extreme seasonality and high-energy disturbance events (variation).
| phenomenon | process | primary driver | effect on catch diversity (mechanism) | projected ecosystems affecteda | observed example(s) |
|---|---|---|---|---|---|
| range shifts | movement of thermal optima in depth and space | trend | increase or decrease in species richness due to immigration and emigration modified by changing predation and competition | all, but leading to decreased richness at low latitudes and increased richness at high latitudes | North Sea (Perry et al. 2005; Dulvy et al. 2008; Hiddink & ter Hofstede 2008) |
| declining production | increased thermal stratification | trend | decrease in species richness owing to lower environmental heterogeneity, lower temporal variation or fewer potential stable statesb | all (except upwelling areas), fisheries losses may be most severe in temperate areas | tropical latitudes (Behrenfeld et al. 2006) |
| growth rates | increase or decline in thermal performance | trend | increase or decrease in species richness due to change in niche structure | increase in high latitude systems and decrease in low latitude systems | North Atlantic (Dutil & Brander 2003) |
| habitat loss | physical destruction of critical habitat from storms and coral bleaching | variation | decrease in species richness due to reduced habitat heterogeneity | coral reefs; mangroves; intertidal zones; kelp forests, ice areas | Western Indian Ocean (Graham et al. 2008) |
| declining recruitment | decoupling in the timing of spawning and nutrients; chemical cue failures | variation | decrease in species richness due to decreased larval survivorship | coral reefs; ice areas; shelf areas; pelagic zones | North Atlantic (Planque & Frédou 1999); Great Barrier Reef (Munday et al. 2010) |
aExcluding ecosystems below 200 m which are less fished than other areas.
bFrom Chase & Liebold (2002).
(a). Adapting to change
It is critical for human societies to understand how to adapt to changes in fisheries resources. The impacts on society will depend on their vulnerability, with outcomes heavily influenced by environmental, social, economic, political and cultural considerations. Vulnerability can be conceptualized as having three key components: exposure, sensitivity and adaptive capacity (figure 2; Adger 2006). Exposure is the degree to which a system is stressed, combining the level of human presence in climate-affected areas with the magnitude, frequency and duration of a climatic disturbance event (Cutter 1996; Adger 2006). Sensitivity is the level of susceptibility to harm from climate change and is affected by the level of resource-dependence (Adger 2006; Cinner et al. 2009d). Adaptive capacity helps to offset impacts and includes preconditions that enable adaptation such as flexibility, learning, social organization and assets, all of which are necessary for successful adaptation (Nelson et al. 2007; Cinner et al. 2009b). Both adaptation and adaptive capacity occur at multiple scales and successful adaptation often requires linkages across scales (Adger et al. 2005; Ford et al. 2007). These components define our framework for understanding the social impact of climate change in marine fisheries.
Figure 2.
Conceptual framework for understanding components of social vulnerability to climate change. Exposure, sensitivity and the four components of adaptive capacity operate at local, community, national and regional scales to varying degrees, depending on conditions. Adaptive capacity includes aspects of governance, education, health and wealth as aspects of the four components. Based on Cinner et al. (2009b).
Here we consider potential effects of climate change on the biodiversity and productivity of the world's marine fisheries. We employ three case studies to illustrate the kinds of ecosystem change expected in tropical, temperate and polar (Arctic) LMEs, discussing how societies can adapt and outlining key social aspects of vulnerability. Although many negative effects are predicted there may also be benefits (Brander 2010), with ecological and economic winners and losers (Arnason 2007; Cheung et al. 2010).
2. Tropical fisheries: the western indian ocean
The greatest fisheries losses from climate change are likely to occur among reef-based fisheries. Because reef-building corals exist in low-variation tropical conditions near their upper thermal tolerances (figure 1b; Hughes et al. 2003; Tewksbury et al. 2008), they are susceptible to acute thermal stress that causes them to expel symbiotic zooxanthellae (coral bleaching) and, eventually, die (Hoegh-Guldberg et al. 2007). Hard corals are critical for many reef fishes, providing habitat for settlement, physical protection and food (MacNeil et al. 2009), while reef fish help corals dominate macroalgae through numerous functional roles (e.g. Wilson 2004; Ledlie et al. 2007; Bonaldo & Bellwood 2009). Predicted increases in the magnitude and frequency of acute disturbance events (McClanahan 2002) will probably be responsible for the main climate impacts on tropical fisheries over coming decades, as they destroy reef structure and degrade function (Munday et al. 2008).
The potential effects of reef degradation on human societies are substantial. Many societies depend on reef-based fisheries for food and livelihoods and are therefore especially vulnerable (McClanahan et al. 2009). Tropical fishers make up more than 90 per cent of the estimated 3.5 million fishermen in the world (Badjeck et al. 2010), often in countries twice as dependent on fisheries for dietary protein than other regions (Allison et al. 2009). Although the widespread overexploitation in many reef fisheries (Newton et al. 2007) may have resulted in communities tolerant to additional climate-change effects (Vinebrooke et al. 2004), such effects are not ubiquitous and many reef fisheries remain vulnerable.
Analysis of previous large-scale climatic events may help to anticipate climate effects in tropical fisheries of the future. In 1998, reefs throughout the Western Indian Ocean (WIO) experienced the most severe bleaching event on record, owing to an El Niño Southern Oscillation phase that co-occurred with the Indian Ocean Dipole (Saji et al. 1999). The size and scope of the event, combined with widespread ecological monitoring across the WIO, provide a unique, large-scale example of acute disturbance effects in tropical fisheries.
Bleaching occurred across many WIO reefs during 1998, with coral mortality from 1 to 95 per cent depending on local conditions (Graham et al. 2008; McClanahan et al. 2008). Fish-community effects were apparent throughout the WIO but were patchily distributed, reflecting the dependencies of individual species on corals. In the most severely impacted locations of the northern Indian Ocean, fish diversity declined by 50 per cent, with corallivorous and planktivorous fishes dependent on corals for food and shelter declining by at least 76 and 68 per cent, where coral cover declined by more than 50 per cent (figure 3a,b; Graham et al. 2008; MacNeil & Graham 2010).
