Abstract
The relative number of seeds produced by competing species can influence the community structure; yet, traits that influence seed production, such as pollinator attraction and floral colour, have received little attention in community ecology. Here, we analyse floral colour using reflectance spectra that include near-UV and examined the phylogenetic signal of floral colour. We found that coflowering species within communities tended to be more divergent in floral colour than expected by chance. However, coflowering species were not phylogenetically dispersed, in part due to our finding that floral colour is a labile trait with a weak phylogenetic signal. Furthermore, while we found that locally rare and common species exhibited equivalent floral colour distances from their coflowering neighbours, frequent species (those found in more communities) exhibited higher colour distances from their coflowering neighbours. Our findings support recent studies, which have found that (i) plant lineages exhibit frequent floral colour transitions; and (ii) traits that influence local population dynamics contribute to community structure.
Keywords: ecological character displacement, floral colour, phylogenetic community structure, pollinator competition
1. Introduction
Floral colour is thought to play an important role in the attraction of pollinators to patches and may mediate competition or facilitation for pollinators within patches (Levin & Anderson 1970; Ghazoul 2006). Indeed, colour is among the most recognizable signals in the identification of ‘pollination syndromes’ relating to the perception and preference of pollinators (Faegri & van der Pijl 1979; Gumbert et al. 1999; Fenster et al. 2004; Lazaro et al. 2008). For example, blue is typically associated with bee pollination, while red floral colour is related to hummingbird pollination (Faegri & van der Pijl 1979). Whether coflowering species exhibit similar or divergent pollination syndromes has received little attention (but see Armbruster 2002). While a disproportionate number of species with a similar pollination syndrome may indicate the prevalence of a certain pollinator guild (Sargent & Vamosi 2008; Arnold et al. 2009), it may also lead to pollinator competition (Caruso 2000; Botes et al. 2008). Therefore, if pollinators do not exhibit fidelity to a particular species (i.e. are generalized), the neighbouring coflowering community influences the fitness associated with a particular floral trait and can thus potentially influence the evolution of floral traits and pollination syndromes.
Plant species that do not adhere closely to any particular syndrome (i.e. generalists that employ a variety of pollinators) can still be effectively pollinated if pollinators are relatively constant in their attentions to a particular species within a single foraging bout (i.e. they exhibit floral constancy). While floral constancy has been observed to break down when coexisting species have similar floral colours (Chittka et al. 1999), more attention has been paid in community-level studies to traits such as inflorescence height, nectar production, corolla length, corolla width and flower number (Waddington 1979; Petit & Freeman 1995; Caruso 2000; Jordan & Harder 2006; Rodgriguez-Girones & Santamaria 2007). The relative neglect of the role of floral colour may arise due to complexities in including the entire perceptual range of pollinators (300–660 nm, Altshuler 2003). Many previous studies investigating floral colour in natural communities (Weiss 1991; Wilson & Stine 1996; Bosch et al. 1997; Jones & Reithel 2001; Schemske & Bierzychudek 2001; Ishii 2006) have measured colour exclusively with the human perception of colour (e.g. chroma/hue metrics, wavelength range: 420–600 nm) and thus may be categorizing some species as similar when, from a pollinators perspective, they are quite different.
While many plant competition studies examine processes of competition in terms of growth rates (Cahill et al. 2008), the outcome of competition is often determined in subsequent generations by the relative number of viable seeds produced by competing species, which depends on pollination (Heilbuth et al. 2001; Wilson & Harder 2003; Vamosi et al. 2007). Scaling up from these local community dynamics are examinations of how local communities are assembled from regional species pools (Chave 2004; Tilman 2004), which depend upon rates of dispersal and ecological filtering (Myers & Harms 2009) as well as competition (Inouye et al. 1980; Crawley & May 1987; Tilman 1994; Coomes & Grubb 2003). If local assemblages are non-random collections of species from the regional species phylogeny (Webb 2000; Cavender-Bares et al. 2004), we may infer that species coexistence depends upon traits that are evolving at varying rates upon the phylogeny. Because these community phylogenetic analyses typically examine larger time scales (Swenson et al. 2006), many previous studies have operated under the implicit assumption that immigration events of every generation are effectively summed and species are not dispersal-limited (i.e. some seeds of every species should arrive at every local site over periods of thousands of years). Over ecological time scales however, several generations of competition can occur within a local neighbourhood—for instance, most meadow herbs disperse the majority (99%) of their seeds less than or equal to 15 m from the parental plant (Vittoz & Engler 2007). Therefore, community assemblages are the net result of processes happening at different time scales, composed of species exhibiting inherited traits that were locally successful in previous generations (Kembel 2009).
Despite the reduction in seed set that may occur from interspecific pollen transfer, many coexisting species benefit from pollinator sharing by increasing the visitation rate of pollinators (Feldman et al. 2004; Moeller 2004; Ghazoul 2006; Sargent & Ackerly 2008). If increasing the visitation rate outweighs the costs of any interference from interspecific pollen transfer (Morales & Traveset 2008), then floral traits within communities should be, on average, more similar than chance would predict. Furthermore, whether floral characters are important determinants of competition or facilitation of pollination may be affected by the local abundance of a population (Spigler & Chang 2008). The abundance or density of a species within a community directly determines the amount of conspecific pollen that is available for pollination (Spigler & Chang 2008). Rare species will receive more interspecific pollen owing to the relative abundance of coflowering species and therefore may only persist in a community if they exhibit increased divergence in floral traits than do common species (Ishihama et al. 2006). Recent models (Sargent & Otto 2006) and field studies (Gumbert et al. 1999; Spigler & Chang 2008) indicate that rare species are under more pressure to have divergent floral traits and to evolve specialist pollination systems than are common species. Conversely, plant–pollinator network studies have documented that rare species are pollinated by generalist or super-generalist pollinators (Bascompte et al. 2006; Vazquez et al. 2007; Petanidou et al. 2008). If this were generally true, we may expect that rare species will show less divergence in floral colour than their neighbours, as they likely share pollinators with at least one attractive common species (Feldman et al. 2004).
