Abstract
A better understanding of animal emotion is an important goal in disciplines ranging from neuroscience to animal welfare science. The conscious experience of emotion cannot be assessed directly, but neural, behavioural and physiological indicators of emotion can be measured. Researchers have used these measures to characterize how animals respond to situations assumed to induce discrete emotional states (e.g. fear). While advancing our understanding of specific emotions, this discrete emotion approach lacks an overarching framework that can incorporate and integrate the wide range of possible emotional states. Dimensional approaches that conceptualize emotions in terms of universal core affective characteristics (e.g. valence (positivity versus negativity) and arousal) can provide such a framework. Here, we bring together discrete and dimensional approaches to: (i) offer a structure for integrating different discrete emotions that provides a functional perspective on the adaptive value of emotional states, (ii) suggest how long-term mood states arise from short-term discrete emotions, how they also influence these discrete emotions through a bi-directional relationship and how they may function to guide decision-making, and (iii) generate novel hypothesis-driven measures of animal emotion and mood.
Keywords: emotion, mood, core affect, cognition, decision-making
1. Introduction
A better understanding of animal emotion is highly desirable in disciplines including neuroscience, psychopharmacology, pain research and animal welfare science. To date, much animal emotion research, like Darwin's (1872/2009) pioneering writings on the subject, has focused on ‘discrete’ emotions. Researchers have investigated how animals respond to situations assumed to induce specific emotional states. For example, there is a whole industry devoted to the study of fear and anxiety, largely based on the development of tests designed to induce these states. This approach has yielded detailed information on candidate behavioural and physiological indicators of discrete emotions (e.g. Boissy 1995; Ramos & Mormede 1998; Forkman et al. 2007), and on their putative neural and neurochemical substrates (e.g. LeDoux 1996; Panksepp 1998; Berridge 2003; Dalgleish 2004).
Furthermore, it has been argued that, at least in mammals, there are ‘basic’ discrete emotional systems (e.g. fear, rage, panic, play) rooted in the neural circuitry of particular brain areas, serving specific adaptive functions, and representing the fundamental building blocks of all emotional reactions (Ekman 1992; Panksepp 1998). For example, Panksepp (1998) argues that an evolutionarily ancient ‘fear system’, linking the amygdala and periaqueductal grey (PAG) of the midbrain, coordinates coherent fear responses to challenging situations, and is also the source of subjective feelings of fear. Other systems may have distinct or overlapping neurophysiological substrates, and each is thought to play a causal role in generating discrete and adaptive emotional responses to specific challenges, facilitating survival of the animal.
The discrete emotions approach, however, has inevitably been piecemeal, leaving some emotional states, including positive ones (Boissy et al. 2007), under-studied. It also lacks an overarching framework or ‘structure of emotion’ that can integrate the wide range of possible emotional states, and provide a priori predictions, applicable across species, for how these states are manifest, and hence how they can be measured. Such a framework could be offered by ‘dimensional’ theories that have become increasingly prominent in the study of human emotion (Russell & Barrett 1999; Watson et al. 1999; Carver 2001; Russell 2003). These are largely based on reports of subjective emotional experiences and the temporal relationships between different reported states, and suggest that these states can be represented as locations in two- or three-dimensional space (figure 1). Different theories propose slightly different dimensional axes, but a ‘valence’ dimension or dimensions (positivity/negativity) is central to all. Some theorists argue that these dimensions, and not basic emotions, are the core building blocks of all emotional experience, are underpinned by specific brain systems and serve important adaptive functions such as the control of approach and avoidance behaviour (e.g. Watson et al. 1999; Carver 2001; Posner et al. 2005; Barrett 2006; Barrett et al. 2007).
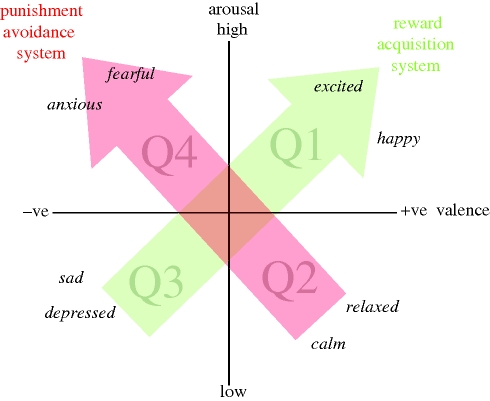
Figure 1.
Core affect represented in two-dimensional space. Words in italics indicate possible locations of specific reported affective states (including discrete/basic emotions). Positive affective states are in quadrants Q1 and Q2, and negative states in quadrants Q3 and Q4. Arrows indicate putative biobehavioural systems associated with reward acquisition and the Q3–Q1 axis of core affect (green), and punishment avoidance and the Q2–Q4 axis of core affect (red). Adapted from Russell (e.g. Russell & Barrett 1999) and Panksepp (e.g. Burgdorf & Panksepp 2006).
There is ongoing debate between proponents of the discrete/basic and dimensional views of emotion (e.g. Ortony & Turner 1990; Ekman 1992; Barrett 2006; Izard 2007; Panksepp 2007). It is not our aim to argue in support of one or other. Rather, we believe that they can be brought together to provide a conceptual framework for studying animal emotions that: (i) offers a structure for integrating disparate discrete emotions, providing a functional perspective on the adaptive value of different emotional states, (ii) suggests how ‘free-floating’ mood states arise from short-term emotional responses to events, and how they may function to guide decision-making, and (iii) yields novel hypothesis-driven measures of animal emotion.
In developing this framework, we aim to bring together and build upon the ideas of many other researchers of human and animal emotions (e.g. Lang et al. 1990; Cabanac 1992; Gray 1994; Panksepp 1998; Cacioppo et al. 1999; Russell & Barrett 1999; Watson et al. 1999; Nesse 2000, 2004, 2005; Carver 2001; Ellsworth & Scherer 2003; Rolls 2005; Nesse & Ellsworth 2009). We start by outlining the dimensional approach, which has received limited attention in animal emotion research (but see Gray 1994), then briefly summarize some disagreements between discrete and dimensional theorists before suggesting how the two approaches may be brought together. We end by considering implications for the assessment of animal emotion, including the development of new measures that may be particularly useful for assessing long-term mood states.
2. A dimensional view of the structure of emotion: core affect
(a). Core affect and subjective emotional experience
Emotions interest us because of their distinctive conscious manifestation—the feelings of joy, relief, anxiety or depression. The conscious subjective experience of emotions is what we are ultimately concerned about when we consider human and animal welfare, and it is these subjective experiences that define the field of emotion research. In humans, the ‘gold standard’, albeit indirect, method for measuring these experiences is linguistic self-report. Statistical analyses of these reports suggest that emotions can be defined in terms of two fundamental underlying dimensions (e.g. Stanley & Meyer 2009). Emotional experiences are valenced—they are perceived as positive or negative, rewarding or punishing, pleasant or unpleasant—neutral states are not emotional states. Emotional experiences also vary in reported activation or arousal. For example, the states of elation and contentment are both positively valenced, but the former involves a higher degree of arousal than the latter.
