Abstract
Natural selection operates throughout the life cycle of an organism. Correlative studies typically fail to consider the effects of viability selection prior to trait expression. A 3-year field experiment on the wildflower Mimulus guttatus demonstrates that this unmeasured component of selection can be very strong. As in previous studies, we find that fecundity is positively related to flower size. However, survival to flowering is much lower in large-flowered genotypes than in small-flowered genotypes. Aggregating viability and fecundity, lifetime fitness through female function generally favoured smaller flowered genotypes. This result differs from the great majority of field studies, which suggest strong positive selection on flower size. It has important cautionary implications for studies of natural and sexual selection on adult characters generally, in both plants and animals.
Keywords: flower size, invisible fraction, Mimulus guttatus, natural selection, selection gradients
1. Introduction
Measuring natural selection in wild populations is a major endeavour for evolutionary biologists. Since the publications of Lande (1979) and Lande & Arnold (1983), estimates of natural selection on behavioural, morphological, physiological and life-history traits have accumulated from many taxa (Endler 1986; Kingsolver et al. 2001). This abundance of data permits testing of broad taxonomic hypotheses. For example, strong positive correlations between body size and fecundity have been documented in many species (Kingsolver & Pfennig 2004). Interpreted literally, these estimates suggest a mechanism for Cope's rule (the tendency for lineages to evolve larger body size over time; Cope 1896; Bonner 1988).
In plants, many studies have considered selection on flower size. The survey of Kingsolver et al. (2001) includes nine estimates, seven of which indicate significant positive directional selection on flower size. Moreover, the review of Kingsolver et al. (2001) includes only a fraction of the abundant literature suggesting that natural selection favours larger flowers (e.g. Medel et al. 2003; Armbruster et al. 2005; Wright & Stanton 2007; Sandring & Agren 2009). Despite apparently overwhelming directional selection for larger flowers, populations retain high genetic variation in flower size. Artificial selection can greatly increase mean flower size (Worley & Barrett 2000; Lendvai & Levin 2003; Delph et al. 2004; Lehtila & Brann 2007; Kelly 2008), indicating that natural populations are not at maximum values for this trait.
The majority of estimates for natural selection come from correlative studies. Generally, investigators identify individuals within a field population, measure traits and then monitor these individuals to determine their reproductive success. Natural selection is inferred from the association of trait values (e.g. flower size) with reproductive success (e.g. seed set). The overall selection on a quantitative trait is most naturally estimated as S, the selection differential (Falconer & Mackay 1996), which equals the covariance of trait values with relative fitness (typically positive for flower size). S includes both direct selection on the measured trait, as well as indirect selection on correlated traits. Many studies report selection gradients, which describe the ‘direct selection’ on a character. Selection gradients are the partial regression coefficients obtained when a fitness component is regressed on multiple traits simultaneously (Lande & Arnold 1983). Like selection differentials, flower size gradients are usually positive. However, there is greater variability in estimates for these quantities. Selection gradients on floral size measurements depend strongly on the identity of other plant traits that are included in the multiple regression (see §4).
While the correlative approach is straightforward and broadly applicable, selection estimates are prone to several well-known biases (Mitchell-Olds & Shaw 1987; Wade & Kalisz 1990; Rausher 1992; Willis 1996). Direct interpretation of correlative estimates requires that included individuals are a random sample of the population. To be included in the calculation of a selection differential or gradient, an individual must express the trait and then yield an estimate for fitness. Individuals who die before expressing the trait (e.g. before flowering) will be excluded. If the likelihood of flowering is correlated with the ‘latent’ value for the trait—formally, the genotypic value for the trait (Falconer & Mackay 1996)—then a component of selection is missed. Grafen (1988) referred to this population of individuals who die before expressing the trait of interest as the ‘invisible fraction’.
The difficulty in detecting viability selection prior to trait expression is relevant to any correlative selection analysis of adult traits. However, the quantitative importance of this effect is not widely appreciated. Bennington & McGraw (1995) showed that selection on plant height of jewelweed is significantly altered when the invisible fraction is taken into account. Sinervo & McAdam (2008) assessed the survival of side-blotched lizards before sexual maturity and revealed non-random mortality prior to clutch-size expression. Hadfield (2008) recently considered a variety of invisible fraction scenarios, and questioned whether any non-manipulative method can accurately estimate selection when individuals die before trait expression.
