Abstract
Theory and empirical evidence show that intraspecific competition can drive selection favouring the use of novel resources (i.e. niche expansion). The evolutionary response to such selection depends on genetic variation for resource use. However, while genetic variation might facilitate niche expansion, genetically diverse groups may also experience weaker competition, reducing density-dependent selection on resource use. Therefore, genetic variation for fitness on different resources could directly facilitate, or indirectly retard, niche expansion. To test these alternatives, we factorially manipulated both the degree of genetic variation and population density in flour beetles (Tribolium castaneum) exposed to both novel and familiar food resources. Using stable carbon isotope analysis, we measured temporal change and individual variation in beetle diet across eight generations. Intraspecific competition and genetic variation acted on different components of niche evolution: competition facilitated niche expansion, while genetic variation increased individual variation in niche use. In addition, genetic variation and competition together facilitated niche expansion, but all these impacts were temporally variable. Thus, we show that the interaction between genetic variation and competition can also determine niche evolution at different time scales.
Keywords: niche evolution, genetic diversity, intraspecific competition, resource niche expansion, individual specialization, individual variation
1. Introduction
Niche diversification can generate and maintain biodiversity by facilitating ecological speciation, or by promoting coexistence of distinct species (Chesson 1991; Schluter 2000; Rundle & Nosil 2005). Hence, understanding the factors that promote or hinder niche diversification is a major goal of ecological and evolutionary research. Theory predicts that intraspecific competition for shared resources can promote niche diversification by generating directional or disruptive selection related to niche use (Wilson & Turelli 1986; Abrams et al. 2008). This was shown experimentally in Drosophila populations maintained at high densities (high intraspecific competition), which evolved tolerance to food containing toxic cadmium chloride more rapidly than populations at lower densities (Bolnick 2001). Experimentally elevated intraspecific competition also led to niche expansion in stickleback fish kept in enclosures in natural habitat, though the shift was behavioural rather than genetic (Svanbäck & Bolnick 2007). Further, studies with spadefoot toads and sea otters show that intraspecific competition facilitates variation in resource use (Pfennig et al. 2007; Tinker et al. 2008; Martin & Pfennig 2009). Thus, it is clear that intraspecific competition can generate selection for niche expansion within populations.
Classical population genetics theory suggests that the rate of adaptive evolution (increase in mean fitness) is proportional to the additive genetic variation for fitness (Fisher 1930). Various laboratory experiments with Drosophila and Tribolium provide support for this prediction: populations with greater genetic variation (either derived from hybrid lines or generated by radiation-induced mutations) had significantly greater productivity in and faster adaptation to new environments (e.g. Ayala 1965; Crenshaw 1965). Thus, if intraspecific competition generates strong selection for niche expansion, then intraspecific genetic variation in niche use should facilitate adaptive niche expansion. This leads to the simple prediction that in a novel habitat, genetically diverse populations facing high resource competition should show the fastest rates of evolutionary resource niche expansion. Conversely, populations with low competition and little genetic variation should exhibit lower niche change. In other words, we expect that genetic variation and competition should interact synergistically to increase the rate of niche expansion.
This expected synergy between competition and genetic variation presumes that the only effect of genetic variation is to facilitate a response to selection. However, genetic variation may also directly alter the strength of selection: genetically dissimilar individuals may compete less than genetically similar individuals (Dempster 1955; Lewontin & Matsuo 1963; Maynard Smith 1978). Although evidence favouring this hypothesis is equivocal, experiments with plants (Allard & Adams 1969; Cheplick & Kane 2004; Reusch et al. 2005; Boyden et al. 2008), Drosophila (Pérez-Tomé & Toro 1982; Fowler & Partridge 1986; Martin et al. 1988; López-Suárez et al. 1993), territorial salmon (Griffiths & Armstrong 2001) and fire-bellied toads (Jasienski 1988) show that genetically heterogeneous groups of individuals have greater productivity than genetically similar groups, potentially due to more efficient niche partitioning within genetically diverse groups. Consequently, at a given population density genetically diverse populations may be subject to weaker selection for niche diversification compared with less diverse populations. For instance, under high competition, heterogeneous groups of Drosophila larvae (from multiple sires) exhibit higher productivity and develop faster than homogeneous groups (from a single sire; Martin et al. 1988).
We therefore propose that genetic variation can both hinder and facilitate niche expansion in a novel habitat, and the net effect of genetic variation depends on the time scale over which niche expansion is examined. Specifically, we consider evolution of the dietary niche in the presence of a novel resource. Over the very short term, genetic variation should have a negative effect on diet expansion by reducing intraspecific resource competition and weakening selection for using a novel resource. Given a specific selection pressure for diet expansion, however, genetic variation should facilitate long-term evolutionary niche expansion. However, if genetic variation lowers intraspecific resource competition (and therefore decreases the strength of selection for diet expansion), genetic variation for niche use could potentially impede niche evolution even in the long term. Here, we describe an experimental test of our hypothesis that genetic variation and intraspecific competition interact to affect the rate of niche expansion, and that this interaction may vary with time scale.
Previous work with the red flour beetle, Tribolium castaneum, suggests that genetic relatedness within groups can increase the strength of intraspecific competition and reduce productivity (Jasienski et al. 1988; Garcia & Toro 1992). Furthermore, T. castaneum harbours additive genetic variation for resource use that is necessary to respond to selection for dietary niche expansion (Bell 1969; Sokoloff 1977; Dawson & Riddle 1983). Therefore, both positive and negative effects of genetic variation on niche expansion discussed above could operate in this species. At high density, beetle excreta and toxins ‘condition’ the flour, reducing its nutritive value; this is associated with decreased fecundity and greater cannibalism-related mortality (Sokoloff 1977). Therefore, beetles compete for nutrition as well as space at high density, generating directional selection for expanding the resource niche onto a novel resource. We factorially manipulated both the level of resource competition (population density) and genetic variation in T. castaneum populations, in habitats containing both the ancestral wheat resource and a novel corn resource. Because niche expansion may occur via individual specialization or generalization (Bolnick et al. 2003), we also quantified the temporal change in both average niche use and among-individual variation in niche use across eight generations. Genetic variation within our populations was correlated with phenotypic variation in resource use (see §2b). Therefore, we assume that overall genetic similarity between individuals predicts similarity in their resource use and determines resource competition. We show that genetic variation and intraspecific competition interact positively to facilitate niche expansion, but they act on different components of niche evolution at different time scales.
