Abstract
In many butterfly species, males compete over areas advantageous for encountering females. Rules for contest settlement are, however, largely unknown and neither morphological nor physiological traits can reliably predict the contest outcome. Here, we test the hypothesis that contests are settled in accordance with a motivation asymmetry. We staged contests between males of Pararge aegeria and after removing the resident, the non-resident was allowed (i) either to interact with a non-receptive female for 30 min (n = 30) or (ii) to spend 30 min alone in the cage (n = 30), after which the initial resident was reintroduced. The results show that males that had interacted with a female had a higher probability of becoming dominant and reversing contest outcome. Moreover, males that were faster to take over a vacant territory when the resident was removed were more likely to become dominant. Here, we show for the first time, to our knowledge, that frequent encounters with a mated female can increase male motivation to persist in a territorial contest in a butterfly. Further, we suggest that variation in intrinsic motivation reflects male eagerness to take over vacant territory. This study indicates that variation in resource value and motivational asymmetries are important for settling contests in butterflies.
Keywords: sexual selection, Lepidoptera, mate locating behaviour, loser effect, resource-holding potential
1. Introduction
Variation in the ability to take over and defend valuable resources often results in variation in fitness and is thus a keystone in understanding the evolution of animal behaviour. How disputes between a territory resident and an intruder are settled is crucial to understand the distribution of matings, and thereby the evolutionary processes within the population. Customarily, the resident wins contests against intruders and the possible explanations for this have gained considerable scientific attention (Alcock 2001; Kemp & Wiklund 2001). A common predictor for contest outcome is an asymmetry in fighting ability (cf. resource holding potential; Parker 1974), where one of the combatants is better able to fight and defend the resource. The asymmetry in fighting ability is often correlated with morphological and physiological attributes, such as body size, weaponry and ornaments (Huntingford & Turner 1987). But theory suggests that resident–intruder asymmetry per se can also be used as an arbitrary cue and the contest may then be settled by the convention ‘resident wins, intruder retreats’ (Maynard Smith & Parker 1976). A third hypothesis in how animals settle conflicts is an asymmetry in how the resident and the intruder value the resource, where one of the combatants may value the resource more highly than its opponent does (Enquist & Leimar 1987).
Many butterfly species are territorial, where the males defend areas where the probability of encountering receptive females is particularly high (Kemp & Wiklund 2001). Territorial contests are remarkably similar across butterfly species and consist of non-contact aerial contests in which the two males circle near each other until one of them gives up (Kemp & Wiklund 2001, 2004). The circling flight component can be found in male–male interactions in many territorial butterflies and can last up to several minutes (e.g. Wickman & Wiklund 1983; Rutowski 1992; Kemp & Wiklund 2004; Kemp et al. 2006a; Takeuchi 2006a; Takeuchi & Honda 2009). Detailed studies of many butterfly species suggest that the circling flight is to be considered as the true ‘war of attrition’ contest component (Kemp & Wiklund 2001, 2004). The circling flight is often followed by a horizontal pursuit in which the winner pursues the loser and chases him away from the contested area before he eventually returns (Kemp & Wiklund 2001, 2004). The two components of the territorial contest, the circling flight and the horizontal pursuit, are clearly distinguishable and easy to tell apart (Knapton 1985; Kemp 2000; Kemp & Wiklund 2004). Territorial contests are usually initiated when an intruder flies into another male's occupied area, and often result in the above described two contest phases; however, if one of the males is reluctant to engage in a circling flight, the contest can be settled solely by a horizontal chase (Kemp & Wiklund 2004).
Contests settlement in butterflies has been extensively studied over the last decades and several hypotheses have been empirically tested. Davies (1978) tested if resident and intruder roles are used as conventional cues for rapid settlement of contests in Pararge aegeria and found that resident males virtually always won contests with intruding males. Later studies have, however, shown that the resident does not always win (Wickman & Wiklund 1983; Stutt & Willmer 1998; Kemp & Wiklund 2004), even though residency has proved to be a good predictor of contest outcome in nature. Although many studies indicate that in butterfly contests there is an asymmetry between individuals not only in resident/intruder roles, but also in physiology/morphology, there is still no real consensus on how different physical attributes affect contest outcome in butterflies. For instance, in some species there is a positive correlation between body size and contest outcome, where larger males are more successful in territorial contests (Rosenberg & Enquist 1991; Martínez-Lendech et al. 2007; Peixoto & Benson 2008), while in other species the opposite pattern prevails (Hernández & Benson 1998). However, there are also several species, including P. aegeria, where body size does not affect contest outcome (Lederhouse 1982; Kemp 2000; Takeuchi 2006a,b; Bergman et al. 2007). Age can also influence the outcome of territorial contest, with older males having higher contest persistence in some species (Kemp 2002), while younger males have an advantage in other species (Kemp 2003). Yet, in some other species, including P. aegeria, age has no or little effect on contest resolution (Kemp et al. 2006a; Takeuchi 2006b; Bergman et al. 2007).
