Abstract
Mutualisms between reef-building corals and endosymbiotic dinoflagellates are particularly sensitive to environmental stress, yet the ecosystems they construct have endured major oscillations in global climate. During the winter of 2008, an extreme cold-water event occurred in the Gulf of California that bleached corals in the genus Pocillopora harbouring a thermally ‘sensitive’ symbiont, designated Symbiodinium C1b-c, while colonies possessing Symbiodinium D1 were mostly unaffected. Certain bleached colonies recovered quickly while others suffered partial or complete mortality. In most colonies, no appreciable change was observed in the identity of the original symbiont, indicating that these partnerships are stable. During the initial phases of recovery, a third species of symbiont B1Aiptasia, genetically identical to that harboured by the invasive anemone, Aiptasia sp., grew opportunistically and was visible as light-yellow patches on the branch tips of several colonies. However, this symbiont did not persist and was displaced in all cases by C1b-c several months later. Colonies with D1 were abundant at inshore habitats along the continental eastern Pacific, where seasonal turbidity is high relative to offshore islands. Environmental conditions of the central and southern coasts of Mexico were not sufficient to explain the exclusivity of D1 Pocillopora in these regions. It is possible that mass mortalities associated with major thermal disturbances during the 1997–1998 El Niño Southern Oscillation eliminated C1b-c holobionts from these locations. The differential loss of Pocillopora holobionts in response to thermal stress suggests that natural selection on existing variation can cause rapid and significant shifts in the frequency of particular coral–algal partnerships. However, coral populations may take decades to recover following episodes of severe selection, thereby raising considerable uncertainty about the long-term viability of these communities.
Keywords: climate change, coral bleaching, eastern Pacific, holobiont, natural selection, Symbiodinium
1. Introduction
Reef corals and the ecosystems they support have exhibited extreme sensitivity to environmental disturbances produced by changes in global climate (Hoegh-Guldberg 1999; Coles & Brown 2003). Severe or prolonged exposure to abnormally high or low temperatures may cause physiological stress that disrupts the intracellular symbiosis between photosynthetic dinoflagellates in the genus Symbiodinium (symbiont) and reef-building corals (Fitt et al. 2001; Hoegh-Guldberg et al. 2005). For example, increases of a few degrees above normal sea surface temperatures (SSTs) have induced large numbers of symbiotic corals to perish around the world after losing their symbionts (mass bleaching; Glynn 1993; Hoegh-Guldberg 1999). These episodes have a long-lasting effect on the general health and sustainability of coral reef ecosystems (Hughes et al. 2003). Such sensitivity to environmental stress raises questions about how coral symbioses, and the ecosystems they support, were able to persist through major changes in global climate, and consequently how will they respond to the warming trends that are predicted in the coming decades (IPCC 2007).
Reef corals are obligate symbioses constrained by the combined physiological limitations of both partners and as functional units may exhibit greater sensitivity to environmental stressors (Iglesias-Prieto & Trench 1997; Fitt et al. 2001). While morphological and physiological variation within and between coral species can determine life and death responses to environmental stress (Loya et al. 2001; Baird et al. 2009), just as important is the identity of the resident symbiont and its capacity to tolerate thermal stress and/or extreme irradiance (Rowan 2004; Berkelmanns & van Oppen 2006; Warner et al. 2006). Therefore, the resident species of symbiont can significantly influence the longevity of a coral colony exposed to environmental change.
The degree to which symbiotic corals exhibit specificity and/or variability in their symbiotic associations may significantly influence their survival of rising and fluctuating SSTs. The potential for acquisition of a physiologically tolerant symbiont through the differential growth of a resident background population referred to as symbiont ‘shuffling’ (Rowan et al. 1997; Berkelmanns & van Oppen 2006; Jones et al. 2008), or from external environmental sources referred to as symbiont ‘switching’ (Lewis & Coffroth 2004), are thought to constitute mechanisms of rapid physiological change (Baker 2003). However, high specificity and stability may limit the extent to which ‘shuffling' and ‘switching' can occur (Goulet 2006). In this case, existing, or new, partner combinations tolerant of the prevailing environmental conditions may require some amount of time to increase in prevalence and/or evolve as the climate changes.
Few studies have monitored the long-term stability of Symbiodinium populations in a host colony (Goulet & Coffroth 2003; LaJeunesse et al. 2005; Thornhill et al. 2006a,b; Stat et al. 2008) and whether certain host–symbiont combinations persist during, and following, severe stress events (Glynn et al. 2001; Goulet et al. 2008; Jones et al. 2008; LaJeunesse et al. 2009). While numerous bleaching episodes have occurred in recent decades, rarely have colonies been monitored to determine the actual importance of the resident symbiont to the coral's survival (Glynn et al. 2001; Goulet et al. 2008; Jones et al. 2008; Sampayo et al. 2008). For corals exhibiting polymorphic associations with more than one symbiont species, replacement of the dominant symbiont population by a second compatible species can occur when colonies are transplanted to a different environment (Rowan et al. 1997; Baker 2001; Berkelmanns & van Oppen 2006). Observed responses of coral populations to acute warming, however, indicate that differential mortality, more so than symbiont ‘switching' and/or ‘shuffling', causes lasting change in the relative frequency of particular host–symbiont combinations (Sampayo et al. 2008; but see Jones et al. 2008). It remains unknown to what extent natural selection can shift the frequency of stress-tolerant combinations in a large and widely distributed coral population. The potential for broad changes in holobiont composition is of particular importance in regions that are dominated by only a few coral species like the eastern Pacific.
The first reports of large-scale bleaching and mortality were published in the early 1980s and involved coral communities of the eastern Pacific (Glynn 1983, 1993). Among the species most affected were colonies of the ecologically dominant Pocillopora spp. During 1997–1998 El Niño Southern Oscillation (ENSO), patchy distributions of ‘bleached’ white colonies next to ‘healthy’ unbleached colonies were subsequently related to different symbiont species harboured by affected and unaffected colonies (Glynn et al. 2001). Pocillopora spp. harbouring Symbiodinium Clade D from Uva Island in the Gulf of Chiriqui, Panama, appeared to be tolerant of thermal stress, while colonies with Symbiodinium Clade C bleached (Glynn et al. 2001). The measured change in the proportion of colonies harbouring Clade D 4 years later could not be attributed to a particular ‘adaptive' mechanism (i.e. ‘switching', ‘shuffling' or differential survival) cold-water because individual colonies had not been tagged and monitored before and after the ENSO event (Baker et al. 2004). The coexistence in the eastern Pacific of two host–symbiont combinations in populations of an ecologically dominant coral can serve as a model to infer how more complex coral communities elsewhere may respond to changing climate and episodes of environmental stress.