2. Material and methods
(a). Experimental populations
Experimental populations were initiated at two densities (200 and 400 adults for low and high competition, respectively) crossed with two levels of genetic variation (two or four strains of T. castaneum; table 1). Although the differences in effective population size in the two competition treatments would lead to varying rates of genetic drift, its impact on evolution should be minimal within the eight experimental generations. Populations were maintained in identical 240 ml plastic containers, with 20 g wheat flour (+5% yeast) and 20 g corn flour in adjacent patches that allowed free movement between patches (see the electronic supplementary material, figure S1). This led to population densities of 10 and 20 adults per gram of wheat, respectively, in the low and high competition treatments. The populations were effectively panmictic: during the experiment, adults and larvae were observed moving across patches. Wheat flour (+5% yeast; henceforth W) is the ancestral resource for T. castaneum (for more than 20 years in the laboratory), while corn flour (henceforth WC) represents a novel suboptimal resource (Agashe 2009). Populations were maintained in incubators at 33°C (±1°C) and 70 per cent humidity.
Table 1.
Experimental design. The four T. castaneum strains are denoted C (Col-2), P (Pak-3), Z (Z-7) and T (Tiw-5). Numbers in parentheses denote the number of replicate populations; only two replicates from each strain combination were used for isotope analysis.
| genetic variation (number of strains; possible strain combinations) | intraspecific competition for wheat |
|
|---|---|---|
| low (10 adults per g wheat) | high (20 adults per g wheat) | |
| low (2; CP/PT/TZ/CZ/PZ/CT) | 6 × (3) = 18 | 6 × (3) = 18 |
| high (4; CPTZ) | 1 × (3) = 3 | 1 × (3) = 3 |
We initiated the first experimental generation using adults chosen randomly from stock populations of each of the four strains (‘generation 0’). Stocks had a sex ratio of approximately 1 : 1 (measured by sexing 50 randomly chosen adults per population). We allowed adults to mate and oviposit for one week, then removed all adults and stored them at −80°C for diet analysis (described below). The number of eggs laid depends on the number of ovipositing adults (Sokoloff 1977); hence, the number of founding adults determined the degree of competition experienced by larvae as they developed. After larvae had developed for four weeks, we counted the number of new adults in each population. From these, a random sample of 200 or 400 adults was added to fresh flour to start the next generation, and excess adults were frozen for isotope analysis. This cycle was repeated for eight generations.
Populations in the high competition treatment often produced fewer than the 400 adults per generation required to start the next generation (see the electronic supplementary material, figure S2; mean number of adults (±s.e.): in W = 407 ± 48, in WC = 330 ± 54). In these cases, all live adults were used to initiate the next generation and none were frozen. Lower productivity could have resulted from increased cannibalism and/or decreased fecundity due to flour conditioning at high density (Sokoloff 1977). Because we aimed to test the impact of genetic variation on niche evolution within closed populations, we did not supplement these founding adults with beetles from stock populations. Therefore, high-competition populations were sometimes founded with less than the intended 400 adults per generation, and the number of adults per generation varied between populations according to their productivity. Regardless, the number of founding adults used for high-competition populations was larger (more than 300) than that used for low-competition populations (which produced 466 ± 41 and 356 ± 50 adults in W and WC, respectively, only 200 of which were used per habitat to start the next generation). Therefore, populations in the high competition treatment were effectively maintained at higher competition, although the difference between high and low competition densities was less than intended due to the unexpected within- and between-population variation in productivity.
(b). Genetic and phenotypic trait variation
We posit that genetic diversity can (i) directly facilitate niche expansion by providing heritable trait variation for selection to act on, or (ii) indirectly inhibit niche expansion by mitigating the strength of intraspecific competition. Both hypotheses require that genetic variation gives rise to variance in resource use among individuals. The four T. castaneum strains we used differed significantly for various fitness measures on wheat and corn (fecundity, survival and behavioural resource choice), as shown in the electronic supplementary material, table S1 (also see Agashe 2009). All these traits have large additive genetic components in T. castaneum (see references in §1), with the exception of larval behavioural resource preference, for which heritability is unknown. Therefore, we assume that our four-strain populations had greater additive genetic variation for fitness-related traits on wheat and corn, compared with two-strain populations.
It is possible that epistatic and dominance effects could weaken the assumed association between number of founding strains and additive genetic variation. However, our assumption is supported by various lines of evidence from previous work with the same strain combinations. First, ecologically relevant variation in these combinations was large enough to cause significantly different population dynamics and persistence in a comparable time period in WC habitats (Agashe 2009). Second, under strong directional selection in a corn-only habitat, four-strain combinations showed a significantly faster increase in fecundity on corn (Agashe et al. in preparation). Finally, the within-population variance in fecundity of two-strain combinations was significantly lower than that of the four-strain combination (measured using six females per population as described in Agashe 2009; in wheat: t = −2.05, d.f. = 5, p = 0.047; in corn: t = −2.16, d.f. = 5, p = 0.042). Therefore, our genetic variation treatments effectively increased phenotypic variance in fecundity—a major fitness component—on both resources.