Here, we describe a genotypic manipulation experiment demonstrating that viability selection on the invisible fraction can be strong enough to completely reverse the overall direction of selection. The focus of our work is Mimulus guttatus (yellow monkeyflower). Research on this species is a substantial component of the flower size/natural selection literature. At least six previous studies report estimates from a multitude of populations and years of study (Fenster & Ritland 1994; Willis 1996; van Kleunen & Ritland 2004; Hall & Willis 2006; Fishman & Willis 2008; Murren et al. 2009). In all cases, the overall selection on floral size traits appears to be significantly, and often strongly, positive.
We employ a field transplant experiment of small-, medium- and large-flowered genotypes of M. guttatus to estimate both viability and fecundity selection on flower size. This approach allows us to ‘visualize’ the invisible fraction through prior knowledge about the phenotypes of the transplanted individuals. As expected from previous studies, we find that fecundity is positively related to flower size among adult plants. However, the genotypic transplant allows us to estimate the relative viability of flower size genotypes prior to expressing the trait in the field. We find that viability is negatively related to the genotypic value for flower size. When survivorship and female reproductive success are both taken into account, natural selection generally favours smaller flowers.
2. Material and methods
(a). Study system
The yellow monkeyflower, M. guttatus (Phrymaceae), is a self-compatible, mostly outcrossing, monoecious plant that inhabits a wide range of habitats from alpine (annual) to coastal (perennial). The study was carried out from 2007 to 2009 within a single annual alpine population at the Browder Ridge trailhead (Oregon, USA; 44º22′7.3194″ N, 122º2′57.84″ W). At this site, Mimulus is annual or winter annual. Seeds germinate either in the autumn (and persist as seedlings under the snow) or during the spring immediately following snowmelt in late May or early June. They develop rapidly and the peak of flowering is typically from mid- to late June. These predominantly bumble-bee pollinated plants set seed by mid-July in most years (J.P. Mojica, personal observation).
(b). Genotypic field transplant experiment
We transplanted seedlings from three distinct genotypic groups into the field site. Each group was a random selection of plants from one of three divergent populations produced via nine generations of artificial selection on corolla width, a measure of flower size (Kelly 2008). Low, control and high populations were initiated from a common source population, derived from a single natural population located at Iron Mountain in central Oregon (Willis 1996). Importantly, the selection experiment was conducted using very large population sizes: 200 adult plants were selected in each population to constitute the next generation. As a consequence, the high, low and control plants are actually genotypic classes. Each class retains high internal genetic variation (see table 3 of Kelly 2008). The difference between high, low and control plants in mean flower size is due to the cumulative effects of many quantitative trait loci (QTL). Lee (2009) mapped flower size QTL on all 14 M. guttatus chromosomes (most chromosomes have multiple QTLs) using parental plants from these same low and high populations.
We pooled seed from approximately 70 distinct families within each population. Seedlings were germinated in separate flats in the University of Oregon greenhouse during May in three successive years (2007, 2008 and 2009). We transplanted 14-day-old seedlings (n2007 = 675, n2008 = 750, n2009 = 450) into 5-m transects at the Browder Ridge site in the first week of June (coincident with the seedling stage of the native Mimulus). Browder Ridge is close to the Iron Mountain site (source population) and experiences approximately the same climatic environment. Any transplants that died within a week of transplant were replaced to reduce the effect of transplant shock on survivorship data. We monitored transplants subsequently and recorded the day when the first flower opened (flowering time) and measured corolla width in millimetres using a steel ruler. At the end of the growing season (mid-July), we collected and counted all the seeds produced by each flowering plant.