Motivational asymmetry is a well-known phenomenon in animal fighting theory but has gained little attention in butterfly contest research. Theory postulates that resident individuals will win frequently because they stand to gain a higher pay-off if successful, because of the time and energy invested in establishing and defending the resource (cf. the ‘dear enemy’ phenomenon, Temeles 1994). Residents might also win more frequently because of an asymmetry in information of resource value, where the resident is often better informed about the resource than the intruder, and places greater subjective value on the contested area (Enquist & Leimar 1987). Kemp & Wiklund (2001) argued that the latter might be potentially relevant in territorial butterfly species. Resident males could conceivably assess the value of the contested area if there are reliable indicators of the potential rate of encountering receptive females. Such indicators could be the encounter frequencies of females or conspecific males (Kemp & Wiklund 2001). In crickets, it is well known that motivation is of great importance in contest resolution (Hofmann & Stevenson 2000; Hofmann & Schildberger 2001; Brown et al. 2007). Male variation in motivation to compete for a resource is probably influenced by intrinsic factors, but has also been shown to be strongly correlated to extrinsic factors such as encounters with females and previous mating success (Killian & Allen 2008).
Males of the speckled wood butterfly, P. aegeria, establish territories in large sunspots on the forest floor in open forest habitat (Davies 1978; Wickman & Wiklund 1983; Shreeve 1987; Van Dyck et al. 1997; Bergman & Wiklund 2009a). Males engage in contests over these well-defined sunlit areas. While the winner becomes resident at the sunspot, the loser is chased away and will continue his search for a suitable sunspot; if suitable sunspots are in short supply, the male will alight in a smaller, suboptimal sunspot (Bergman & Wiklund 2009b). Ever since Davies (1978) tested the uncorrelated asymmetry hypothesis using the speckled wood butterfly as focal species, P. aegeria has become something of a model species for studies on territorial behaviour in butterflies and some of the most cutting-edge theories about contest evolution have been empirically tested using this species (e.g. Wickman & Wiklund 1983; Stutt & Willmer 1998; Kemp & Wiklund 2004; Kemp et al. 2006a,b; Bergman et al. 2007).
Here, we use the speckled wood butterfly (P. aegeria) as a model to test the effect of motivational state on contest outcome by manipulating the perceived value of the territory for a male by allowing one group of males to repeatedly encounter a female during a 30 min territorial residency, while another group of males spent the 30 min territorial residence alone, and thereafter testing the effect on contest outcome and duration.
2. Material and methods
(a). Experimental cages
The experiments were performed in outdoor cages, located at Kronängen, approximately 100 km south of Stockholm in central Sweden. The cages were semi-cylindrical, tunnel shaped, with an 8 × 8 m base and a 4 m radius to the roof. The roof was covered with a net and a green tarpaulin. In each cage, we removed a 2 × 2 m section of the tarpaulin to create one large sunspot on the cage floor. We additionally removed several smaller 0.2 × 0.2 m sections of the tarpaulin to create a mosaic of smaller sun flecks on the cage floor. The floor of the cages consisted of unmown native grass.