(c). Population productivity
If genetic variation led to greater resource partitioning and lower competition within populations, we would expect that genetically diverse populations would have higher per capita productivity each generation relative to genetically depauperate populations, within each competition treatment. To test this, we used population census data (see above) to calculate per capita productivity of each population (the number of eclosed adults per generation per founding individual, averaged over the entire eight generations). To also test the impact of genetic diversity on productivity under benign conditions in the ancestral homogeneous habitat, we initiated and maintained a set of control populations in 40 g W using the same design as in WC (with equivalent numbers of founding adults), but with two replicates per strain combination instead of three. Because this control habitat contained 40 g wheat flour compared with the experimental populations' 20 g wheat flour, the ‘low’ and ‘high’ density treatments in the two habitats resulted in different degrees of competition for the ancestral wheat resource. However, flour volume and depth both affect beetle fecundity; therefore, it was important to keep them constant across control (W) and experimental (WC) populations.
Populations of two strain combinations (CP and CZ) went extinct between generations 4 and 8, both at high and low densities. Hence, data from these populations were not included in the analysis described below, reducing the number of data points to four low-diversity combinations and one high-diversity combination, with two replicates per strain combination (table 1). Strains C and Z both have low growth rates and slower development; hence it is probable that the strain combination CZ could not maintain a sufficiently high growth rate to avoid extinction within the enforced four-week generation time. In CP populations, the sudden decrease in the number of larvae after generation 4 was associated with the growth of mould in the containers causing the flour to clump. While the exact effects of the mould on beetle growth and survival are not clear, it is probable that the mould contributed to the extinction of CP populations despite the high fecundity of strain P individuals.
(d). Stable carbon isotope analysis
To quantify temporal change in resource use in WC populations, we measured the stable carbon isotope ratio of 10 whole beetles frozen at generations 4 and 8 (reflecting lifetime resource use during larval and adult stages), from two of three replicate populations of each strain combination (sample size was constrained by the high cost of isotope analysis). The isotope ratio of a sample is measured against a standard as δ13C = [(Rsample/Rstandard) − 1] × 1000, where R = 13C : 12C. Beetle δ13C is a measure of the dietary proportion of corn, since δ13Cwheat = −22.74 (±0.08), δ13Ccorn = −11.84 (±0.16), and δ13C of beetles varies largely linearly with percentage of corn (Focken 2007). Prior to the experiment, all beetles were maintained on wheat resource and had the same isotope ratio (δ13C) of −22.74. Therefore, a mean δ13C ratio closer to −11.84 indicates greater consumption of corn flour by beetles. To infer actual corn use from beetle δ13C ratios, we used the regression equation for percentage of corn versus δ13C (percentage of corn = 1.911 + 0.08 × δ13C) from beetles reared on pure corn (n = 13) and pure wheat (n = 15). For each population, the isotope ratio of beetles at generation 4 (or 8) minus the initial wheat ratio represents the degree of niche expansion that occurred in four (or eight) generations. Similarly, variance in δ13C of the 10 beetles from each population was used to measure change in individual variation in resource use.
We also measured isotope ratios of adults one week after exposure to the experimental WC habitat (‘generation 0’), to quantify the degree of immediate behavioural niche expansion by founding adults. By behavioural niche expansion, we mean that individuals were willing to include a completely unfamiliar resource (corn) in their diet. However, some fraction of beetle tissues will not turn over isotopically within one week (retaining the isotope signature of the ancestral wheat resource used during larval development), whereas fast-turning tissues and gut contents should accurately represent any rapid change in diet. As a result, the measured behavioural niche expansion is probably an underestimate, and some of the across-generation niche expansion during the first four generations may be confounded by behavioural niche expansion. However, change in isotope ratios between generations 4 and 8 represents evolutionary niche expansion, since both ratios reflect lifetime resource use.
(e). Data analysis
Because the experimental design was unbalanced (4 versus 1 strain combinations in each genetic variation treatment), our data could not be analysed with standard parametric tests such as regression or ANOVA. Therefore, we conducted Monte Carlo permutations of the data in R (R Development Core Team 2008) to test whether genetic variation and competition interacted to affect: (i) niche expansion for first four generations (response: difference in mean δ13C, generation 4 − wheat); (ii) niche expansion for last four generations (response: difference in mean δ13C, generation 8 − 4); (iii) total niche expansion (response: difference in mean δ13C, generation 8 − wheat); and (iv) behavioural niche expansion (response: mean δ13C of generation 0 after one week minus the initial δ13C in W = −22.74). In each case the measurement of interest is a difference in isotope ratio, averaged for two replicate populations to give a single value for each strain combination (four with low genetic variation and one with high genetic variation). For each test, null distributions of effect sizes were calculated using 50 000 permuted datasets. As an example, the test used for the degree of behavioural niche expansion is given in electronic supplementary material, appendix A. The same procedure was used for each of the other three tests (see above) using the appropriate measured variable (see table 2).
Table 2.
Results of permutation test for evolutionary change in resource use. p-values from permutation tests are shown for each response variable for each contrast. GV = genetic variation; Comp = competition; σ2 = variance; W = wheat (see §2e). ‘interaction group’ indicates the four treatment groups (table 1 and electronic supplementary material, appendix A).
| response variable | effect | effect size | contrasted generations |
Δ effect (4−W versus 8−4) | |||
|---|---|---|---|---|---|---|---|
| 4−W | 8−4 | 8−W | 0−W | ||||
| Δ mean δ13C | GV | Δhigh GV—Δlow GV | 0.31 | 0.26 | 0.70 | 0.36 | 0.29 |
| Comp | Δhigh Comp—Δlow Comp | 0.55 | 0.03 | 0.63 | 0.50 | 0.06 | |
| GV × Comp | σ2 (Δinteraction group) | 0.68 | 0.03 | 0.99 | 0.51 | 0.42 | |
| Δ variance δ13C | GV | Δhigh GV—Δlow GV | 0.25 | 0.02 | 0.12 | 0.40 | 0.004 |
| Comp | Δhigh Comp—Δlow Comp | 0.15 | 0.35 | 0.65 | 0.32 | 0.17 | |
| GV × Comp | σ2 (Δinteraction group) | 0.26 | 0.25 | 0.49 | 0.38 | 0.28 | |
Effects of genetic variation can arise either through synergistic interactions among genotypes (e.g. niche complementarity) or via a simple sampling effect where diverse populations are more likely to have particular genotypes (a portfolio effect). To test the impact of particular strain combinations on niche expansion, we conducted separate ANOVAs using data for replicates of each strain combination, for each contrast tested above (Δ mean δ13C ∼ strain combination). We conducted similar tests for change in among-individual variance in niche use.