Subjective experiences that can be characterized in terms of these valence and arousal dimensions have been labelled core affect (Russell 2003; Barrett et al. 2007). They can be represented in two-dimensional space as shown in figure 1. Positive affective states lie in the right half of this space (quadrants Q1 and Q2), and negative affective states in the left half (Q3 and Q4). Core affect can thus be conceptualized as the fundamental subjectively reportable manifestation of any emotion or mood state, and core affect space provides us with a way of conceptualizing the structure of subjective emotional experiences. Discrete emotions such as fear, sadness and happiness are located somewhere in this space, although their location per se does not fully encapsulate their subjective qualities. For example, a highly aroused negatively valenced state (Q4) accompanied by an urge to flee may characterize fear while a state of the same valence and arousal accompanied by an urge to attack may characterize anger.
The core affect concept is rooted in an understanding of the subjective experience of emotion. While this may lie at the heart of our interests in animal emotion, it raises challenges given that direct measurement of subjective states in another human, let alone another species, is not currently possible. However, the reported subjective experiences of core affect in humans are accompanied by neural, behavioural, physiological and cognitive changes, such as alterations in brain activity, facial expressions, heart rate and attention to threat. These changes can be measured objectively. Together with subjective experience, they make up the components of emotional or affective states. Researchers can study these measurable components of animal emotions, and attempt to identify those components that are reliably associated with particular locations in core affect space.
Of course, even if we can use measurable components of emotional responses to locate an animal's position in core affect space, we cannot be certain that they experience the conscious component too. Whether or not, and to what extent, different species experience conscious affective states remains an area of intense and unresolved debate (e.g. Wemelsfelder 1997; Macphail 1998; Baars 2001; Rolls 2005). For the purposes and scope of this paper, however, we must leave this to one side, discussing animal emotions as states that may or may not be experienced consciously.
(b). Acquiring rewards and avoiding punishment: a functional view of core affect
Could animals have states that equate to core affect (regardless of whether these are consciously experienced)? We think this is plausible because of the likely evolutionary advantage of systems that can represent the organism's overall experience of reward and punishment. Emotional states occur in response to stimuli or situations that are actually, or potentially, rewarding or punishing. Reward and punishment thus lie at the heart of all emotional states and determine emotional valence (e.g. Lang et al. 1990; Gray 1994; Cacioppo et al. 1999; Watson et al. 1999; Carver 2001; Rolls 2005; Barrett et al. 2007; Nesse & Ellsworth 2009). Inherently rewarding or punishing stimuli include those that enhance fitness (rewards—food, water, shelter, mates, etc.) and those that threaten fitness (punishers—predator attack, thermal damage, etc.). The affective responses of different animal species to these stimuli are thought to have developed over evolutionary time, and to act as proximate mechanisms guiding and coordinating the organism to achieve two principal survival goals: maximizing acquisition of fitness-enhancing rewards and minimizing exposure to fitness-threatening punishers (e.g. Rolls 2005; Burgdorf & Panksepp 2006; Nesse & Ellsworth 2009).
Position in core affect space is widely believed to reflect this functionality. Theoretical and empirical studies suggest that positive high arousal affective states in quadrant Q1 (e.g. excitement, happiness) are associated with appetitive motivational states, and function to facilitate seeking and obtaining rewards (Cabanac 1992; Carver 2001; Custers & Aarts 2005; Rolls 2005; Burgdorf & Panksepp 2006). In contrast, negative low arousal states in Q3 (e.g. sadness, depression) are associated with experiences of loss or lack of reward, and may promote low activity and conservation of energy in conditions where resources are lacking (Nesse 2000). Thus, affective states along the Q3–Q1 axis appear to be related primarily to acquiring fitness-enhancing rewards, and the success or otherwise of this endeavour (figure 1). Several researchers propose that an individual's position along this axis may be associated with the activity of underlying, perhaps primitive, biobehavioural systems (‘positive activation’, ‘behavioural activation’ (BAS) or ‘approach process’ systems; Gray 1994; Watson et al. 1999; Carver 2001) concerned with the control of approach behaviour and resource acquisition. The mesolimbic dopaminergic system and its role in appetitive and ‘wanting’ behaviours may be an important neural substrate of such systems (Berridge 1996, 2007; Panksepp 1998; McNaughton & Corr 2008). In humans, activation of left anterior cortical areas may also reflect enhanced activity of these systems (Davidson 1998; Davidson & Irwin 1999).
Negative high-arousal affective states in quadrant Q4 (e.g. fear) are thought to be principally associated with, and to coordinate appropriate responses to, the presence of threat or danger (Gray 1994; Carver 2001; Rolls 2005; Burgdorf & Panksepp 2006). In contrast, positive low-arousal affective states in Q2 (e.g. calm, relaxed) are associated with experience of low levels of threat (Carver 2001), perhaps facilitating the expression of maintenance, consolidation and recovery activities. Affective states along the Q2–Q4 axis thus appear to be primarily related to the need to avoid fitness-threatening punishers (figure 1). An individual's position on the Q2–Q4 axis is principally determined by the perceived presence of danger or threat, and has been associated with the activation of underlying biobehavioural systems (‘negative activation’, ‘fight flight flee system’ (FFFS), ‘avoidance process’; Gray 1994; Watson et al. 1999; Carver 2001) that have evolved to facilitate the avoidance of punishment. Gray (1994) also suggests that a ‘behavioural inhibition system’ (BIS) underpins anxiety states in conditions of conflicting threat/reward; see McNaughton & Corr 2008). Brain structures including the PAG, amygdala, anterior cingulate and ventral prefrontal cortex may form the neural substrate of such systems (McNaughton & Corr 2008). In humans, activation of the right anterior cortical areas may also reflect enhanced activity of these systems (Davidson 1998; Davidson & Irwin 1999).
In summary, position in core affect space appears to be functionally related to the experience of success or failure in acquiring rewards and avoiding punishment, and biobehavioural systems have evolved to serve these two fundamentally important activities. It is worth mentioning that some states (e.g. severe depression) may sometimes be the result of brain pathologies unrelated to experience or, while reflecting the organism's experiences, may be so extreme as to have limited functional value to the organism (i.e. represent a non-adaptive side effect of the proximate mechanisms involved). The border between functionality and pathology in states such as severe depression remains a topic of much debate (Nesse 2000).