(b). Experimental trials
The experiments were performed during June to September 2008 and May to June 2009. In the experiments, we used a population of P. aegeria, originating from Madeira, Portugal. The butterflies were reared as a laboratory stock population at Stockholm University and brought to Kronängen in coolers. The microclimate conditions during the experiments, with a temperature range from 18°C to 31°C and a mean temperature of 25°C, corresponds to the temperatures naturally occurring on Madeira and is within the temperature range reported in earlier field studies of territorial behaviour in P. aegeria (Shreeve & Smith 1992; Jones et al. 1998). The experimental trials were performed following a three-step programme. In the first step, we introduced two males simultaneously in the large sunspot. Invariably, the two males engaged in a territorial contest, whereupon one of the males would establish himself as resident in the large sunspot, while the other male would be chased away from the large sunspot. However, not all interactions ended in a clear winner/loser outcome, and so to ascertain the true dominance relationship between the two males, we used a minimum of five won contests in a row before one male was considered to be dominant over the other. This meant that the total number of interactions before a resident–non-resident relationship was established varied between dyads of males. We staged territorial contests between a total of 120 males and recorded the duration of each contest between these 60 dyads of males using a stopwatch. When we in this study refer to contest duration, this implies only the circling flight component, i.e. the escalated contest phase, which is the true war of attrition phase in butterfly conflicts (Kemp & Wiklund 2004). In the second step, we removed the resident male and placed him in a cooler and applied our experimental treatment by assigning males to one of the two treatments, by allowing the loser male either to (i) interact with a female during a 30 min period, or (ii) to spend the 30 min period alone in the cage. In the first group, 30 males were allowed to interact with a mated female; this group of males is referred to as the ‘female encounter treatment group’. The males were free to interact with the female for 30 min, but if the male did not encounter the female within 5 min, we brought the female to the male to initiate an interaction. We did this every fifth minute, if the male had not interacted with the female during the preceding 5 min period. By doing this, we made certain that the male encountered the female at least five times during the 30 min period. In the other group of 30 males, referred to as the control group, the original loser was alone in the cage, following the removal of the original winner, for a 30 min period. In both groups we recorded the time it took for a non-resident male to alight in the large sunspot after the removal of the resident male. In the third, and final step, we reintroduced the original resident male into the cage; we did this to ascertain whether the original resident would regain ownership of the large sunspot, and to see whether the treatment of the original losers had had any effect on the contest outcome after the original loser had spent 30 min as sole male in the cage, and had either met a female every 5 min, or had been all alone in the cage. In the third step of each experimental trial, we recorded the duration of each contest and, as before during the first step, ruled that a male was considered resident of the large sunspot after winning five contests in a row. Although the number of interactions between males in dyads in the first step was variable, it had no effect on the probability of a reversed contest outcome in the third experimental step (Mann–Whitney U-test: Z = −0.30; p = 0.76). To make certain that time of day or season did not bias the experiments, we consistently alternated between control group trials and female encounter group trials during each day when we performed the experiments.
(c). Statistical analysis
The data on contest duration were log-transformed to meet the assumptions of normality. To analyse the differences in contest duration between the two groups (female encounter group and control group) and between the first and second male–male encounter periods, we used a repeated-measure ANOVA with contest duration as the dependent variable, male–male encounter period as a repeated measure and group as a categorical factor. Here, we used the mean duration of all escalated contests in one male–male encounter period.
The data on the time it took for a non-resident male to alight in the large sunspot after the resident males had been removed did not meet the assumption of normality, and so the non-parametric Mann–Whitney U-test was applied for the analysis.
3. Results
(a). Contest outcome
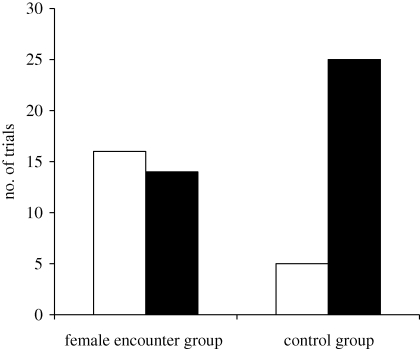
Males that interacted with a female for 30 min were more likely to later defeat the original resident male (figure 1; Fisher's exact two-tailed: p = 0.0061). In 16 of 30 trials there was a reversed contest outcome after the 30 min of interactions with a female. In the control group, only five of 30 trials ended with a reversed contest outcome, where the original non-resident male became resident (figure 1).
Figure 1.
The outcome of contests between males of P. aegeria during the second contest period when the original winner had been reintroduced, and after the original losers had either interacted with a female during 30 min (female encounter group, n = 30) or been alone for 30 min (control group, n = 30); ‘reversal’ (open bars) denotes that the male that lost the contest during the first contest period reversed the outcome and won the contest against the original winner in the second contest period, and ‘no reversal’ (filled bars) denotes that the same male won in both contest periods.
