Abstract
Theory suggests that habitat structure and population density profoundly influence the phenotypic development of animals. Here, we predicted that reduced rearing density and increased structural complexity promote food search ability, anti-predator response and the ability to forage on novel prey, all behavioural skills important for surviving in the wild. Brown trout were reared at three densities (conventional hatchery density, a fourth of conventional hatchery density and natural density) in tanks with or without structure. Treatment effects on behaviour were studied on trout fry and parr, whereupon 20 trout from each of the six treatment groups were released in an enclosed natural stream and recaptured after 36 days. Fry reared at natural density were faster to find prey in a maze. Moreover, parr reared at natural density were faster to eat novel prey, and showed more efficient anti-predator behaviour than fish reared at higher densities. Furthermore, parr reared at reduced densities were twice as likely to survive in the stream as trout reared at high density. In contrast, we found no clear treatment effects of structure. These novel results suggest that reduced rearing densities can facilitate the development of behavioural life skills in captive animals, thereby increasing their contribution to natural production.
Keywords: density, foraging, anti-predator behaviour, survival, hatchery supplementation
1. Introduction
Behavioural flexibility allows animals to respond more rapidly to environmental changes and should therefore confer fitness advantages when shifting to new habitats (Sol et al. 2002; Watters & Meehan 2007; Sutter & Kawecki 2009). Poor shifts between captive and natural habitats is a key problem explaining the failure of many reintroduction and supplementation programmes, as the captive environment often does little to prepare individuals for transition to the wild (Griffin et al. 2000; Brown & Day 2002; Brännäs & Johnsson 2008). It is therefore of critical importance to understand how early life experience influences learning and behavioural development (Brown & Day 2002). Recently, researchers have outlined various ways to prepare captive animals for release (reviewed by Brown & Laland 2001; Griffin 2004). However, attempts to train animals, for example to avoid predators or to forage on live prey, have yielded inconsistent results (e.g. Brown et al. 2003; Vilhunen & Hirvonen 2003; Hawkins et al. 2008), and it has been proposed that the ability to respond adequately to new information may be more important than trained specific skills, which often are context-dependent (Braithwaite & Salvanes 2005). Such general behavioural flexibility may be enhanced by enrichment of the captive environment.
The ability to use spatial information to find shelters (Markel 1994) or food patches (Noda 2004) is directly related to fitness. It is well established that spatial complexity stimulates behavioural flexibility, as well as learning and memory in mammals and birds (Young 2003). Recent studies on fish suggest similar effects, where structural enrichment in hatchery tanks have been found to improve foraging performance (Brown et al. 2003), stability of social hierarchies (Galhardo et al. 2008), exploratory behaviour (Braithwaite & Salvanes 2005; Lee & Berejikian 2008), learning (Odling-Smee & Braithwaite 2003; Odling-Smee et al. 2006), memory (Brydges et al. 2008) and neural development (Kihslinger & Nevitt 2006). However, it is still unclear to what extent structural complexity can help captive reared individuals to survive in the wild (Brockmark et al. 2007).
The behavioural development of animals may also be influenced by rearing density. First, the potential for developing recognition-based social systems is likely reduced in a crowded environment (Cubitt et al. 2008), as the ability to recognize specific individuals should decrease with increasing group size owing to cognitive constraints, i.e. the theory of limited attention (reviewed by Dukas 2002). Moreover, resource defence theory predicts that the cost of territorial defence should increase as the density of competitors increases (Grant 1997). Thus, there is an expectation that territoriality ceases at very high densities.
We recently showed that high conventional hatchery densities restrain the ability to cope with social interactions, including the ability to defend contested resources, with subsequent effects of reduced growth and survival in a natural stream section (Brockmark & Johnsson 2010). Increased density may also impair individual learning by inducing sensory overload and/or altering the trade-off between reliance on public and private information (Laland 2004), where high density may facilitate schooling (Chapman et al. 2008) at the cost of a reduced ability to learn individual skills (Sumpter et al. 2008; Ward et al. 2008). These possibilities are addressed in the present study.
Salmonid species are commonly used in hatchery programmes aimed to supplement or restore declining wild populations (Levin et al. 2001). However, hatcheries generally keep fish at high densities in plain rearing tanks, where they receive plenty of nutritious pellets, so that there is no need for hatchery fish to actively search for food. Thus, the hatchery environment may deprive the juvenile trout of the specific stimulation necessarily for the development of life skills important in the wild. While some recent studies have investigated the effects of structural stimuli on behaviour (Berejikian et al. 2000, 2001; Brown et al. 2003; Braithwaite & Salvanes 2005; Salvanes & Braithwaite 2005; Lee & Berejikian 2008), none has explored density-related effects on the development of individual behavioural skills of captive animals aimed for release.
The model species for this study, anadromous brown trout (Salmo trutta), is frequently used in hatchery programmes. Wild anadromous brown trout spend their first years in freshwater streams, where they compete to monopolize feeding territories or form hierarchical groups depending on population density and habitat structure (Elliott 1994). Young trout are opportunistic feeders (Bridcut & Giller 1995), and preferably use riffles areas with substrate of pebbles or gravel (Haury et al. 1999). Anadromous brown trout migrate to sea in spring at the age of 1–3 years, and normally return to spawn in their native stream after 1–2 years at sea (Elliott 1994).
Based on the theories discussed above, we test the predictions that reduced rearing density and increased structural complexity in conventional hatchery tanks promote the development of adaptive behaviour, resulting in increased post-release survival in the wild. Brown trout were reared from egg stage at three densities in tanks with or without in-water structure. Trout from these six treatment groups were tested for foraging skills, habitat preference, anti-predator behaviour, and growth and survival in the wild.
