Abstract
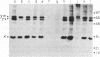
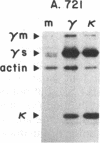
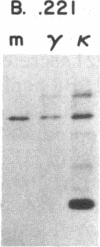
B-lymphoblastoid cell line (LCL) 721.221 lacks HLA-A, -B, and -C class I antigens and transcripts as a result of gamma-ray-induced mutations. LCL 721, from which mutant .221 was derived, produces membrane and secreted forms of IgG1(kappa). In contrast, IgG expression in .221 had these characteristics: (i) gamma 1 heavy chains were diminished by 98% but were detectable with chain-specific antibodies in cell lysates; (ii) kappa light chains were present at normal levels in cell lysates and free kappa chains were secreted; (iii) cell-surface-associated IgG and secreted IgG were absent. Mutants that had partially reduced amounts of class I antigens continued to secrete IgG; however, both the absolute amount of IgG secreted and the relative amount of kappa vs. intact IgG secreted were abnormal in such partially class I-deficient cells. The failure to export IgG and the deficiency of HLA-A, -B, and -C were not merely coincidental in mutant .221, since production of IgG was restored by transferring a functional HLA-A, -B, or -C gene into .221. Cell surface antigen expression of cloned HLA-A, -B, and -C transgenes introduced into .221 was comparable to that of the same genes in their normal chromosomal locations. These observations reveal a relation between production of HLA class I gene products and production of IgG.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankert R. B., Repasky E. A., Mazzaferro P. K., Kubo R. T. Generation and use of an antigen-specific hybrid to study B-cell function. J Mol Cell Immunol. 1987;3(5):279–291. [PubMed] [Google Scholar]
- Bevan M. J. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975 Dec 1;142(6):1349–1364. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham W. J., Pan M. H., Mason B., Ceman S., Sollinger H. W. Induction of antiidiotypic antibodies to donor HLA-A2 following blood transfusions in a highly sensitized HLA-A2+ recipient. Transplantation. 1988 Jun;45(6):1066–1071. doi: 10.1097/00007890-198806000-00013. [DOI] [PubMed] [Google Scholar]
- Clayberger C., Parham P., Rothbard J., Ludwig D. S., Schoolnik G. K., Krensky A. M. HLA-A2 peptides can regulate cytolysis by human allogeneic T lymphocytes. Nature. 1987 Dec 24;330(6150):763–765. doi: 10.1038/330763a0. [DOI] [PubMed] [Google Scholar]
- DeMars R., Rudersdorf R., Chang C., Petersen J., Strandtmann J., Korn N., Sidwell B., Orr H. T. Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8183–8187. doi: 10.1073/pnas.82.23.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due C., Simonsen M., Olsson L. The major histocompatibility complex class I heavy chain as a structural subunit of the human cell membrane insulin receptor: implications for the range of biological functions of histocompatibility antigens. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6007–6011. doi: 10.1073/pnas.83.16.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert T. D., Wacholtz M. C., Davis L. S., Lipsky P. E. Activation of human T4 cells by cross-linking class I MHC molecules. J Immunol. 1988 Apr 1;140(7):2155–2164. [PubMed] [Google Scholar]
- Goverman J., Hunkapiller T., Hood L. A speculative view of the multicomponent nature of T cell antigen recognition. Cell. 1986 May 23;45(4):475–484. doi: 10.1016/0092-8674(86)90279-5. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hendershot L. M., Kearney J. F. A role for human heavy chain binding protein in the developmental regulation of immunoglobin transport. Mol Immunol. 1988 Jun;25(6):585–595. doi: 10.1016/0161-5890(88)90081-8. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Levitt D. Differential regulation of membrane and secretory mu chain synthesis in human beta cell lines. Regulation of membrane mu or secreted mu. J Exp Med. 1982 Dec 1;156(6):1622–1634. doi: 10.1084/jem.156.6.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavathas P., Bach F. H., DeMars R. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4251–4255. doi: 10.1073/pnas.77.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Mazzaferro P. K., Repasky E. A., Black J., Kubo R. T., Bankert R. B. Biochemical and ultrastructural characterization of a novel cell structure associated with immunoglobulin secretion in B-lymphocytes. J Mol Cell Immunol. 1987;3(5):293–306. [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., DeMars R. Class I-like HLA genes map telomeric to the HLA-A2 locus in human cells. Nature. 1983 Apr 7;302(5908):534–536. doi: 10.1038/302534a0. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Rajan T. V., Dick R. F., 2nd, Bluestone J. A. Substitution at residue 227 of H-2 class I molecules abrogates recognition by CD8-dependent, but not CD8-independent, cytotoxic T lymphocytes. Nature. 1989 Jan 5;337(6202):73–75. doi: 10.1038/337073a0. [DOI] [PubMed] [Google Scholar]
- Schmitt K., Däubener W., Bitter-Suermann D., Hadding U. A safe and efficient method for elimination of cell culture mycoplasmas using ciprofloxacin. J Immunol Methods. 1988 Apr 22;109(1):17–25. doi: 10.1016/0022-1759(88)90437-1. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Schlessinger J., Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984 Feb;98(2):725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman R. K., van Rood J. J., Vossen J. M., Schellekens P. T., Feltkamp-Vroom T. M., Doyer E., Gmelig-Meyling F., Visser H. K. Failure of lymphocyte-membrane HLA-A and -B expression in two siblings with combined immunodeficiency. Clin Immunol Immunopathol. 1979 Dec;14(4):418–434. doi: 10.1016/0090-1229(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Sharon M., Gnarra J. R., Baniyash M., Leonard W. J. Possible association between IL-2 receptors and class I HLA molecules on T cells. J Immunol. 1988 Nov 15;141(10):3512–3515. [PubMed] [Google Scholar]
- Shimizu Y., DeMars R. Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur J Immunol. 1989 Mar;19(3):447–451. doi: 10.1002/eji.1830190306. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989 May 1;142(9):3320–3328. [PubMed] [Google Scholar]
- Shimizu Y., Geraghty D. E., Koller B. H., Orr H. T., DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Koller B., Geraghty D., Orr H., Shaw S., Kavathas P., DeMars R. Transfer of cloned human class I major histocompatibility complex genes into HLA mutant human lymphoblastoid cells. Mol Cell Biol. 1986 Apr;6(4):1074–1087. doi: 10.1128/mcb.6.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia R., Neuberger M. S., Milstein C. Regulation of membrane IgM expression in secretory B cells: translational and post-translational events. EMBO J. 1987 Dec 20;6(13):3969–3977. doi: 10.1002/j.1460-2075.1987.tb02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern S. O., Swain S. L., Dutton R. W. Induction of the H-2 D antigen during B cell activation. J Immunol. 1989 Jan 1;142(1):336–342. [PubMed] [Google Scholar]
- Touraine J. L., Betuel H., Souillet G., Jeune M. Combined immunodeficiency disease associated with absence of cell-surface HLA-A and -B antigens. J Pediatr. 1978 Jul;93(1):47–51. doi: 10.1016/s0022-3476(78)80598-8. [DOI] [PubMed] [Google Scholar]
- Wall R., Kuehl M. Biosynthesis and regulation of immunoglobulins. Annu Rev Immunol. 1983;1:393–422. doi: 10.1146/annurev.iy.01.040183.002141. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. H-2 compatability requirement for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. Different cytotoxic T-cell specificities are associated with structures coded for in H-2K or H-2D;. J Exp Med. 1975 Jun 1;141(6):1427–1436. doi: 10.1084/jem.141.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]