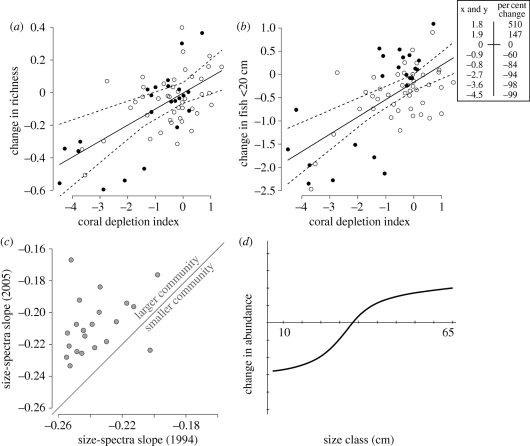
Figure 3.
Selected effects of 1998 bleaching event on reef fish community structure in the Western Indian Ocean (WIO) calculated from before (1993–1995) versus after (2005) the 1998 El Niño Southern Oscillation. Observed effects include a lower (a) species richness and (b) fewer small (<20 cm TL) fish, where corals were depleted between the two time periods; open circles indicate fished areas, closed circles have protected status; both axes are scaled relative change indices at 66 locations in eight countries; results include mean trends (solid lines) with Bayesian 95% credible intervals (dashed lines); methods provided in Graham et al. (2008). (c) Observed decrease in size-spectral slopes at the majority of WIO locations in 2005. Conceptually, these observations reflect (d) the growth of the existing fish assemblage from 1994 (abscissa) to 2005 (curved line) coupled with substantial losses of small fish owing to lack of coral cover affecting protection and recruitment (data from Graham et al. 2007).
As newly recruited coral reef fishes rely heavily on the complexity of coral reefs to provide shelter from predation (Pratchett et al. 2008), small fishes were particularly affected by the 1998 bleaching event. The severity of losses among small- and medium-sized fishes was clear from pre- and post-bleaching community size spectra (figure 3c), with higher post-bleaching size-spectral slopes reflecting widespread losses of small fish, even when large herbivorous fishes increased in abundance (figure 3d; Graham et al. 2007). These losses were substantial, affecting both small species and small size classes of larger fishery target species (Graham et al. 2007). Because reef fish can be exceptionally long-lived (Choat & Robertson 2002), considerable time lags are expected between the loss of live coral, collapse of the reef structure and declines in remnant fish biomass (Graham et al. 2007; Paddack et al. 2009). We hypothesize that a positive feedback between losses of coral and losses of fish will generate a higher risk of species extinction among reef-fish communities than elsewhere.
Surprisingly, there appeared to be little immediate impact of the 1998 bleaching event on fish biomass (Grandcourt & Cesar 2003). However, many studies were conducted only several years post-bleaching, with potentially little time for impacts to affect community dynamics. Long-term studies (7–10+ years) are detecting declines in fishery catch consistent with lagged impacts of benthic disturbance in reef fisheries (Pistorius & Taylor 2009). Importantly, many fishers shifted to seagrass-associated species prior to 1998 owing to heavy exploitation (McClanahan et al. 2008), thereby buffering local catches from the event. Such resource-shifts may prove critical in areas affected by increased disturbance and overexploitation, although often as less-desirable fisheries with fewer species and lower prices (McClanahan in press).
The capacity of WIO societies to respond to sustained reef fishery losses is heterogeneous; while some groups have strengths in aspects of adaptive capacity, they frequently lack others. Social and governance systems tend to have a reasonable degree of flexibility, such as diverse livelihoods among economic sectors (Cinner & Bodin 2010) and diversity between and within occupations (e.g. the use of several fishing gears) that allow people to switch among economic activities and targets (Turner et al. 2007). Parts of the WIO have developed flexible and decentralized management systems that permit rapid implementation of locally appropriate management rules (Cinner et al. 2009c), however rigid taboos and social norms may constrain adaptation options (Cinner et al. 2009b).
At both national and local scales, many WIO countries lack assets to effectively navigate planned or autonomous adaptations. In Kenya for instance, where low capital is combined with few livelihood options, fishers readily fall into poverty traps, whereby the absence of credit or savings makes continuing to fish the only option (Cinner et al. 2009d). At the national scale, high levels of corruption and political conflict can hamper organization and delivery of services during periods of disturbance and climate stress (Barnett & Adger 2007). Although the coral reefs in the WIO region are well studied, feedback about the condition of corals and fisheries is seldom provided to community managers and low levels of education may limit the capacity to synthesize scientific and local knowledge. In sum, many WIO fisheries are likely to be highly vulnerable to climate change owing to significant asset gaps.
3. Temperate fisheries: the north sea
Temperate fisheries typically experience higher levels of seasonal variation than those in the tropics (figure 1b), with species distributed close to the centre of their thermal tolerance range. These factors will typically reduce warming susceptibility, giving temperate fishes greater capacity for range changes. Temperate changes in species composition will thus be driven primarily by poleward movement of boreal fishes out of mid-latitudes and the arrival of warm-water species from lower latitudes. Rapid climate-driven community turnover (i.e. decades; Perry et al. 2005) has already been reported from temperate North Atlantic LMEs, currently among the fastest warming in the marine environment (Sherman et al. 2009).
The North Sea provides a well-studied example of warming-driven biodiversity change in temperate fisheries, being a shallow basin (<40 m in many places) that has heated up more rapidly than waters at similar latitude. Between 1982 and 2006, North Sea temperatures rose by 1.31°C (Sherman et al. 2009), including a dramatic 0.9°C increase in 1989 (Dulvy et al. 2008) that led to major biogeographical shifts in community structure. From 1977 to 2001, almost two-thirds of fished species in the North Sea changed their spatial distribution in response to warming (e.g. figure 4b,d), driven primarily by northward shifts in faster growing species exploiting warmer northerly waters (Perry et al. 2005; Hiddink & ter Hofstede 2008). Because the northward emigration of larger, cold-water species progressed more gradually (Perry et al. 2005), diversity increased within the North Sea (figure 4e,f). Southward movements of warm-water species such as sole Solea solea and scaldfish Arnoglossus laterna into the shallow southern North Sea have also occurred owing to earlier springtime warming (Dulvy et al. 2008).