2. Material and methods
(a). Experimental fish
We used offspring of sea-ranched anadromous brown trout (Salmo trutta) originating from the River Dalälven (for strain information see Johnsson et al. 1996). Trout were artificially fertilized at the Swedish board of Fisheries in Älvkarleby on 31 October 2006, when eggs from 11 females were fertilized by milt from 11 males. In early January 2007, eggs were transported to the research station of the Swedish Board of Fisheries in Kälarne where the experiment was conducted. The eggs were incubated in hatchery trays flowed with lake water (8 l min−1, 1.9°C).
On 26 March, eyed eggs were randomly assigned between the treatment hatchery tanks (1 × 1 m2). In the structural treatment, henceforth called (S), we added a submerged structure consisting of seven dark green plastic bags (17 l). Each bag was sliced up to resemble water plants and provided with a stone ballast to keep it in place. The bags provided protective shelter for the fish and more heterogeneous water flow dynamics compared with the barren tanks. In addition, visual contact with conspecifics was reduced. The structure reduced visibility of the bottom from above by 50–70%. Fish were reared at three densities: conventional hatchery density (according to local practice, H: 2500 individuals per m2), approximately a fourth of conventional hatchery density (M: 600 individuals per m2) and natural density (L: 150 individuals per m2). The natural density was based on density measures for migratory brown trout in a natural stream (Elliott 1994). Structure (S) and density treatments (H, M, L) were combined in a 2 × 3 design generating the following combinations: H, HS, M, MS, L and LS. Each treatment was replicated five times, except for one HS replicate, which was excluded because of irregular water flow. Mean water depth in the rearing tanks was 0.17 m until the middle of July and thereafter 0.67 m. The height of the plastic bags was regulated with the water level. After yolk sac absorption, fish were fed with commercial trout food, 2 per cent of wet weight per individual per day. The amount of food was regulated each week as fish grew. On three occasions (13 July, 20 September and 21 October), 150 fish per treatment (900 fish in total) were anaesthetized with 2-phenoxyethanol (0.5 ml l−1), and fork length and wet weight was measured for each individual. Fish were released back into the treatment tanks once measured.
Throughout the experiments, the indoor light regime was maintained at 12 L : 12 D. In all experiments, test fish were sampled in a pseudo-randomized fashion to ensure equal representation from the 29 hatchery tanks.
(b). Behavioural experiments on fry
Two experiments on fry were conducted between 27 June and 19 July 2007 using the same individuals throughout (in total 120 individuals, 20 per treatment). We used 12 replicate aquaria for each of 10 runs for each experiment. All six treatments were equally represented in each run, and tank observation order was randomized.
(i). Novel prey and habitat preference
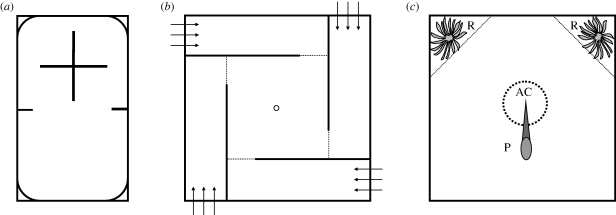
In the first experiment, the ability to forage on novel prey and habitat preference was studied. At 17–18 h, 120 juvenile trout were randomly netted from the treatment tanks and transferred to individual observation aquaria. Each aquarium (0.42 × 0.21 × 0.10 m) was divided into a barren and a structural area (figure 1a). The structural area consisted of four rooms screened-off by grey opaque polyvinylchloride (PVC) walls and the sides of each aquarium were covered with dark grey plastic. Fish had access to all areas and rooms during the experiment. Following an acclimatization period of 38 h, each fish was presented a novel prey item (calf-liver, 2% of wet weight per individual). Food was manually delivered to the middle of the barren area from behind a hide. The observations were made during a 10 min trial starting at 09.00 h. Time to capture the prey was recorded manually. Any remaining food was carefully removed after the novel prey trial. Subsequently, spatial exploration behaviour was recorded (digital Sony DCR-SR32). From these recordings (1 h), spatial exploration and the position of the fish (barren or structured area) were scored with point observations every 30 s. After the observations, test fish were singly transferred to the closed section in the middle of the maze for acclimatization over two nights (figure 1b).
Figure 1.
Schematic figures presenting dorsal views of experimental areas used in the following experiments: (a) habitat preference and novel prey-foraging. Two small walls separate the barren area from the structural area. The structural area is divided with walls into four rooms. The liver item was placed in the middle of the barren area, (b) food search ability in a maze. The dotted lines are movable opaque PVC doors shielding entries to the four maze arms. The arrows represent the water inflow and the ring shows the water outflow, (c) escape response under a simulated predator attack. P represents the heron model, AC the acclimatization chamber (broken line) and the R's represent the refuge areas (delimited with dotted lines) with ‘water plants’. AC was withdrawn before the simulated attack from P. In all figures, the solid lines represent opaque PVC walls.
(ii). Food search ability in a maze
Food search ability was assessed using an experimental maze (figure 1b). The maze (0.31 × 0.31 m) consisted of four similar rectangular maze arms (0.25 × 0.06 m) placed around a central area (0.25 × 0.25 m). Each of the four maze arms could be closed with a white opaque PVC door. The bottom of the maze was covered with brown–black folio to mimic natural substrate. After one night of acclimatization, a piece of calf-liver (2% of wet weight per individual and per day) was placed next to the water-inflow of a randomly selected maze arm. The liver was fixed to a steel wire to keep it in place just under the water surface. Subsequently, a pre-training trial took place by opening the PVC doors, whereupon the fish were allowed to explore the maze for 8 h. The maze entries were then closed and the fish was housed in the closed central area over the next night. The following day, each fish was observed for two 20 min trials, the first starting at 09.00 h, and the second starting at 13.00 h. Food was placed in the same maze arm as during the pre-training trial. The time needed to find the food (contact with the mouth) was recorded manually. At the end of each experiment, fish were anaesthetized, and wet weight and fork length were measured.