3. Integrating discrete and dimensional approaches
A major disagreement between proponents of discrete and dimensional approaches relates to the relative primacy of discrete emotional systems or dimensional core affect systems in generating felt emotional states. Core affect theorists propose that ongoing core affective state (valence and arousal) is combined with evaluations or appraisals of current context/environmental conditions to generate a subjective state that can be described in discrete emotion terms (e.g. Barrett 2006). The experience of specific emotions is thus a product of core affect and not vice versa. Discrete/basic emotion theorists take the opposite view and argue that core affect emerges as a cognitive ‘distillation’ of the overall affective impact of the experience of specific emotions (e.g. Panksepp 2007; see also Tellegen et al. 1999). Given that there is psychological, neural and behavioural evidence for both types of system, an alternative view is to hypothesize that both systems may be present in humans and many animals, that they interact in some way and that they serve different functions. Such a synthesis has been proposed by, among others, Panksepp (2007) and Izard (2007) and we develop it further here.
We first briefly consider the causes and functions of discrete emotions. We then suggest that: (i) core affective components of discrete emotions and other affective states provide a ‘common currency’ that may function to prioritize actions, (ii) discrete emotions, generated by events, influence position in core affect space, (iii) cumulative experience of location in core affect space underlies longer term mood, and (iv) core affect mood states can, in turn, influence decision-making and discrete emotions. Thus, a bi-directional relationship between discrete emotion and core affect systems is proposed.
(a). Causes and functions of discrete emotions
Discrete emotions arise in response to anticipation or experience of rewarding or punishing events. They are thus event-focused (or object-focused) and usually short-lasting. Appraisal theorists suggest that a process of ‘stimulus checks’ of a number of key characteristics (e.g. valence, predictability, familiarity) of eliciting circumstances generates emotions (Ellsworth & Scherer 2003). For example, appraisal of a stimulus as intrinsically unpleasant (punishing), sudden, unpredictable and unfamiliar is likely to induce a ‘fear’ (Q4) emotion (Scherer 2001). In humans, situations may be appraised in numerous ways depending on, for example, the characteristics of the situation and the subject's previous experiences and current motivations. Consequently, appraisals may result in many different emotional states, including varied nuances of discrete emotions (Ellsworth & Scherer 2003). Human appraisals may involve cognitive processes such as memory (underpinning familiarity/novelty appraisals) and anticipation (underpinning appraisals of predictability), but they can also be simple, rapid and ‘automatic’ (subconscious; Zajonc 1980; Grandjean & Scherer 2008). It is thus conceivable that similar processes occur in animals (Desiré et al. 2002), and act to trigger discrete emotional states which engage specific neurobehavioural systems.
These discrete emotion systems probably function to organize short-term responses to the eliciting circumstances, recruiting appropriate physiological resources, motivating relevant behaviours and thus facilitating the organism's immediate survival (e.g. Frijda 1994; Rolls 2005). Different discrete emotion systems have evolved to deal with different types of challenge. For example, Panksepp (1998) postulates that a distinct ‘panic’/‘separation distress’ system functions to maintain social bonds between separated individuals by triggering vocalization and search behaviour.
(b). Discrete emotions, sensations and motivations have core affective characteristics that may function as a common currency in decision-making
Discrete emotions have an underlying valenced structure—this is what characterizes them as part of the affective/emotional system—and can thus be located in core affective space. Other states such as sensations and motivations also share these characteristics. Physical sensations mediated by direct neural connections between the sensory apparatus (e.g. receptors for touch, taste, smell) and the brain can be inherently rewarding or punishing and hence also located in core affect space. For example, a taste may give rise to a positively valenced affective response of ‘pleasure’ that may be located at some point in the Q1 quadrant and function to influence subsequent wanting of the stimulus (Berridge 2007).
Wanting states—motivation for a specific reward—can also be located in core affect space. They are determined by internal changes reflecting current physiological need, and external stimuli that have become strongly associated with reward through the animal's developmental or evolutionary past, and, in humans, can be experienced as high-arousal positive states (Q1) associated with reward-seeking behaviour.
Location in core affect space thus represents a common currency (cf. McNamara & Houston 1986; Cabanac 1992; Spruijt et al. 2001) that may function to allow comparisons and trade-offs between disparate discrete emotions, sensations and motivations when behavioural decisions are being made. For example, a hungry and thirsty animal needs to be able to weigh up the relative reward values of searching to obtain food or water at any one time, and also to calculate when searching behaviour becomes too dangerous because of heightened threats from punishers such as predator attack. Emotions and motivations can only be made use of in such ‘expected utility’ type decisions if they incorporate the core affective feature of valence that can function as a common currency (cf. Cabanac 1992).
(c). Discrete emotions and other states influence location in core affect space
Following from the above, discrete emotions, sensations and motivations can be conceptualized as generating short-term changes in an animal's location in core affect space. For example, the onset of feeding motivation may involve the animal moving towards an aroused, positive, seeking state (Q1). Detection of a food item may then lead to a higher arousal state of excitement with successful capture of prey perhaps leading to a temporary state of elation (Q1), followed by a lower arousal consummatory state of sensory pleasure during eating and an even lower arousal post-consummatory positive state of contentment or satisfaction (Q2). On the other hand, failure to detect or obtain a food item may initially lead to a temporary high-arousal negative state of frustration (Q4), which may then subside to a lower arousal negative state of disappointment or sadness (Q3). These trajectories, related to the acquisition of fitness-enhancing rewards (one can imagine similar trajectories related to the search for mates, positive social interactions, etc.), are illustrated in figure 2. Although, as discussed earlier, they tend to inhabit the Q3–Q1 axis, it is clear that relative success or failure can lead to states in the Q2 and Q4 quadrants too.
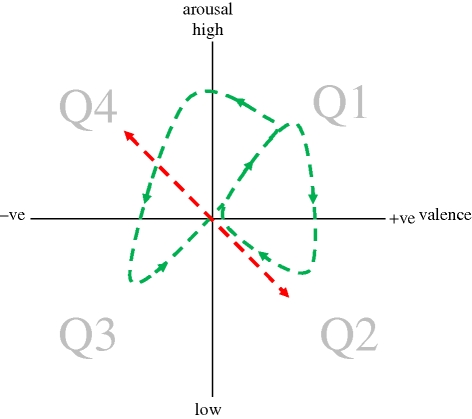
Figure 2.
Hypothetical examples of how an organism's core affective state may change with time. The right-hand green loop represents changes during successful cycles of reward acquisition. The left-hand green loop represents changes when reward acquisition is unsuccessful. The red line represents changes in response to the presence (quadrant Q4) or successful avoidance (Q2) of threats or punishers. See text for details.