The degree that floral colour determines phylogenetic community structure depends to some degree on the lability of the trait in question (Cavender-Bares et al. 2004; Kraft et al. 2007; Kembel 2009). A previous investigation of the phylogenetic distribution of colour in Iochroma (Smith et al. 2008) found that floral traits such as brightness, chroma and hue were distributed randomly with respect to phylogeny indicating that these traits are highly labile. Since floral colour acts as a cue for pollinators (Weiss 1991) and is not directly responsible for the placement of pollen, as are other floral parts, floral colour may be relatively less constrained (Whittal et al. 2006). Other studies, however, have found floral colour to be constrained (Chittka 1997), possibly when pigments have dual roles in pollinator attraction and herbivory defence (Chittka et al. 2001). Depending on the level of sympatry between related species, there may be selection for divergence of floral colour among close relatives to attract different pollinators, or promote pollinator constancy, without sacrificing adaptive floral morphology traits (Armbruster et al. 1999; Ashman & Majetic 2006; Smith & Rausher 2008). The rapid divergence in a trait between closely related species may erase any signal for phylogenetic divergence in communities (Brooks & McLennan 1991; Losos 1996; Sargent & Ackerly 2008; Cavender-Bares et al. 2009).
Considering the potential importance of floral colour in promoting pollinator fidelity and angiosperm diversification, we examined the mean divergence in terms of floral colour and relatedness in tandem to investigate whether trait or phylogenetic similarity more keenly influenced inclusion within flowering communities. We examined whether coflowering communities exhibit (i) convergent or divergent floral colour distributions when compared with randomly assembled communities (ii) phylogenetic clustering or evenness, (iii) patterns of convergence or divergence that depend on the abundance or frequency of individual species. Finally, we examine the strength of phylogenetic signal in floral colour, while converting floral colour to a reflectance spectrum metric that incorporates the wider range of wavelengths observed by potential pollinators. This joint examination of the influence and lability of floral colour, may provide insight into how coflowering communities are assembled and how variation in communities may affect the diversification and evolution of angiosperms.
2. Material and methods
(a). Study areas, sampling and reflectance readings
Five subalpine meadows in the Kananaskis Region near Calgary, Alberta, Canada were selected for survey and sampling during the months of July and August 2008. Each study site was an open dry meadow habitat dominated by grasses and surrounded by mixed Picea spp., Pinus spp., Pseudotsuga menziesii, and Populusspp. forest. Each meadow was situated on a south-facing slope. The approximate area of the open habitats ranged from 1.3 to 4 km2, and the elevations between sites ranged from 1500 to 1700 metres. Within each study site, a sampling area of approximately 75 m2 in size was set up for identification and collection of specimens. All coflowering species within the sampling area were identified and their relative abundance measured. For species with less than 30 individuals in the study area, individual plants were numbered and five randomly selected plants had single flowers removed. For species with greater than or equal to 30 individuals in the study area, single flowers were haphazardly sampled from separate individuals. Variation in floral colour spectra within species and between habitats was low.
Per cent transmission of flower petals was obtained with an Ocean Optics USB 2000 spectrometer, an Ocean Optics DH-2000 Deuterium Tungsten Halogen light source and a BaSO4 white standard for every 0.22 nm between 300 and 700 nm wavelengths. The light source, spectrometer probe and petals were covered prior to readings to prevent ambient light from entering the readings. Reflectance readings were obtained from the dominant colour pattern of flower petals (or showy bracts) after a scan of the petals with the spectrometer for differing petal colour patterns undetectable by the human eye from 300 to 700 nm wavelength. When flowers were too small to obtain a reading, multiple flowers from the same individual were placed overlapping each other to facilitate measurement of reflectance. The mean per cent reflectance from 300 to 700 nm for the flowers of each species was subtracted from the raw per cent reflectance values to correct for brightness and allow for a comparison of spectral quality differences (colour) between species (Cuthill et al. 1999).
(b). Statistical analyses
The analysis of divergence or convergence of colour traits within communities was assessed in a similar manner as an assessment of phylogenetic community structure (Webb 2000) to compare whether phylogenetic distance generates a good approximation of traits perceived to be important in competition. Statistical analyses were conducted using the general functions from R Statistical Software (R Development Core Team 2008) along with specialized functions of the picante package (Kembel et al. 2010). The raw reflectance values from all 44 species found flowering in the five communities were used to generate a Euclidean distance matrix between species, which was then used in hierarchical cluster analysis to generate a cluster dendrogram representing the regional colour distribution (similar to the functional diversity (FD) dendrograms used in other studies (Petchey & Gaston 2007; Cadotte et al. 2009)). We then compared the mean pair-wise distance within each community to the colour distribution of 10 000 randomly assembled communities from the regional species pool (using the ‘phylogeny shuffle’ option). Fisher's combined probability test was applied to these results to test for an overall tendency of divergence or convergence of floral colour within communities. We then performed the same technique to assess the mean phylogenetic distance of coflowering communities (Webb 2000), this time using a regional phylogeny constructed with all species found in all communities (44 species in total). Phylogenetic data were obtained from Phylomatic (Webb & Donoghue 2005) and from the angiosperm phylogeny website (Stevens 2001 onwards).
The abundance of plants in each community was classified into three categories: low, medium or high abundance. Abundances of less than or equal to 10 individuals were classified as having low abundance, greater than 10 but less than 50 individuals per species were classified as having medium abundance, and greater than or equal to 50 individuals were classified as having high abundance. High abundance species were later classified together with medium abundance species in a common species category, as there were not enough high abundance species to conduct contrasts. For each community the reflectance values at every 10 nm from the flowers of each species were reduced to the first component from principal components analysis (PC1). PC1 was then used as the responding variable to test if rare species tend to reflect in different parts of the spectrum than common species in their communities. Each community was analysed separately to maintain independence of data points, as many species were present in more than one community. Fisher's combined probability test was conducted on the community-specific results to detect an overarching relationship between abundance and floral colour divergence. Because there was little variation in floral colour of any given species between meadows, mean PC1 score for a species was calculated as well to perform a phylogenetic GLM between floral colour reflectance PC1 and abundance using the regional phylogeny of all species combined.