(c). Behavioural experiments on parr
The experiments on parr were conducted between 21 September and 26 October 2007. Parr were not tested more than once. At the end of each experiment, fish were anaesthetized and measured for wet weight and fork length.
(i). Novel prey-foraging
Novel prey-foraging was tested using live maggots (mean length of 9 mm; pinkies, Fibe AB, Överkalix, Sweden). Each parr was randomly netted from the treatment tanks, singly transferred and placed in one of the 12 observational aquaria (0.42 × 0.21 × 0.15 m water depth). All sides except the top of the aquaria were covered with black plastic to avoid disturbance. Fish were allowed to acclimatize to the new environment for a period of ca 38 h, and then observed twice during the observation day for 2 h with point checks every 10 min, starting 09.00 h and 13.00 h, respectively. At the start of each trial, a live maggot was delivered to the middle of the aquarium from behind a hide. During each trial, the foraging behaviour of the fish was manually recorded. Ten fish per treatment were tested (60 fish in total). Order of observation was randomized among treatments. An individual was classified as successful in the novel prey task, if it ate the novel prey during at least one occasion.
(ii). Escape response under a simulated predator attack
The escape responses of trout (19 per treatment, 114 in total) to a simulated predator attack using a heron model (natural predator of trout) were studied using a method modified from Barber et al. (2004). At the start of each trial, a single fish was netted from the treatment tanks and transferred to a circular acclimatization chamber (ø0.24 m) placed in the middle of the experimental tank (1 × 1 × 0.1 m water depth; figure 1c). After a 1 min acclimatization period, we waited until fish remained immobile for 10 s before the trial was started by gently lifting the chamber. Once the chamber was fully lifted, the predator model was immediately withdrawn, the beak was plunged in the water just above the fish and the escape response was monitored for 10 min. The acclimatization chamber and the predator model were controlled manually from behind a hide. Two green plastic bags (similar to those used in the rearing tanks) were used as cover and placed in the two corners opposite from were the heron attacked. In each corner, a triangular area (sides 0.4, 0.4 and 0.6 m) was defined as refuge area (figure 1c). The immediate escape response and movement into refuges were recorded using a Sony DCR-SR32 video camera positioned above the tank.
(d). Release of the fish
On 21 September, 20 fish from each treatment (120 in total) were sampled for release into a closed natural stream section. These numbers were chosen to mimic high natural densities of brown trout (1 parr per m2: Elliott 1994). All fish were anaesthetized, measured for wet weight and fork length, and individually tagged using a passive integrated transponder (PIT) inserted into the body cavity. Fish were left overnight in holding tanks to recover. The next morning, they were released in the middle of a divided section of the river Aneråsån, Kälarne (62°58′33 N, 16°06′18 E). The 95 m long stream section (approx. 120 m2, water depth 0.7 m) was closed off in both ends with stainless steel mesh, allowing the passage of water and invertebrate prey, but preventing fish from passing through. The stream section contains three pools with riffles in-between and is surrounded by deciduous trees. Fish in the stream had only access to naturally occurring food and were exposed to natural predation from mink (Mustela vison) and heron (Ardea cinerea) (own observations). On 25 October, all fish in the experimental stream were recaptured using electric fishing. The section was fished five times to ensure that no fish remained in the stream. Recaptured fish were anaesthetized, and measured for wet weight and fork length.
(e). Data treatment and statistics
The effects of density (Dens), tank structure (Str) and tank origin (random factor) on the response variables body mass and length were analysed using a mixed analysis of variance (ANOVA) model.
Habitat preference (proportion of time spent in the open) and growth in the stream after release were analysed using an analysis of covariance (ANCOVA) model with the same independent variables as in the ANOVA model and initial body size added as a continuous covariate. The data for habitat preference were transformed (arcsine) to meet requirements for normal distribution. Recapture rate and novel prey-foraging success were binominal variables analysed with logistic regression using the same independent variables as in the ANCOVA model.
We used semi-parametric Cox regression models (survival analysis) to analyse food search ability in the maze (time to find food) and escape response (time to escape) to a simulated predator attack, again with independent variables as in the ANCOVA model.
To increase the statistical power, the models were reduced by leaving out (Dens × Str × Tank), (Dens × Str) or initial body size when these were not significant. Categorical main effects (p < 0.1) were further investigated using standard post hoc tests (Wald χ2). To simplify presentation, p-values above 0.1 are not presented below. All statistical analyses were conducted using SPSS 16.
3. Results
(a). Behavioural experiment—fry
(i). Novel prey and habitat preference
Neither density nor structure affected habitat preference or the foraging success on novel prey. Smaller fish spent more time in the open area (F = 4.6, d.f. = 1, p = 0.035). No other effects were found.