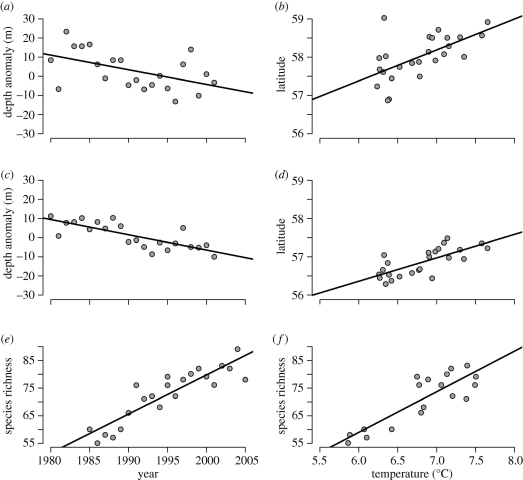
Figure 4.
Climate-induced changes in North Sea community structure. Change in anglerfish Lophius piscatorius showing (a) greater depth distribution through time and (b) higher mean latitude with 5-year mean winter bottom temperature; change in cod Gadus morhua showing (c) greater depth distribution through time and (d) higher mean latitude with 5-year mean winter bottom temperature (data from Dulvy et al. 2008; Perry et al. 2005). Increases in North Sea species richness (e) through time and (f) with 5-year mean temperatures (data from Hiddink & ter Hofstede 2008). All lines are normal linear regressions run in R, presented to illustrate general trends.
Despite such spatial changes, the most consistent response of North Sea fishes to warming has been a deepening of the assemblage. On average, the assemblage has deepened by 3.6 m decade−1, belying exceptional changes in species such as cod Gadus morhua (figure 4c) and anglerfish Lophius piscatorius (figure 4a; Dulvy et al. 2008). A widespread change in depth is a predictably efficient response for species as tracking the movement of their thermal optima requires smaller movements with depth or elevation than for latitude (Colwell et al. 2008). It is important to note however that the distribution of fishing mortality throughout the North Sea may play a role in these patterns that is difficult to assess.
Movement of thermal optima are not the only process determining fish diversity in the North Sea, as thermally driven shifts in prey base may also be an important factor. Between 1960–1981 and 1988–2001, a 10°C thermal boundary linked to differences in copepod community structure in northwest Atlantic waters moved from the edge of the English Channel into the central basin as community structure changed (Beaugrand et al. 2008). While a change in prey base could influence competition among predatory fishes, primary production ultimately drives fisheries production, and changes in total North Sea production are expected to broadly track changes in primary production as in other systems (Chassot et al. 2010; Jennings & Brander 2010). Thus, if warming increases thermal stratification and reduces primary production (Behrenfeld et al. 2006) there may be a decline in North Sea fisheries yield.
As some of the least fisheries-dependent nations in the world, North Sea fishing nations are among those least vulnerable to climate impacts (Allison et al. 2009). Fleets are technologically advanced, being able to change target species through spatial movement and gear changes (Catchpole et al. 2005) and fishers have demonstrated capacity to maintain high catch per unit effort even as stocks decline (Hentrich & Salomon 2006; Villasante 2010). This is partly because of European Common Fisheries Policy (CFP) historically supporting overcapacity in the region, allowing both sub-optimal fishing and marginal profitability to develop in overexploited stocks (Hentrich & Salomon 2006). Although numbers of boats and fishers have declined in recent years (Villasante 2010), European negotiators have also brokered access to West African or Indo-Pacific fisheries that have buoyed EU fishing capacity.
Fishers in the North Sea are well poised in terms of adaptive capacity to adjust targets to match projected changes in catch. Fisheries have already developed for red mullet Mullus barbatus, whose distribution has rapidly expanded into the North Sea during the past 20 years (Beare et al. 2004). Households within the EU also have higher than average levels of social and economic flexibility that, albeit often reluctantly, allowed many to leave fishing as catches have declined (Stead 2005). Furthermore, Northern Europe has among the highest levels of scientific and financial assets in the world, with hundreds of fisheries scientists working to understand the effects of climate variability on North Sea stocks. A strong asset and learning base combined with the social flexibility to switch fisheries has allowed some North Sea fishing societies to persist through periods of low catches and reduced quotas.
The greatest gaps in adaptive capacity for North Sea fisheries are therefore regulatory, primarily owing to the inflexibility of EU CFP regulations in responding to changing fishing opportunities (Hentrich & Salomon 2006). To sustainably target fisheries developing as a result of climate, both the industry and management system will need to be made more flexible (Perry et al. 2010). In the North Sea context, this requires a management system that is better able to assess and support new sustainable fishing opportunities, rather than being locked in to assessing quotas for a series of populations that provide a falling share of total catch. Changes in population distribution could also lead to challenging negotiations with non-EU countries in the area (e.g. Norway and Iceland), if they assert rights to stocks migrating further out of EU waters. For species of potential commercial value appearing in North Sea waters, simple approaches based on life-history characteristics could be used to provide a first assessment of potential productivity (Beddington & Kirkwood 2005) and in determining whether fishery development could be supported. Such an approach would reduce transitional barriers for fishers exploiting newly arrived species (McIlgorm et al. 2010) and may reduce conflicts over jurisdiction and fishing opportunities likely to arise from shifting stocks (Vilhjálmsson et al. 2005).
4. Arctic fisheries: the bering sea
Of the world's 66 LMEs, the Arctic Ocean ecosystem is changing most rapidly owing to systematic losses of multi-year sea ice that defines the environment (Sherman et al. 2009). Marginal sea ice—the transitional seasonal ice linking multi-year sea ice and open water—is currently the primary source of production for the Arctic benthic food web, with intense algal growth in spring and summer generating high levels of spatially concentrated primary production (Usher et al. 2005). Declines in total sea ice across the Arctic are well-documented (NSIDC 2008) and forecasts suggest that warming may soon generate ice-free summers (Overpeck et al. 2005). Arctic marine organisms are particularly susceptible to these effects because they are adapted to life in a low variation environment at the low end of ocean temperatures (figure 1b), conditions that will cease to exist in an ice-free Arctic.
The loss of multi-year ice cover will profoundly affect Arctic ecology and will probably lead to positive fisheries effects. While some primary and secondary production will be lost from a potential match–mismatch along marginal sea ice, Arctic primary production is severely light limited by multi-year sea ice. New open-water areas will probably experience an explosion of primary productivity, leading to increased zooplankton abundance and higher fish biomass throughout the region (Loeng et al. 2005; Behrenfeld et al. 2006; Sherman et al. 2009).