We also examined whether mean trait and phylogenetic divergence depending upon abundance using the icomdist function in Phylocom (Webb et al. 2008) that provides a matrix of the pair-wise branch length between species in each community. From this matrix, we calculated a mean distance from all other coflowering neighbours for every species. We ran this function on the colour dendrogram (estimating mean colour distance) and the species phylogeny (estimating mean phylogenetic distance) and examined whether abundance (categorized here as ‘rare’ or ‘common’) was an important factor determining mean trait and/or phylogenetic divergence. Finally, we also examined whether mean trait and species divergence was higher or lower in frequent species (species that appeared in more communities). With the last procedure, we accounted for differences in species richness between communities (that may affect mean pair-wise distances (MPD)) by standardizing all distance metrics; i.e. subtracting the mean distance of the null communities and dividing by the standard deviation (e.g. -(MPD-MPDnull)/SD(MPDnull)), effectively turning each distance metric into a metric analogous to the dimensionless net relatedness index values, described in Webb (2000) and Kembel & Hubbell (2006). With the community-wide MPD values, we also tested for a relationship between mean overall tree balance and frequency.
To test the hypothesis that floral colour is an evolutionarily labile trait, the first and second principal component score (PC1, PC2) for each species was analysed by calculating the maximum-likelihood estimated λ (Pagel 1999; Freckleton et al. 2002) and examining whether it was significantly different from 0 or 1 using a phylogenetic glm procedure kindly provided by R. Freckleton for R Statistical Software (R Development Core Team 2008). We also examined whether local abundance and frequency exhibited a phylogenetic signal by the same method and performed phylogeny-corrected GLM of rarity versus PC1.
3. Results
Communities consisted of 11–21 coflowering species. In total, we found 44 species flowering in our five subalpine meadows of Kananaskis, Alberta in July and August 2008 (figure 1), with floral colour ranging from greenish white to bright purple (according to human perceptive abilities). Principal component analysis on floral colour revealed that colour could be adequately described with the first two principal components (PC1: 69.3% of the variance; PC2: 19.6% of the variance). Within each study site, the variance that the first principal component explained ranged from 70.2 to 90.1 per cent.
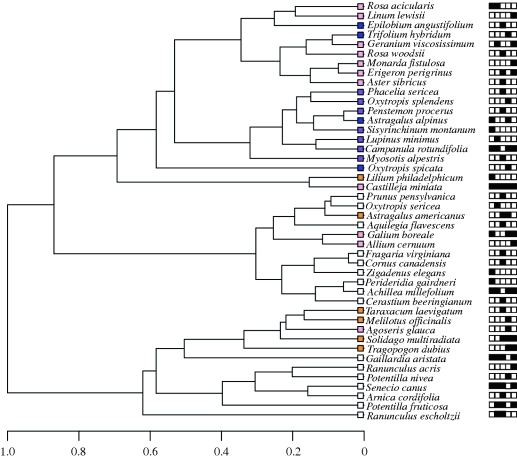
Figure 1.
Hierarchical cluster dendrogram based on the reflectance spectra for all 44 flowering species found in the five communities representing the regional colour distribution used in the analysis, showing general clustering according to the human visual spectrum. Coloured squares represent the PC1 scores of the species, matched approximately to our human perception of colour. PC1 separates white/yellow flowers from those reflecting increasing amounts of purple–UV wavelengths and represented approximately 72% of the variance in colour reflectance. PC1 scores matched approximately to our human perception of colour unless the species reflected UV wavelengths. Black squares represent the community presence matrix for all sites 1–5 (order key for squares: 1st = BHM, 2nd =HJN, 3rd = MCA, 4th =MTA and 5th = SBM).
(a). Trait structuring in subalpine meadow communities
Coflowering species had divergent reflectance floral colour spectra in four of five communities, and convergent reflectance spectra in one community (table 1) when compared with random colour distributions drawn from the regional colour pool (figure 1). One community had species with significantly divergent reflectance spectra, while other communities exhibited marginally significant divergence or no significant divergence from randomly assembled communities (table 1). However, the combined probability of floral colour distributions within communities was significantly more divergent than randomly assembled communities (Fisher's combined probability test: χ2 = 24.67, d.f. = 10, p = 0.006).
Table 1.
Summary statistics for the standard effect size of the mean pair-wise distance between species to test for divergence (high p-value) or clustering (low p-value) of colour within communities when compared with the regional colour distribution (using the ‘phylogeny shuffle’ randomization technique).
| study site | species richness | mean pair-wise distance (MPD) | random MPD | p-value |
|---|---|---|---|---|
| Bighorn Meadows | 13 | 1.528 | 1.547 ± 0.076 | 0.316 |
| Highwood Junction | 11 | 1.666 | 1.546 ± 0.089 | 0.985 |
| Mist Creek | 21 | 1.603 | 1.547 ± 0.046 | 0.935 |
| Mt. Allan | 14 | 1.617 | 1.548 ± 0.070 | 0.878 |
| Sibbald Meadows | 15 | 1.624 | 1.547 ± 0.065 | 0.946 |
(b). Phylogenetic structuring in subalpine meadow communities
There were trends towards phylogenetic clustering of coflowering communities in four of five communities, and phylogenetic dispersion in one community (table 2). Phylogenetic clustering was significant in one community, while the other four coflowering communities were not significantly different from random. Overall, there was significant phylogenetic clustering of coflowering communities (Fisher's combined probability test: χ2 = 23.54, d.f. = 10, p = 0.009). The opposite patterns found in community structure between floral colour traits and phylogeny can occur because of the frequent phylogenetic transitions observed in floral colour (no phylogenetic signal for either PC1 or PC2, electronic supplementary material, table S1).
Table 2.