Recently, coral communities in the Gulf of California were subjected to a severe event beginning in February 2008 (figure 1a). Differential bleaching and mortality among Pocillopora spp. at two long-term study sites initiated an investigation to understand why some colonies responded differently and whether symbiont ‘shuffling' or ‘switching' was common during the recovery of affected individuals. The results of this investigation were then related to broader geographic patterns in host–symbiont demographics observed among Pocillopora communities across the eastern Pacific and how the dominance of particular holobiont combinations relates to local environmental conditions (principally temperature and water clarity) and regional differences in mass mortality during the 1997–1998 ENSO event.
Figure 1.
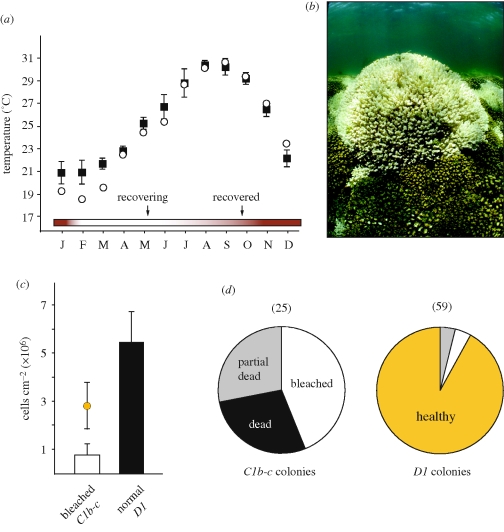
Recent coral ‘bleaching’ and mortality in the Gulf of California caused by low-water temperatures. (a) Average monthly SSTs for the southern Gulf of California between 2003 and 2007 (black squares) relative to temperatures in 2008 (open circles). The brown and white colour shaded bar at the bottom of the graph indicates the period when colonies bleached and recovered, respectively, and arrows indicate approximate sampling times. (b) A large bleached colony of Pocillopora photographed in May 2008 is surrounded by unbleached colonies in the Gulf of California. (c) Average symbiont cell densities in colonies harbouring either Symbiodinium ‘C1b-c’ or ‘D1’ sampled in May 2008. The coloured circle above the mean value calculated for bleached colonies is the average cell density in colonies harbouring ‘C1b-c’ under normal conditions. Error bars represent 1 s.d. (d) Percentage of Pocillopora colonies harbouring either Symbiodinium ‘C1b-c’ or ‘D1’ that were healthy, bleached, partially dead or entirely dead. The numbers in parentheses above the pie graphs indicate the total number of permanently tagged colonies whose symbiont has been monitored since 2006.
2. Material and methods
(a). Oceanographic data
Values for monthly SSTs (°C) and chlorophyll a (mg m−2) between January 2003 and December 2007 were acquired from the Giovanni online data system, developed and maintained by the NASA Goddard Earth Sciences (GES) Data and Information Center (DISC). MODIS/Aqua monthly measurements were averaged over approximately 24 km2 from waters adjacent to sampling sites in the Gulf of California, Banderas Bay, western Gulf of Tehuantepec, Gulf of Panama, San Benedicto Island and Clipperton Atoll.
(b). Surveys of colony condition
The pigmentation and health of 84 tagged colonies distributed along six 25 m transects from two study sites in the southern Gulf of California were evaluated and biopsies taken in mid May 2008. The genetic identity of Symbiodinium in these colonies had been monitored since May 2006 (LaJeunesse et al. 2008). Colonies with normal looking pigmentation were scored as healthy and those with extremely pale or white tissues were designated as bleached. Colonies with greater than 90 per cent mortality were scored as dead, while those with dead regions comprising 10–90% of the colony were defined as having experienced partial mortality. In addition to the 84 colonies, 50 additional colonies were selected that displayed significant paling in May 2008, and used for additional genetic analysis of symbionts following the bleaching event. Branches from 10 ‘healthy’ colonies and 10 ‘bleached’ colonies were collected from the original 84 colonies and used for symbiont cell density calculations. Chlorophyll a fluorescence was recorded with a pulse amplitude modulated fluorometer (Diving PAM, Walz-USA) following acclimation to dim light (less than 5.0 µmol photons m−2 s−1) for 20 min and total darkness for subsequent 5 min.
(c). Calculation of symbiont cell densities
Coral tissue was removed from the skeleton using a WaterPik. Total volume extract was measured and a 1 ml aliquot was removed for five replicate cell counts on a haemacytometer. The bare coral skeletons were dried at room temperature for two weeks and the surface area of each branch was calculated using the hot wax method (Stimson 1997). A standard curve was constructed using squares of Pocillopora skeleton cut to produce surface areas of various sizes. As described above, the wax covering only the natural surface of each square was measured and graphed. From these data, a linear trend line was calculated (R = 0.97) and used in the equation to calculate cells per centimetre squared:
(d). Sample collection for molecular genetic analysis
All material for genetic analyses was preserved in a high salt and 20 per cent DMSO buffer (Seutin et al. 1991). From the Gulf of California, sampling was conducted in mid May 2008 at the start of recovery and late September 2008 when colonies appeared normally pigmented. In May 2008, samples were collected from 25 ‘C1b-c’ and 59 ‘D1’ colonies previously tagged and monitored for symbiont identity since May 2006. At this point in time, an additional 50 well-bleached colonies were sampled in order to identify the repopulating symbiont (bringing the total number of bleached colonies whose recovery was monitored to 75). In late September 2008, colonies that had survived bleaching and recovered were again sampled. Multiple samples (n = 4–5) were collected from several colonies to determine the extent to which the symbiont populations were homogeneous for a particular symbiont species.
The biogeographic data from other eastern Pacific regions were based on the collections made between 2007 and 2009. Colonies of Pocillopora were sampled from the Clipperton Atoll, San Beneticto Island and various locations within Banderas Bay (four sites) in April 2007. Five sites were surveyed in May 2008 from the western Gulf of Tehuantepec, Oaxaca. Two locations were visited and samples collected in the Gulf of Panama in January 2009. In each location, colonies with differing morphologies were sampled along linear transects at depths in the range of 2–10 m. Previous studies indicate that there is no clear relationship between the depth of collection and species of Symbiodinium (C1b-c or D1) harboured by Pocillopora except in extremely shallow habitats (LaJeunesse et al. 2008).