(c). Estimation of phenotypic selection on flower size
We used corolla width as our measure of flower size. After square-root transformation, this variable is normally distributed within genotypic classes and has been used in previous studies (Kelly 2008 and references therein). For all plants that flowered, we estimated the effects of genotype, year and their interaction on corolla width using a two-way ANOVA (normal residuals). Generalized linear models were used to assess year and genotype effects on survivorship and seed counts. For survivorship, the response variable is dichotomous (0/1) and we used the standard Logit link function. Seed number of surviving plants (fecundity) was highly right-skewed and we employed an overdispersed Poisson model with the Log link function. We used the same statistical model for total female fitness, which is the seed production of all transplants (including zero values for plants that died before flowering). Finally, we also used the Poisson model for the regression of fecundity onto observed flower sizes with the year included as a categorical predictor. The estimates from this model yield a selection gradient for corolla width. These analyses were conducted using JMP v. 8 (SAS Corporation).
3. Results
(a). Phenotypic variation among flower size genotypes
The significant differences in flower size among low, control and high genotypes that had been previously documented in the greenhouse (see table 2 of Kelly 2008) were reiterated in the field (F2,580 = 157.3, p < 0.001). Across years, the estimated mean corolla width was 2.96 among lows (s.d. = 0.34, n = 406), 3.55 among controls (s.d. = 0.40, n = 153) and 4.30 among highs (s.d. = 0.51, n = 30). The phenotypic distribution of control plants is representative of the ancestral Iron Mountain population and the native plants at Browder Ridge. Relative to the control distribution, the mean floral size of high plants was about 1.9 s.d. greater than the control mean, while the mean floral size of the lows was about 1.5 s.d. less (figure 1a).
Figure 1.
Means are reported for (a) corolla width, (b) survivorship to flowering, (c) fecundity of survivors and (d) total fitness for each Mimulus guttatus genotype, pooled across years. (a) Corolla width is square-root transformed. (d) Total fitness is the average of absolute seed set of all transplants of a genotype. Error bars are 95 per cent confidence intervals of each mean.
(b). Viability and fecundity selection on flower size
Death owing to transplant shock was low across years and unrelated to genotype. After transplant establishment, mortality was uniformly low for all genotypes until the final ‘dry down’ in each field season. All plants eventually desiccated as snowmelt diminished, although the interval of this final drought differed among years (20–30 July in 2007, 5–15 August in 2008 and 25 July to 4 August in 2009). However, the number of plants that matured fast enough to flower and set seed varied greatly with genotype and year. Across years, survivorship to flowering of low genotypes was 12-fold higher than that of high genotypes (figure 1b). Control genotypes consistently showed intermediate survivorship to flowering. Likelihood ratio tests confirm the significant viability effects of genotype (χ2 = 486.6, d.f. = 2, p < 0.001), of year (χ2 = 68.0, d.f. = 2, p < 0.001) and of genotype-by-year interaction (χ2 = 29.6, d.f. = 4, p < 0.001). Despite the interaction, the rank order of genotypes was consistent across years (low survivorship > control > high).
Among plants surviving to flower, fecundity was positively related to flower size genotype (figure 1c; χ2 = 8.25, d.f. = 2, p = 0.016). There was also a large effect of year (χ2 = 59.0, d.f. = 2, p < 0.001). The genotype-by-year interaction was non-significant and dropped from the model. The combined effects of viability and fecundity were evaluated by considering total seed production per transplant (table 1). There were significant effects of genotype (χ2 = 39.3, d.f. = 2, p < 0.001) and year (χ2 = 112.7, d.f. = 2, p < 0.001) on this measure of total female fitness. The interaction was non-significant and was excluded from the model, but the rank order of estimated genotype means for total fitness was not completely consistent across years. The high genotype was always lowest, but control had the highest mean in 2009 (table 1).
Table 1.