Summary statistics for the standard effect size of the mean pair-wise phylogenetic distance between species to test for divergence (high p-value) or clustering (low p-value) of phylogenetic relationships of coflowering communities when compared with the regional distribution of species (using the ‘phylogeny shuffle’ randomization technique).
| study site | species richness | mean pair-wise distance (MPD) | random MPD | p-value |
|---|---|---|---|---|
| Bighorn Meadows | 13 | 1.57 | 1.526 ± 0.095 | 0.669 |
| Highwood Junction | 11 | 1.46 | 1.528 ± 0.107 | 0.272 |
| Mist Creek | 21 | 1.47 | 1.527 ± 0.062 | 0.197 |
| Mt. Allan | 14 | 1.25 | 1.527 ± 0.089 | 0.001 |
| Sibbald Meadows | 15 | 1.46 | 1.526 ± 0.084 | 0.216 |
(c). Floral colour and abundance
There was a significant relationship between local abundance and floral colour in three of five study sites, with rare species exhibiting higher PC1 scores for floral colour (i.e. they were more purple) than common species (electronic supplementary material, table S2). Overall, rare species had significantly higher PC1 scores for floral colour than common ones (Fisher's combined probability test: χ2 = 29.36, d.f. = 10, p = 0.001). Examining the relationship between rarity and PC1 in all communities combined in a phylogenetically corrected analysis, we still find that rare species have higher PC1 scores (F1,42 = 7.26; p = 0.01; figure 2).
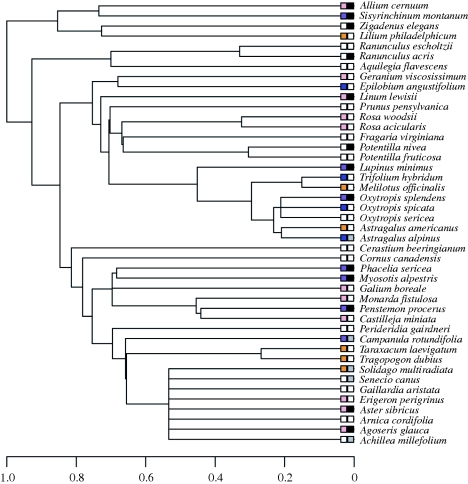
Figure 2.
Regional phylogeny of species found in the five subalpine meadows. Boxes show values for PC1 of floral colour (exhibiting little phylogenetic signal (see electronic supplementary material, table S1)), displayed with the best match possible with human perception (as in figure 1). Note that PC1 separated UV, purple, blue, yellow and white colours very well but did not separate pink or red from blue or yellow (as PC2 did). Species that were rare (dark squares) tended to have higher PC1 scores than other species in the community (i.e. had spectral reflectance tending towards the purple/UV end of the spectrum versus the white/yellow end). Grey squares indicate species that were observed to be locally rare in some communities and abundant in others.
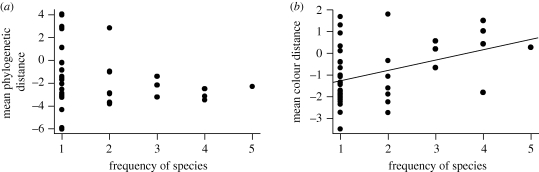
Rare species exhibited no significant difference from common species in floral colour divergence from coflowering neighbours (electronic supplementary material, figure S1; all communities pooled; F1,42 = 0.41; p = 0.52, or in relatedness (all communities pooled; F1,42 = 0.41; p = 0.52). However, the more frequent a species was, the more it was consistently divergent in colour from its neighbours (F2,42 = 7.78, p = 0.008; figure 3b), although there was no relationship between frequency and phylogenetic distance to neighbours (F2,42 = 0.98, p = 0.33; figure 3a). We found no evidence to suggest that frequent species were drawn from more imbalanced local phylogenies than infrequent species (mean phylodistance of the communities did not change with increasing frequency; F2,42 = 0.2882; p = 0.60). Removing the four species (Achillea millefolium, Astragalus alpinus, Campanula rotundifolia and Solidago multiradiata) that were not consistently rare or common, we found a weak positive relationship between local abundance and frequency of occurrence (F1,38 = 3.79; p = 0.047), but no phylogenetic signal for either trait (electronic supplementary material, table S1).
Figure 3.
Relationship between frequency of occurrence (measured as the number of communities a species was found in) and (a) standardized mean phylogenetic distance mean or (b) standardized colour distance from coflowering neighbours.
4. Discussion
While alpine meadow communities are noted for their vibrant displays of disparate colours, the colours are only moderately more divergent than expected by chance, even when the spectra are examined without the limitations of human perception. We find a consistent general tendency for floral colour dispersion, although it was weak in all but one community, lending some support for the hypothesis that communities are structured to some degree by floral colour. The divergence of floral colour suggests that visual cues have a role in distinguishing flowers in the community, functioning to attract broad categories of dominant pollinators (e.g. bird versus bee) as well as eliciting constancy within pollinator classes (Wilson & Stine 1996; Gegear 2005). Floral colour diversity may thus provide a good approximate representation of the FD in pollinator resources of the community (Petchey & Gaston 2007). Furthermore, our alpine meadows maintained colour dispersion despite showing an opposing tendency for phylogenetic clustering (close relatives coexisting more often by chance). The lack of concordance between trait and phylogenetic community structuring is due to the remarkable evolutionary flexibility in floral colour (little to no phylogenetic signal). Admittedly, our phylogeny was not completely resolved (e.g. a large polytomy remains among Asteraceae), which may have decreased our ability to detect a phylogenetic signal. Nevertheless, the lability of floral colour may represent a ‘line of least evolutionary resistance’ for character displacement compared with morphological changes, such as evolution of differing floral shape or symmetry (Whittal et al. 2006). Similar arguments have been made for phenological changes (Gleeson 1981), a transition that may be more common in areas with longer flowering seasons.