(e). Molecular genetic analyses of symbiont populations
Nucleic acid extractions on Pocillopora symbionts were conducted using a modified Promega Wizard genomic DNA extraction protocol (LaJeunesse et al. 2008). Denaturing gradient gel electrophoresis (DGGE) was used to fingerprint the partial 5.8S and the internal-transcribed spacer (ITS) region 2 (LaJeunesse 2002; LaJeunesse & Pinzón 2007; Sampayo et al. 2009). This region was amplified using a touch-down thermal cycle profile with the primers ‘ITS 2 clamp’ and ‘ITSintfor2’ (LaJeunesse & Trench 2000). PCR products were electrophoresed on denaturing gradient gels (45–80% of 7 M urea and 40% formamide) using a CBScientific system (Del Mar, CA, USA) for 16 h at 115 V. The resulting ITS-DGGE fingerprints were matched with previous analyses of symbionts from eastern Pacific corals (LaJeunesse et al. 2008). In the case where a distinctly different fingerprint was found, the dominant band was excised, re-amplified and directly sequenced.
Three microsatillite loci Si15, Si34 and 4.86 (Santos et al. 2004; Pettay & LaJeunesse 2007) were amplified and the flanker regions sequenced from samples containing Symbiodinium B1 to determine the extent to which these matched with the B1 populations found in the small brown anemone, Aiptasia sp., common to the area.
(f). Statistical analyses
The statistical significance of a particular symbiont in determining the response of a colony to thermal stress was calculated using a Fisher's exact test of independence. This test was also used for comparing the proportions of bleached colonies harbouring a particular symbiont in the initial phase of recovery versus when the symbiont populations in these colonies had recovered four months later. The Kruskal–Wallis test, a non-parametric version of one-way analysis of variance, was used to calculate the significance of cell densities (particularly in colonies harbouring C1b-c) measured in colonies before bleaching with densities in bleached colonies.
3. Results
(a). Spring bleaching event
By late February 2008, numerous Pocillopora colonies in the Gulf of California, near La Paz, Mexico, bleached after regional SSTs decreased from January to March by up to 2°C below historical averages measured by remote-sensing (figure 1a) and by up to 4°C in situ at our sampling locations (data not shown). Colony colour (pigmentation), symbiont cell densities, photosynthetic capacity of photosystem II and mortality were used to evaluate the condition of 84-tagged colonies in May 2008, a month after temperatures returned to normal. The identity of the dominant resident symbiont in these colonies was monitored for 2 years prior to the bleaching event (electronic supplementary material, figure S1a,b; LaJeunesse et al. 2008).
Analysis of active chlorophyll fluorescence by PAM fluorometry indicated no significant change in the maximal quantum yield of photosystem II (Fv/Fm) (Warner et al. 1996) in bleached and unbleached colonies relative to measurements taken during the same season in preceding years (data not shown), indicating that the symbionts in bleached corals had recovered physiologically. One hundred per cent of colonies harbouring Symbiodinium C1b-c were bleached, with Symbiodinium numbers significantly lower relative to densities calculated before bleaching and to colonies harbouring D1 symbionts (figure 1b,c; p < 0.01; Kruskal–Wallis test). Of these C1b-c colonies, 56 per cent suffered partial or total mortality (figure 1d). In contrast, one of the 59 colonies harbouring Symbiodinium D1 before the coldwater episode bleached mildly and one other showed signs of minimal mortality at the tips of its branches, indicating a significant difference in the response to thermal stress by each host–symbiont combination (i.e. holobiont; p = 0.000; Fisher's exact test of independence). Therefore, six months following the start of bleaching (February), mortality in seven out of 25 C1b-c colonies (by September small areas, less than 5%, of living tissue existed in three of these colonies) created nearly a 14 per cent shift in the proportion of tagged D1 colonies relative to C1b-c.
The colonies of other common coral genera in the region (e.g. Porites, Pavona) remained pigmented and were apparently unaffected by the cold temperatures. These corals associate with specific species of Clade C Symbiodinium not harboured by Pocillopora and are also not known to associate with Symbiodinium D1 (LaJeunesse et al. 2008).
(b). Monitoring the recovery phase
The genetic identities of Symbiodinium from 75 recovering Pocillopora colonies (25 of which were monitored since 2006; electronic supplementary material, figure S1a,b) were assessed early in the recovery phase in May 2008 and again in September 2008 when normal pigmentation had returned to each colony (figure 2b). Ultimately, the percentage of colonies that were recovering with C1b-c in May increased slightly from 85 to 90 per cent by September, while 4 per cent of colonies harboured high proportions of both symbiont species (figure 2b). On average, 5 per cent (and sometimes as much as 20%) of colonies at a particular site contain mixtures of D1 and C1b-c populations (LaJeunesse et al. 2008).
Figure 2.
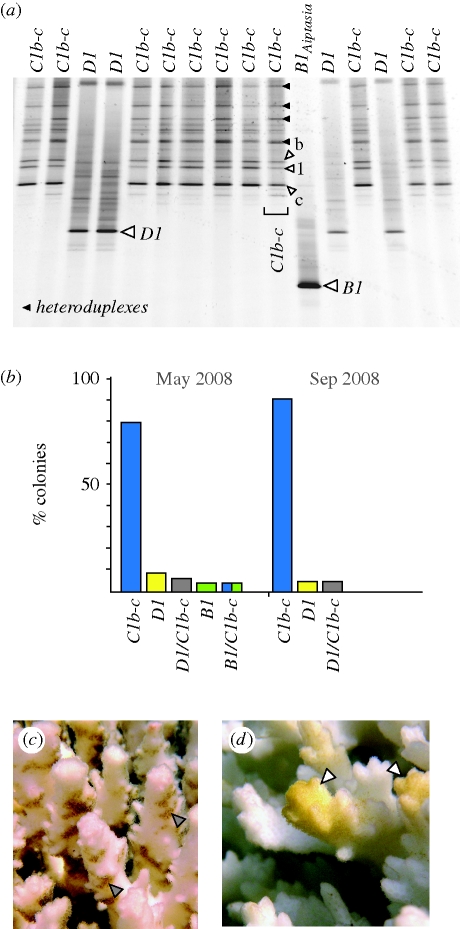
(a) The genetic identification of Symbiodinium in bleached and recovering colonies using ITS-DGGE rDNA fingerprinting identified three Symbiodinium spp., C1b-c, D1 and B1Aiptasia. (b) Proportions of tagged bleached colonies with Symbiodinium C1b-c, D1 and/or B1Aiptasia that were in the early stages of recovery (May 2008), and then four months later (September) when they had recovered completely. (c) Normal patterns of pigmentation in recovering colonies on the sides and bases of branches (grey arrows). (d) The light golden-yellow pigment observed on the apical portion of branches from several unusual colonies containing Symbiodinium B1Aiptasia (white arrows).