The average seed set of each Mimulus guttatus genotype in each year of study is given (n is the sample size, s.e.m. is the standard error of the mean and s.d. is the standard deviation).
| genotype | n | mean | s.e.m. | s.d. |
|---|---|---|---|---|
| 2007 | ||||
| low | 259 | 3.66 | 0.65 | 10.46 |
| control | 159 | 1.08 | 0.49 | 6.15 |
| high | 257 | 0.01 | 0.01 | 0.12 |
| 2008 | ||||
| low | 269 | 23.70 | 3.33 | 54.62 |
| control | 257 | 17.57 | 3.68 | 59.02 |
| high | 224 | 6.25 | 2.50 | 37.36 |
| 2009 | ||||
| low | 150 | 3.82 | 0.81 | 9.91 |
| control | 150 | 5.61 | 1.57 | 19.22 |
| high | 150 | 1.27 | 0.74 | 9.02 |
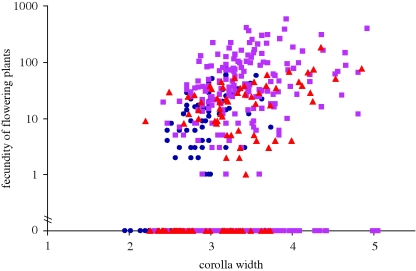
The relationship between observed flower sizes and fecundity is illustrated in figure 2. The Poisson regression model estimates expected seed set as C × exp(0.93 z), where z is the corolla width (square-root transformed) and C is a constant dependent on year. The positive relationship is highly significant: χ2 = 54.0, d.f. = 1, p < 0.001. The univariate selection gradient (i.e. the linear regression of relative fitness onto trait value) is 0.93. Gradients can be standardized in a variety of ways (Hereford et al. 2004) given the trait mean ( = 3.18) and standard deviation (σz = 0.217 after factoring out differences among years). The variance–standardized gradient is (0.217)(0.93) = 0.20. The mean–standardized gradient is (3.18)(0.93) = 2.96. These values are comparable to gradient estimates from other plants and animals for size-related traits (see figs 1 and 2 of Kingsolver & Pfennig 2004; fig. 3 of Hereford et al. 2004).
= 3.18) and standard deviation (σz = 0.217 after factoring out differences among years). The variance–standardized gradient is (0.217)(0.93) = 0.20. The mean–standardized gradient is (3.18)(0.93) = 2.96. These values are comparable to gradient estimates from other plants and animals for size-related traits (see figs 1 and 2 of Kingsolver & Pfennig 2004; fig. 3 of Hereford et al. 2004).
Figure 2.
The linear regression of survivor fecundity onto corolla width of Mimulus guttatus is depicted. Corolla width is square-root transformed. Blue circle, 2007; purple square, 2008; red triangle, 2009.
4. Discussion
(a). Seeing the invisible fraction
Figures 1 and 2 demonstrate the potentially dominant effects of selection on the invisible fraction: the collection of individuals that die prior to trait expression (Grafen 1988). Viability and fecundity selection on flower size were conflicting in each of the three years of the study, but only the latter process would be evident in a correlative study of natural selection. We observed a strong positive relationship between fecundity and flower size (figure 2), a result consistent both with the extensive field data from this species and from flowering plants generally (§1). However, here we were also able to measure pre-flowering selection by using genotypic groups known a priori to differ in flower size. The mortality data (figure 1b) identifies strong viability selection prior to trait expression. The invisible fraction is composed disproportionately of large-flowered genotypes that did not mature fast enough to reach flowering. As a consequence, the overall direction of selection was usually for smaller flowers (table 1). Given that the intrinsic features of the invisible fraction are usually unknown, the conclusion that natural selection habitually favours larger flowers should be viewed with caution.
In this experiment, the differential mortality of flower size genotypes was determined by differences in development rate. Across genotypes, mortality was minimal until the terminal drought period in each year. During this drought interval, all plants died. Survivorship to flowering was highest for low genotypes because they mature faster than control genotypes. Control genotypes reach flowering faster than high genotypes. Across years, mean time to first flower was 45.1 days for lows (s.d. = 17.0, n = 424), 55.9 days for controls (s.d. = 15.3, n = 170), and 68.6 days for highs (s.d. = 16.1, n = 31). Flower size exhibits a positive genetic correlation with time to flower mainly because plants that delay flowering have greater vegetative biomass when they do flower (see fig. 6 of Kelly 2008). Larger flowers have greater reproductive capacity, producing more pollen and more ovules (Kelly 2008; Lee 2009), and may also recruit more pollinators (Martin 2004; Sandring & Agren 2009). However, the delay in flowering associated with larger flower size can be costly in alpine field sites such as Browder Ridge where the availability of water diminishes over the season and drought is the primary cause of mortality.