(ii). Food search ability in a maze
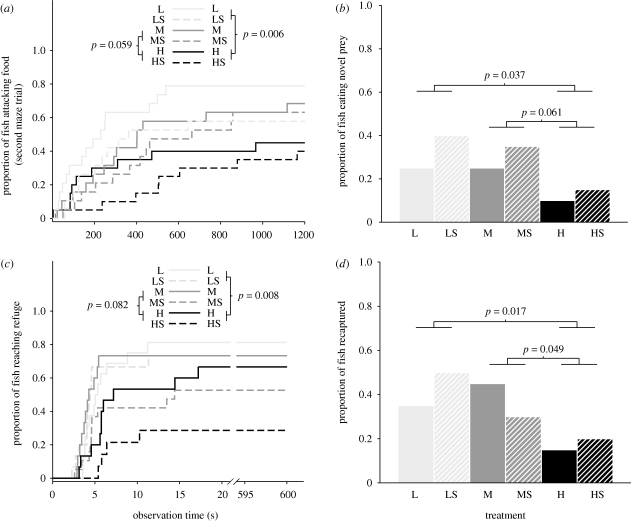
During the first trial, smaller fish tended to find the prey faster than larger conspecifics (Wald χ2 = 4.2, d.f. = 1, p = 0.040), but there was no treatment effects on food search ability. During the second trial, however, density significantly affected the time to find the prey (Wald χ2 = 7.7, d.f. = 2, p = 0.021). Post hoc tests revealed that fish from high densities (H + HS) were slower to find the prey than fish from lower density treatments (L + LS; Wald χ2 = 7.6, d.f. = 1, p = 0.006; figure 2a), and tended to need more time when compared with individuals from the medium densities (M + ML; Wald χ2 = 3.6, d.f. = 1, p = 0.059).
Figure 2.
Performance of brown trout reared under six different conditions: density treatments are indicated as natural density (L), approximately a third of conventional hatchery density (M) and conventional hatchery density (H), while the presence of structure is noted with S. (a) The proportion of fry attacking the food in the maze during the second trial as a function of observation time. (b) The proportion of parr eating a novel prey (i.e. maggot). (c) The proportion of parr reaching a refuge after a simulated predator attack as a function of observation time. (d) The proportion of parr recaptured after release in a natural stream (i.e. 35 days). All p-values indicate significance values for post hoc tests for the three-level categorical main effect density. In addition, the time to reach a refuge was longer for fish reared in structured tanks (p = 0.042).
(b). Behavioural experiment—parr
(i). Novel prey
Density tended to influence the foraging success on novel prey (maggots, Wald χ2 = 4.8, d.f. = 2, p = 0.091; figure 2b). Post hoc tests showed that fish from high density (H + HS) consumed less novel prey than fish from lower density treatments (L + LS; Wald χ2 = 4.3, d.f. = 1, p = 0.037; figure 2b), and tended to consume less novel prey than fish from medium densities (M + MS; Wald χ2 = 3.5, d.f. = 1, p = 0.061). No other effects were found.
(ii). Escape response under a simulated predator attack
About 65 per cent (L: 81%, LS: 80%, M: 67%, MS: 55%, H: 67%, HS: 43%) of the fish reacted to the attack from the model heron by fleeing to either of the two refuges available. The remaining 35 per cent responded either by freezing, or did not respond at all. Time to first movement was longer for fish reared in structure (Wald χ2 = 4.0, d.f. = 1, p = 0.045), but was not influenced by density. Time to flee (reaching the refuge) was longer for fish reared in structured tanks, and also influenced by rearing density (Wald χ2 = 7.1, d.f. = 2, p = 0.028). Post hoc tests showed that fish from high density (H + HS) required more time to reach the refuge area when compared with fish from low density (L + LS; Wald χ2 = 7.1, d.f. = 1, p = 0.008; figure 2c), and tended to require more time than fish from medium density (M + MS; Wald χ2 = 3.0, d.f. = 1, p = 0.082).
(c). Growth in the hatchery
During the first growth period (13 July–20 September), fish in low densities (L + LS) grew faster than high-density fish (W: F2,22 = 4.1, p = 0.030; FL: F2,22 = 11.1, p < 0.001). However, growth was not significantly affected by structure. During the second growth period (20 September–21 October), we found no significant treatment effects on growth. The size data from the three sampling occasions: 13 July, 20 September and 21 October are presented in table 1.
Table 1.
Mean (±s.e.) weight and fork length of juvenile brown trout in six treatment groups (H, HS, M, MS, L and LS) in the hatchery and after release and recapture in a stream. Fish were released in the stream on 20 September and recaptured on 25 October.
| H | HS | M | MS | L | LS | |
|---|---|---|---|---|---|---|
| hatchery | n = 150 | n = 150 | n = 150 | n = 150 | n = 150 | n = 150 |
| 13 June | ||||||
| weight (g) | 2.07 ± 0.04 | 2.24 ± 0.04 | 1.83 ± 0.04 | 2.17 ± 0.04 | 1.73 ± 0.04 | 1.87 ± 0.04 |
| fork length (mm) | 57.0 ± 0.34 | 58.4 ± 0.34 | 55.2 ± 0.33 | 58.1 ± 0.33 | 54.0 ± 0.34 | 55.1 ± 0.33 |
| 20 September | ||||||
| weight (g) | 12.1 ± 0.09 | 11.9 ± 0.06 | 12.0 ± 0.09 | 13.6 ± 0.10 | 13.07 ± 0.11 | 14.1 ± 0.11 |
| fork length (mm) | 99.7 ± 0.24 | 100.4 ± 0.16 | 100.1 ± 0.24 | 104.9 ± 0.23 | 101.7 ± 0.23 | 105.7 ± 0.27 |
| 21 October | ||||||
| weight (g) | 16.9 ± 0.54 | 16.7 ± 0.60 | 17.22 ± 0.54 | 18.9 ± 0.54 | 19.1 ± 0.54 | 18.2 ± 0.54 |
| fork length (mm) | 112.0 ± 1.05 | 111.7 ± 1.16 | 112.8 ± 1.03 | 117.1 ± 1.02 | 115.2 ± 1.06 | 116.4 ± 1.02 |
| stream | n = 3 | n = 5 | n = 8 | n = 5 | n = 7 | n = 10 |
| 25 October | ||||||
| weight (g) | 15.8 ± 0.24 | 15.3 ± 0.39 | 14.01 ± 0.54 | 20.6 ± 0.61 | 16.2 ± 0.42 | 16.8 ± 0.42 |
| fork length (mm) | 111.3 ± 1.07 | 112.8 ± 0.75 | 108.6 ± 1.22 | 127.0 ± 1.21 | 113.9 ± 0.85 | 116.0 ± 1.04 |
(d). Performance in the stream
Out of 120, 39 released fish (32.5%) were recaptured. Density influenced recapture rate significantly (Wald χ2 = 6.04, d.f. = 2, p = 0.049; figure 2d). Post hoc tests showed that fish from low density (L + LS; Wald χ2 = 5.7, d.f. = 1, p = 0.017) and medium density (M + MS; Wald χ2 = 3.9, d.f. = 1, p = 0.049 had higher recapture rates than fish from high density (H + HS; figure 2d). In contrast, there was no effect of structure on recapture rate. Growth rate in the stream was not affected by density or structure treatment.