The kinds of positive effects of warming expected in the Arctic have already been demonstrated on Arcto-Norweigian cod distributions and abundance. This population shows stronger year classes in warm years and poor year classes in cold, and warming has led to a northern range expansion in Norway and Greenland (Drinkwater 2006, 2009). As a result of warming, yields are predicted to increase by approximately 20 per cent for the most important cod and herring Clupea harengus stocks in Iceland, and approximately 200 per cent in Greenland over the next 50 years (Arnason 2007). Climate-driven fish invasions into the Arctic are expected to exceed any other LME (Cheung et al. 2009).
Despite the generally positive effects of climate-warming predicted for Arctic fisheries (excepting the loss of the current marine ecosystem), how invading species interact in this newly habitable environment and what sorts of ecosystems develop remain major ecological unknowns. This uncertainty has been recognized by, for example, the North-Pacific Fisheries Management Council, which has closed extensive shelf areas to fishing in order to conduct baseline research and to protect critical crab habitat from the northward expansion of trawlers into newly ice-free waters (Stram & Evans 2009). This precautionary approach is an important first step towards achieving a viable fishery, but it remains to be determined whether new Arctic fisheries can be developed in a sustainable and equitable way.
Bering Sea fisheries provide 50 per cent of United States domestic seafood production and are the largest contributor to US seafood exports, with species such as Pacific halibut Hippoglossus stenolepis, various ground-fish and salmon, sablefish Anoplopoma fimbria and crab (NMFS 2008). One-third of the total US crab catches originate here, as do an annual 2 billion kg of pollock Theragra chalcogramma (Woodby et al. 2005), and these resources are important for the diets and livelihoods of rural and indigenous people in Alaska, Chukotka, Northern Canada and elsewhere. Participation in the Bering Sea's commercial and subsistence fisheries is vital for coastal livelihoods, and their management has been well regarded in terms of sustainability (Woodby et al. 2005).
Most fisheries in the Bering Sea are managed under a limited access privilege system that provides a substantial flexibility for fishers responding to weather and ocean conditions, though not equally among fishers (Loring et al. in press). Faced with the impacts of climatic change, some participants have more room to adapt than others (Ford 2009; Loring & Gerlach 2010). Often large commercial operations can afford to fish in inclement weather, absorb fuel price shocks and follow large-scale movements of stocks, while small-scale fishers are vulnerable to small increases in fuel price that can limit time on the water. With such high levels of exposure, the majority of rural subsistence hunters and fishers suffer from asset-limited adaptive capacity.
In the past, northern peoples have enjoyed high adaptive capacity and success responding to environmental variability through social organizations that share resources and spread risk among individuals and communities (Moran 1981; Ford 2009); through local ecological knowledge and expertise that informs hunting and fishing practices while minimizing risks (Kawagley 1995); and through high levels of mobility that allow switching among hunting, fishing and gardening activities between seasons and years (Berkes & Jolly 2001; Loring & Gerlach 2010). However, many of these adaptive capacity components have been reduced by severe changes in sea ice conditions (Berkes & Jolly 2001); restrictive hunting and fishing seasons; jurisdictional conflicts among state, federal and private lands; and single-species management rather than an ecosystem-based approach (Schumann & Macinko 2007; Loring et al. in press). This combination of environmental and regulatory factors has drastically increased the vulnerability of Native hunters and fishers in ways that have had limited impact on large-scale commercial interests.
Although options for Native fishers are currently declining, cultural and intellectual capacity for adaptation persists at a high level, presenting a rare opportunity for indigenous peoples to benefit from climate change, even after the irreplaceable loss of traditional livelihoods. Tools and livelihood strategies in the region are highly advanced for regional and climatic adaptation (Moran 1981) and these components of adaptive capacity can be directed towards development of sustainable Arctic fisheries. Developing such a fishery requires government involvement, whereby local small-scale fishers are given regulatory authority through resource co-management arrangements (Huntington 2000; Loring et al. in press), as the asset wealth and low vulnerability of large-scale commercial interests would dominate entry into new fisheries. There is a clear moral imperative for local and Native control, as the climate warming that will create open-water Arctic fisheries is also responsible for eliminating their traditional livelihoods.
5. Conclusions
The future of marine fisheries will develop from a complex interaction of oceanographic conditions, physiological tolerances and thermally induced distribution shifts that cannot be predicted accurately. Although outcomes span a range of positive and negative outcomes among latitudes, all jurisdictions require forethought and planning to avoid the most negative effects of climate change on their fisheries. As it is highly unlikely that substantive emission reductions will occur in the medium term, and given that changes in fisheries driven by long-term temperature trends are already being observed, fisheries managers must plan and act to adapt to climate change.
(a). Adaptation strategies
Successful adaptation to climate change will depend heavily on local social and environmental conditions, with some societies being more flexible because of economic and cultural factors (Cinner et al. 2009b). Although reducing negative impacts such as overfishing will help reduce effects on fisheries (Brander 2010), nations with low adaptive capacity may find it difficult to change course, and small-scale fishers readily fall into poverty traps that greatly limit their ability to adapt (Sherman et al. 2009). Although the effects of climate change will ultimately reveal if existing livelihoods and management systems are resilient (Badjeck et al. 2010), there are several strategies likely to help societies adapt to the ecosystem consequences of widespread change.
1. Divert effort: as many fish communities are expected to change composition or decline in biomass, or both, fishers and fisheries regulators must prepare to shift target species from traditional stocks to new or underutilized ones, and to aquaculture. Most fisheries are location-specific and operate at utilization levels that make them inflexible (McIlgorm et al. 2010); however, they must be made flexible if they are to continue to support local communities. People need the ability to adjust not just where and when, but what they harvest. In those relatively rare circumstances in which existing fisheries select only a proportion of the available resource, bet-hedging through modest development of alternative fisheries less likely to decline should be adopted. For instance, promoting a gradual shift from reef-associated to pelagic species by establishing near-reef fish aggregation devices may help many Pacific Island states sustain local protein demands and livelihoods when the productivity of their reef fisheries decline (Bell et al. 2009). Options must be weighed carefully, however, as short-term adaptations targeting new species must account for their ecological impacts and effects on existing resource-users.
2. Protect key functional groups: local action to protect key functional groups may increase resilience to climate change effects. The clearest example is on coral reefs, where a variety of fishes play key functional roles in maintaining coral dominance over macroalgae, and these species are more vulnerable to fishing than to climate-driven habitat loss (Graham et al. submitted). Changing gear use or banning gears with large impacts on key species has been suggested to help maintain ecological function (Cinner et al. 2009a). Herbivory plays a key functional role on reefs, and herbivores are protected in some jurisdictions, such as herbivore fishing bans in Herbivore Fisheries Management Areas of Hawaii, that can help determine the wider applicability of such a focused management approach.