The fact that flower size is a direct function of body size in annual monkeyflowers suggests a broader relevance of these results. Strong positive correlations between body size and fecundity are frequently observed in both plants and animals (Kingsolver & Pfennig 2004), but there are several reasons why the overall direction of selection may favour small or intermediate size. Most relevant to the present study is the increased mortality of juveniles owing to longer development. In animals, longer development means elongated exposure to predation, parasitism or starvation before reproductive maturity (Blackenhorn 2000). Also, mate attraction traits are often correlated with adult size and such traits routinely exhibit strong positive correlations with mating success (Hews 1990; Grether 1996; Preziosi & Fairbairn 1996; Burrowes 2000). However, if attraction traits are genetically correlated with development time, then viability selection on the invisible fraction might impede sexual selection. At the very least, a failure to account for pre-adult viability can lead to a misleading characterization of natural selection.
(b). Genotypic manipulation as a tool for measuring natural selection
Our experimental design is a variant of the genotypic transplant method (Rausher 1992; Willis 1996). Measuring selection on individuals of known genotype, or of known ancestry within a breeding design, was suggested to address the problem of environmentally induced covariances between traits and fitness measures (Rausher & Simms 1989; Stinchcombe et al. 2002). Bias is reduced if trait breeding values are used to predict fitness instead of phenotypic values. Breeding value estimates can be obtained by averaging individuals from a genotype or family. Given that this sample must be random, the breeding value regression is also subject to the problem of viability selection prior to trait measurement, although averaging probably reduces the magnitude of this difficulty (see Hadfield 2008 for a detailed consideration). In this study, each of our ‘genotypes’ was a genetically diverse collection of plants classified a priori for flower size. Flower size means observed in the field study followed our prior expectation (low < control < high).
This experiment does not identify causal relationships between particular traits and fitness because our genotypes differ in multiple features simultaneously (e.g. overall plant size, flower size and development time; see table 2 of Kelly 2008). In principle, putative causal relationships can be distinguished statistically. Selection gradients estimate the ‘direct’ effect of the trait on fitness while statistically controlling for other measured characters (Lande & Arnold 1983). Fenster & Ritland (1994) measured overall plant size in a study of selection on floral traits of M. guttatus. In two populations (Guenoc and Hough Springs), these authors obtained positive selection differentials on corolla width and corolla length, indicating a positive overall association of flower size with fecundity. However, when overall plant size was included with other traits in a multiple regression predicting seed set, the selection gradients on the flower size traits became significantly negative. It is possible that the data of figure 2 might produce a negative selection gradient on corolla width if plant size or ovule number were included as additional predictors in the regression.
From a genetic perspective, the observed negative correlation between female reproductive success and viability probably represents an example of antagonistic pleiotropy. While genetic correlations can also result from linkage disequilibria (Falconer & Mackay 1996), several features of this system favour pleiotropy. Most notably, there is a clear developmental connection between flower size and development rate. Antagonistic pleiotropy has been invoked as an explanation for the evolution of late-life fecundity (Rauser et al. 2006), age and size of sexual maturation (Basolo 2008), senescence (Curtsinger et al. 1994; Williams & Day 2003), and the maintenance of genetic variation (Charlesworth & Hughes 2000). Given that flower size exhibits abundant genetic variation within the Iron Mountain population and that this variation cannot be explained by mutation–selection balance (Kelly & Willis 2001; Kelly 2003; Lee 2009), antagonistic pleiotropy emerges as a potentially important mechanism. Beyond Mimulus, this study contributes to the very limited field data on antagonistic pleiotropy.