Change in symbiont dominance among individual colonies was rare and did not result in appreciable net gains in the frequency of one symbiont over another (p = 0.234, Fisher's exact test of independence; figure 2b). Presumed ‘shuffling’ from Symbiodinium C1b-c (May) to D1 or to a mixture involving both (September) was observed in only five colonies. Likewise, a similar number of colonies (n = 6) with D1 in May subsequently harboured C1b-c or a D1/Clb-c mixture by September (electronic supplementary material, figures S1a,b). Therefore, symbiont ‘shuffling’ in colonies of Pocillopora did not contribute to substantive shifts in the relative frequency of D1 in the Gulf of California.
(c). Observations of unusual symbioses during early recovery
During the early recovery period in May, several bleached colonies possessed light yellow-brown pigmentation located at the tips of branches (electronic supplementary material, figure S1a). This unusual coloration and odd apical location were very different from coloration patterns observed among the majority of recovering colonies (figure 2c versus d). Molecular genetic analyses (ITS-DGGE and microsatellite flanker sequencing) of the symbionts in these branch tips matched it with Symbiodinium B1Aiptasia, harboured normally by the sea anemone Aiptasia sp. In late September, B1Aiptasia was no longer detected in colonies where this symbiont occurred in abundance during the early recovery phase (figure 2b and electronic supplementary material, figure S1a). Quantitative PCR analyses of multiple independent samples from individual colonies (Correa et al. 2009) were unable to detect these symbionts at background levels (data not shown).
(d). Biogeographic patterns of Pocillopora–Symbiodinium holobionts in relation to regional environments and mass mortality during the 1997–1998 ENSO
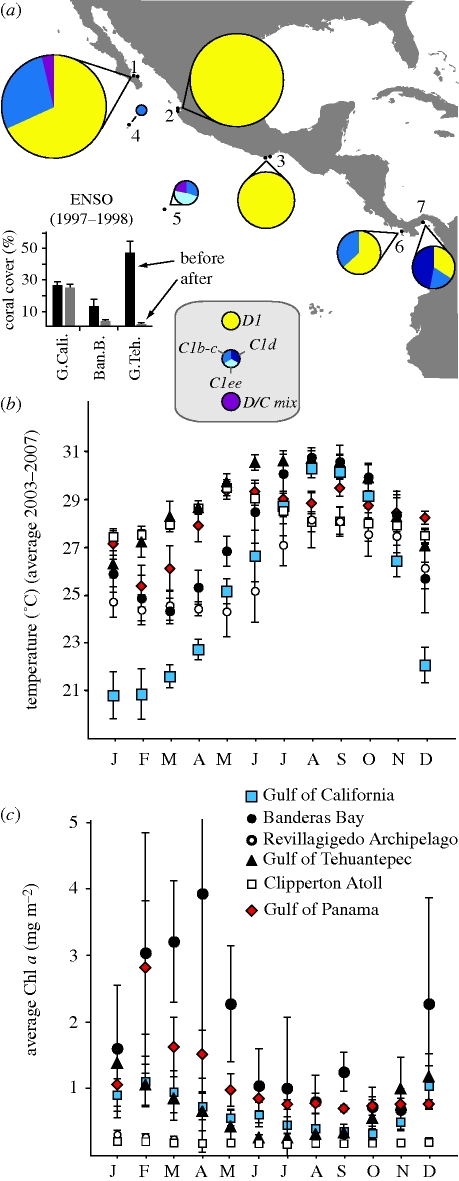
The prevalence of colonies with Symbiodinium D1 differed markedly among communities surveyed throughout the eastern Pacific (figure 3a). The relative frequency of D1 colonies was lowest at the Clipperton Atoll and not detected in the three colonies sampled at San Benedicto Island of the Revillagigedo Archipelago. One hundred per cent of Pocillopora harboured only Symbiodinium D1 at various sites in Banderas Bay and western Gulf of Tehuantepec in Oaxaca. The proportion of colonies with D1 in the Gulf of Panama was lower than was found in previous surveys in the Gulf of Chiriqui, Panama (Glynn et al. 2001). The geographic distribution and prevalence of Symbiodinium D1 does not appear to correspond with average monthly temperatures or seasonal fluctuations (figure 3b), but may relate to conditions of turbidity that differ markedly between inshore and offshore environments (figure 3c). Analysis of average monthly chlorophyll a (turbidity) and temperature from 2003 to 2007 shows marked differences in maximal levels throughout the year across these sites (figure 3b,c).
Figure 3.
(a) Geographic patterns in the relative dominance of colonies with Symbiodinium D1 surveyed a decade after the 1997–1998 ENSO. Analyses of individual Pocillopora from (1) The Gulf of California (n = 201); (2) Banderas Bay (n = 179); (3) western Gulf of Tehuantepec, Oaxaca (n = 82); (4) Revillagigedo Archipelago (n = 3); (5) Clipperton Atoll (n = 21); (6) Gulf of Chiriqui (Uva Island), Panama (n = 41; Baker et al. 2004); and (7) Gulf of Panama (n = 42). Loss of live coral cover was most severe among communities of Pocillopora from central (Banderas Bay) and southern Mexico (Gulf of Tehuantepec) sites during the 1997–1998 ENSO (inset modified from Reyes-Bonilla et al. 2002). The average monthly values (±s.d.) between 2003 and 2007 of SSTs (b) and chlorophyll a concentrations (a proxy for turbidity) for each location based on satellite imaging.
4. Discussion
Symbiont ‘shuffling' and/or ‘switching’ would constitute a rapid and potentially significant physiological change without the significant loss of individual coral colonies. However, following a severe cold-water event in the Gulf of California, an increase in the relative frequency of a stress-tolerant symbiotic partnership occurred essentially through the loss of colonies with stress-sensitive symbionts and not through the replacement of one symbiont by another within surviving colonies (figures 1c and 2b). These data suggest that symbiont ‘shuffling’ or ‘switching’ may not lead to widespread partner recombination as a result of bleaching.