3. Results
(a). Population productivity
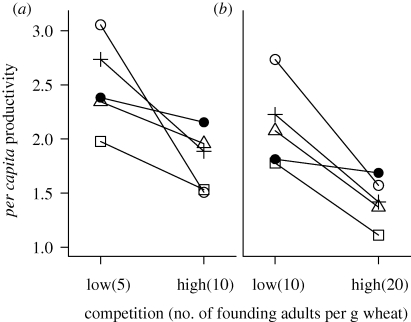
We manipulated founding population density in each generation to control the degree of intraspecific competition within developing larvae. However, genetic variation could mitigate the negative effects of competition on fecundity or larval mortality, resulting in larger numbers of eclosed adults. We tested this possibility by measuring the per capita productivity of genetically diverse and depauperate populations within each competition treatment. Within low-diversity populations, combinations of strains with high growth rate in monoculture maintained greater productivity (PT populations in figure 1; see the electronic supplementary material, table S1). Founding adult density had no effect on the productivity of high-diversity populations in either habitat (paired t-test with replicate high-diversity populations, p = 0.85). In contrast, productivity of low-diversity populations decreased as a function of founding population density, both in the control W (figure 1a; paired t-test, p = 0.04) and in the WC treatment (figure 1b; paired t-test, p = 0.014). This difference in the effect of competition on low- and high-diversity populations was significant in the WC habitat (one-sample t-test for difference in productivity of low-diversity populations, with μ = difference in productivity of high-diversity populations = −0.12: t = −6.27, d.f. = 3, p = 0.008) but not in the W habitat (μ = −0.23, t = −2.16, d.f. = 3, p = 0.12).
Figure 1.
Mean population productivity. Mean per capita productivity of populations is shown as a function of competition in (a) control W and (b) experimental WC habitats. Square, CT; white circle, PT; triangle, PZ; plus symbol, ZT; black circle, CPZT. Note that populations in both habitats were founded with equal numbers of adults (200 for low competition and 400 for high competition) in equal quantities of total resource.
To summarize, in the WC habitat, increased population density reduced the per capita productivity of populations with low genetic diversity, but did not affect productivity of high genetic diversity populations. Thus, increased genetic diversity could mitigate the effect of competition, hindering the process of niche expansion in response to competition.
(b). Resource niche expansion and treatment effects
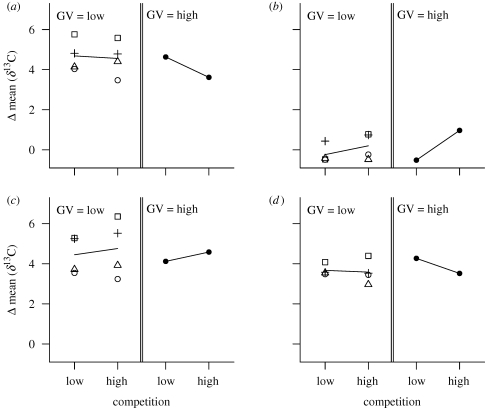
The novel corn resource comprised 37 per cent of beetle diet within the first four experimental generations (significant increase, one-sample t-test of mean change: p = 0.002), and 39 per cent by the end of the experiment (not a significant increase, one-sample t-test of mean change, p = 0.9). During the first four generations, all populations increased their use of corn, and neither genetic variation nor competition had significant effects on the magnitude of increase in corn use (table 2; figure 2a). Subsequently, between generations 4 and 8, corn use increased in some populations but decreased in others. Populations that showed an increase in corn use had greater genetic variation and higher competition (i.e. the two treatments interacted to facilitate niche expansion; table 2; figure 2b). Additionally, genetic variation and competition alone appear to have significant but opposite effects on niche expansion during the first and last four generations (figure 2a,b). Although this temporal difference in their opposing effects is not significant (table 2), it may explain the lack of significant treatment effects on the overall degree of niche expansion from the start to the end (figure 2c; table 2). Data for generation 4 were therefore critical to detect the dynamics of niche expansion and transient treatment effects.
Figure 2.
Temporal change in mean resource use of populations. Each data point is the average value of mean resource use of two replicate populations with a specific strain combination and treatment (square, CT; white circle, PT; triangle, PZ; plus symbol, ZT; black circle, CPZT). Lines connect means of competition treatments. GV = genetic variation. Panels show change in mean resource use (a) in generations 0–4, (b) in generations 4–8, (c) in generations 0–8 and (d) within generation 0.
Behavioural niche expansion accounted for a large fraction of the total niche expansion observed during the experiment: founding-generation adults consumed, on average, at least 30 per cent corn within a week of exposure to the novel habitat, representing 81 per cent of the change in corn use observed over the first four generations. Note that the behavioural niche expansion measured here is a minimal estimate (see §2d); hence it is possible that actual behavioural effects accounted for an even greater fraction of early niche expansion (up to generation 4). Clearly, however, immediate behavioural changes in resource use accounted for the greatest niche change in the experimental populations (figure 2d; one-sample t-test for mean δ13C at generation 0 with μ = −23.82, p < 0.001). As with total niche expansion, behavioural niche expansion was not affected by genetic variation, competition or their interaction (table 2). In addition, strain combinations of low-genetic-variation populations did not significantly affect any phase of niche expansion (ANOVA, effect of strain combination: p > 0.07 in each case).