3. Invest: the societies best able to adapt to climate change are likely to be well-informed, well-capitalized and able to shift to alternative fisheries or activities (Brander 2010). Fishers in poorly capitalized, developing nations are least likely to be able to divert effort towards new or under-used resources that could meet local resource demands (Allison et al. 2009). Judicious capital investment in landing sites, engines and boats, and alternative gears would serve to improve access to other resources, but risk subsidizing overfishing. Recovery from acute disturbances such as storms requires public or private investment in insurance schemes that allow fishers to quickly return to fishing (Badjeck et al. 2010) and transitioning from traditional to novel fisheries requires interim support. Local scientific capacity to understand ecosystem ecology and regulate fisheries requires national and international investment in field and computing resources, and in the human capital needed to conduct research.
4. Monitoring and indicators: deciding what, where and when to monitor are among the most important decisions in the development of any fisheries adaptation plan. To act, regulators must understand the current state of the ecosystem (Vilhjálmsson et al. 2005) and this understanding can only come through monitoring of indicators showing changes in state (King & McFarlane 2006) that can be related to unfavourable thresholds (Mumby et al. 2007) and show how close the system is to reaching them (Scheffer & Carpenter 2003). Often quite simple biological indicators such as population variance (Brooks et al. 2006) or current abundance as a fraction of unfished abundance (Worm et al. 2009) can provide useful information but many of these are underused (Brander 2010). Tailoring indicators to the system being fished requires careful, well-informed thought about what provides the greatest level of contrast near transitions between ecosystem states. Development of social indicators and reference points is also an important, but as yet undeveloped, aspect of adaptation. For instance, measuring fishers income and the levels at which they fall into and get out of poverty traps could prove critical in helping to maintain coastal livelihoods.
5. Decoupling from fisheries: although fisheries have often provided a refuge for impoverished peoples (Tietze et al. 2000), fisheries in some areas are expected to collapse in response to repeated acute disturbance and increasing temperature. Programmes to deal with the effects of such losses would ideally be in place before they occur. Societies that fail to prepare for the threat of fisheries collapse may experience severe food shortages while those that prepare to divert nutritional needs and livelihoods to land or freshwater-based sources will fare better. Unfortunately, some coastal tropical regions most likely to collapse also have the least capacity to undertake such preparations.
A key challenge for future fisheries management is to determine in which combination these adaptation options should be applied to suit particular fisheries. Climate change is an environmental problem that forces individual fisheries jurisdictions to deal with the local and regional effects of factors beyond their control. The challenge for managers is to spread risks and remain nimble enough to ensure that fisheries are efficient, sustainable and productive, even as they undergo unprecedented change.
Acknowledgements
We wish to thank Maria Dornales and Anne Magurran for their invitation to participate in the Biological diversity in a changing world discussion meeting, as well as John Pinnegar and an anonymous reviewer for their thoughtful and thorough reviews of our draft manuscript.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Biological diversity in a changing world’.
References
- Adger W. N.2006Vulnerability. Glob. Environ. Change 16, 268–281 (doi:10.1016/j.gloenvcha.2006.02.006) [Google Scholar]
- Adger W. N., Arnell N. W., Tompkins E. L.2005Successful adaptation to climate change across scales. Glob. Environ. Change 15, 77–86 (doi:10.1016/j.gloenvcha.2004.12.005) [Google Scholar]
- Allison E., et al. 2009Vulnerability of national economies to the impacts of climate change on fisheries. Fish Fish. 10, 173–196 (doi:10.1111/j.1467-2979.2008.00310.x) [Google Scholar]
- Arnason R.2007Climate change and fisheries, assessing the economic impact in Iceland and Greenland. Nat. Resour. Modell. 20, 163–197 (doi:10.1111/j.1939-7445.2007.tb00205.x) [Google Scholar]
- Badjeck M., Allison E., Halls A., Dulvy N.2010Impacts of climate variability and change on fishery-based livelihoods. Mar. Policy 34, 375–383 (doi:10.1016/j.marpol.2009.08.007) [Google Scholar]
- Barnett J., Adger W. N.2007Climate change, human security and violent conflict. Polit. Geogr. 26, 639–655 (doi:10.1016/j.polgeo.2007.03.003) [Google Scholar]
- Beare D. J., Burns F., Greig A., Jones E. G., Peach K., Kienzle M., McKenzie E., Reid D. G.2004Long-term increases in prevalence of North Sea fishes having southern biogeographic affinities. Mar. Ecol.-Progr. Ser. 284, 269–278 (doi:10.3354/meps284269) [Google Scholar]
- Beaugrand G., Edwards M., Brander K., Luczak C., Ibanez F.2008Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecol. Lett. 11, 1157–1168 (doi:10.1111/j.1461-0248.2008.01218.x) [DOI] [PubMed] [Google Scholar]
- Beddington J., Agnew D., Clark C.2007Current problems in the management of marine fisheries. Science 316, 1713–1716 (doi:10.1126/science.1137362) [DOI] [PubMed] [Google Scholar]
- Beddington J. R., Kirkwood G. P.2005The estimation of potential yield and stock status using life-history parameters. Phil. Trans. R. Soc. B 360, 163–170 (doi:10.1098/rstb.2004.1582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrenfeld M., et al. 2006Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 (doi:10.1038/nature05317) [DOI] [PubMed] [Google Scholar]