The differential mortality reported by this study is consistent with evidence that most coral colonies maintain long-term stable relationships with specific Symbiodinium sp. even after bleaching (Sampayo et al. 2008; Stat et al. 2009; Thornhill et al. 2009). Indeed, repeated temporal samplings indicate that most partnerships remain stable even when a colony is transplanted to a different habitat and/or experiences stress-induced ‘bleaching’ (Baker 2001; Goulet & Coffroth 2003; Goulet 2006; Thornhill et al. 2006a,b; Jones et al. 2008; Sampayo et al. 2008). Experimental field manipulations have induced a stable replacement of a once dominant symbiont by a second symbiont species (Rowan et al. 1997; Baker 2001; Berkelmanns & van Oppen 2006), but such exchanges involved symbionts known to naturally associate with the particular species of host under study.
Symbiont replacement, when documented, often appears to be induced by physiological stress; for example, following transplantation to a new environment (Berkelmanns & van Oppen 2006; but see Thornhill et al. 2006b). While novel combinations may be induced experimentally (Schoenberg & Trench 1980; Lewis & Coffroth 2004), there is little evidence that such processes readily occur naturally and/or are permanent. The finding of B1Aiptasia in some severely bleached colonies during early recovery shows that physiological stress may cause the opportunistic establishment of a heterologous symbiont (LaJeunesse et al. 2009), even among hosts with closed modes of symbiont acquisition, as the symbionts in these corals are maternally inherited during egg development (vertically transmitted; figure 2d). It has become increasingly common to observe unusual or atypical Symbiodinium in chronically stressed, recently bleached and/or diseased colonies (Toller et al. 2001; Thornhill et al. 2006b; Jones et al. 2008; Sampayo et al. 2008). However, when conditions return to normal, many of these ‘opportunistic’ symbionts do not persist and/or appear to be competitively displaced during or soon after recovery by the homologous symbiont (LaJeunesse et al. 2009). The lack of detectable concentrations of B1Aiptasia among colonies having fully recovered from bleaching, even at background levels (5.0–0.1%), is in agreement with this scenario (figure 2b; quantitative PCR data not shown).
Existing ecological, phylogenetic and population genetic data indicate that Symbiodinium D1 is a unique species in Clade D (LaJeunesse et al. 2008, 2010; T. C. LaJeunesse & D. T. Pettay 2009–2010, unpublished data). In contrast to Symbiodinium trenchi (D1a), D1 is specific to the genus Pocillopora in the Pacific and there is no indication that it can spread opportunistically to other host taxa during episodes of stress (LaJeunesse et al. 2008, 2009). A conventional species characterization of Symbiodinium D1 (assuming this symbiont is culturable) will probably not offer the precision and practicality that genetic data have in delineating closely related species of morphologically cryptic eukaryotic microbes (Adl et al. 2007). The binomial ‘Symbiodinium glynni’ will, therefore, be assigned to this organism and used in future correspondence.
The long-term viability of coral communities in the eastern Pacific is uniquely dependent on the responses of Pocillopora populations to climate change. This is because Pocillopora often constitute greater than 90 per cent of the live coral cover in the eastern Pacific (Glynn & Ault 2000). A biogeographic survey of the eastern Pacific was, therefore, conducted to determine to what extent different environmental conditions and bleaching history have affected the relative dominance of Pocillopora harbouring C1b-c or D1 at various locations.
Temperature and water clarity are extremely variable among locations in the eastern Pacific (Glynn & Ault 2000). While Pocillopora harbouring D1 or C1b-c occurred at all latitudes along the eastern Pacific, their relative ecological dominance at a particular location appeared to be influenced by environmental conditions characteristic of a particular location (figure 3a–c). Symbiodinium D1 was most abundant in regions with comparatively high seasonal turbidity including the Gulf of California, Banderas Bay and Gulf of Panama (figure 3a,c). As a group, Clade D Symbiodinium, when present, are most common in corals living in near-shore turbid environments (Toller et al. 2001; van Oppen et al. 2005; Mostafavi et al. 2007; LaJeunesse et al. 2010). While more sampling is required from offshore islands, C1b-c was most frequent among colonies living at locations where water quality is persistently clear (figure 3a). However, its existence in the Gulf of California indicates that C1b-c is tolerant of the highest seasonal range in temperature found at any of the locations surveyed and can also cope with high seasonal turbidity (figure 3b,c; LaJeunesse et al. 2008). Why were colonies with C1b-c apparently absent from habitats in central and southern Mexico? Annual rainfall in these areas is higher than for the Gulf of California, and this may create episodes of extreme coastal turbidity that is not reported by satellite data. A second possibility relates to historical thermal events in the region that may have fragmented the distribution of Pocillopora C1b-c holobionts.
Hot and cold-water anomalies have impacted eastern Pacific coral communities in recent decades (Glynn 1983; Reyes-Bonilla 2001). For areas in Mexico, SSTs in1997 were considerably warmer and of longer duration than in previous ENSO years (Reyes-Bonilla 2001). Pocillopora colonies harbouring C1b-c may have been eliminated from central and southern Mexico during the mass mortality events that occurred in 1997 and 1998 (figure 3a inset). Populations in these regions experienced mortalities as high as 95 per cent, and live coral cover was reduced from as high as 50 per cent before the event to less than five per cent a year later (Reyes-Bonilla et al. 2002). The 1997–1998 ENSO was far less severe in the Gulf of California and Panama, where Pocillopora communities experienced minimal bleaching and/or mortality (Glynn et al. 2001; Reyes-Bonilla et al. 2002). While high and long-lasting temperatures in 1997 killed most colonies in Banderas Bay, severe reductions in coral cover from Oaxaca were probably the result of unusually cold temperatures attributed to the anti-El Niño (La Niña) that followed in 1998 (figure 3a inset). While it is reasonable to presume that colonies harbouring C1b-c were eliminated from central and southern Mexico a decade ago, data prior to 1997–1998 are needed to substantiate this claim. Regardless, the biogeographic information provided here establishes a ‘baseline’ upon which to evaluate the impact of future thermal disturbances—information that is badly needed for many coral communities throughout the world.