(c). Among-individual variance in niche use and niche specialization
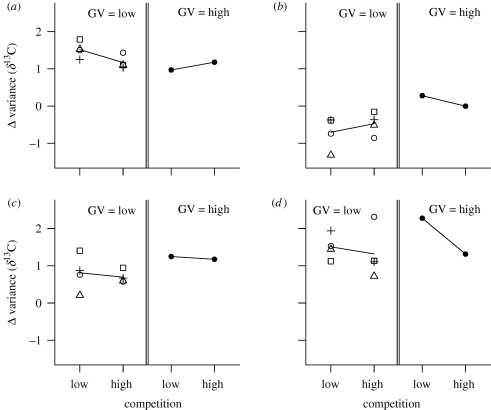
We tested whether the competition and genetic variation treatments altered individual variation in niche use during niche expansion. Among-individual variance in resource use increased within the founding generation in all populations (figure 3d), and was not affected by experimental treatments (table 2). However, populations with low genetic variation subsequently lost much of this individual variation in resource use, while genetically diverse populations retained it. By the end of the experiment, genetically more diverse populations had significantly greater among-individual variance in resource use compared with less diverse populations (figure 3c; Welch two-sample t-test, p = 0.007). This positive effect of genetic variation was only observed between generations 4 and 8 (table 2). During this period, among-individual variance in resource use declined significantly in populations with low diversity (figure 3b; mean change in variance δ13C < 0); in contrast, populations with high diversity maintained among-individual variation in resource use (mean change in variance δ13C = 0). Thus, during the last four generations, genetic variation within populations determined the degree of individual variation in resource use. For populations with low genetic variation and low competition, strain combination also affected the total change in among-individual variance in resource use (ANOVA, effect of strain combination: p = 0.006; for all other contrasts, p > 0.7). At low competition, populations of strain combination CT had maximum individual variation in niche use, while those with combination PZ had almost no individual variation in resource use. This result may be due to the large difference in fitness on corn between C and T strains (see the electronic supplementary material, table S1), which would cause greater variation in resource use at low competition.
Figure 3.
Temporal change in among-individual variance in resource use of populations. Each data point is the average value of among-individual variance in resource use of two replicate populations with a specific strain combination and treatment. Symbols and panel information are as in figure 2.
In summary, during generations 4–8, competition significantly increased niche expansion, whereas genetic variation prevented the loss of among-individual variation in niche use. These transient dynamics ultimately determined total evolutionary niche expansion and maintenance of individual variation in niche use in the populations. These results together imply that genetic variation and competition affect different components of niche expansion and place different constraints on niche evolution.
4. Discussion
Genetic variation may have both positive and negative implications for resource niche expansion. Genetic variation for resource use can increase the response to selection arising from intraspecific competition (Fisher 1930) and therefore facilitate niche expansion. On the other hand, such variation can lower the degree of intraspecific competition (Maynard Smith 1978) and hence weaken selection for niche expansion. Our experiment shows for the first time that both positive and negative effects of genetic variation occur, but they occur during different stages of niche expansion. Our results also indicate that genetic variation largely determines the maintenance of individual variance in niche use, whereas resource competition (generating selection for resource niche expansion) may be the major driver of niche change.
Below we discuss these observed effects in turn: (i) as predicted, genetic variation alters resource competition within populations; (ii) genetic variation and competition differentially affect niche expansion across different time scales; (iii) genetic variation and competition interact transiently to facilitate niche expansion; and (iv) genetic variation alters the degree of individual variation in niche use.
(a). Genetic variation, competition and their influence on productivity in different habitats
Quantifying productivity was important in this experiment because it established that genetic diversity lowered intraspecific competition in the novel WC habitat (figure 1). This confirms that genetic variation could have both negative (via decreased competition) and positive (via increased response to selection) impacts on niche expansion. In both W and WC habitats, genetic variation within populations decreased larval competition and maintained productivity at high density (figure 1), in accord with previous results (Garcia & Toro 1992). Further experiments with a wider range of population densities in each habitat could shed light on the exact mechanism through which genetic variation influences the relationship between productivity and population density in the WC habitat (e.g. via density-dependent fecundity or larval mortality). The low per capita productivity of high-competition populations (figure 1b) led to a discrepancy between the planned and actual difference in population density between competition treatments (see §2 and the electronic supplementary material, figure S2), although it was still higher than the low-density treatments (300 versus 200). Hence, the competition effect measured by this experiment was probably underestimated due to within-population (temporal) and between-population variation in the number of founding adults in high-density populations. A true twofold difference in population density may thus have a greater impact on niche evolution than that measured in this experiment.
(b). Behavioural and evolutionary niche expansion
Many studies show that genetic variation has a greater impact on population parameters under strong selection (reviewed in Wise et al. 2002; Armbruster & Reed 2005; Charmantier & Garant 2005). Our results contribute to this literature by showing that the impact of genetic variation also depends on the degree of intraspecific resource competition. Genetic variation aided exploitation of the novel corn resource when high population density generated strong selection for niche expansion (figure 2). In a separate experiment, we found that under extreme selection in a corn-only habitat, genetic variation aided niche shifts onto corn (Agashe et al. in preparation). Thus, genetic variation can facilitate exploitation of novel resources as long as selection is sufficiently strong.