The appearance of a fitness-threatening stimulus can intrude into these reward acquisition cycles at any time and rapidly shift the animal's position in core affect space into the Q4 quadrant of high-arousal negative fear and anxiety states (figure 2). These states are associated with appropriate responses to danger aimed at avoiding punishers. Successful responses will result in the removal of threat and a lower arousal positive state of relief or calm (Q2). Such trajectories, related to the avoidance of fitness reduction, thus primarily inhabit the Q2–Q4 axis and, owing to their potentially life-threatening sequelae, generally assume primacy over Q1–Q3 states associated with the acquisition of fitness-enhancing rewards (e.g. Haselton & Nettle 2006; cf. Dawkins & Krebs 1979).
In this view, movement through core affect space is driven by discrete emotions, sensations and motivations, and represents the organism's experiences of success and failure in acquiring rewards and avoiding punishers. We suggest that it forms the basis for longer term mood states.
(d). The causes of longer term core affect ‘mood’ states
In humans, core affective states do not only occur in response to specific events or stimuli. They also occur in the absence of, and without being directed at, any particular object. In this case, they are usually referred to as free-floating moods. Moods are typically longer lasting than discrete emotions, sensations or motivations, and are a relatively ‘pure’ form of core affect, lacking the action tendencies and appraisal-induced responses to emotion-eliciting situations that characterize discrete emotions. They include longer term states of ‘happiness’ or ‘sadness’ and, in their more extreme forms, states such as chronic anxiety (Q4) or depression (Q3) or mania (Q1). At any one time, an individual's core affective state can be conceptualized as a combination of their longer term background mood state and their reactions to current emotion-inducing events. Thus, a chronically anxious individual may experience temporary states of positive affect under certain circumstances (e.g. when eating a particularly delicious meal) despite their ‘background’ state of anxiety. Consequently, ongoing mood states may be most easily revealed when an individual is not currently exposed to strong emotion-inducing events (which may lead to ‘ceiling-effect’ responses that mask background mood state) or when novel or ambiguous events occur whose affective salience is not immediately apparent. They can be conceptualized as the background core affect state that the individual will revert to when specific emotion-inducing events are absent.
In our view, mood states probably reflect a cumulative function of the experience of shorter term emotional episodes (e.g. discrete emotions, sensations, motivations). For example, if an animal is in an environment in which it experiences frequent threatening events, and hence its emotional state is often in the Q4 quadrant, it may develop a longer term high-arousal negative mood state that mirrors this cumulative experience. If it is frequently successful at avoiding these events, or it is in a generally safe environment, a longer term low-arousal positive mood state (Q2: ‘relaxed’/‘calm’) may result. On the other hand, if it is in a plentiful environment and successful at acquiring fitness-enhancing rewards, it is likely to exhibit a mood state that is centred on the Q1 quadrant, whereas a low-resource environment and failure to acquire rewards will lead to a predominantly Q3 mood (cf. Carver 2001).
In relation to the trajectories shown in figure 2, we propose that mood states can be likened to a ‘running mean’ of positions occupied within core affect space over a preceding time period, and thus continually (albeit slowly) change as the result of novel events and experiences. This view of mood states as representing past experience chimes with the findings that, for example, chronic anxiety and depression states usually arise from exposure to specific environmental and emotional circumstances (e.g. chronic stress, major life events; e.g. Eysenck et al. 2006; Young & Korszun 2009).
At a neural level, the above proposal requires that there is cross-talk between the activity of discrete emotional systems (e.g. sub-cortical structures such as the PAG) and those that become activated across a broad range of rewarding or punishing events (e.g. BAS/BIS/FFFS). Widely dispersed neuromodulatory systems (e.g. dopaminergic, serotonergic, noradrenergic, opioidergic) appear to be prime candidates for the latter reward/punishment systems (Gray 1994; Burgdorf & Panksepp 2006; Berridge 2007; Leknes & Tracey 2008), and it is possible that they, and higher cortical areas (e.g. anterior cingulate cortex, orbitofrontal cortex), somehow integrate or distil the activity of discrete emotion systems across time in terms of overall positive and negative experience (cf. Spruijt et al. 2001; Panksepp 2007). Differences in the sensitivity of such systems, whether determined by genetic or experiential factors, may underlie individual predispositions to particular mood states (Corr 2008).
(e). Functions of mood states: their influence on decision-making, appraisals and discrete emotions
We suggest that mood states provide information about the type of environment the organism is living in—the presence (or probability) of threats and reward opportunities—and how well it is coping (see also Carver 2001, 2003; Prinz 2004). This information plays an important role in guiding animals' decisions when appraising new situations or stimuli, especially if there is a degree of ambiguity in their potentially rewarding or punishing consequences (Davidson 1994; Mendl et al. 2009). For example, if an individual is living in an environment where it has experienced high levels of threat, its mood state has a greater likelihood of being in the Q4 quadrant. In such an environment where probability of danger is high, it would make adaptive sense to appraise ambiguous stimuli such as a rustle in the grass as more likely to predict a negative event (e.g. predator), and hence to take safety-first avoidant action, in comparison to an individual living in a low threat environment with a mood state in the Q2 quadrant for whom a negative judgement is likely to result in wasted time and energy (Nesse 2005).
Different responses to ambiguity may also be observed in individuals whose background mood state is predominantly in quadrant Q1 (resulting from experience of an environment with high probability of opportunity for gaining fitness-enhancing rewards) or Q3 (experience of low reward-opportunity environments). Those in Q1 are likely to benefit from judging ambiguous stimuli as indicating a positive event (e.g. prey), thus facilitating reward-seeking behaviour, relative to those in Q3 mood states who may benefit from inhibiting reward-seeking behaviour in order to conserve energy until environmental conditions change (Nesse 2000).
Thus, we suggest that Q1 moods are associated with decisions appropriate to high reward-opportunity environments, reflecting a high ‘expectation’ of positive events, and Q2 moods with decisions reflecting low expectation of negative events. These can be termed ‘optimistic’ biases in decision-making (e.g. judging ambiguous stimuli positively). Conversely, Q3 moods are associated with low expectation of positive events and Q4 moods with high expectation of negative events (‘pessimistic’ biases). Clearly, environments may not be as simple as this. Some complexity can be added by considering combinations of environments with low, intermediate and high probabilities of opportunity and threat to generate a broader range of predictions linking experience of environment, mood and expectations/decision-making as indicated in table 1.
Table 1.