Blackenhorn (2000) motivated his review of natural selection on body size with the question ‘what keeps organisms small?’ Our study provokes the opposite question: what keeps flowers large? Across years, the large flower genotypes consistently had the lowest overall fitness. The low genotypes had the highest fitness average in two years, but the controls were actually highest in one year. Environmental fluctuations may determine the evolutionary balance between viability and fecundity selection (e.g. Schluter et al. 1991; Childs et al. 2004). Also, we admit that our study did not include one critical component of fitness: outcross siring success. Few studies have considered this variable, but the available data suggest that differential siring success may generate selection on flower size (Bell 1985; Stanton et al. 1986; van Kleunen & Burczyk 2008). Also, because M. guttatus is a self-compatible hermaphrodite, female fecundity may include both outcrossed and self-fertilized seed. If flower size influences the partitioning of reproductive effort between outcrossing and selfing, this may be another avenue of natural selection on this trait. Experiments are ongoing to determine the effect of flower size on outcross siring success and selfing rate.
Acknowledgements
We thank Helen Alexander, Alison Scoville, Vanessa Koelling and two very thorough anonymous reviewers for their helpful comments on the manuscript. We are also grateful to Alan Kelly, Kendra Koch, Shane Easter, Cory Wallace, Terra Lubin and Marcia Trenary for assistance with field or laboratory work. This research was supported by NIH grant GM073990 and NSF grant DEB-0543052.
References
- Armbruster W. S., Antonsen L., Pelabon C.2005Phenotypic selection on Dalechampia blossoms: honest signalling affects pollination success. Ecology 86, 3323–3333 [Google Scholar]
- Basolo A. L.2008Evolution of pleiotropic alleles for maturation and size as a consequence of predation. Biol. Lett. 4, 200–203 (doi:10.1098/rsbl.2007.0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G.1985On the function of flowers. Proc. R. Soc. Lond. B 224, 223–265 (doi:10.1098/rspb.1985.0031) [Google Scholar]
- Bennington C. C., McGraw J. B.1995Phenotypic selection in an artificial population of Impatiens pallida: the importance of the invisible fraction. Evolution 49, 317–324 (doi:10.2307/2410342) [DOI] [PubMed] [Google Scholar]
- Blackenhorn W. U.2000The evolution of body size: what keeps organisms small? Q. Rev. Biol. 75, 385–407 [DOI] [PubMed] [Google Scholar]
- Bonner J. T.1988The evolution of complexity. Princeton, NJ: Princeton University Press [Google Scholar]
- Burrowes P. A.2000Parental care and sexual selection in the Puerto Rican cave-dwelling frog, Eleutherodactylus cooki. Herpetologica 56, 375–386 [Google Scholar]
- Charlesworth B., Hughes K. A.2000The maintenance of genetic variation in life history traits. In Evolutionary genetics from molecules to morphology (eds Singh R. S., Krimbas C. B.), pp. 369–392 Cambridge, UK: Cambridge University Press [Google Scholar]
- Childs D. Z., Rees M., Rose K. E., Grubb P. J., Ellner S. P.2004Evolution of size-dependent flowering in a variable environment: construction and analysis of a stochastic integral projection model. Proc. R. Soc. Lond. B 271, 425–434 (doi:10.1098/rspb.2003.2597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope E. D.1896The primary factors of organic evolution. Chicago, IL: Open Court Publishing [Google Scholar]
- Curtsinger J. W., Service P. M., Prout T.1994Antagonistic pleiotropy, reversal of dominance, and genetic polymorphism. Am. Nat. 144, 210–228 (doi:10.1086/285671) [Google Scholar]
- Delph L. F., Gehring J. L., Frey F. M., Arntz A. M., Levri M.2004Genetic constraints on floral evolution in a sexually dimorphic plant revealed by artificial selection. Evolution 58, 1936–1946 (doi:10.1111/j.0014-3820.2004.tb00481.x) [DOI] [PubMed] [Google Scholar]