In this experiment, the strength of selection on niche use was determined by intraspecific competition, but previous work suggests that competition could in turn be altered by genetic variation (Jasienski et al. 1988; Garcia & Toro 1992). This complex interaction between genetic variation and competition gave rise to temporal variation in genetic variation's effect on niche expansion. Between generations 0 and 4, genetic variation did not significantly affect the magnitude of niche expansion: it decreased larval competition for the ancestral resource (figure 2a) and presumably reduced selection for niche expansion, but this effect was probably weak compared with the extensive behavioural niche expansion. Therefore, the positive impact of genetic variation for increasing response to selection was only apparent after a few generations (here, between generations 4–8). Because selection is greater under strong competition (Martin et al. 1988), genetic variation only increased niche expansion in populations with high competition (figure 2), as reflected in the significant interaction term between genetic variation and competition (table 2). In low-competition populations, genetic variation did not affect niche expansion, probably because resource competition was too low, and so selection for niche expansion was weak or absent. Thus, both positive and negative impacts of genetic variation played a role in determining niche dynamics in our experiment.
Our experiment also shows that the competitive advantage conferred by genetic variation depends on the environment. While genetic variation may confer short-term competitive benefits, these benefits can only translate into a longer-term advantage under strong selection (in this case, mediated by high population density in a heterogeneous habitat). Conversely, genetic variation may impart a competitive disadvantage only in a benign environment (a ‘genetic load’). For instance, in ambient CO2, Arabidopsis thaliana populations with diverse genotypes have lower fitness than monocultures; however, under elevated CO2, genotype mixtures perform better than single-genotype stands (Andalo et al. 2001).
Our competition and genetic variation treatments affected founding-generation beetles only for one week, and only in their adult stage. On the other hand, the treatments altered the genetic and competitive environment of each subsequent cohort for four weeks throughout their development from eggs to mature adults. Therefore, the impact of the competition and genetic variation treatments on change in resource use would be expected to increase in later generations. In agreement with this prediction, the experimental treatments facilitated evolutionary niche expansion, but had no effect on the degree of behavioural niche expansion within the founding adult population (table 2 and figure 2). Rapid behavioural adaptation to novel habitats via niche shifts has been frequently documented in earlier studies (e.g. Ghalambor et al. 2007). The pervasive behavioural niche expansion that we observed regardless of experimental treatment (figure 2) shows that such behavioural plasticity can overcome constraints imposed by standing genetic variation or by weak selection on niche use.
It should also be noted that increased genetic variation could only affect niche use if variation at loci relevant to resource use increases (resulting in greater phenotypic variance in resource use)—that is, when genetic variation is ecologically significant. In addition, phenotypic variance in combinations of two phenotypically extreme strains may be larger than that in four-strain combinations, even though average phenotypic variance in four-strain combinations is greater. In other words, the specific combinations of strains may matter more than the number of strains. However, in our experiment we found no impact of strain identity on niche expansion at any stage. An experiment manipulating phenotypic variance rather than number of distinct starting strains would be able to directly address the impact of phenotypic variation on niche evolution.
(c). Among-individual variance in niche use and individual specialization
An increase in population niche width can occur via an increase in either within-individual or between-individual variation in niche use (Roughgarden 1972; Bolnick et al. 2003). In the first case, all individuals increase their niche width to a similar extent, using a greater variety of resources. In the second case, population niche width increases because individuals use different resources to different extents, though individuals continue to be relatively specialized. A key result from this study is that the degree of founding genetic variation facilitated the long-term maintenance of individual variation in resource use in experimental populations (figure 3 and table 2).
While it seems obvious that greater genetic variation in resource use should allow for greater individual variation in niche use, it is not necessarily true, for the following reasons. First, sexual recombination or selection can erode individual variation unless other factors promote the maintenance of genetic polymorphism (such as habitat heterogeneity; e.g. Hedrick 1986). Second, individual resource specialization may largely be an outcome of behavioural or physiological plasticity (Bolnick et al. 2003) or learning (Estes et al. 2003), rather than heritable variation for fitness on different resources. Lastly, mathematical models predict that individual specialization is typically difficult to maintain unless promoted by functional trade-offs in resource use (Roughgarden 1972; Taper & Case 1985; Ackermann & Doebeli 2004). Without trade-offs, a single generalist genotype (or single most-fit specialist) is expected to reach fixation, eliminating among-individual variance in resource use.
Despite these reasons for genetic variation to be dissociated from individual variation in resource use, the impact of genetic variation on the maintenance of individual niche variation hitherto remained empirically untested. The degree of individual variation in niche use has significant implications for population ecological and evolutionary dynamics (see Bolnick et al. 2003 for a review). For instance, individual niche variation can substantially alter population dynamics (Lomnicki 1978; Kendall & Fox 2002) and species coexistence (Lichstein et al. 2007). Depending on other conditions such as niche-based assortative mating (Snowberg & Bolnick 2008), niche variation can allow populations to undergo subsequent adaptive diversification and speciation (Dieckmann & Doebeli 1999). Our finding that genetically diverse populations maintain greater individual variance in resource use shows that such heritable variation can maintain the within-population phenotypic diversity that is critical for generating species diversity. This is further demonstrated by the observation that, within populations with low genetic variation, strain pairs with the most divergent fitness on the two resources maintained maximum among-individual niche variation.
Previous work suggests that niche expansion most commonly occurs via individual niche specialization rather than generalization (the niche variation hypothesis; Van Valen 1965). Empirical evidence for the niche variation hypothesis has been debated at length (reviewed in Bolnick et al. 2003), but recently Bolnick et al. (2007) reported widespread support for it in natural populations of diverse taxa, including gastropods, fish, frogs and lizards. In our experiment, however, the total degree of niche expansion was not associated with among-individual variance in niche use at generation 8 (Pearson's product–moment correlation = 0.43, t = 1.34, d.f. = 8, p = 0.22; both variables were normally distributed: Shapiro–Wilk normality test, p > 0.6). Therefore, our results do not support the NVH prediction that larger niche shifts occur primarily via increased individual variation in niche use.