We observed substantial variation in the degree of floral colour dispersion. One contribution to this variation can be attributed to the differences in results between frequent and infrequent species. When using either the ‘independent swap’ (Gotelli & Entsminger 2003) or ‘trial swap’ (Miklos & Podani 2004) routines that constrain the null distributions to maintain species frequencies to be the same as observed communities, we found that the degree of colour divergence decreased (data not shown). Species that were very frequently observed in meadow communities (e.g. Castilleja miniata) were, on average, more colour dispersed from their neighbours than are less frequently observed species. This provides some evidence for the ‘Narcissus effect’ that arguably makes controlling for frequencies statistically overconservative when a trait (such as floral colour) plays roles in determining frequency and community structure (Colwell & Winkler 1984; Kembel & Hubbell 2006). We venture that the evolution into ‘rare colour space’ may be advantageous and contributes to the success of species such as Castilleja miniata (Webb & Pitman 2002). Admittedly, the unusual coloration of some of the alpine's more widespread species could be circumstantial and more research into this pattern is warranted.
Another avenue of colour divergence variation comes from the degree of phylogenetic divergence. Our communities exhibited slight tendencies for phylogenetic clustering much like other studies in plants and animals (Webb 2000; Cavender-Bares et al. 2009; Vamosi et al. 2009). While we were not expecting to find evidence of ecological sorting in our communities because all species in our study were tolerant of subalpine environments, we find a tendency for an inverse relationship between floral colour pattern and coflowering phylogenetic community structure (tables 1 and 2). The divergence of floral colour between closely related species may provide an explanation for how closely related angiosperms coexist in phylogenetically clustered communities (Kraft et al. 2007; Vamosi et al. 2009) and is similar to the chemical defence overdispersion observed in previous studies (Becerra 2007). The converse may also be true: processes that affect phylogenetic community structure will undoubtedly affect selection on floral colour from local pollinator competition. It may be that floral colour divergence is selected only when communities are phylogenetically clustered, thus the processes that affect phylogenetic community structure may alter the context of floral colour evolution.
The lack of any phylogenetic signal in floral colour when considering the whole spectrum supports the notion that floral colour is an evolutionarily labile trait, consistent with previous studies that have found frequent colour changes with flowering plant clades (Beardsley et al. 2003; Smith et al. 2008). Furthermore, the absence of phylogenetic signal suggests that colour cues of various pollination syndromes (e.g. bee-, bird-, fly-pollinated) are generally not as well conserved as the pollinator syndromes themselves (Sargent & Vamosi 2008). While floral colour has been associated with pollination syndromes (Faegri & van der Pijl 1979), we should note that all our species were insect-visited to some degree. While some red–orange species were present (Castilleja miniata, Lilium philadelphicum) and this colour is associated with bird pollination, evidence suggests that insects regularly visit these species. We posit that divergent colours within communities do not simply divide the community among the various pollinating orders (bees, flies, moths, birds) but rather operate more commonly to elicit pollinator constancy within pollinator classes.
The tendency for locally rare species to reflect different floral colours than the average of all the other colours reflected by common species in the community (rare species tended to have purple/UV flowers (or high values of PC1)) may at first seem to support the notion that rare species are more divergent in floral colour when compared with common species (Gumbert et al. 1999; Sargent & Otto 2006). However, rare species actually tended to be closer in colour space to their neighbours than common species, though this trend was not significant. Thus, even though rare species tend to be purple, they are present in communities where at least one other common species is also purple. This finding would support the claim of facilitation in pollination more than competition (Feldman et al. 2004). Other studies have found that white/yellow flowers are visited by fewer species (i.e. are more specialized), while purple flowers are more generalized in alpine environments (Lazaro et al. 2008). With rare species being more purple/blue, our results may then indicate that these rare, purple-flowered species benefit from sharing generalist pollinators with common purple species, despite experiencing high amounts of heterospecific pollen (Sargent & Otto 2006; Spigler & Chang 2008). While recent studies have indicated a role of mutualisms in phylogenetically structured extinction cascades (Rezende et al. 2007), common species may act as magnets for pollinators of rare species; a pattern that should result in stabilizing forces maintaining diversity within communities.
In summary, our subalpine meadows showed moderate tendencies for phylogenetic community clustering in rough agreement with past studies (Vamosi et al. 2009). However, because floral colour was so labile, the likelihood that pollinators transfer pollen between two close relatives is reduced, increasing the probability of coexistence. From this perspective, it seems that floral colour may be similar to behavioural traits that have been observed to be labile in animal lineages (Blomberg et al. 2003) and would be designated as an alpha-trait (a trait that determines within-habitat alpha-diversity) in the recent phylogenetic community structure literature (Silvertown et al. 2006; Emerson & Gillespie 2008). The lack of phylogenetic signal in floral colour may be indicative that character displacement has occurred (Levin 1985) and further study performing detailed examinations of the fitness of a variable plant species flowering in different flowering communities would help decipher the relative frequencies of character displacement versus competitive exclusion. Furthermore, our observations on abundance appear to indicate that rare species may tolerate poor-quality visits (those with a high-proportion of heterospecific pollen) in exchange for increased total number of visits. While such a facilitating relationship may be stable, we envision that character displacement away from common colours may be associated with increases in abundance, a process that would result in correlations between abundance and species richness (Webb & Pitman 2002). Rapid transitions in traits such as rarity and floral colour are indicative of how abundance and traits are central to our understanding of the importance of phylogeny in community ecology and should be important components of future studies aimed at investigating the interaction between community assembly and adaptation.
Acknowledgements
The authors thank E. M. Schuett and C. M. Hepp for their field assistance, S. M. Vamosi for statistical advice and R. Freckleton for providing us with the pglm R routine, and two anonymous reviewers for their insightful suggestions. This work was funded by a University of Calgary Starter Grant and NSERC Discovery Grant to J.C.V.