- Bell J. D., Kronen M., Vunisea A., Nash W. J., Keeble G., Demmke A., Pontifex S., Andrefouet S.2009Planning the use of fish for food security in the Pacific. Mar. Policy 33, 64–76 (doi:10.1016/j.marpol.2008.04.002) [Google Scholar]
- Berkes F., Jolly D.2001Adapting to climate change, social-ecological resilience in a Canadian western Arctic community. Conserv. Ecol. 5, 1–18 [Google Scholar]
- Bonaldo R., Bellwood D.2009Dynamics of parrotfish grazing scars. Mar. Biol. 156, 771–777 (doi:10.1007/s00227-009-1129-x) [Google Scholar]
- Brander K.2009Impacts of climate change on marine ecosystems and fisheries. J. Mar. Biol. Assoc. India 51, 1–13 [Google Scholar]
- Brander K.2010Impacts of climate change on fisheries. J. Mar. Syst. 79, 389–402 (doi:10.1016/j.jmarsys.2008.12.015) [Google Scholar]
- Brooks T., Mittermeier R., Da Fonseca G., Gerlach J., Hoffmann M., Lamoreux J., Mittermeier J., Pilgrim J., Rodrigues A.2006Global biodiversity conservation priorities. Science 313, 58–61 (doi:10.1126/science.1127609) [DOI] [PubMed] [Google Scholar]
- Chase J. M., Liebold M. A.2002Spatial scale dictates the productivity–biodiversity relationship. Nature 416, 427–430 (doi:10.1038/416427a) [DOI] [PubMed] [Google Scholar]
- Chassot E., Bonhommeau S., Dulvy N. K., Mélin F., Watson R., Gascuel D., Le Pape O.2010Marine primary production constrains world fish catch. Ecol. Lett. 13, 495–505 (doi:10.1111/j.1461-0248.2010.01443.x) [DOI] [PubMed] [Google Scholar]
- Catchpole T. L., Frid C. L. J., Gray T. S.2005Discards in North Sea fisheries, causes, consequences and solutions. Mar. Policy 29, 421–430 (doi:10.1016/j.marpol.2004.07.001) [Google Scholar]
- Cheung W., Lam V., Sarmiento J., Kearney K., Watson R., Pauly D.2009Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 10, 235–251 (doi:10.1111/j.1467-2979.2008.00315.x) [Google Scholar]
- Cheung W., Lam V., Sarmiento J., Kearney K., Watson R., Zeller D., Pauly D.2010Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Glob. Change Biol. 16, 24–35 (doi:10.1111/j.1365-2486.2009.01995.x) [Google Scholar]
- Choat J. H., Robertson D. R.2002Age-based studies on coral reef fishes. In Coral reef fishes, dynamics and diversity in a complex ecosystem (ed. Sale P.), pp. 57–80 New York, NY: Academic Press [Google Scholar]
- Cinner J., Bodin 2010Livelihood diversification in tropical coastal communities: a network-based approach to analyzing ‘livelihood landscapes.’ PLoS ONE 5, e11999 (doi:10.1371/journal.pone.0011999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinner J., McClanahan T., Graham N., Pratchett M., Wilson S., Raina B.2009aGear-based fisheries management as a potential adaptive response to climate change and coral mortality. J. Appl. Ecol. 46, 724–732 (doi:10.1111/j.1365-2664.2009.01648.x) [Google Scholar]
- Cinner J., Fuentes M. M. P. B., Randriamahazo H.2009bExploring social resilience in Madagascar's marine protected areas. Ecol. Soc. 14, 1–41 [Google Scholar]
- Cinner J., Wamukota A., Randriamahazo H., Rabearisoa A.2009cToward community-based management of inshore marine resources in the Western Indian Ocean. Mar. Policy 33, 489–496 (doi:10.1016/j.marpol.2008.11.001) [Google Scholar]
- Cinner J., Daw T. M., McClanahan T. R.2009dSocioeconomic factors that affect artisanal fishers' readiness to exit a declining fishery. Conserv. Biol. 23, 124–130 (doi:10.1111/j.1523-1739.2008.01041.x) [DOI] [PubMed] [Google Scholar]
- Colwell R., Brhem G., Cardeĺus C., Gilman A., Longino J.2008Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 322, 258–261 (doi:10.1126/science.1162547) [DOI] [PubMed] [Google Scholar]
- Cutter S. L.1996Vulnerability to environmental hazards. Progr. Hum. Geogr. 20, 529–539 (doi:10.1177/030913259602000407) [Google Scholar]
- Drinkwater K.2006The regime shift of the 1920s and 1930s in the North Atlantic. Progr. Oceanogr. 68, 134–151 (doi:10.1016/j.pocean.2006.02.011) [Google Scholar]
- Drinkwater K.2009Comparison of the response of Atlantic cod (Gadus morhua) in the high-latitude regions of the North Atlantic during the warm periods of the 1920s–1960s and the 1990s–2000s. Deep-Sea Res. II 56, 2087–2096 (doi:10.1016/j.dsr2.2008.12.001) [Google Scholar]
- Dulvy N., Rogers S., Jennings S., Stelzenmüller V., Dye S., Skjoldal H.2008Climate change and deepening of the North Sea fish assemblage, a biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039 (doi:10.1111/j.1365-2664.2008.01488.x) [Google Scholar]
- Dutil J.-D., Brander K.2003Comparing productivity of North Atlantic cod (Gadus morhua) stocks and limits to growth production. Fish. Oceanogr. 12, 502–512 (doi:10.1046/j.1365-2419.2003.00243.x) [Google Scholar]
- Edwards M., Richardson A.2004Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (doi:10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- FAO 2008The state of world fisheries and aquaculture. Technical Report of the Food and Agricultue Organization of the United Nations, Rome, Italy [Google Scholar]
- Ford J.2009Vulnerability of Inuit food systems to food insecurity as a consequence of climate change, a case study from Igloolik, Nunavut. Regional Environ. Change 9, 83–100 (doi:10.1007/s10113-008-0060-x) [Google Scholar]
- Ford J., Pearce T., Smit B., Wandel J., Allurut M., Shappa K., Ittusujurat H., Qrunnut K.2007Reducing vulnerability to climate change in the Arctic, the case of Nunavut, Canada. Arctic 60, 150–166 [Google Scholar]
- Freitas V., Campos J., Fonds M., Van der Veer H.2007Potential impact of temperature change on epibenthic predator–bivalve prey interactions in temperate estuaries. J. Thermal Biol. 32, 328–340 (doi:10.1016/j.jtherbio.2007.04.004) [Google Scholar]