(a). Recognizing units of selection among coral–algal symbioses and their significance in an era of climate change
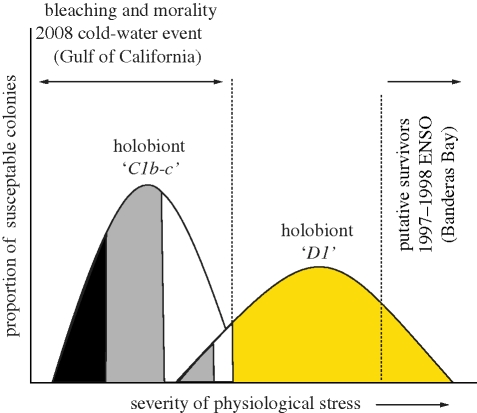
Natural selection acts on variation in a population. While the 2008 cold-water episode differentially affected ‘C1b-c Pocillopora’ holobionts relative to the D1 holobionts in the Gulf of California (figure 1d), colonies harbouring C1b-c responded differently, as some individuals died rapidly while adjacent colonies recovered with no apparent adverse effects. This suggests that genotypic variation among colonies and/or the resident species of symbiont may be critical in the evolutionary response of these symbioses to climate change (Barshis et al. 2010).
Figure 4 depicts a conceptual representation of how natural selection impacts the two populations of Pocillopora harbouring different symbiont species. For each species combination, differences in individual genotypes among host and symbiont likely affect the overall physiological performance (e.g. the weakest genotypic combinations of C1b-c and D1 holobionts suffered partial or total mortality during the cold-water event). More severe episodes of stress, such as prolonged high temperatures associated with the 1997–1998 ENSO likely affected many D1 colonies in Banderas Bay with only the most tolerant host–symbiont genotypic combinations surviving (figure 4). Populations of Symbiodinium D1 in the eastern Pacific comprise numerous recombinant genotypes (i.e. strains) and some may possess physiologies that are significantly different (D. T. Pettay & T. C. LaJeunesse 2009, unpublished data). However, the high mortality has been costly to these populations as live coral cover at sites around Banderas Bay remains well below levels that existed prior to the 1997–1998 ENSO (H. Reyes-Bonilla 2007, personal observation; Reyes-Bonilla et al. 2002). The long-term viability under continued climate change of these populations is, therefore, uncertain.
Figure 4.
Conceptual representation depicting ranges in the stress responses of two host–symbiont combinations (i.e. holobionts). In the winter of 2007–2008, colonies of Pocillopora with C1b-c in the Gulf of California were more sensitive to thermal stress (e.g. cold water) than colonies with D1. Within each combination, different genotypes of host and symbiont probably influence overall physiological performance. The weakest genotypic combinations among ‘C1b-c' and ‘D1' holobionts suffered partial (grey shading) or total mortality (black). Only the most tolerant genotypic combinations might survive severe episodes of physiological stress such as the high and prolonged temperatures experienced in Banderas Bay during the 1997–1998 ENSO.
Many species of reef-building coral are long-lived and have endured major oscillations in temperature over geological time (Budd 2000). The significant warming or cooling of SSTs, which corresponded to the endings and/or beginnings of the last few geological periods, may have initiated major rearrangements in host–symbiont partnerships (LaJeunesse 2005) and explains why many distantly related hosts harbour closely related symbionts and vice versa (Rowan & Powers 1991; LaJeunesse 2005). The process of evolving new and stable partnerships may be relatively sudden on a geological time scale, but still requires spans of time involving thousands and, perhaps, millions of years (LaJeunesse 2005; but see LaJeunesse et al. 2010).
There is considerable uncertainty about the capacity for coral symbioses to evolve over decadal time scales (Maynard et al. 2008). The possibility that rapid evolution, acting over several generations, might increase the thermal tolerance of coral communities through the selection of certain partner combinations involving specific species and/or genotypes of animal and dinoflagellate requires much more attention (Carroll et al. 2007). Coral communities throughout the Indo-Pacific and Atlantic differ markedly in diversity and species composition of hosts (Veron 2000) and symbionts (LaJeunesse 2005; LaJeunesse et al. 2010). While intraspecific variation in host–symbiont combinations is limited at local scales, many host species display a broad diversity of partnerships over wide geographic ranges (e.g. Loh et al. 2001; LaJeunesse et al. 2004, 2010). As SSTs continue to warm, certain regions and coral species may initially fare better than others simply because they possess greater numbers of physiologically tolerant combinations having already evolved in response to pre-existing environmental conditions (e.g. eastern Pacific Pocillopora, LaJeunesse et al. 2008; the Persian Gulf; Mostafavi et al. 2007; Southeast Asia, LaJeunesse et al. 2010). The severity and geographic scope of a single disturbance, or frequency of repeated disturbances, will likely dictate the extent to which differential survival changes the community composition of coral–algal symbioses. However, if conditions continue to worsen as projected (IPCC 2007), substantial coral mortality will ultimately threaten the functional integrity of these ecosystems (Hoegh-Guldberg et al. 2007).
Acknowledgements
We thank Mara Wills for logistical support in La Paz, and Sergio Flores Ramirez and the faculty and staff at the Universidad Autonoma de Baja California Sur Marine Station at Pichilingue. This research was supported by the National Science Foundation (IOB 544854 to T.C.L. and IOB 544765 to M.E.W.), Pennsylvania State University and the University of Delaware.