4. Discussion
Consistent with the predictions, we found positive effects of reduced densities on food location ability, novel prey-foraging and escape response following a predator attack. Moreover, these density effects on behavioural life skills were apparently translated to increased survival in the wild. However, in contrast to the results of some previous studies, the effects of structure were minor and inconsistent. This may be due to differences in species-specific behaviours, including ontogenetic effects on sociality and habitat use. However, it should be kept in mind that only few studies have demonstrated effects of physical structure per se (Kihslinger & Nevitt 2006; Lee & Berejikian 2008), whereas others have found effects of structure in combination with additional modifications (e.g. Braithwaite & Salvanes 2005; Salvanes & Braithwaite 2005). The discussion below will thus mainly focus on the novel findings of density-dependent effects on behaviour and post-release performance.
(a). Behaviours
Trout reared at reduced densities consumed more live novel prey, fled more rapidly to refuges after a predator attack and were faster to locate food in a maze compared with fish from higher densities. These behavioural life skills are probably of great importance for growth and survival upon release. Before describing the significance of specific behaviours, we will elucidate some potential explanations for the strong density effects on behaviour.
In a previous study (Brockmark & Johnsson 2010), we suggested that high-density environments may constrain the ability to establish social-based recognition, and coordinate behavioural interactions with specific individuals (Griffiths 2003; Griffiths et al. 2004). The sensory overload in a crowded environment (Dukas 2002), together with the associated spatial restriction of activity (Ruxton 1995) and perception may in fact constrain the development of both social and individual behaviour. An alternative, not mutually exclusive explanation is that high-density conditions may alter the trade-off between using private versus public information (Laland 2004; Brown et al. 2006). Indeed, human studies demonstrate that individuals with long-time experience of crowding gradually reduce individual control (Bell et al. 2001; Camazine et al. 2001). Theoretical analysis (Rogers 1988; Giraldeau et al. 2002) and empirical studies (van Bergen et al. 2004) suggest that the combination of private and public information sources is the optimal base for adaptive decision-making. Thus, density conditions that constantly favour the use of public information during development might over time lead to conformity through more or less irreversible losses of capacity for independent decision-making. Note that the use of public information does not require individual recognition, so that the ability to interact with specific individuals could still be impaired at high densities, as discussed above. Further experimental studies are necessary to evaluate the relative importance of these candidate explanations.
(i). Food searching
In nature, food resources are often highly variable in time and space (Warburton 2003). To minimize energy costs and movement-associated predation risk while searching for food, animals need to find their way efficiently (Odling-Smee & Braithwaite 2003). Previous studies show that a range of environmental factors can influence the food search of animals (Odling-Smee et al. 2006). Here, we found that fry reared at low densities were faster to find prey in a maze compared with trout reared at higher densities. Fish reared in natural densities are more able to move around freely which, as discussed above, may promote the capability of independent decision-making and a flexible behavioural repertoire, allowing fish to match their orientation strategy to a variable environment on the basis of experience.
(ii). Novel prey-foraging
In this study, we found that density conditions are important for the ability to forage on novel prey. Trout were tested for novel prey twice during their first seven months after hatching. When tested at the fry stage (i.e. after four months), fish were equally willing to consume novel prey (i.e. liver) independent of treatment. However, when tested three months later, trout parr reared at natural densities consumed more novel live prey items (i.e. maggots) compared with fish from higher densities. There may be several explanations for these stage- and prey-specific effects. For example, it may be more demanding to switch from pellets to moving maggots than from pellets to liver. Alternatively, the density effects on individual foraging may have developed gradually during ontogeny. Hatchery-reared fish, which traditionally feed on a diet of pellets, often have difficulties to switch to live novel prey after release (Ellis et al. 2002). Indeed, hatchery brown trout have been shown to be slower than wild-reared conspecifics to learn to feed on novel prey (i.e. crickets, Sundström & Johnsson 2001).