Postulated links between prevailing environmental conditions (in terms of reward acquisition opportunities and threat of punishers), resulting predominant core affective mood state (quadrants Q1–Q4) and biases in expectation of rewarding (+ve) or punishing (−ve) events (↑, increased expectation; ↓, decreased expectation) that drive optimistic or pessimistic decision-making. See text for details.
| low reward-opportunity environment (leads to Q3 state) | ‘intermediate’ reward-opportunity environment | high reward-opportunity environment (leads to Q1 state) | |
|---|---|---|---|
| low-threat environment (leads to Q2 state) | Q2/Q3, ↓ expect. of −ve, ↓ expect. of +ve | Q2, ↓ expect. of −ve, ‘optimism about −ve’ | Q1/Q2, ↑ expect. of +ve, ↓ expect. of −ve, ‘full optimism’ |
| ‘intermediate’-threat environment | Q3, ↓ expect. of +ve, ‘pessimism about +ve’ | ‘neutral state’, no (or baseline) bias | Q1, ↑ expect. of +ve, ‘optimism about +ve’ |
| high-threat environment (leads to Q4 state) | Q3/Q4, ↓ expect. of +ve, ↑ expect. of −ve, ‘full pessimism’ | Q4, ↑ expect. of −ve, ‘pessimism about −ve’ | Q1/Q4, ↑ expect. of +ve, ↑ expect. of −ve |
Consistent with these ideas, there is a large body of research with humans showing that background mood state does indeed appear to influence decision-making (e.g. Schwarz & Clore 1983; Bechara et al. 2000; Loewenstein et al. 2001), including in ways similar to those predicted. People in negative states tend to judge ambiguous stimuli negatively (e.g. MacLeod & Byrne 1996). They also more readily attend to threatening stimuli and recall negative memories than people in positive mood states (see Mineka et al. 1998; Mogg & Bradley 1998). Furthermore, there is evidence that people with a long-term tendency towards (trait) anxiety and/or current (state) anxiety (Q4) judge ambiguous stimuli as more likely to be negative, while people in states of sadness or depression (Q3) judge them as less likely to be positive in line with predictions outlined above (e.g. MacLeod et al. 1997; Stober 2000; MacLeod & Salaminiou 2001). There is also evidence that people in positive moods show optimistic forms of these so-called ‘cognitive biases’ (e.g. Nygren et al. 1996).
Mood state may thus act as a heuristic device influencing cognitive processes and facilitating appropriate decision-making behaviour. Because appraisals of situations/events may themselves involve cognitive processes, mood states can therefore also affect these appraisals and the resulting short-term emotional responses. The causal link between short-term discrete emotions and longer term core affect mood is thus bi-directional. It is possible to envisage positive feedback loops in which, for example, a Q4 mood state enhances anticipation of negative events and negative interpretation of ambiguity, and this leads to further negative short-term emotional experiences which, in turn, intensify the Q4 mood. Such processes are implicated in the aetiology of chronic anxiety and depression in humans (Beck 1967, 2008). In the natural environment, they may function to help animals escape from or cope with difficult or threatening conditions (Nesse 2000), until circumstances change and the experience of more positive events leads to a gradual alteration in mood state.
4. Implications for the measurement of animal emotion
Discrete emotion approaches rely on identifying situations or tests that are assumed to induce a particular emotional state and then measuring behavioural and physiological responses as putative indicators of that state. Such tests may be quick to carry out and, if they accurately identify naturalistic situations that reliably induce a particular state, they may be very useful and ecologically valid. However, there are also some disadvantages to this ad hoc approach. For example, different individuals may perceive (appraise) the same situation differently, and different situations thought to induce the same emotion may not do so. This will decrease the likelihood that consistent behavioural and physiological responses are detected. Indeed, in the extensive literature on fear testing, studies sometimes find good cross-test agreement in how they rank order individuals (i.e. in terms of ‘fearfulness’), but disagreement is common (e.g. Ramos & Mormede 1998; Miller et al. 2006; Forkman et al. 2007) and behavioural and physiological responses often vary considerably across tests which are all ostensibly designed to measure fear (e.g. Forkman et al. 2007).
One potential solution to this problem is to develop a priori hypotheses, based on the conceptual framework outlined above, for the types of event or situation that will generate affective states in each of quadrants Q1–Q4. At a simple level, we can hypothesize that Q1 states occur when a reward is signalled or presented, Q4 states occur when a punisher is signalled or presented, Q3 states occur when a reward is removed or omitted and Q2 states occur when a punisher is removed or omitted (Rolls 2005). Thus, if we can accurately identify species-relevant rewarding and punishing stimuli, we can measure behavioural and physiological responses to their presentation or removal and identify those responses, or response profiles, that occur reliably and hence may be good indicators of the corresponding affective state (e.g. Reefmann et al. 2009).
Of particular relevance to the measurement of free-floating mood states that exist in the absence of specific situations or events, we can use the a priori predictions summarized in table 1 for how expectations of rewarding or punishing events, and related decision-making, are likely to be associated with an organism's position in core affect space. For example, we would predict that individuals that show enhanced expectation of negative events will be in a Q4 state, while individuals that show decreased expectation of positive events (a different type of pessimism) will be in a Q3 state. There is thus the potential to use objective and quantifiable measures of these affect-induced cognitive biases as indicators of the more elusive affective states that influence them. Furthermore, as mentioned previously, mood-dependent cognitive biases are likely to influence appraisals of emotion-inducing stimuli and hence the resulting short-term emotions (e.g. negative cognitive biases may underlie a negative appraisal of an event). Consequently, short-term emotional responses (e.g. behavioural and physiological changes identified as described in the preceding paragraph) may vary in animals exposed to the same event, but experiencing different background mood states, and hence themselves be a useful indicator of underlying mood.
We have recently developed assays of cognitive bias in animals and started to investigate whether manipulations designed to alter affective states (e.g. living in an unpredictable or threatening environment) are linked to cognitive bias in the ways predicted, and as in humans (Paul et al. 2005). Our ‘judgement bias’ task (first developed by Harding et al. 2004) involves training an animal to perform a particular response (e.g. press left lever) when presented with a particular stimulus (e.g. tone A) to obtain a positive outcome (e.g. food), and to perform a different response (e.g. press right lever) when presented with a different stimulus (e.g. tone C) to avoid a relatively negative outcome (e.g. no food; noise). The subject is then presented with intermediate, ambiguous stimuli (e.g. tone B), and we hypothesize that, for example, animals in a positive emotional state (Q1/Q2) will be more likely to judge these stimuli as predicting the better outcome (e.g. by pressing the left lever; an optimistic judgement bias), compared with animals in a negative state (Q3/Q4). Recent studies of species including rats (Harding et al. 2004; Burman et al. 2008, 2009; Enkel et al. 2010), sheep (Doyle et al. 2010), starlings (Bateson & Matheson 2007; Matheson et al. 2008), rhesus monkeys and dogs (see Mendl et al. 2009 for a review) have found evidence in support of these hypotheses, indicating that this new approach for assessing emotional state in animals holds promise.