References
- Altshuler D. J.2003Flower color, hummingbird pollination, and habitat irradiance in four neotropical forests. Biotropica 35, 344–355 [Google Scholar]
- Armbruster W. S.2002Can indirect selection and genetic context contribute to trait diversification? A transition-probability study of blossom-colour evolution in two genera. J. Evol. Biol. 15, 468–486 (doi:10.1046/j.1420-9101.2002.00399.x) [Google Scholar]
- Armbruster W. S., Di Stilio V. S., Tuxill J. D., Flores T. C., Velasquez Runk J. L.1999Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a re-evaluation of Berg's correlation-pleiades concept. Am. J. Bot. 86, 39–55 (doi:10.2307/2656953) [PubMed] [Google Scholar]
- Arnold S. E. J., Savolainen V., Chittka L.2009Flower colours along an alpine altitude gradient, seen through the eyes of fly and bee pollinators. Arthropd Plant Interac. 3, 27–43 (doi:10.1007/s11829-009-9056-9) [Google Scholar]
- Ashman T.-L., Majetic C. J.2006Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96, 343–352 (doi:10.1038/sj.hdy.6800815) [DOI] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Olesen J. M.2006Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433 (doi:10.1126/science.1123412) [DOI] [PubMed] [Google Scholar]
- Beardsley P. M., Yen A., Olmstead R. G.2003AFLP phylogeny of Mimulus section Erythranthe and the evolution of hummingbird pollination. Evolution 57, 1397–1410 [DOI] [PubMed] [Google Scholar]
- Becerra J. X.2007The impact of herbivore–plant coevolution on plant community structure. Proc. Natl Acad. Sci. USA 104, 7483–7488 (doi:10.1073/pnas.0608253104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg S. P., Garland T., Jr, Ives A. R.2003Testing for phylogenetic signal in comparitive data: behavioural traits are more labile. Evolution 57, 717–745 [DOI] [PubMed] [Google Scholar]
- Bosch J., Retana J., Cerda X.1997Flowering phenology, floral traits and pollinator composition in a herbaceous mediterranean plant community. Oecologia 109, 583–591 (doi:10.1007/s004420050120) [DOI] [PubMed] [Google Scholar]
- Botes C., Johnson S. D., Cowling R. M.2008Coexistence of succulent tree aloes: partitioning of bird pollinators by floral traits and flowering phenology. Oikos 117, 875–882 (doi:10.1111/j.0030-1299.2008.16391.x) [Google Scholar]
- Brooks D. R., McLennan D. A.1991Phylogeny, ecology, and behavior: a research program in comparative biology. Chicago, IL: University of Chicago Press [Google Scholar]
- Cadotte M. W., Cavender-Bares J., Tilman D., Oakley T. H.2009Using phylogenetic, functional and trait diversity to understand patterns of plant community productivity. PLoS ONE 4, e5695 (doi:10.1371/journal.pone.0005695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J. F., Kembel S. W., Lamb E. G., Keddy P. A.2008Does phylogenetic relatedness influence the strength of competition among vascular plants? Perspect. Plant Ecol. Evol. Syst. 10, 41–50 [Google Scholar]
- Caruso C. M.2000Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution 54, 1546–1557 [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J., Ackerly D., Baum D. A., Bazzaz F. A.2004Phylogenetic overdispersion in Floridean oak communities. Am. Nat. 163, 823–843 (doi:10.1086/386375) [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J., Kozak K., Fine P., Kembel S.2009The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 693–715 (doi:10.1111/j.1461-0248.2009.01314.x) [DOI] [PubMed] [Google Scholar]
- Chave J.2004Neutral theory and community ecology. Ecol. Lett. 7, 241–253 (doi:10.1111/j.1461-0248.2003.00566.x) [Google Scholar]
- Chittka L.1997Bee color vision is optimal for coding flower colors, but flower colors are not optimal for being coded—why? Israel J. Plant Sci. 45, 115–127 [Google Scholar]
- Chittka L., Thomson J. D., Waser N. M.1999Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86, 361–377 (doi:10.1007/s001140050636) [Google Scholar]
- Chittka L., Spaethe J., Schmidt A., Hickelsberger A.2001Adaptation, constraint, and chance in the evolution of flower color and pollinator color vision. In Cognitive ecology of pollination (eds Chittka L., Thomson J. D.), pp. 106–126 Cambridge, UK: Cambridge University Press [Google Scholar]
- Colwell R. K., Winkler D. W.1984A null model for null models in biogeography. In Ecological communities: conceptual issues and the evidence. (eds Strong D. R., Simberloff D., Abele L. G., Thistle A. B.), pp. 344–359 Princeton, NJ: Princeton University Press [Google Scholar]
- Coomes D. A., Grubb P. J.2003Colonization, tolerance, competition, and seed-size variation within functional groups. Trends Ecol. Evol. 18, 283–291 (doi:10.1016/S0169-5347(03)00072-7) [Google Scholar]
- Crawley M. J., May R. M.1987Population dynamics and plant community structure: competition between annuals and perennials. J. Theor. Biol. 125, 475–489 (doi:10.1016/S0022-5193(87)80215-1) [Google Scholar]
- Cuthill I. C., Bennett A. T. D., Partridge J. C., Maier E. J.1999Plumage reflectance and the objective assessment of avian sexual dichromatism. Am. Nat. 160, 183–200 [DOI] [PubMed] [Google Scholar]
- Emerson B. C., Gillespie R. G.2008Phylogenetic approaches to community assembly and structure over space and time. Trends Ecol. Evol. 23, 619–630 (doi:10.1016/j.tree.2008.07.005) [DOI] [PubMed] [Google Scholar]
- Faegri K., van der Pijl L.1979The principles of pollination ecology. Oxford, UK: Pergamon Press [Google Scholar]
- Feldman T. S., Morris W. F., Wilson W. G.2004When can two plant species facilitate each other's pollination? Oikos 105, 197–207 [Google Scholar]