(b). Contest duration
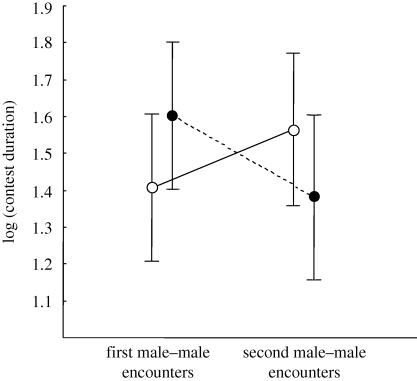
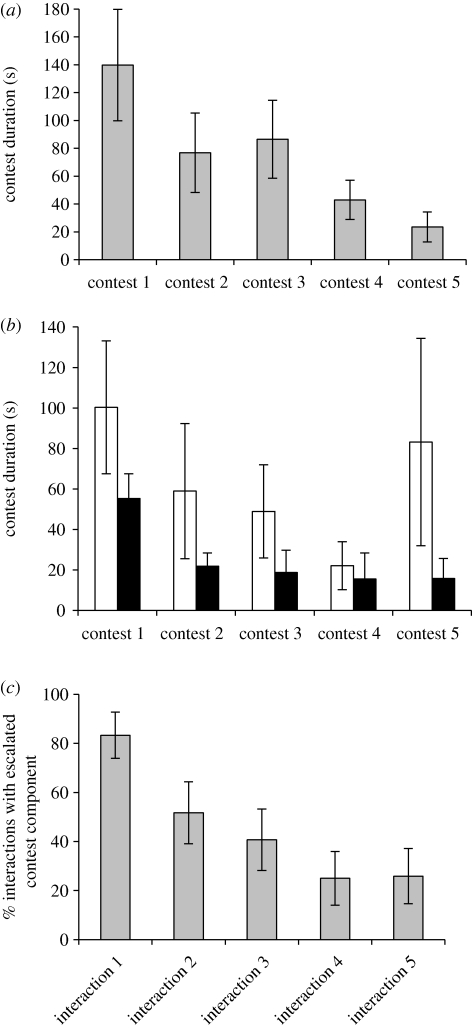
There was an effect of the female encounter treatment on contest duration, whereas contest duration between male dyads in the control group was shorter during the second period of male–male encounters, contest duration between male dyads increased in the female encounter treatment group (figure 2; repeated-measure ANOVA: male–male encounter period: F1,50 = 0.68, p = 0.41; group: F1,50 = 0.11, p = 0.74; male–male encounter period × group: F1,50 = 5.26, p = 0.026). Since we used a minimum of five contests won in a row before one male was considered dominant, males in the dyads engaged in at least five contests, but usually in more. During the first period of encounters between males in a dyad there was a decrease in contest duration with the first contest lasting on average 139.7 ± 40.1 s and the fifth contest lasting on average 23.5 ± 10.7 s (figure 3a). During the second period of encounters between males in a dyad, there was a decrease in contest duration as well, with the first contest lasting the longest (figure 3b). There was also a decrease in the proportion of interactions that included the true contest component as the number of interactions that consisted solely of a horizontal pursuit increased (figure 3c); in the first interaction, 17 per cent of all trials were settled by a short horizontal chase only, while in the fifth interaction between two males, 75 per cent of the trials were settled by a short horizontal chase only.
Figure 2.
The duration of contests during two contest periods; the second period after the males had interacted with a female for 30 min (female encounter group, open circles) or had been alone for 30 min (control group, filled circles). Values are given as mean and 95% CI.
Figure 3.
(a) Contest duration in five subsequent contests between males of P. aegeria. Since not all dyads in the experiment (n = 60) included five escalated contests there is variation in sample size: ncontest 1 = 60; ncontest 2 = 48; ncontest 3 = 35; ncontest 4 = 24; ncontest 5 = 19. Values are given as mean ± s.e. (b) Contest duration in five subsequent contests between males of P. aegeria in the second contest period, after the males had interacted with a female for 30 min (female encounter group, open bars) or been alone for 30 min (control group, filled bars). Since not all dyads in the experiment included five escalated contests, the sample sizes for the female encounter group are: ncontest 1 = 28; ncontest 2 = 22; ncontest 3 = 14; ncontest 4 = 8; ncontest 5 = 5, and the sample size for the control group are: ncontest 1 = 24; ncontest 2 = 18; ncontest 3 = 11; ncontest 4 = 6; ncontest 5 = 5. Values are given as mean ± s.e. (c) The proportion of trials that included a true, escalated, contest component of circling flights during the five first interactions between males of P. aegeria. If no circling flight occurred, the contests were settled only by a shorter horizontal pursuit. Values are given as proportions ± 95% CI.