To date, the studies have not systematically looked for differences in judgement bias that would discriminate between, for example, different types of positive (Q1 versus Q2) or negative (Q3 versus Q4) states, but the approach has the potential to do this. It adds to existing behavioural and physiological measures of animal emotion (e.g. open field; forced swim; sympathetic-adrenal or hypothalamic–pituitary–adrenal activity) and addresses some of the problems with these, including: (i) that they may reflect arousal but not valence (e.g. heart rate and alertness/activity may increase in high-arousal positive and negative situations and hence fail to distinguish Q1 and Q4 states), (ii) a lack of theoretical frameworks and a priori hypotheses for many measures resulting in post hoc interpretations and presenting a barrier to easy transfer of measures between species, (iii) a lack of measures of positive affective states, and (iv) a lack of measures of free-floating mood state owing to a focus on object-based discrete emotions (Paul et al. 2005).
5. Conclusions
Does the conceptual framework outlined here help us to better understand and assess affective states in animals? We believe it does in a number of ways.
The framework is grounded in an understanding of the structure of core subjective affective states in humans. It thus links what we are ultimately interested in but cannot yet investigate directly—subjective affective experience—with biologically relevant and tractable phenomena and concepts. This allows predictions to be made about the behavioural, physiological and cognitive changes that may accompany particular affective states, and hence enables the development of novel measures of these states.
The framework attempts to integrate discrete and dimensional approaches to the study of emotion, providing hypotheses for how they interact in a reciprocal fashion. It illustrates how discrete emotions, sensations and motivations contribute to core affect, and how core affect in turn may influence decision-making, including discrete emotional responses.
The framework brings together all types of affective states. To date, the study of animal emotions has focused largely on Q4 affective states such as fear and anxiety, although there is a substantial literature on the neurobiology of reward processes that underpin Q1 states in particular, and interest in Q3 states such as depression. Q2 states have received very little attention and yet their role in recovery processes and the general well-being of animals may be significant (cf. Porges 2001). Moreover, these different states have generally been studied independently with little reference to each other, or to how they may interact. The framework encourages us to investigate them as a whole and to understand how the animal may move between states according to experience.
The framework emphasizes that affective states are strongly influenced by the organism's environment and its experiences within it. It suggests how environmental events can profoundly alter affective state.
By taking a functional perspective on the structure of core affective states (cf. Nesse 2000, 2004, 2005; Carver 2001), the framework allows us to make predictions about the types of situation that will lead to a particular affective state, and also the sorts of response that may be a good indicator of that affective state. In particular, the framework emphasizes the role of longer term mood states in decision-making processes. We believe that the links between affective and cognitive processes, which have been extensively studied in humans, have an important role to play in furthering our understanding of, and ability to assess, affective states in animals.
Acknowledgements
We thank Richard Parker for discussing these topics with us, Lorenz Gygax and another, anonymous, referee for their comments, and BBSRC and UFAW for supporting our work.
References
- Baars B. J.2001There are no known differences in brain mechanisms of consciousness between humans and other mammals. Anim. Welfare 10, S31–S40 [Google Scholar]
- Barrett L. F.2006Are emotions natural kinds? Perspect. Psychol. Sci. 1, 28–58 (doi:10.1111/j.1745-6916.2006.00003.x) [DOI] [PubMed] [Google Scholar]
- Barrett L. F., Mesquita B., Ochsner K. N., Gross J. J.2007The experience of emotion. Ann. Rev. Psychol. 58, 373–403 (doi:10.1146/annurev.psych.58.110405.085709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M., Matheson S. M.2007Performance on a categorisation task suggests that removal of environmental enrichment induces ‘pessimism' in captive European starlings (Sturnus vulgaris). Anim. Welfare 16, 33–36 [Google Scholar]
- Bechara A., Damasio H., Damasio A. R.2000Emotion, decision-making and the orbitofrontal cortex. Cereb. Cortex 10, 295–307 (doi:10.1093/cercor/10.3.295) [DOI] [PubMed] [Google Scholar]
- Beck A. T.1967Depression, clinical, experimental and theoretical aspects. New York, NY: Harper Row [Google Scholar]
- Beck A. T.2008The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry 165, 969–977 (doi:10.1176/appi.ajp.2008.08050721) [DOI] [PubMed] [Google Scholar]
- Berridge K. C.1996Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 20, 1–25 (doi:10.1016/0149-7634(95)00033-B) [DOI] [PubMed] [Google Scholar]
- Berridge K. C.2003Comparing the emotional brains of humans and other animals. In Handbook of affective sciences (eds Davidson R. J., Scherer K. R., Goldsmith H. H.), pp. 25–51 Oxford, UK: Oxford University Press [Google Scholar]
- Berridge K. C.2007The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology 191, 391–431 (doi:10.1007/s00213-006-0578-x) [DOI] [PubMed] [Google Scholar]
- Boissy A.1995Fear and fearfulness in animals. Q. Rev. Biol. 70, 165–191 (doi:10.1086/418981) [DOI] [PubMed] [Google Scholar]
- Boissy A., et al. 2007Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 92, 375–397 (doi:10.1016/j.physbeh.2007.02.003) [DOI] [PubMed] [Google Scholar]
- Burgdorf J., Panksepp J.2006The neurobiology of positive emotions. Neurosci. Biobehav. Rev. 30, 173–187 (doi:10.1016/j.neubiorev.2005.06.001) [DOI] [PubMed] [Google Scholar]
- Burman O. H. P., Parker R., Paul E. S., Mendl M.2008A spatial judgement task to determine background emotional state in laboratory rats, Rattus norvegicus. Anim. Behav. 76, 801–809 (doi:10.1016/j.anbehav.2008.02.014) [Google Scholar]