- Fenster C. B., Armbruster W. S., Wilson P., Thomson J. D., Dudash M. R.2004Pollination syndromes and floral specialization. Ann. Rev. Ecol. Evol. Syst. 35, 375–403 (doi:10.1146/annurev.ecolsys.34.011802.132347) [Google Scholar]
- Freckleton R. P., Harvey P. H., Pagel M.2002Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- Gegear R. J.2005Multicomponent floral signals elicit selective foraging in bumblebees. Naturwissenschaften 92, 269–271 (doi:10.1007/s00114-005-0621-5) [DOI] [PubMed] [Google Scholar]
- Ghazoul J.2006Floral diversity and the facilitation of pollination. J. Ecol. 94, 295–304 (doi:10.1111/j.1365-2745.2006.01098.x) [Google Scholar]
- Gleeson S. K.1981Character displacement in flowering phenologies. Oecologia 51, 294–295 (doi:10.1007/BF00540618) [DOI] [PubMed] [Google Scholar]
- Gotelli N., Entsminger G.2003Swap algorithms in null model analysis. Ecology 84, 532–535 (doi:10.1890/0012-9658(2003)084[0532:SAINMA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Gumbert A., Kunze J., Chittka L.1999Floral colour diversity in plant communities, bee colour space and a null model. Proc. R. Soc. Lond. B 266, 1711–1716 (doi:10.1098/rspb.1999.0836) [Google Scholar]
- Heilbuth J. C., Ilves K. L., Otto S. P.2001The consequences of dioecy for seed dispersal: modeling the seed-shadow handicap. Evolution 55, 880–888 (doi:10.1554/0014-3820(2001)055[0880:TCODFS]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Inouye R. S., Byers G. S., Brown J. H.1980Effects of predation and competition on survivorship, fecundity, and community structure of desert annuals. Ecology 61, 1344–1351 (doi:10.2307/1939043) [Google Scholar]
- Ishihama F., Ueno S., Tsumura Y., Washitami I.2006Effects of density and floral morph on pollen flow and seed reproduction of an endangered heterosylous herb, Primula sieboldii. J. Ecol. 94, 646–855 [Google Scholar]
- Ishii H. S.2006Floral display size influences subsequent plant choice by bumblebees. Funct. Ecol. 20, 233–238 (doi:10.1111/j.1365-2435.2006.01106.x) [Google Scholar]
- Jones K. N., Reithel J. S.2001Pollinator-mediated selection on a flower colour polymorphism in experimental populations of Antirrhinum (Scrophulariaceae). Am. J. Bot. 88, 447–454 (doi:10.2307/2657109) [PubMed] [Google Scholar]
- Jordan C. Y., Harder L. D.2006Manipulation of bee behaviour by inflorescence architecture and its consequences for plant mating. Am. Nat. 167, 496–509 (doi:10.1086/501142) [DOI] [PubMed] [Google Scholar]
- Kembel S. W.2009Disentangling niche and neutral influences on community assembly: assessing the performance of community phylogenetic structure tests. Ecol. Lett. 12, 949–960 (doi:10.1111/j.1461-0248.2009.01354.x) [DOI] [PubMed] [Google Scholar]
- Kembel S., Hubbell S. P.2006The phylogenetic structure of a neotropical forest tree community. Ecology 87, 86–99 (doi:10.1890/0012-9658(2006)87[86:TPSOAN]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Kembel S., Ackerly D., Blomberg S., Cowan P., Helmus M., Webb C.2010Picante: tools for integrating phylogenies and ecology. Bioinformatics 0, btq166v1–btq166 [DOI] [PubMed] [Google Scholar]
- Kraft N. J. B., Cornwell W. K., Webb C. O., Ackerly D. D.2007Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am. Nat. 170, 271–283 (doi:10.1086/519400) [DOI] [PubMed] [Google Scholar]
- Lazaro A., Hegland S. J., Totland O.2008The relationships between floral traits and specificity of pollination systems in three Scandinavian plant communities. Oecologia 157, 249–257 (doi:10.1007/s00442-008-1066-2) [DOI] [PubMed] [Google Scholar]
- Levin D. A.1985Reproductive character displacement in Phlox. Evolution 39, 1275–1281 (doi:10.2307/2408784) [DOI] [PubMed] [Google Scholar]
- Levin D. A., Anderson W. W.1970Competition for pollinators between simultaneously flowering species. Am. Nat. 104, 455–467 (doi:10.1086/282680) [Google Scholar]
- Losos J. B.1996Phylogenetic perspective on community ecology. Ecology 77, 1344–1354 (doi:10.2307/2265532) [Google Scholar]
- Miklos I., Podani J.2004Randomization of presence-absence matrices: comments and new algorithms. Ecology 85, 86–92 (doi:10.1890/03-0101) [Google Scholar]
- Moeller D. A.2004Facilitative interactions among plants via shared pollinators. Ecology 85, 3289–3301 (doi:10.1890/03-0810) [Google Scholar]
- Morales C. L., Traveset A.2008Interspecific pollen transfer: magnitude, prevalence, and consequences for plant fitness. Crit. Rev. Plant Sci. 27, 221–238 (doi:10.1080/07352680802205631) [Google Scholar]
- Myers J. A., Harms K. E.2009Seed arrival, ecological filters, and plant species richness: a meta-analysis. Ecol. Lett. 12, 1250–1260 (doi:10.1111/j.1461-0248.2009.01373.x) [DOI] [PubMed] [Google Scholar]
- Pagel M.1999The maximum likelihood approach to reconstruction ancestral character states of discrete characters on phylogenies. Syst. Biol. 48, 612–622 [Google Scholar]
- Petanidou T., Kallimanis A. S., Tzanopoulous J., Sgardelis S. P., Pantis J. D.2008Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure, and implications for estimates of specialization. Ecol. Lett. 11, 564–575 (doi:10.1111/j.1461-0248.2008.01170.x) [DOI] [PubMed] [Google Scholar]
- Petchey O. L., Gaston K. G.2007Dendrograms and measuring functional diversity. Oikos 116, 1422–1426 (doi:10.1111/j.0030-1299.2007.15894.x) [Google Scholar]
- Petit S., Freeman E.1995Nectar production of two sympatric species of columnar cacti. Biotropica 29, 175–183 (doi:10.1111/j.1744-7429.1997.tb00022.x) [Google Scholar]