(c). Courtship
An interaction between a male and a mated female invariably started with the male detecting the female in flight and the male taking off in pursuit of the flying female; these pursuits lasted on average 3.75 ± 0.73 s and ended with the female alighting, often as a vertical drop into the grass on the cage floor. These pursuits were much shorter than those that involve virgin females, which last on average 15.9 ± 4.8 s (M. Bergman 2010, unpublished data). The male would alight in close association to the female and start courting her for on average 69.7 ± 10.6 s. Since each trial in the female encounter treatment group consisted of at least five male–female interactions, we noticed a decrease in courtship duration where the first courtship was longest, on average 109.1 ± 19.9 s, while the fifth courtship was the shortest with an average of 22.5 ± 5.5 s. However, there was no difference in courtship duration (averaged over the five encounters) for males that later won the following contests with the original winner and those that did not (Nreversal = 16; Nno reversal = 13; t-test: t = 0.21, p = 0.84).
(d). Establishment as a new resident
When we removed the resident male from the large sunspot, there was some variation in how long it took for the non-resident male to alight in the large sunspot and establish himself as a new resident. Some males alighted in the large sunspot within a few seconds, while other males waited several minutes, indeed up to 23 min, before settling in the large sunspot. Nevertheless, all non-resident males eventually landed in the large sunspot. In the female encounter treatment group, males that later reversed contest outcome and took over the large sunspot from the former resident were quicker to establish themselves in the large sunspot than males that did not reverse contest outcome and lost contests with the former resident also during the second period of male–male encounters (Mann–Whitney U-test: Z = −2.90; p = 0.004). There was also an overall difference between the two groups, where males in the female encounter treatment group were faster in establishing themselves as new residents in the large sunspot (Mann–Whitney U-test: Z = −2.97; p = 0.003).
4. Discussion
Here, we show that encounters with a mated female increase a male's motivation to persist and ultimately win territorial contests in a butterfly. About half of the males that were allowed repeated interactions with a female managed to reverse the dominance relationship and become new residents when interacting with the former dominant male. In the control group that did not interact with a female and spent a 30 min period as an isolated male in the experimental cage, only 17 per cent of the males reversed the outcome in a new territorial fight with the initial dominant male. Hence, the repeated interactions with a female increased the motivation of the former subordinate males to persist in territorial contests.
It is known when two males interact, the outcome of previous fights and interactions with other individuals could have an impact on future behaviour. In a system with resident and intruder roles, there is often an information asymmetry where the resident is better informed about the quality of the territory (Enquist & Leimar 1987). The ability to adjust the level of aggression in relation to the resource value should then be strongly selected for. The higher subjective value an individual places on a resource, the greater the cost the animal is prepared to pay to gain the resource (Enquist & Leimar 1987; Arnott & Elwood 2008). In P. aegeria, the rate at which a resident male encounters other butterflies is a good indicator of the probability of also encountering receptive virgin females, i.e. a good predictor of territory quality. Davies (1978) found that in nature, resident males encountered mated egg-laying females at a higher rate than non-resident males. Furthermore, a recent study has shown that resident males of P. aegeria were more likely to pursue, court and successfully mate with a female compared with a subordinate, non-resident male (Bergman et al. 2007). It is likely that a resident male will then place a greater subjective value on high-quality territories and be prepared to engage in longer contests over their control. We believe that this explains the results in our experiments. When the original non-resident male in our experiment established himself as new resident and was allowed to interact with a mated female, this most likely increased the male's assessment of the sunspot as being a high quality one; hence, the male most likely put a higher subjective value on the sunspot and was more motivated to persist in the coming interaction with the original resident. Similar effects, where female interactions increase the perceived territory value, have been demonstrated in other insects. Subordinate males of the cricket Acheta domesticus that were allowed to interact with a female become more aggressive and also become dominant (Killian & Allen 2008).