- Burman O. H. P., Parker R. M. A., Paul E. S., Mendl M. T.2009Anxiety-induced cognitive bias in non-human animals. Physiol. Behav. 98, 345–350 (doi:10.1016/j.physbeh.2009.06.012) [DOI] [PubMed] [Google Scholar]
- Cabanac M.1992Pleasure—the common currency. J. Theor. Biol. 155, 173–200 (doi:10.1016/S0022-5193(05)80594-6) [DOI] [PubMed] [Google Scholar]
- Cacioppo J. T., Gardner W. L., Berntson G. G.1999The affect system has parallel and integrative processing components: form follows function. J. Pers. Soc. Psychol. 76, 839–855 (doi:10.1037/0022-3514.76.5.839) [Google Scholar]
- Carver C. S.2001Affect and the functional bases of behavior: on the dimensional structure of affective experience. Pers. Soc. Psychol. Rev. 5, 345–356 (doi:10.1207/S15327957PSPR0504_4) [Google Scholar]
- Carver C. S.2003Pleasure as a sign you can attend to something else: placing positive feelings within a general model of affect. Cogn. Emotion 17, 241–261 (doi:10.1080/02699930302294) [DOI] [PubMed] [Google Scholar]
- Corr P. J.2008The reinforcement sensitivity theory of personality. Cambridge, UK: Cambridge University Press [Google Scholar]
- Custers R., Aarts H.2005Positive affect as implicit motivator: on the nonconscious operation of behavioral goals. J. Pers. Soc. Psychol. 89, 129–142 (doi:10.1037/0022-3514.89.2.129) [DOI] [PubMed] [Google Scholar]
- Dalgleish T.2004The emotional brain. Nat. Rev. Neurosci. 5, 582–589 (doi:10.1038/nrn1432) [DOI] [PubMed] [Google Scholar]
- Darwin C.1872/2009The expression of the emotions in man and animals. London, UK: Harper Perennial [Google Scholar]
- Davidson R. J.1994On emotion, mood, and related affective constructs. In The nature of emotion (eds Ekman P., Davidson R. J.), pp. 51–55 New York, NY: Oxford University Press [Google Scholar]
- Davidson R. J.1998Affective style and affective disorders: perspectives from affective neuroscience. Cogn. Emotion 12, 307–330 (doi:10.1080/026999398379628) [Google Scholar]
- Davidson R. J., Irwin W.1999The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 3, 11–21 (doi:10.1016/S1364-6613(98)01265-0) [DOI] [PubMed] [Google Scholar]
- Dawkins R., Krebs J. R.1979Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511 (doi:10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- Desiré L., Boissy A., Veissier I.2002Emotions in farm animals: a new approach to animal welfare in applied ethology. Behav. Proc. 60, 165–180 (doi:10.1016/S0376-6357(02)00081-5) [DOI] [PubMed] [Google Scholar]
- Doyle R. E., Fisher A. D., Hinch G. N., Boissy A., Lee C.2010Release from restraint generates a positive judgement bias in sheep. Appl. Anim. Behav. Sci. 122, 28–34 (doi:10.1016/j.applanim.2009.11.003) [Google Scholar]
- Ekman P.1992Are there basic emotions. Psychol. Rev. 99, 550–553 (doi:10.1037/0033-295X.99.3.550) [DOI] [PubMed] [Google Scholar]
- Ellsworth P. C., Scherer K. E.2003Appraisal processes in emotion. In Handbook of affective sciences (eds Davidson R. J., Scherer K. R., Goldsmith H. H.), pp. 572–595 Oxford, UK: Oxford University Press [Google Scholar]
- Enkel T., Gholizadeh D., von Bohlen und Halbach O., Sanchis-Segura C., Hurlemann R., Spanagel R., Gass P., Vollmayr B.2010Ambiguous-cue interpretation is biased under stress and depression-like states in rats. Neuropsychopharmacology 35, 1008–1015 (doi:10.1038/npp.2009.204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M. W., Payne S., Santos R.2006Anxiety and depression: past, present, and future events. Cogn. Emotion 20, 274–294 (doi:10.1080/02699930500220066) [Google Scholar]
- Forkman B., Boissy A., Meunier-Salauen M. C., Canali E., Jones R. B.2007A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 92, 340–374 (doi:10.1016/j.physbeh.2007.03.016) [DOI] [PubMed] [Google Scholar]
- Frijda N.1994Emotions are functional, most of the time. In The nature of emotion (eds Ekman P., Davidson R. J.), pp. 112–122 New York, NY: Oxford University Press [Google Scholar]
- Grandjean D., Scherer K. R.2008Unpacking the cognitive architecture of emotion processes. Emotion 8, 341–351 (doi:10.1037/1528-3542.8.3.341) [DOI] [PubMed] [Google Scholar]
- Gray J. A.1994Three fundamental emotion systems. In The nature of emotion (eds Ekman P., Davidson R. J.), pp. 243–247 New York, NY: Oxford University Press [Google Scholar]
- Harding E. J., Paul E. S., Mendl M.2004Animal behavior—cognitive bias and affective state. Nature 427, 312 (doi:10.1038/427312a) [DOI] [PubMed] [Google Scholar]
- Haselton M. G., Nettle D.2006The paranoid optimist: an integrative evolutionary model of cognitive biases. Pers. Soc. Psychol. Rev. 10, 47–66 (doi:10.1207/s15327957pspr1001_3) [DOI] [PubMed] [Google Scholar]
- Izard C. E.2007Basic emotions, natural kinds, emotion schemas and a new paradigm. Perspect. Psychol. Sci. 2, 260–280 (doi:10.1111/j.1745-6916.2007.00044.x) [DOI] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., Cuthbert B. N.1990Emotion, attention and the startle reflex. Psychol. Rev. 97, 377–395 (doi:10.1037/0033-295X.97.3.377) [PubMed] [Google Scholar]
- LeDoux J.1996. In The emotional brain. New York, NY: Simon and Schuster [Google Scholar]
- Leknes S., Tracey I.2008Science & society—a common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 9, 314–320 (doi:10.1038/nrn2333) [DOI] [PubMed] [Google Scholar]
- Loewenstein G. F., Weber E. U., Hsee C. K., Welch N.2001Risk as feelings. Psychol. Bull. 127, 267–286 (doi:10.1037/0033-2909.127.2.267) [DOI] [PubMed] [Google Scholar]
- MacLeod A. K., Byrne A.1996Anxiety, depression, and the anticipation of future positive and negative experiences. J. Abnorm. Psychol. 105, 286–289 (doi:10.1037/0021-843X.105.2.286) [DOI] [PubMed] [Google Scholar]
- MacLeod A. K., Salaminiou E.2001Reduced positive future-thinking in depression: cognitive and affective factors. Cogn. Emotion 15, 99–107 (doi:10.1080/0269993004200006) [Google Scholar]
- MacLeod A. K., Tata P., Kentish J., Jacobsen H.1997Retrospective and prospective cognitions in anxiety and depression. Cogn. Emotion 11, 467–479 (doi:10.1080/026999397379881) [Google Scholar]
- Macphail E. M.1998The evolution of consciousness. Oxford, UK: Oxford University Press [Google Scholar]
- Matheson S. M., Asher L., Bateson M.2008Larger, enriched cages are associated with ‘optimistic' response biases in captive European starlings (Sturnus vulgaris). Appl. Anim. Behav. Sci. 109, 374–383 (doi:10.1016/j.applanim.2007.03.007) [Google Scholar]