- R Development Core Team 2008R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Rezende E. L., Lavabre J. E., Guimaraes P. R., Jordano P., Bascompte J.2007Non-random coextinctions in phylogenetically structured mutualistic networks. Nature 448, 925–928 (doi:10.1038/nature05956) [DOI] [PubMed] [Google Scholar]
- Rodgriguez-Girones M. A., Santamaria L.2007Resource competition, character displacement, and the evolution of deep corolla tubes. Am. Nat. 170, 455–464 [DOI] [PubMed] [Google Scholar]
- Sargent R. D., Ackerly D. D.2008Plant–pollinator interactions and the assembly of plant communities. Trends Ecol. Evol. 23, 123–130 [DOI] [PubMed] [Google Scholar]
- Sargent R. D., Otto S. P.2006The role of local species abundance in the evolution of pollinator attraction in flowering plants. Am. Nat. 167, 67–80 (doi:10.1086/498433) [DOI] [PubMed] [Google Scholar]
- Sargent R. D., Vamosi J. C.2008The influence of canopy position, pollinator syndrome, and region on evolutionary transitions in pollinator guild size. Int. J. Plant. Sci. 169, 39–47 (doi:10.1086/523359) [Google Scholar]
- Schemske D. W., Bierzychudek P.2001Perspective: evolution of flower color in the desert annual Linanthus parryae Wright revised. Evolution 55, 1269–1282 [DOI] [PubMed] [Google Scholar]
- Silvertown J., McConway K., Gowing D., Dodd M., Fay M. F., Joseph J. A., Dolphin K.2006Absence of phylogenetic signal in the niche structure of meadow plant communities. Proc. R. Soc. B 273, 39–44 (doi:10.1098/rspb.2005.3288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Rausher M. D.2008Selection for character displacement is constrained by the genetic architecture of floral traits in the ivyleaf morning glory. Evolution 62, 2829–2841 (doi:10.1111/j.1558-5646.2008.00494.x) [DOI] [PubMed] [Google Scholar]
- Smith S. D., Ane C., Baum D. A.2008The role of pollinator shifts in the floral diversification of Iochroma (Solanaceae). Evolution 62, 1–14 [DOI] [PubMed] [Google Scholar]
- Spigler R. B., Chang S. M.2008Effects of plant abundance on reproductive success in the biennial Sabita angularis (Gentianaceae): spatial scale matters. J. Ecol. 96, 323–333 (doi:10.1111/j.1365-2745.2007.01335.x) [Google Scholar]
- Stevens P. F.2001onwards. Angiosperm phylogeny website, version 9, 2008. See http://www.mobot.org/MOBOT/research/APweb/ [Google Scholar]
- Swenson N. G., Enquist B. J., Pither J., Thompson J., Zimmerman J. K.2006The problem and promise of scale dependency in community phylogenetics. Ecology 87, 2418–2424 (doi:10.1890/0012-9658(2006)87[2418:TPAPOS]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Tilman D.1994Competition and biodiversity in spatially structured habitats. Ecology 75, 2–16 (doi:10.2307/1939377) [Google Scholar]
- Tilman D.2004Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc. Natl Acad. Sci. USA 101, 10 854–10 861 (doi:10.1073/pnas.0403458101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamosi J. C., Zhang Y., Wilson W. G.2007Animal dispersal dynamics promoting dioecy over hermaphroditic. Am. Nat. 170, 485–491 (doi:10.1086/519856) [DOI] [PubMed] [Google Scholar]
- Vamosi S. M., Heard S. B., Vamosi J. C., Webb C. O.2009Emerging patterns in the comparative analysis of phylogenetic community structure. Mol. Ecol. 18, 572–592 (doi:10.1111/j.1365-294X.2008.04001.x) [DOI] [PubMed] [Google Scholar]
- Vazquez D. P., Melian C. J., Williams N. M., Bluthgen N., Krasnov B. R., Poulin R.2007Species abundance and asymmetric interaction strength in ecological networks. Oikos 116, 1120–1127 (doi:10.1111/j.0030-1299.2007.15828.x) [Google Scholar]
- Vittoz P., Engler R.2007Seed dispersal distances: a typology based on dispersal modes and plant traits. Bot. Helv. 117, 109–124 (doi:10.1007/s00035-007-0797-8) [Google Scholar]
- Waddington K. D.1979Divergence in inflorescence height: an evolutionary response to pollinator fidelity. Oecologia 40, 43–50 (doi:10.1007/BF00388809) [DOI] [PubMed] [Google Scholar]
- Webb C. O.2000Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 156, 145–155 (doi:10.1086/303378) [DOI] [PubMed] [Google Scholar]
- Webb C. O., Donoghue M. J.2005Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes 5, 181 See http://www.phylodiversity.net/phylomatic/. (doi:10.1111/j.1471-8286.2004.00829.x) [Google Scholar]
- Webb C. O., Pitman N. C. A.2002Phylogenetic balance and ecological evenness. Syst. Biol. 51, 898–907 (doi:10.1080/10635150290102609) [DOI] [PubMed] [Google Scholar]
- Webb C. O., Ackerly D., Kembel S.2008Software for the analysis of community phylogenetic structure and character evolution, version 4.0.1. See http://www.phylodiversity.net/phylocom [DOI] [PubMed] [Google Scholar]
- Weiss M. R.1991Floral colour changes as cues for pollinators. Nature 354, 227–229 (doi:10.1038/354227a0) [Google Scholar]
- Whittal J. B., Voelckel C., Kliebenstein D. J., Hodges S. A.2006Convergence, constraint and the role of gene expression during adaptive radiation: floral anthocyanins in Aquilegia. Mol. Ecol. 15, 4645–4657 (doi:10.1111/j.1365-294X.2006.03114.x) [DOI] [PubMed] [Google Scholar]
- Wilson W. G., Harder L. D.2003Reproductive uncertainty and the relative competitiveness of simultaneous hermaphroditism versus dioecy. Am. Nat. 162, 220–241 (doi:10.1086/376584) [DOI] [PubMed] [Google Scholar]
- Wilson P., Stine M.1996Floral constancy in bumble bees: handling efficiency or perceptual conditioning? Oecologia 106, 493–499 (doi:10.1007/BF00329707) [DOI] [PubMed] [Google Scholar]