References
- Adl S. M., et al. 2007Diversity, nomenclature, and taxonomy of Protists. Syst. Biol. 56, 684–689 (doi:10.1080/10635150701494127) [DOI] [PubMed] [Google Scholar]
- Baird H. A., Bhagooli R., Ralph P. J., Takahashi S.2009Coral bleaching: the role of the host. Trends Ecol. Evol. 24, 16–20 (doi:10.1016/j.tree.2008.09.005) [DOI] [PubMed] [Google Scholar]
- Baker A. C.2001Reef corals bleach to survive change. Nature 411, 765–766 (doi:10.1038/35081151) [DOI] [PubMed] [Google Scholar]
- Baker A. C.2003Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34, 661–689 (doi:10.1146/annurev.ecolsys.34.011802.132417) [Google Scholar]
- Baker A. C., Starger C. J., McClanahan T. R., Glynn P. W.2004Corals' adaptive response to climate change. Nature 430, 741 (doi:10.1038/430741a) [DOI] [PubMed] [Google Scholar]
- Barshis D. J., Stillman J. H., Gates R. D., Toonen R. J., Smith L. W., Birkeland C.2010Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Mol. Ecol. 19, 1705–1720 (doi:10.1111/j.1365-294X.2010.04574.x) [DOI] [PubMed] [Google Scholar]
- Berkelmanns R., van Oppen M. J. H.2006Flexible partners in coral symbiosis: a ‘nugget of hope' for coral reefs in an era of climate change. Proc. R. Soc. B 273, 2305–2312 (doi:10.1098/rspb.2006.3567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd A.2000Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs 19, 25–35 (doi:10.1007/s003380050222) [Google Scholar]
- Carroll S. P., Hendry A. P., Reznick D. N., Fox C. W.2007Evolution on ecological time-scales. Funct. Ecol. 21, 387–393 (doi:10.1111/j.1365-2435.2007.01289.x) [Google Scholar]
- Coles S. L., Brown B. E.2003Coral bleaching—capacity for acclimatization and adaptation. Adv. Mar. Biol. 46, 183–223 (doi:10.1016/S0065-2881(03)46004-5) [DOI] [PubMed] [Google Scholar]
- Correa A. M. S., McDonald M. D., Baker A. C.2009Development of clade-specific Symbiodinium primers for quantitative PCR (qPCR) and their application to detecting clade D symbionts in Caribbean corals. Mar. Biol. 156, 2403–2411 (doi:10.1007/s00227-009-1263-5) [Google Scholar]
- Fitt W. K., Brown B. E., Warner M. E., Dunne R. P.2001Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20, 51–65 (doi:10.1007/s003380100146) [Google Scholar]
- Glynn P. W.1983Extensive ‘bleaching’ and death of reef corals on the Pacific coast of Panamá. Environ. Conserv. 10, 149–154 (doi:10.1017/S0376892900012248) [Google Scholar]
- Glynn P. W.1993Coral bleaching: ecological perspectives. Coral Reefs 12, 1–17 (doi:10.1007/BF00303779) [Google Scholar]
- Glynn P. W., Ault J. S.2000A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19, 1–23 (doi:10.1007/s003380050220) [Google Scholar]
- Glynn P. W., Mate J. L., Baker A. C., Calderon M. O.2001Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño-Southern oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull. Mar. Sci. 69, 79–109 [Google Scholar]
- Goulet T. L.2006Most corals may not change their symbionts. Mar. Ecol. Prog. Ser. 321, 1–7 (doi:10.3354/meps321001) [Google Scholar]
- Goulet T. L., Coffroth M. A.2003Stability of an octocoral–algal symbiosis over time and space. Mar. Ecol. Prog. Ser. 250, 117–124 (doi:10.3354/meps250117) [Google Scholar]
- Goulet T. L., LaJeunesse T. C., Fabricius K.2008Symbiont specificity and bleaching susceptibility among soft corals during the 1998 GBR mass coral bleaching event. Mar. Biol. 154, 795–804 (doi:10.1007/s00227-008-0972-5) [Google Scholar]
- Hoegh-Guldberg O.1999Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 50, 839–866 (doi:10.1071/MF99078) [Google Scholar]
- Hoegh-Guldberg O., Fine M., Skirving W., Johnstone R., Dove S., Strong A.2005Coral bleaching following wintry weather. Limnol. Oceanogr. 50, 265–271 [Google Scholar]
- Hoegh-Guldberg O., et al. 2007Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- Hughes T. P., et al. 2003Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (doi:10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- Iglesias-Prieto R., Trench R. K.1997Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll-protein complexes to different photon-flux densities. Mar. Biol. 130, 23–33 (doi:10.1007/s002270050221) [Google Scholar]
- IPCC 2007Climate change synthesis report. Contribution of working groups I, II, and III to the fourth assessment of the Intergovernmental Panel on Climate Change (eds Pachauri R., Reisinger A.), pp. 104 Geneva [Google Scholar]
- Jones A. M., Berkelmans R., van Oppen M. J. H., Mieog J. C., Sinclair W.2008A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc. R. Soc. B 275, 1359–1365 (doi:10.1098/rspb.2008.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse T. C.2002Community structure and diversity of symbiotic dinoflagellate populations from a Caribbean coral reef. Mar. Biol. 141, 387–400 [Google Scholar]
- LaJeunesse T. C.2005‘Species’ radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene–Pliocene transition. Mol. Biol. Evol. 22, 570–581 (doi:10.1093/molbev/msi042) [DOI] [PubMed] [Google Scholar]
- LaJeunesse T. C., Pinzón J.2007Screening intragenomic rDNA for dominant variants can provide a consistent retrieval of evolutionary persistent ITS (rDNA) sequences. Mol. Phylogenet. Evol. 45, 417–422 (doi:10.1016/j.ympev.2007.06.017) [DOI] [PubMed] [Google Scholar]
- LaJeunesse T. C., Trench R. K.2000The biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal anemone, Anthopleura elegantissima (Brandt). Biol. Bull. 199, 126–134 [DOI] [PubMed] [Google Scholar]
- LaJeunesse T. C., Bhagooli R., Hidaka M., deVantier L., Done T. J., Schmidt G. W., Fitt W. K., Hoegh-Guldberg O.2004Closely related Symbiodinium spp. differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Mar. Ecol. Prog. Ser. 284, 147–161 (doi:10.3354/meps284147) [Google Scholar]
- LaJeunesse T. C., Lee S., Bush S., Bruno J. F.2005Persistence of non-Caribbean algal symbionts in Indo-Pacific mushroom corals released to Jamaica 35 years ago. Coral Reefs 24, 157–159 (doi:10.1007/s00338-004-0436-4) [Google Scholar]
- LaJeunesse T. C., Reyes-Bonilla H., Warner M. E., Wills M., Schmidt G. W., Fitt W. K.2008Specificity and stability in high latitude eastern Pacific coral–algal symbioses. Limnol. Oceanogr. 53, 719–727 [Google Scholar]