- McNamara J. M., Houston A. I.1986The common currency for behavioral decisions. Am. Nat. 127, 358–378 (doi:10.1086/284489) [Google Scholar]
- McNaughton N., Corr P. J.2008The neuropsychology of fear and anxiety: a foundation for reinforcement sensitivity theory. In The reinforcement sensitivity theory of personality (ed. Corr P. J.), pp. 44–94 Cambridge, UK: Cambridge University Press [Google Scholar]
- Mendl M., Burman O. H. P., Parker R. M. A., Paul E. S.2009Cognitive bias as an indicator of animal emotion and welfare: emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 118, 161–181 (doi:10.1016/j.applanim.2009.02.023) [Google Scholar]
- Miller K. A., Garner J. P., Mench J. A.2006Is fearfulness a trait that can be measured with behavioural tests? A validation of four fear tests for Japanese quail. Anim. Behav. 71, 1323–1334 (doi:10.1016/j.anbehav.2005.08.018) [Google Scholar]
- Mineka S., Watson D., Clark L. A.1998Comorbidity of anxiety and unipolar mood disorders. Ann. Rev. Psychol. 49, 377–412 (doi:10.1146/annurev.psych.49.1.377) [DOI] [PubMed] [Google Scholar]
- Mogg K., Bradley B. P.1998A cognitive-motivational analysis of anxiety. Behav. Res. Ther. 36, 809–848 (doi:10.1016/S0005-7967(98)00063-1) [DOI] [PubMed] [Google Scholar]
- Nesse R. M.2000Is depression an adaptation? Arch. Gen. Psychiat. 57, 14–20 (doi:10.1001/archpsyc.57.1.14) [DOI] [PubMed] [Google Scholar]
- Nesse R. M.2004Natural selection and the elusiveness of happiness. Phil. Trans. R. Soc. Lond. B 359, 1333–1347 (doi:10.1098/rstb.2004.1511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse R. M.2005Natural selection and the regulation of defenses—a signal detection analysis of the smoke detector principle. Evol. Hum. Behav. 26, 88–105 (doi:10.1016/j.evolhumbehav.2004.08.002) [Google Scholar]
- Nesse R. M., Ellsworth P. C.2009Evolution, emotions, and emotional disorders. Am. Psychol. 64, 129–139 (doi:10.1037/a0013503) [DOI] [PubMed] [Google Scholar]
- Nygren T. E., Isen A. M., Taylor P. J., Dulin J.1996The influence of positive affect on the decision rule in risky situations. Org. Behav. Hum. Decis. Process 66, 79–91 (doi:10.1006/obhd.1996.0038) [Google Scholar]
- Ortony A., Turner T. J.1990What's basic about basic emotions. Psychol. Rev. 97, 315–331 (doi:10.1037/0033-295X.97.3.315) [DOI] [PubMed] [Google Scholar]
- Panksepp J.1998. In Affective neuroscience. The foundations of human and animal emotion. New York, NY: Oxford University Press [Google Scholar]
- Panksepp J.2007Neurologizing the psychology of affects. How appraisal-based constructivism and basic emotion theory can coexist. Perspect. Psychol. Sci 2, 281–296 (doi:10.1111/j.1745-6916.2007.00045.x) [DOI] [PubMed] [Google Scholar]
- Paul E. S., Harding E. J., Mendl M.2005Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci. Biobehav. Rev. 29, 469–491 (doi:10.1016/j.neubiorev.2005.01.002) [DOI] [PubMed] [Google Scholar]
- Porges S. W.2001Is there a major stress system at the periphery other than the adrenals? In Coping with challenge. Welfare in animals including humans (ed. Broom D. M.), pp. 135–149 Berlin, Germany: Dahlem University Press [Google Scholar]
- Posner J., Russell J. A., Peterson B. S.2005The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 17, 715–734 (doi:10.1017/S0954579405050340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz J. J.2004Gut reactions. A perceptual theory of emotion. Oxford, UK: Oxford University Press [Google Scholar]
- Ramos A., Mormede P.1998Stress and emotionality: a multidimensional and genetic approach. Neurosci. Biobehav. Rev. 22, 33–57 (doi:10.1016/S0149-7634(97)00001-8) [DOI] [PubMed] [Google Scholar]
- Reefmann N., Wechsler B., Gygax L.2009Behavioural and physiological assessment of positive and negative emotion in sheep. Anim. Behav. 78, 651–659 (doi:10.1016/j.anbehav.2009.06.015) [Google Scholar]
- Rolls E. T.2005Emotion explained. Oxford, UK: Oxford University Press; (doi:10.1093/acprof:oso/9780198570035.001.0001) [Google Scholar]
- Russell J. A.2003Core affect and the psychological construction of emotion. Psychol. Rev. 110, 145–172 (doi:10.1037/0033-295X.110.1.145) [DOI] [PubMed] [Google Scholar]
- Russell J. A., Barrett L. F.1999Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J. Pers. Soc. Psychol. 76, 805–819 (doi:10.1037/0022-3514.76.5.805) [DOI] [PubMed] [Google Scholar]
- Scherer K. R.2001Appraisal considered as a process of multi-level sequential checking. In Appraisal process in emotion: theory, methods, research (eds Scherer K. R., Schorr A., Johnstone T.), pp. 92–120 Oxford, UK: Oxford University Press [Google Scholar]
- Schwarz N., Clore G. L.1983Mood, misattribution and judgements of well-being: informative and directive functions of affective states. J. Person. Soc. Psychol. 45, 513–523 (doi:10.1098/rstb.2004.1511) [Google Scholar]
- Spruijt B. M., van den Bos R., Pijlman F. T. A.2001A concept of welfare based on reward evaluating mechanisms in the brain: anticipatory behaviour as an indicator for the state of reward systems. Appl. Anim. Behav. Sci. 72, 145–171 (doi:10.1016/S0168-1591(00)00204-5) [DOI] [PubMed] [Google Scholar]
- Stanley D. J., Meyer J. P.2009Two-dimensional affective space: a new approach to orienting the axes. Emotion 9, 214–237 (doi:10.1037/a0014612) [DOI] [PubMed] [Google Scholar]
- Stober J.2000Prospective cognitions in anxiety and depression: replication and methodological extension. Cogn. Emotion 14, 725–729 (doi:10.1080/02699930050117693) [Google Scholar]
- Tellegen A., Watson D., Clark L. A.1999On the dimensional and hierarchical structure of affect. Psychol. Sci. 10, 297–303 (doi:10.1111/1467-9280.00157) [Google Scholar]
- Watson D., Wiese D., Vaidya J., Tellegen A.1999The two general activation systems of affect: structural findings, evolutionary considerations, and psychobiological evidence. J. Pers. Soc. Psychol. 76, 820–838 (doi:10.1037/0022-3514.76.5.820) [Google Scholar]
- Wemelsfelder F.1997The scientific validity of subjective concepts in models of animal welfare. Appl. Anim. Behav. Sci. 53, 75–88 (doi:10.1016/S0168-1591(96)01152-5) [Google Scholar]
- Young E., Korszun A.2009Sex, trauma, stress hormones and depression. Mol. Psychiatry 15, 23–28 (doi:10.1038/mp.2009.94) [DOI] [PubMed] [Google Scholar]
- Zajonc R. B.1980Feeling and thinking—preferences need no inferences. Am. Psychol. 35, 151–175 (doi:10.1037/0003-066X.35.2.151) [Google Scholar]