5. Conclusions
Our experiment identifies a previously untested interaction between genetic variation and intraspecific competition as a factor promoting resource niche expansion under directional selection. Our results further indicate that intraspecific competition may be a major factor driving the rate of niche evolution, while genetic variation in niche use may largely determine the maintenance of individual niche variation. Perhaps most interestingly, our results show that the two factors have different impacts during various stages of niche evolution. Intraspecific competition and genetic variation for niche use are ubiquitous attributes of natural populations, and both have important implications for population ecology and evolution. Experimental evidence of their interaction and impact of competition and genetic variation during niche evolution is a step towards enhancing our understanding of how such intrinsic properties shape population and niche dynamics.
Acknowledgements
We thank Hussain Khan, Maradona Truong, Rob Arthur and Jay Falk for laboratory assistance; Jay Falk for the electronic supplementary material, figure S1; and Christine Parent, Will Stutz and Rose Carlson for comments on the manuscript. This research was supported by an NSF DDIG grant (DEB 0808356); a Packard Foundation Grant to D.I.B.; and research grants to D.A. from the Society for Integrative and Comparative Biology and the Section of Integrative Biology at the University of Texas at Austin.
References
- Abrams P. A., Rueffler C., Kim G.2008Determinants of the strength of disruptive and/or divergent selection arising from resource competition. Evolution 62, 1571–1586 (doi:10.1111/j.1558-5646.2008.00385.x) [DOI] [PubMed] [Google Scholar]
- Ackermann M., Doebeli M.2004Evolution of niche width and adaptive diversification. Evolution 58, 2599–2612 [DOI] [PubMed] [Google Scholar]
- Agashe D.2009The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. Am. Nat. 174, 255–267 (doi:10.1086/600085) [DOI] [PubMed] [Google Scholar]
- Agashe D., Falk J., Bolnick D. I.In preparation Genetic variation facilitates adaptation to a novel habitat. [Google Scholar]
- Allard R. W., Adams J.1969Population studies in predominantly self-pollinating species XIII. Intergenotypic competition and population structure in barley and wheat. Am. Nat. 103, 621 (doi:10.1086/282630) [Google Scholar]
- Andalo C., Goldringer I., Godelle B.2001Inter- and intragenotypic competition under elevated carbon dioxide in Arabidopsis thaliana. Ecology 82, 157–164 [Google Scholar]
- Armbruster P., Reed D. H.2005Inbreeding depression in benign and stressful environments. Heredity 95, 235–242 (doi:10.1038/sj.hdy.6800721) [DOI] [PubMed] [Google Scholar]
- Ayala F. J.1965Evolution of fitness in experimental populations of Drosophila serrata. Science 150, 903–905 (doi:10.1126/science.150.3698.903) [DOI] [PubMed] [Google Scholar]
- Bell A. E.1969The nature of selection responses in Tribolium. Japan. J. Genet. 44(Suppl. 1), 299–309 [Google Scholar]
- Bolnick D. I.2001Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature 410, 463–466 (doi:10.1038/35068555) [DOI] [PubMed] [Google Scholar]
- Bolnick D. I., Svanback R., Fordyce J. A., Yang L. H., Davis J. M., Hulsey C. D., Forister M. L.2003The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 [DOI] [PubMed] [Google Scholar]
- Bolnick D. I., Svanback R., Araujo M. S., Persson L.2007Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc. Natl Acad. Sci. USA 104, 10 075–10 079 (doi:10.1073/pnas.0703743104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden S., Binkley D., Stape J. L.2008Competition among Eucalyptus trees depends on genetic variation and resource supply. Ecology 89, 2850–2859 (doi:10.1890/07-1733.1) [DOI] [PubMed] [Google Scholar]
- Charmantier A., Garant D.2005Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B 272, 1415–1425 (doi:10.1098/rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheplick G. P., Kane K. H.2004Genetic relatedness and competition in Triplasis purpurea (Poaceae): resource partitioning or kin selection? Int. J. Plant Sci. 165, 623–630 (doi:10.1086/386556) [Google Scholar]
- Chesson P.1991A need for niches. Trends Ecol. Evol. 6, 26–28 (doi:10.1016/0169-5347(91)90144-M) [DOI] [PubMed] [Google Scholar]
- Crenshaw J. W.1965Radiation-induced increases in fitness in the flour beetle Tribolium confusum. Science 149, 426–427 (doi:10.1126/science.149.3682.426) [DOI] [PubMed] [Google Scholar]
- Dawson P. S., Riddle N. C.1983Genetic variation, environmental heterogeneity, and evolutionary stability. In Population biology: retrospect and prospect (eds King C. E., Dawson P. S.), pp. 147–170 New York, NY: Columbia University Press [Google Scholar]
- Dempster E. R.1955Maintenance of genetic heterogeneity. Cold Spring Harbor Symp. Quant. Biol. 20, 25–32 [DOI] [PubMed] [Google Scholar]
- Dieckmann U., Doebeli M.1999On the origin of species by sympatric speciation. Nature 400, 354–357 (doi:10.1038/22521) [DOI] [PubMed] [Google Scholar]
- Estes J. A., Riedman M. L., Staedler M. M., Tinker M. T., Lyon B. E.2003Individual variation in prey selection by sea otters: patterns, causes and implications. J. Anim. Ecol. 72, 144–155 (doi:10.1046/j.1365-2656.2003.00690.x) [Google Scholar]
- Fisher R. A.1930The genetical theory of natural selection. Oxford, UK: Clarendon Press [Google Scholar]