There are a few possible mechanisms explaining why residents may put a higher value on a contested territory (Kemp & Wiklund 2001). The subjective value might be higher because of an information asymmetry, where the resident is better informed about the quality of the territory (Enquist & Leimar 1987). Resident males might also value the territory higher because they have a higher pay-off to gain if successful. There is often a considerable cost for intruders to establish a territory owing to relations with neighbouring territory residents, where it takes time to establish territory borders. A resident male then has a relatively lower cost in defending the territory (the ‘dear enemy’ phenomenon), and the subjective value of the territory should then be correlated to residency experience. However, Kemp & Wiklund (2001) argued that this would be rare in nature since butterfly territories are small and discrete and individuals appear unable to recognize neighbours. However, males of the lycaenid butterfly Chrysozephyrus smaragdinus that had been residents for prolonged periods did tend to win more contests (Takeuchi & Honda 2009). Nevertheless, the two mechanisms might be difficult to disentangle in field studies, since long residency times enable males to secure better information on territory quality. Our experimental design, with a residency time of 30 min in both the female encounter group and the control group, allows us to conclude that the increase in motivation in our experiments is caused by male interactions with a female, and not by residency time.
There was also some contest outcome reversals in the control group, where the second male–male encounter settled with the original non-resident as winner in five of the 30 trials (figure 1). Such shifts of territory ownership have also been observed in the field. Wickman & Wiklund (1983) found that when the resident P. aegeria male left the sunspot, another male often settled in the vacant sunspot. When the original resident later returned, he invariably won back the sunspot territory from the intruder. Reversals of contest outcome in subsequent encounters between the same two individuals have also been documented in experimental studies with a similar experimental procedure as used in this study. Kemp & Wiklund (2004) conducted two-stage experiments to test the uncorrelated asymmetry hypothesis (cf. Maynard Smith & Parker 1976; Davies 1978; Kemp & Wiklund 2001), where the winner of a first encounter was allowed to adopt the intruder role in a second encounter. In agreement with our results, and in clear contradiction to the uncorrelated asymmetry hypothesis, they found that in 25 of 26 trials, the initial winner regained the territory after a period where the initial non-resident was granted sole ownership of the territory. The mechanism by which the initial subordinate male later can become dominant over the same opponent is unknown, but it is likely that the experimental design used here and by Kemp & Wiklund (2004) generates residency experience effects, where a time of sole ownership of the territory changes the motivational state of the initial loser.
We also found that encounters with females affected contest duration. In ‘war of attrition’ type of contests, as in butterflies, the contest is settled when one of the combatants surrenders and flees the area. The contest duration therefore reflects the intrinsic fighting ability and motivational state of the loser (Kemp 2000, 2003; Kemp et al. 2006a). Effects on contest duration have been documented in field studies. Wickman & Wiklund (1983) found that when a resident male returned to a sunspot from a voluntary absence, if the sunspot had been occupied by an intruder male, the contests were significantly longer than contests between the resident and a trespasser. A similar pattern has also been found in the butterfly C. smaragdinus, where males with long residency experience were more motivated to persist in territorial contests than males with a short residency experience (Takeuchi 2006a). Increased motivation to persist in contests over territories owing to long residency experience has also been shown in the tarantula hawk wasp, Hemipepsis ustulata (Alcock & Bailey 1997).
It is known that previous experience in territorial contests can have an effect on future contest success. Experience in winning might increase aggression and the probability of winning future contests, whereas the experience of losing might decrease aggression and the probability of winning future contests (Otronen 1988; Dugatkin 1997; Whitehouse 1997; Rutte et al. 2006). One possible explanation for the decrease in contest duration in this study (figure 3) is such a loser effect, where the experience in the previous contest affects the motivation to persist in the following contest. Each contest bout with the same outcome probably reinforces the asymmetry and increases the difference in the motivational state of the two males even further. A probable adaptive function of the loser effect is that a male can use previous contest experience to estimate his own contest ability in relation to other males in the population to avoid the costs of contests he is not likely to win (Whitehouse 1997; Rutte et al. 2006). For a subordinate butterfly male, it would be beneficial to avoid prolonged contests in subsequent interactions with a dominant male. Effects of previous contest experience have been found in earlier studies using P. aegeria. Kemp & Wiklund (2004) conducted trials where prior losers were staged in contests against each other and prior winners were staged in contests against each other, and they found that contests between prior winners were significantly longer than contests between prior losers. Furthermore, we contend that a loser effect also can explain the increased probability of reaching settlement by a shorter horizontal chase only (figure 3c). When interactions consist only of a horizontal chase, one of the males (usually the intruder) is evidently not prepared to fight for a territory but instead gives up quickly (Kemp & Wiklund 2001). The reinforcement of the motivational asymmetry owing to subsequent contests between two males is likely to generate such an effect in P. aegeria. A subordinate male's avoidance of further costly contests would be predicted by a loser effect and has been demonstrated in other insects (e.g. Iwasaki et al. 2006).