(iii). Anti-predator response
There is a strong selection pressure for early detection and avoidance of predation threats (Lima & Dill 1990). According to the economic flight hypothesis (Ydenberg & Dill 1986), the decision to flee depends on several factors, including nearness to refuge, prey cryptic coloration, type of predator species and group size (Lima & Dill 1990; Godin 1997). In the present study, we found that trout reared at lower densities were faster to escape and hide after a predator attack compared with fish from high densities. Moreover, trout reared in barren hatchery tanks responded more rapidly to a simulated predator attack than trout from tanks containing structure. In a recent study (Kihslinger & Nevitt 2006), fish reared in hatchery tanks containing stones showed lower movement activity than fish from barren tanks. Assuming that fish in the present study respond in a similar way, low activity would explain why structured-reared fish responded to the predator attack with freezing rather than fleeing. However, the ability to find and use shelter is important in the wild, especially during early life stages, when juveniles are subjected to mortality risks from a wide range of predators.
(b). Performance in the stream
In this study, trout reared at reduced densities survived better in the natural stream compared with trout reared at conventional hatchery density. Thus, the development of behavioural skills in the hatchery appears to be critical for survival upon release. Positive density-dependent effects were found when trout were reared at a fourth of conventional hatchery densities, but were even clearer in high natural densities. Habitat shifts often cause high mortality rates due to predation (Biro et al. 2003; Byström et al. 2003). The mortality in the present study was probably mainly caused by predation from mink and heron, both common in the area. Mortality from starvation is less likely to have occurred over the limited time period fish spent in the stream (see Johnsson & Bohlin 2006). Also, up- or downstream fences prevent fish migration and repeated electric fishing made sure few or any fish were escaped from being recaptured (see §2).
(c). Implications for captive rearing
An increasing number of studies demonstrate that fish are able to learn and integrate multiple pieces of information (Braithwaite 2006; Brown et al. 2006). Moreover, fish behaviour is strongly species- and stage-specific, including ontogenetic effects on sociality and habitat use (Godin 1997; Magnhagen et al. 2008). Our results suggest that more nature-like rearing densities promote behavioural skills and post-release survival in hatchery salmonids (see also Brockmark & Johnsson 2010). However, further field studies are needed to evaluate the generality of these findings. If these effects turn out to be consistent, the overall socioeconomic benefits of reducing rearing densities will depend on whether the benefits of increased adult returns will compensate the increased production requirements for hatchery juveniles, a question that requires further long-term full-scale experiments and economic analyses. Finally, ecologically based rearing methods are not only important to increase the success of supplementary and conservation hatcheries. There is also a more general ethical aspect mirrored by the increasing public demands that captive animals should be allowed to express their natural behaviour (Shumway 1999; Branson 2008).
Acknowledgements
The experiment was approved by the Ethical Committee for Animal Research in Göteborg (license 132/2005), and comply with current laws in Sweden.
We thank Torleif Andersson and staff at the Swedish Board of Fisheries Research Station in Kälarne for valuable assistance during the experiment and for fish care. Financial support for this project was provided by Helge Ax:son Johnson stiftelse (S.B.), Rådman och fru Ernst Collianders stiftelse (S.B.), Stiftelsen Lars Hiertas Minne (B.A.) and the Royal Swedish Academy of Sciences (B.A.). J.I.J. was financed by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning.
References
- Barber I., Walker P., Svensson P. A.2004Behavioural responses to simulated avian predation in female three spined sticklebacks: the effect of experimental Schistocephalus solidus infections. Behaviour 141, 1425–1440 (doi:10.1163/1568539042948231) [Google Scholar]
- Bell P. A., Greene T. C., Fisher J. D., Baum A.2001Environmental psychology. Orlando, FL: Harcourt College Publishers [Google Scholar]
- Berejikian B. A., Tezak E. P., Flagg T. A., LaRae A. L., Kummerow E., Mahnken C. V. W.2000Social dominance, growth, and habitat use of age-0 steelhead (Oncorhynchus mykiss) grown in enriched and conventional hatchery rearing environments. Can. J. Fish. Aquat. Sci. 57, 628–636 (doi:10.1139/cjfas-57-3-628) [Google Scholar]
- Berejikian B. A., Tezak E. P., Park L., LaHood E., Schroder S. L., Beall E.2001Male competition and breeding success in captively reared and wild coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci. 58, 804–810 (doi:10.1139/cjfas-58-4-804) [Google Scholar]
- Biro P. A., Post J. R., Parkinson E. A.2003Population consequences of a predator-induced habitat shift by trout in whole-lake experiments. Ecology 84, 691–700 (doi:10.1890/0012-9658(2003)084[0691:PCOAPI]2.0.CO;2) [Google Scholar]
- Braithwaite V. A.2006Cognitive ability in fish. In Behaviour and physiology of fish (eds Sloman K. A., Wilson R. W., Balshine S.). San Diego, CA: Elsevier Academic Press [Google Scholar]