- Endler J. A.1986Natural selection in the wild. Princeton, NJ: Princeton University Press [Google Scholar]
- Falconer D. S., Mackay T. F. C.1996Introduction to quantitative genetics. London, UK: Prentice Hall; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster C. B., Ritland K.1994Evidence for natural-selection on mating system in Mimulus (Scrophulariaceae). Int. J. Plant Sci. 155, 588–596 (doi:10.1086/297197) [Google Scholar]
- Fishman L., Willis J. H.2008Pollen limitation and natural selection on floral characters in the yellow monkeyflower, Mimulus guttatus. New Phytol. 177, 802–810 (doi:10.1111/j.1469-8137.2007.02265.x) [DOI] [PubMed] [Google Scholar]
- Grafen A.1988On the uses of data on lifetime reproductive success. In Reproductive success (ed. Clutton-Brock T. H.), pp. 454–471 Chicago, IL: University of Chicago Press [Google Scholar]
- Grether G. F.1996Sexual selection and survival selection on wing coloration and body size in the rubyspot damselfly Hetaerina americana. Evolution 50, 1939–1948 (doi:10.2307/2410752) [DOI] [PubMed] [Google Scholar]
- Hadfield J. D.2008Estimating evolutionary parameters when viability selection is operating. Proc. R. Soc. B 275, 723–734 (doi:10.1098/rspb.2007.1013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. C., Willis J. H.2006Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 60, 2466–2477 (doi:10.1554/05-688.1) [PubMed] [Google Scholar]
- Hereford J., Hansen T. F., Houle D.2004Comparing strengths of directional selection: how strong is strong? Evolution 58, 2133–2143 (doi:10.1554/04-147) [DOI] [PubMed] [Google Scholar]
- Hews D. K.1990Examining hypotheses generated by field measures of sexual selection on male lizards, Uta palmeri. Evolution 44, 1956–1966 (doi:10.2307/2409606) [DOI] [PubMed] [Google Scholar]
- Kelly J. K.2003Deleterious mutations and the genetic variance of male fitness components in Mimulus guttatus. Genetics 164, 1071–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. K.2008Testing the rare-alleles model of quantitative variation by artificial selection. Genetica 132, 187–198 (doi:10.1007/s10709-007-9163-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. K., Willis J. H.2001Deleterious mutations and genetic variation for flower size in Mimulus guttatus. Evolution 55, 937–942 (doi:10.1554/0014-3820(2001)055[0937:DMAGVF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Kingsolver J., Pfennig D. W.2004Individual-level selection as a cause of Cope's rule of phyletic size increase. Evolution 58, 1608–1612 [DOI] [PubMed] [Google Scholar]
- Kingsolver J. G., Hoekstra H. E., Hoekstra J. M., Berrigan D., Vignieri S. N., Hill C. E., Hoang A., Gibert P., Beerli P.2001The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261 (doi:10.1086/319193) [DOI] [PubMed] [Google Scholar]
- Lande R.1979Quantitative genetic analysis of multivariate evolution, applied to brain–body size allometry. Evolution 33, 402–416 (doi:10.2307/2407630) [DOI] [PubMed] [Google Scholar]
- Lande R., Arnold S.1983The measurement of selection on correlated characters. Evolution 37, 1210–1226 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- Lee Y. H.2009Genetic analysis of standing variation for floral morphology and fitness components in a natural population of Mimulus guttatus(common monkeyflower). Durham, NC: Duke University [Google Scholar]
- Lehtila K., Brann K. H.2007Correlated effects of selection for flower size in Raphanus raphanistrum. Can. J. Bot. Rev. Can. Bot. 85, 160–166 (doi:10.1139/B07-007) [Google Scholar]
- Lendvai G., Levin D. A.2003Rapid response to artificial selection on flower size in Phlox. Heredity 90, 336–342 (doi:10.1038/sj.hdy.6800249) [DOI] [PubMed] [Google Scholar]
- Martin N. H.2004Flower size preferences of the honeybee (Apis mellifera) foraging on Mimulus guttatus (Scrophulariaceae). Evol. Ecol. Res. 6, 777–782 [Google Scholar]