- LaJeunesse T. C., Finney J. C., Smith R. T., Oxenford H.2009Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’ event. Proc. R. Soc. B 276, 4139–4148 (doi:10.1098/rspb.2009.1405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse T. C., Pettay D. T., Sampayo E. M., Phongsuwan N., Brown B., Obura D., Hoegh-Guldberg O., Fitt W. K.2010Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J. Biogeogr. 37, 785–800 [Google Scholar]
- Lewis C. L., Coffroth M. A.2004The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science 304, 1490–1492 (doi:10.1126/science.1097323) [DOI] [PubMed] [Google Scholar]
- Loh W. K. W., Loi T., Carter D., Hoegh-Guldberg O.2001Genetic variability of the symbiotic dinoflagellates from the wide ranging coral species Seriatopora hystrix and Acropora longicyathus in the Indo-West Pacific. Mar. Ecol. Prog. Ser. 222, 97–107 (doi:10.3354/meps222097) [Google Scholar]
- Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., van Woesik R.2001Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131 (doi:10.1046/j.1461-0248.2001.00203.x) [Google Scholar]
- Maynard J., Baird A. H., Pratchett M.2008Revisiting the Cassandra syndrome; the changing climate of coral reef research. Coral Reefs 27, 745–749 (doi:10.1007/s00338-008-0432-1) [Google Scholar]
- Mostafavi P. G., Fatemi S. M. R., Shahhosseiny M. H., Hoegh-Guldberg O., Loh W. K. W.2007Predominance of clade D Symbiodinium in shallow-water reef-building corals off Kish and Larak Islands (Persian Gulf, Iran). Mar. Biol. 153, 25–34 [Google Scholar]
- Pettay D. T., LaJeunesse T. C.2007Microsatellites from clade B Symbiodinium spp. specialized for Caribbean corals in the genus Madracis. Mol. Ecol. Notes 7, 1271–1274 (doi:10.1111/j.1471-8286.2007.01852.x) [Google Scholar]
- Pettay D. T., LaJeunesse T. C.2009Microsatellite loci for assessing genetic diversity, dispersal and clonality of coral symbionts in ‘stress-tolerant’ clade D Symbiodinium. Mol. Ecol. Res. 9, 1022–1025 [DOI] [PubMed] [Google Scholar]
- Reyes-Bonilla H.2001Effects of the 1997–1998 El Niño-Southern Oscillation on coral communities of the Gulf of California. Bull. Mar. Sci. 69, 251–266 [Google Scholar]
- Reyes-Bonilla H., Carriquiry J. D., Leyte Morales G. E., Cupul Magana A. L.2002Effects of the El Niño-Southern Oscillation and the anti-El Niño event (1997–1999) on coral reefs of the western coast of México. Coral Reefs 21, 368–372 [Google Scholar]
- Rowan R.2004Thermal adaptation in reef coral symbionts. Nature 430, 742 (doi:10.1038/430742a) [DOI] [PubMed] [Google Scholar]
- Rowan R., Powers D. A.1991Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar. Ecol. Prog. Ser. 71, 65–73 (doi:10.3354/meps071065) [Google Scholar]
- Rowan R., Knowlton N., Baker A., Jara J.1997Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388, 265–269 (doi:10.1038/40843) [DOI] [PubMed] [Google Scholar]
- Sampayo E. M., Ridgeway T., Bongaerts P., Hoegh-Gulberg O.2008Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl Acad. Sci. 105, 10 444–10 449 (doi:10.1073/pnas.0708049105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampayo E., Dove S., LaJeunesse T. C.2009Cohesive molecular genetic data delineate species diversity in the dinoflagellate genus Symbiodinium. Mol. Ecol. 18, 500–519 (doi:10.1111/j.1365-294X.2008.04037.x) [DOI] [PubMed] [Google Scholar]
- Santos S. R., Shearer T. L., Hannes A. R., Coffroth M. A.2004Fine-scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium, Dinophyceae) of the Caribbean. Mol. Ecol. 13, 459–469 (doi:10.1046/j.1365-294X.2003.02058.x) [DOI] [PubMed] [Google Scholar]
- Schoenberg D. A., Trench R. K.1980Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbioses with marine invertebrates. III. Specificity and infectivity of Symbiodinium microadriaticum. Proc. R. Soc. Lond. B 207, 445–460 (doi:10.1098/rspb.1980.0033) [Google Scholar]
- Seutin G., White B. N., Boag P. T.1991Preservation of avian blood and tissue samples for DNA analyses. Can. J. Zool. 69, 82–92 (doi:10.1139/z91-013) [Google Scholar]
- Stat M., Loh W. K. W., Hoegh-Guldberg O., Carter D. A.2008Symbiont acquisition strategy drives host–symbiont associations in the southern Great Barrier Reef. Coral Reefs 27, 763–772 (doi:10.1007/s00338-008-0412-5) [Google Scholar]
- Stat M., Loh W. K. W., LaJeunesse T. C., Hoegh-Guldberg O., Carter D. A.2009Coral-endosymbiont stability following a natural bleaching event. Coral Reefs 28, 709–713 [Google Scholar]
- Stimson J.1997The annual cycle of density of zooxanthellae in the tissues of field and laboratory-held Pocillopora damicornis (Linnaeus). J. Exp. Mar. Biol. Ecol. 214, 35–48 (doi:10.1016/S0022-0981(96)02753-0) [Google Scholar]
- Thornhill D. J., Fitt W. K., Schmidt G. W.2006aHighly stable symbioses among western Atlantic brooding corals. Coral Reefs 25, 515–519 (doi:10.1007/s00338-006-0157-y) [Google Scholar]
- Thornhill D. J., LaJeunesse T. C., Kemp D. W., Fitt W. K., Schmidt G. W.2006bMulti-year seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar. Biol. 148, 711–722 (doi:10.1007/s00227-005-0114-2) [Google Scholar]
- Thornhill D., Xiang Y., Fitt W. K., Santos S. R.2009Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. Plos One 4, e6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toller W. W., Rowan R., Knowlton N.2001Repopulation of zooxanthellae in the Caribbean corals Montastraea annularis and M. faveolata following experimental and disease-associated bleaching. Biol. Bull. 201, 360–373 (doi:10.2307/1543614) [DOI] [PubMed] [Google Scholar]
- van Oppen M. J. H., Mahini A. J., Done T. J.2005Geographic distribution of zooxanthella types in three coral species on the Great Barrier Reef sampled after the 2002 bleaching event. Coral Reefs 24, 482–487 [Google Scholar]
- Veron J. E. N.2000Corals of the world, pp. 1382 Townsville: Australian Institute of Marine Science [Google Scholar]
- Warner M. E., Fitt W. K., Schmidt G. W.1996The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ. 19, 291–299 (doi:10.1111/j.1365-3040.1996.tb00251.x) [Google Scholar]
- Warner M. E., LaJeunesse T. C., Robison J. D., Thur R. M.2006The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol. Oceanogr. 51, 1887–1897 [Google Scholar]