- Braithwaite V. A., Salvanes A. G. V.2005Environmental variability in the early rearing environment generates behaviourally flexible cod: implications for rehabilitating wild populations. Proc. R. Soc. B 272, 1107–1113 (doi:10.1098/rspb.2005.3062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson E. J.2008Fish welfare. Oxford, UK: Blackwell Publishing Ltd [Google Scholar]
- Brännäs E., Johnsson J. I.2008Behaviour and welfare in farmed fish. In Fish behaviour (eds Magnhagen C., Braithwaite V. A., Forsgren E., Kapoor B. G.), pp. 593–627 Enfield, NH: Science Publishers [Google Scholar]
- Bridcut E. E., Giller P. S.1995Diet variability and foraging strategies in brown trout (Salmo trutta): an analysis from subpopulations to individuals. Can. J. Fish. Aquat. Sci. 52, 2543–2552 (doi:10.1139/f95-845) [Google Scholar]
- Brockmark S., Johnsson J. I.2010Reduced hatchery rearing density increases social dominance, post-release growth and survival in brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 67, 288–295 (doi:10.1139/F09-185) [Google Scholar]
- Brockmark S., Neregard L., Bohlin T., Bjornsson B. T., Johnsson J. I.2007Effects of rearing density and structural complexity on the pre- and postrelease performance of Atlantic salmon. Trans. Am. Fish. Soc. 136, 1453–1462 (doi:10.1577/T06-245.1) [Google Scholar]
- Brown C., Day R.2002The future of stock enhancements: bridging the gap between hatchery practice and conservation biology. Fish Fish. 3, 79–94 (doi:10.1046/j.1467-2979.2002.00077.x) [Google Scholar]
- Brown C., Laland K.2001Social learning and life skills training for hatchery reared fish. J. Fish Biol. 59, 471–493 (doi:10.1006/jfbi.2001.1689) [Google Scholar]
- Brown C., Davidson T., Laland K.2003Environmental enrichment and prior experience of live prey improve foraging behaviour in hatchery-reared Atlantic salmon. J. Fish Biol. 63(Suppl. A), 187–196 (doi:10.1046/j.1095-8649.2003.00208.x) [Google Scholar]
- Brown C., Laland K., Krause J.2006Fish cognition and behaviour. Fish and Aquatic Resources Series 11 Oxford, UK: Blackwell Publishing Ltd [Google Scholar]
- Brydges N. M., Colegrave N., Heathcote R. J. P., Braithwaite V. A.2008Habitat stability and predation pressure affect temperament behaviours in populations of three-spined sticklebacks. Anim. Behav. 77, 229–235 (doi:10.1111/j.1365-2656.2007.01343.x) [DOI] [PubMed] [Google Scholar]
- Byström P., Persson L., Wahlström E., Westman E.2003Size- and density-dependent habitat use in predators: consequences for habitat shifts in young fish. J. Anim. Ecol. 72, 156–168 (doi:10.1046/j.1365-2656.2003.00681.x) [Google Scholar]
- Camazine S., Deneubourg J. L., Franks N. G., Sneyd J., Theraulaz G., Bonebeau E.2001Self-organization in biological systems. Princeton, NJ: Princeton University Press [Google Scholar]
- Chapman B. B., Ward A. J. W., Krause J.2008Schooling and learning: early social environment predicts social learning ability in the guppy, Poecilia reticulata. Anim. Behav. 76, 923–929 (doi:10.1016/j.anbehav.2008.03.022) [Google Scholar]
- Cubitt K. F., Winberg S., Huntingford F. A., Kadri S., Crampton V. O., Overli O.2008Social hierarchies, growth and brain serotonin metabolism in Atlantic salmon (Salmo salar) kept under commercial rearing conditions. Physiol. Behav. 94, 529–535 (doi:10.1016/j.physbeh.2008.03.009) [DOI] [PubMed] [Google Scholar]
- Dukas R.2002Behavioural and ecological consequences of limited attention. Phil. Trans. R. Soc. Lond. B 357, 1539–1547 (doi:10.1098/rstb.2002.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. M.1994Quantitative ecology and the brown trout. Oxford series in ecology and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- Ellis T., Hughes R. N., Howell B. R.2002Artificial dietary regime may impair subsequent foraging behaviour of hatchery-reared turbot released into the natural environment. J. Fish Biol. 61, 252–264 (doi:10.1111/j.1095-8649.2002.tb01750.x) [Google Scholar]
- Galhardo L., Correia J., Oliveira R. F.2008The effect of substrate availability on behavioural and physiological indicators of welfare in the African cichlid (Oreochromis mossambicus). Anim. Welfare 17, 239–254 [Google Scholar]
- Giraldeau L. A., Valone T. J., Templeton J. J.2002Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B 357, 1559–1566 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G. J.1997Evading predators. In Behavioural ecology of teleost fishes (ed. Godin J.-G. J.), pp. 191–236 Oxford, UK: Oxford University press [Google Scholar]
- Grant J. W. A.1997Territoriality. In Behavioural ecology of teleost fishes (ed. Godin J. G. J.), pp. 81–103 Oxford, UK: Oxford University Press [Google Scholar]
- Griffin A. S.2004Social learning about predators: a review and prospectus. Learn. Behav. 32, 131–140 [DOI] [PubMed] [Google Scholar]
- Griffin A. S., Blumstein D. T., Evans C.2000Training captive-bred or translocated animals to avoid predators. Conserv. Biol. 14, 1317–1326 (doi:10.1046/j.1523-1739.2000.99326.x) [Google Scholar]
- Griffiths S. W.2003Learned recognition of conspecifics by fishes. Fish Fish. 4, 256–268 (doi:10.1046/j.1467-2979.2003.00129.x) [Google Scholar]
- Griffiths S. W., Brockmark S., Hojesjo J., Johnsson J. I.2004Coping with divided attention: the advantage of familiarity. Proc. R. Soc. Lond. B 271, 695–699 (doi:10.1098/rspb.2003.2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haury J., Ombredane D., Baglinière J. L.1999The habitat of the brown trout (Salmo trutta L.) in water courses. In Biology and ecology of the brown and sea trout (eds Baglinière J. L., Massie G.), pp. 37–90 Heidelberg, Germany: Springer [Google Scholar]