- Focken U.2007Effect of different ratios of wheat to corn flour in the diet on the development and isotopic composition (δC-13, δN-15) of the red flour beetle Tribolium castaneum. Isot. Environ. Health Stud. 43, 143–154 (doi:10.1080/10256010701360520) [DOI] [PubMed] [Google Scholar]
- Fowler K., Partridge L.1986Variation in male fertility explains an apparent effect of genotypic diversity on success in larval competition in Drosophila melanogaster. Heredity 57, 31–36 (doi:10.1038/hdy.1986.83) [Google Scholar]
- Garcia C., Toro M. A.1992Sib competition in Tribolium: a test of the elbow-room model. Heredity 68, 529–536 [Google Scholar]
- Ghalambor C. K., McKay J. K., Carroll S. P., Reznick D. N.2007Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 (doi:10.1111/j.1365-2435.2007.01283.x) [Google Scholar]
- Griffiths S. W., Armstrong J. D.2001The benefits of genetic diversity outweigh those of kin association in a territorial animal. Proc. R. Soc. Lond. B 268, 1293–1296 (doi:10.1098/rspb.2001.1660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick P. W.1986Genetic polymorphism in heterogeneous environments—a decade later. Annu. Rev. Ecol. Syst. 17, 535–566 (doi:10.1146/annurev.es.17.110186.002535) [Google Scholar]
- Jasienski M.1988Kinship ecology of competition—size hierarchies in kin and nonkin laboratory cohorts of tadpoles. Oecologia 77, 407–413 (doi:10.1007/BF00378052) [DOI] [PubMed] [Google Scholar]
- Jasienski M., Korzeniak U., Lomnicki A.1988Ecology of kin and nonkin larval interactions in Tribolium beetles. Behav. Ecol. Sociobiol. 22, 277–284 (doi:10.1007/BF00299843) [Google Scholar]
- Kendall B. E., Fox G. A.2002Variation among individuals and reduced demographic stochasticity. Conserv. Biol. 16, 109–116 (doi:10.1046/j.1523-1739.2002.00036.x) [DOI] [PubMed] [Google Scholar]
- Lewontin R., Matsuo Y.1963Interaction of genotypes determining viability in Drosophila bucksii. Proc. Natl Acad. Sci. USA 49, 270–278 (doi:10.1073/pnas.49.2.270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein J. W., Dushoff J., Levin S. A., Pacala S. W.2007Intraspecific variation and species coexistence. Am. Nat. 170, 807–818 [DOI] [PubMed] [Google Scholar]
- Lomnicki A.1978Individual differences between animals and natural regulation of their numbers. J. Anim. Ecol. 47, 461–475 [Google Scholar]
- López-Suárez C., Toro M. A., Garcia C.1993Genetic heterogeneity increases viability in competing groups of Drosophila hydei. Evolution 47, 977–981 (doi:10.2307/2410203) [DOI] [PubMed] [Google Scholar]
- Martin R. A., Pfennig D. W.2009Disruptive selection in natural populations: the roles of ecological specialization and resource competition. Am. Nat. 174, 268–281 (doi:10.1086/600090) [DOI] [PubMed] [Google Scholar]
- Martin M. J., Pereztome J. M., Toro M. A.1988Competition and genotypic variability in Drosophila melanogaster. Heredity 60, 119–123 (doi:10.1038/hdy.1988.17) [DOI] [PubMed] [Google Scholar]
- Maynard Smith J.1978The evolution of sex. Cambridge, UK: Cambridge University Press [Google Scholar]
- Pérez-Tomé J. M., Toro M. A.1982Competition of similar and non-similar genotypes. Nature 299, 153–154 (doi:10.1038/299153a0) [Google Scholar]
- Pfennig D. W., Rice A. M., Martin R. A.2007Field and experimental evidence for competition's role in phenotypic divergence. Evolution 61, 257–271 (doi:10.1111/j.1558-5646.2007.00034.x) [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2008R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Reusch T. B. H., Ehlers A., Hammerli A., Worm B.2005Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl Acad. Sci. USA 102, 2826–2831 (doi:10.1073/pnas.0500008102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughgarden J.1972Evolution of niche width. Am. Nat. 106, 683–718 [Google Scholar]
- Rundle H. D., Nosil P.2005Ecological speciation. Ecol. Lett. 8, 336–352 (doi:10.1111/j.1461-0248.2004.00715.x) [Google Scholar]
- Schluter D.2000The ecology of adaptive radiation. New York, NY: Oxford University Press [Google Scholar]
- Snowberg L. K., Bolnick D. I.2008Assortative mating by diet in a phenotypically unimodal but ecologically variable population of stickleback. Am. Nat. 172, 733–739 [DOI] [PubMed] [Google Scholar]
- Sokoloff A.1977The biology of tribolium. Oxford, UK: Clarendon Press [Google Scholar]
- Svanbäck R., Bolnick D. I.2007Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. B 274, 839–844 (doi:10.1098/rspb.2006.0198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taper M. L., Case T. J.1985Quantitative genetic models for the coevolution of character displacement. Ecology 66, 355–371 (doi:10.2307/1940385) [Google Scholar]
- Tinker M. T., Bentall G., Estes J. A.2008Food limitation leads to behavioral diversification and dietary specialization in sea otters. Proc. Natl Acad. Sci. USA 105, 560–565 (doi:10.1073/pnas.0709263105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valen L.1965Morphological variation and width of ecological niche. Am. Nat. 99, 377–390 [Google Scholar]
- Wilson D. S., Turelli M.1986Stable underdominance and the evolutionary invasion of empty niches. Am. Nat. 127, 835–850 [Google Scholar]
- Wise C. A., Ranker T. A., Linhart Y. B.2002Modeling problems in conservation genetics with Brassica rapa: genetic variation and fitness in plants under mild, stable conditions. Conserv. Biol. 16, 1542–1554 (doi:10.1046/j.1523-1739.2002.00309.x) [Google Scholar]