- Graham C., Harrod C.2009Implications of climate change for the fishes of the British Isles. J. Fish Biol. 74, 1143–1205 (doi:10.1111/j.1095-8649.2009.02180.x) [DOI] [PubMed] [Google Scholar]
- Graham N., et al. 2008Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS ONE 3, e3039 (doi:10.1371/journal.pone.0003039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham N., et al. Submitted Extinction risk in coral reef fishes. [Google Scholar]
- Graham N. A. J., Wilson S. K., Jennings S., Polunin N. V. C., Robinson J., Bijoux J., Daw T.2007Lag effects in the impacts of mass coral bleaching on coral reef fish, fisheries, and ecosystems. Conserv. Biol. 21, 1291–1300 (doi:10.1111/j.1523-1739.2007.00754.x) [DOI] [PubMed] [Google Scholar]
- Grandcourt E. M., Cesar H. S. J.2003The bio-economic impact of mass coral mortality on the coastal reef fisheries of the Seychelles. Fish. Res. 60, 539–550 (doi:10.1016/S0165-7836(02)00173-X) [Google Scholar]
- Hentrich S., Salomon M.2006Flexible management of fishing rights and a sustainable fisheries industry in Europe. Mar. Policy 30, 712–720 (doi:10.1016/j.marpol.2005.11.003) [Google Scholar]
- Hiddink J., ter Hofstede R.2008Climate induced increases in species richness of marine fishes. Glob. Change Biol. 14, 453–460 (doi:10.1111/j.1365-2486.2007.01518.x) [Google Scholar]
- Hoegh-Guldberg O., et al. 2007Coral reefs under rapid climate change and ocean acidification. Science 317, 1737–1742 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- Hughes T., et al. 2003Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (doi:10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- Huntington H. P.2000Using traditional ecological knowledge, methods and applications. Ecol. Appl. 10, 1270–1274 [Google Scholar]
- Jennings S., Brander K.2010Predicting the effects of climate change on marine communities and the consequences for fisheries. J. Mar. Syst. 79, 418–426 (doi:10.1016/j.jmarsys.2008.12.016) [Google Scholar]
- Kawagley A.1995A Yupiaq world view. Long Grove: Waveland Press [Google Scholar]
- King J., McFarlane G.2006A framework for incorporating climate regime shifts into the management of marine resources. Fish. Manage. Ecol. 13, 93–102 (doi:10.1111/j.1365-2400.2006.00480.x) [Google Scholar]
- Ledlie M. H., Graham N. A. J., Bythell J. C., Wilson S. K., Jennings S., Polunin N. V. C., Hardcastle J.2007Phase shifts and the role of herbivory in the resilience of coral reefs. Coral Reefs 26, 641–653 (doi:10.1007/s00338-007-0230-1) [Google Scholar]
- Loeng H., et al. 2005Marine systems. In Arctic climate impact assessment, pp. 454–538 Cambridge, UK: Cambridge University Press [Google Scholar]
- Loring P., Gerlach S.2010Outpost Gardening in Interior Alaska, Historical dimensions of food system innovation and the traditional and customary practices of crop cultivation. Ethnohistory 57, 83–199 [Google Scholar]
- Loring P., Gerlach S., Arkinson D., Murray M.In press Ways to help and ways to hinder, governance for successful livelihoods in a changing climate. Arctic. [Google Scholar]
- MacNeil M. A., Graham N. A. J.2010Enabling regional management in a changing climate through Bayesian meta-analysis of a large-scale disturbance. Glob. Ecol. Biogeogr. 19, 412–421 (doi:10.1111/j.1466-8238.2009.00515.x) [Google Scholar]
- MacNeil M. A., Graham N. A. J., Polunin N. V. C., Kulbicki M., Galzin R., Harmelin-Vivien M.-L., Rushton S.2009Hierarchical drivers of reef fish metacommunity structure. Ecology 90, 252–264 (doi:10.1890/07-0487.1) [DOI] [PubMed] [Google Scholar]
- McClanahan T. R.2002The near future of coral reefs. Environ. Conserv. 29, 460–483 [Google Scholar]
- McClanahan T. R.In press Effects of fisheries closures and gear restrictions on fishing income in a Kenyan coral reef. Conserv. Biol. (doi:10.1111/j.1523-1739.2010.01530.x) [DOI] [PubMed] [Google Scholar]
- McClanahan T. R., et al. 2008Conservation action in a changing climate. Conserv. Lett. 1, 53–59 (doi:10.1111/j.1755-263X.2008.00008_1.x) [Google Scholar]
- McClanahan T. R., Castilla J. C., White A. T., Defeo O.2009Healing small-scale fisheries by facilitating complex socio-ecological systems. Rev. Fish Biol. Fish. 19, 33–47 (doi:10.1007/s11160-008-9088-8) [Google Scholar]
- McIlgorm A., Hanna S., Knapp G., Le Floc'H P., Millerd F., Pan M.2010How will climate change alter fishery governance? Insights from seven international case studies. Mar. Policy 34, 170–177 (doi:10.1016/j.marpol.2009.06.004) [Google Scholar]
- Moran E. F.1981Human adaptation to Arctic zones. Annu. Rev. Anthropol. 10, 1–25 (doi:10.1146/annurev.an.10.100181.000245) [Google Scholar]
- Mumby P. J., Hastings A., Edwards H. J.2007Thresholds and the resilience of Caribbean coral reefs. Nature 450, 98–101 (doi:10.1038/nature06252) [DOI] [PubMed] [Google Scholar]
- Munday P., Jones G., Pratchett M., Williams A.2008Climate change and the future for coral reef fishes. Fish Fish. 9, 261–285 (doi:10.1111/j.1467-2979.2008.00281.x) [Google Scholar]
- Munday P. L., Dixson D. L., McCormick M. I., Meekan M., Ferrari M. C. O., Chivers D. P.2010Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12 930–12 934 (doi:10.1073/pnas.1004519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Adger W. N., Brown K.2007Adaptation to environmental change: contributions of a resilience framework. Annu. Rev. Environ. Resources 32, 395–419 (doi:10.1146/annurev.energy.32.051807.090348) [Google Scholar]
- Newton K., Coté I. M., Pilling G. M., Jennings S., Dulvy N. K.2007Current and future sustainability of island coral reef fisheries. Curr. Biol. 17, 655–658 (doi:10.1016/j.cub.2007.02.054) [DOI] [PubMed] [Google Scholar]
- NMFS 2008Fisheries of the United States. Silver Springs: National Marine Fisheries Service [Google Scholar]
- NSIDC 2008Arctic sea ice settles at second-lowest, underscores accelerating decline. Boulder, CO: National Sea Ice Data Center; Arctic Sea Ice News and Analysis [Google Scholar]