- Hawkins L. A., Magurran A. E., Armstrong J. D.2008Ontogenetic learning of predator recognition in hatchery-reared Atlantic salmon, Salmo salar. Anim. Behav. 75, 1663–1671 (doi:10.1016/j.anbehav.2007.10.019) [Google Scholar]
- Johnsson J. I., Bohlin T.2006The cost of catching up: increased winter mortality following structural growth compensation in the wild. Proc. R. Soc. B 273, 1281–1286 (doi:10.1098/rspb.2005.3437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson J. I., Petersson E., Jönsson E., Björnsson B. T., Järvi T.1996Domestication and growth hormone alter antipredator behaviour and growth patterns in juvenile brown trout, Salmo trutta. Can. J. Fish. Aquat. Sci. 53, 1546–1554 (doi:10.1139/cjfas-53-7-1546) [Google Scholar]
- Kihslinger R. L., Nevitt G. A.2006Early rearing environment impacts cerebellar growth in juvenile salmon. J. Exp. Biol. 209, 504–509 (doi:10.1242/jeb.02019) [DOI] [PubMed] [Google Scholar]
- Laland K. N.2004Social learning strategies. Learn. Behav. 32, 4–14 [DOI] [PubMed] [Google Scholar]
- Lee J. S. F., Berejikian B. A.2008Effects of the rearing environment on average behaviour and behavioural variation in steelhead. J. Fish Biol. 72, 1736–1749 (doi:10.1111/j.1095-8649.2008.01848.x) [Google Scholar]
- Levin P. S., Zabel R. W., Williams J. G.2001The road to extinction is paved with good intentions: negative association of fish hatcheries with threatened salmon. Proc. R. Soc. Lond. B 268, 1153–1158 (doi:10.1098/rspb.2001.1634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S. L., Dill L. M.1990Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 (doi:10.1139/z90-092) [Google Scholar]
- Magnhagen C., Braithwaite V. A., Forsgren E., Kapoor B. G.2008Fish behaviour. Enfield, NH: Science Publishers [Google Scholar]
- Markel R. W.1994An adaptive value of spatial-learning and memory in the blackeye goby, Coryphopterus nicholsi. Anim. Behav. 47, 1462–1464 (doi:10.1006/anbe.1994.1194) [Google Scholar]
- Noda T.2004Spatial hierarchical approach in community ecology: a way beyond high context-dependency and low predictability in local phenomena. Popul. Ecol. 46, 105–117 (doi:10.1007/s10144-004-0184-x) [Google Scholar]
- Odling-Smee L., Braithwaite V. A.2003The influence of habitat stability on landmark use during spatial learning in the three-spined stickleback. Anim. Behav. 65, 701–707 (doi:10.1006/anbe.2003.2082) [Google Scholar]
- Odling-Smee L., Simpson D. G., Braithwaite V. A.2006The role of learning in fish orientation. In Fish cognition and behaviour (eds Brown C., Laland K., Krause J.), pp. 119–138 Oxford, UK: Blackwell Publishing Ltd [Google Scholar]
- Rogers A. R.1988Does biology constrain culture? Am. Anthropol. 90, 819–831 (doi:10.1525/aa.1988.90.4.02a00030) [Google Scholar]
- Ruxton G. D.1995Foraging in flocks: nonspatial models may neglect important costs. Ecol. Modell. 82, 277–285 (doi:10.1016/0304-3800(94)00098-3) [Google Scholar]
- Salvanes A. G. V., Braithwaite V. A.2005Exposure to variable spatial information in the early rearing environment generates asymmetries in social interactions in cod (Gadus morhua). Behav. Ecol. Sociobiol. 59, 250–257 (doi:10.1007/s00265-005-0031-x) [Google Scholar]
- Shumway C. A.1999A neglected science: applying behavior to aquatic conservation. Environ. Biol. Fishes 55, 183–201 (doi:10.1023/A:1007562023150) [Google Scholar]
- Sol D., Timmermans S., Lefebvre L.2002Behavioural flexibility and invasion success in birds. Anim. Behav. 63, 495–502 (doi:10.1006/anbe.2001.1953) [Google Scholar]
- Sumpter D. J. T., Krause J., James R., Couzin I. D., Ward A. J. W.2008Consensus decision making by fish. Curr. Biol. 18, 1773–1777 (doi:10.1016/j.cub.2008.09.064) [DOI] [PubMed] [Google Scholar]
- Sundström L. F., Johnsson J. I.2001Experience and social environment influence the ability of young brown trout to forage on live novel prey. Anim. Behav. 61, 249–255 (doi:10.1006/anbe.2000.1593) [DOI] [PubMed] [Google Scholar]
- Sutter M., Kawecki T. J.2009Influence of learning on range expansion and adaptation to novel habitats. J. Evol. Biol. 22, 2201–2214 (doi:10.1111/j.1420-9101.2009.01836.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergen Y., Coolen I., Laland K. N.2004Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. Lond. B 271, 957–962 (doi:10.1098/rspb.2004.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhunen S., Hirvonen H.2003Innate antipredator responses of Arctic charr (Salvelinus alpinus) depend on predator species and their diet. Behav. Ecol. Sociobiol. 55, 1–10 (doi:10.1007/s00265-003-0670-8) [Google Scholar]
- Warburton K.2003Learning of foraging skills by fish. Fish Fish. 4, 203–215 (doi:10.1046/j.1467-2979.2003.00125.x) [Google Scholar]
- Ward A. J. W., Sumpter D. J. T., Couzin L. D., Hart P. J. B., Krause J.2008Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953 (doi:10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters J. V., Meehan C. L.2007Different strokes: can managing behavioral types increase post-release success? Appl. Anim. Behav. Sci. 102, 364–379 (doi:10.1016/j.applanim.2006.05.036) [Google Scholar]
- Ydenberg R. C., Dill L. M.1986The economics of fleeing from predators. Adv. Study Behav. 16, 229–249 (doi:10.1016/S0065-3454(08)60192-8) [Google Scholar]
- Young R. J.2003Environmental enrichment for captive animals. Oxford, UK: Blackwell Publishing [Google Scholar]