- Medel R., Botto-Mahan C., Kalin-Arroyo M.2003Pollinator-mediated selection on the nectar guide phenotype in the Andean Monkey flower, Mimulus luteus. Ecology 84, 1721–1732 [Google Scholar]
- Mitchell-Olds T., Shaw R. G.1987Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41, 1149–1161 (doi:10.2307/2409084) [DOI] [PubMed] [Google Scholar]
- Murren C. J., Chang C. C., Dudash M. R.2009Patterns of selection of two North American native and nonnative populations of monkeyflower (Phrymaceae). New Phytol. 183, 691–701 (doi:10.1111/j.1469-8137.2009.02928.x) [DOI] [PubMed] [Google Scholar]
- Preziosi R. F., Fairbairn D. J.1996Sexual size dimorphism and selection in the wild in the waterstrider Aquarius remigis: body size, components of body size and male mating success. J. Evol. Biol. 9, 317–336 (doi:10.1046/j.1420-9101.1996.9030317.x) [Google Scholar]
- Rauser C. L., Tierney J. J., Gunion S. M., Covarrubias G. M., Mueller L. D., Rose M. R.2006Evolution of late-life fecundity in Drosophila melanogaster. J. Evol. Biol. 19, 289–301 (doi:10.1111/j.1420-9101.2005.00966.x) [DOI] [PubMed] [Google Scholar]
- Rausher M. D.1992The measurement of selection on quantitative traits: biases due to the environmental covariances between traits and fitness. Evolution 46, 616–626 (doi:10.2307/2409632) [DOI] [PubMed] [Google Scholar]
- Rausher M. D., Simms E. L.1989The evolution of resistance to herbivory in Ipomoea purpurea. I. Attempts to detect selection. Evolution 43, 563–572 (doi:10.2307/2409059) [DOI] [PubMed] [Google Scholar]
- Sandring S., Agren J.2009Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution 63, 1292–1300 (doi:10.1111/j.1558-5646.2009.00624.x) [DOI] [PubMed] [Google Scholar]
- Schluter D., Price T. D., Rowe L.1991Conflicting selection pressures and life-history trade-offs. Proc. R. Soc. Lond. B 246, 11–17 (doi:10.1098/rspb.1991.0118) [Google Scholar]
- Sinervo B., McAdam A. G.2008Maturation costs of reproduction due to clutch size and ontogenetic conflict as revealed in the invisible fraction. Proc. R. Soc. B 275, 629–638 (doi:10.1098/rspb.2007.1084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M. L., Snow A. A., Handel S. N.1986Floral evolution—attractiveness to pollinators increases male fitness. Science 232, 1625–1627 (doi:10.1126/science.232.4758.1625) [DOI] [PubMed] [Google Scholar]
- Stinchcombe J. R., Rutter M. T., Burdick D. S., Tiffin P., Rausher M. D., Mauricio R.2002Testing for environmentally induced bias in phenotypic estimates of natural selection: theory and practice. Am. Nat. 160, 511–523 (doi:10.1086/342069) [DOI] [PubMed] [Google Scholar]
- van Kleunen M., Burczyk J.2008Selection on floral traits through male fertility in a natural plant population. Evol. Ecol. 22, 39–54 [Google Scholar]
- van Kleunen M., Ritland K.2004Predicting the evolution of floral traits associated with mating system in a natural plant population. J. Evol. Biol. 17, 1389–1399 (doi:10.1111/j.1420-9101.2004.00787.x) [DOI] [PubMed] [Google Scholar]
- Wade M. J., Kalisz S.1990The causes of natural selection. Evolution 44, 1947–1955 (doi:10.2307/2409605) [DOI] [PubMed] [Google Scholar]
- Williams P. D., Day T.2003Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution 57, 1478–1488 [DOI] [PubMed] [Google Scholar]
- Willis J. H.1996Measures of phenotypic selection are biased by partial inbreeding. Evolution 50, 1501–1511 (doi:10.2307/2410887) [DOI] [PubMed] [Google Scholar]
- Worley A. C., Barrett S. C. H.2000Evolution of floral display in Eichhornia paniculata (Pontederiaceae): direct and correlated responses to selection on flower size and number. Evolution 54, 1533–1545 [DOI] [PubMed] [Google Scholar]
- Wright J. W., Stanton M. L.2007Collinsia sparsiflora in serpentine and nonserpentine habitats: using F2 hybrids to detect the potential role of selection in ecotypic diferentiation. New Phytol. 173, 354–366 [DOI] [PubMed] [Google Scholar]