- Overland J., Alheit J., Bakun A., Hurrell J., Mackas D., Miller A.2010Climate controls on marine ecosystems and fish populations. J. Mar. Syst. 79, 305–315 (doi:10.1016/j.jmarsys.2008.12.009) [Google Scholar]
- Overpeck J., Sturm M., Francis J., Perovich D., Serreze M., Benner R., Carmack E., et al. 2005Arctic system on a trajectory to new, seasonally ice-free state. EOS 86, 309–313 (doi:10.1029/2005EO340001) [Google Scholar]
- Paddack M. J., et al. 2009Recent region-wide declines in Caribbean reef fish abundance. Curr. Biol. 19, 590–595 (doi:10.1016/j.cub.2009.02.041) [DOI] [PubMed] [Google Scholar]
- Perry A., Low P., Ellis J., Reynolds J.2005Climate change and distribution shifts in marine fishes. Science 308, 1912–1915 (doi:10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
- Perry R., Cury P., Brander K., Jennings S., Möllmann C., Planque B.2010Sensitivity of marine systems to climate change and fishing, concepts issues and management responses. J. Mar. Syst. 79, 427–435 (doi:10.1016/j.jmarsys.2008.12.017) [Google Scholar]
- Pistorius P. A., Taylor F.2009Declining catch rates of reef fish in Aldabra's marine protected area. Aquat. Conserv., Mar. Freshw. Ecosyst. 19, S2–S9 [Google Scholar]
- Planque B., Frédou T.1999Temperature and the recruitment of atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 56, 2069–2077 (doi:10.1139/cjfas-56-11-2069) [Google Scholar]
- Pörtner O.2010Oxygen- and capacity-limitation of thermal tolerance, a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881–893 (doi:10.1242/jeb.037523) [DOI] [PubMed] [Google Scholar]
- Pratchett M. S., Munday P. L., Wilson S. K., Graham N. A. J., Cinner J. E., Bellwood D. R., Jones G. P., Polunin N. V. C., McClanahan T. R.2008Effects of climate-induced coral bleaching on coral-reef fishes: ecological and economic consequences. Oceanogr. Mar. Biol. Annu. Rev. 46, 251–296 (doi:10.1201/9781420065756.ch6) [Google Scholar]
- Rayner N. A., Parker D. E., Horton E. B., Folland C. K., Alexander L. V., Rowell D. P.2003Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. J. Geophys. Res. 108(D14), 1–37 (doi:10.1029/2002JD002670) [Google Scholar]
- Saji N. H., Goswami B. N., Vinayachandran P. N., Yamagata T.1999A dipole mode in the tropical Indian Ocean. Nature 401, 360–363 (doi:10.1038/43854) [DOI] [PubMed] [Google Scholar]
- Scheffer M., Carpenter S. R.2003Catastrophic regime shifts in ecosystems, linking theory to observation. Trends Ecol. Evol. 18, 648–658 (doi:10.1016/j.tree.2003.09.002) [Google Scholar]
- Schumann S., Macinko S.2007Subsistence in coastal fisheries policy, What's in a word? Mar. Policy 31, 706–718 (doi:10.1016/j.marpol.2006.12.010) [Google Scholar]
- Sherman K., Belkin I., Friedland K., O'Reilly J., Hyde K.2009Accelerated warming and emergent trends in fisheries biomass yields of the world's large marine ecosystems. Ambio 38, 2145–2224 [DOI] [PubMed] [Google Scholar]
- Stead S. M.2005Changes in Scottish coastal fishing communities: understanding socio-economic dynamics to aid management, planning and policy. Ocean Coastal Manage. 48, 670–692 (doi:10.1016/j.ocecoaman.2005.08.001) [Google Scholar]
- Stenseth N. C., Mysterud A., Ottersen G., Hurrell J. W., Chan K.-S., Lima M.2002Ecological effects of climate fluctuations. Science 297, 1292–1296 (doi:10.1126/science.1071281) [DOI] [PubMed] [Google Scholar]
- Stram D. L., Evans D. C. K.2009Fishery management responses to climate change in the North Pacific. ICES J. Mar. Sci. 66, 1633–1639 (doi:10.1093/icesjms/fsp138) [Google Scholar]
- Tewksbury J., Huey R., Deutsch C.2008Putting the heat on tropical animals. Science 320, 1296–1297 (doi:10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- Tietze U., Groenewold G., Marcoux A.2000Demographic change in coastal fishing communities and its implications for the coastal environment. FAO Fish. Tech. Paper 403, 1–151 [Google Scholar]
- Timmermann A., Oberhuber J., Bacher A., Esch M., Latif M., Roeckner E.1999Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature 398, 694–697 (doi:10.1038/19505) [Google Scholar]
- Turner R. E., Cakacaka A., Graham N. A. J., Polunin N. V. C., Pratchett M. S., Stead S. M., Wilson S. K.2007Declining reliance on marine resources in remote South Pacific societies, ecological versus socio-economic drivers. Coral Reefs 26, 997–1008 (doi:10.1007/s00338-007-0238-6) [Google Scholar]
- Usher M., Callaghan T., Gilchrist G., Heal B., Juday G., Loeng H., Magdalena A., Prestrud P.2005Principles of conserving the Arctic's biodiversity. In Arctic climate impact assessment, pp. 539–596 Cambridge, UK: Cambridge University Press [Google Scholar]
- Vilhjálmsson H., et al. 2005Fisheries and aquaculture. In Arctic climate impact assessment, pp. 692–780 Cambridge, UK: Cambridge University Press [Google Scholar]
- Villasante S.2010Global assessment of the European Union fishing fleet: an update. Mar. Policy 34, 663–670 (doi:10.1016/j.marpol.2009.12.007) [Google Scholar]
- Vinebrook R. D., Cottingham K. L., Norberg J., Scheffer M., Dodson S. I., Maberly S. C., Sommer U.2004Impacts of multiple stressors on biodiversity and ecosystem functioning, the role of species co-tolerance. Oikos 104, 451–457 (doi:10.1111/j.0030-1299.2004.13255.x) [Google Scholar]
- Wilson S.2004Growth, mortality and turnover rates of a small detritivorous fish. Mar. Ecol.-Progr. Ser. 284, 253–259 (doi:10.3354/meps284253) [Google Scholar]
- Woodby D., Carlile D., Siddeek S., Funk F., Clark J., Hulbert L.2005. In Commercial fisheries of Alaska. Juneau: Alaska Department of Fish and Game; Special Publication no. 05-09 [Google Scholar]
- Worm B., et al. 2009Rebuilding global fisheries. Science 325, 578–585 (doi:10.1126/science.1173146) [DOI] [PubMed] [Google Scholar]