When we removed the initial resident from the large sunspot, the non-resident claimed the sunspot territory within 23 min in all 60 trials. However, the initial non-residents varied in the time taken to move to the large sunspot. Males in the female encounter group alighted sooner in the large sunspot than males in the control group. This is likely to be a consequence of the female encounters because after meeting and courting a female after the first 5 min of the 30 min period, the male almost invariably settled in the large sunspot, doing so for the first time in many cases; hence, it is conceivable that, following the interaction with a female, the males in the female encounter group were more prone to perch in the large sunspot owing to the same mechanisms affecting contest outcome, namely a higher perceived subjective value on the sunspot area. We also found that in the female encounter group, males that were particularly fast in taking over the vacant sunspot territory were also more likely to later reverse the contest outcome. This might reflect a variation in intrinsic motivation. The fact that not all trials in the female encounter group resulted in a reversed contest outcome (figure 1) implies that other asymmetries, in addition to the female-encounter based motivation asymmetry tested here, influence contest outcome in this species. Since there are no strong effects of asymmetry in intrinsic fighting ability based on morphological–physiological factors (Kemp et al. 2006a,b; Bergman et al. 2007), and since both males were naive with no contest experience (cf. ‘winner/loser effect’), we contend that an intrinsic motivation state can have a profound influence on contest outcome. Our results suggest that a high motivation to take over vacant sunspot territories also entails a high motivation to persist in territorial contests.
The role of motivation is a new and interesting approach in the research of butterfly contests. Even though these ideas have been previously presented and discussed (Kemp & Wiklund 2001) and recently gained some experimental attention (Fisher et al. 2008; Takeuchi & Honda 2009), they are still inadequately empirically tested. Our finding indicates that variation in resource value and motivational state are important factors in contest evolution, although future field studies could help us understand whether differences in motivation often play a role in contest resolution among butterflies.
Acknowledgements
We thank Magne Friberg, Melanie Gibbs, Karl Gotthard and Darrell Kemp for useful comments on the manuscript. This study was funded by a grant from the Swedish Research Council to C.W.
References
- Alcock J.2001Animal behavior: an evolutionary approach, 7th edn.Sunderland, MA: Sinauer [Google Scholar]
- Alcock J., Bailey W. J.1997Success in territorial defence by male tarantula hawk wasps Hemipepsis ustulata: the role of residency. Ecol. Entomol. 22, 377–383 (doi:10.1046/j.1365-2311.1997.00066.x) [Google Scholar]
- Arnott G., Elwood R. W.2008Information gathering and decision making about resource value in animal contests. Anim. Behav. 76, 529–542 (doi:10.1016/j.anbehav.2008.04.019) [Google Scholar]
- Bergman M., Wiklund C.2009aVisual mate detection and mate flight pursuit in relation to sunspot size in a woodland territorial butterfly. Anim. Behav. 78, 17–23 (doi:10.1016/j.anbehav.2009.02.005) [Google Scholar]
- Bergman M., Wiklund C.2009bDifferences in mate location behaviour between residents and nonresidents in a territorial butterfly. Anim. Behav. 78, 1161–1167 (doi:10.1016/j.anbehav.2009.08.003) [Google Scholar]
- Bergman M., Gotthard K., Berger D., Olofsson M., Kemp D. J., Wiklund C.2007Mating success of resident versus non-resident in a territorial butterfly. Proc. R. Soc. B 274, 1659–1665 (doi:10.1098/rspb.2007.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. D., Chimenti A. J., Siebert J. R.2007The payoff of fighting in house crickets: motivational asymmetry increases male aggression and mating success. Ethology 113, 457–465 (doi:10.1111/j.1439-0310.2007.01357.x) [Google Scholar]
- Davies N. B.1978Territorial defence in the speckled wood butterfly (Pararge aegeria): the resident always wins. Anim. Behav. 26, 138–147 (doi:10.1016/0003-3472(78)90013-1) [Google Scholar]
- Dugatkin L. A.1997Winner and loser effects and the structure of dominance hierarchies. Behav. Ecol. 8, 583–587 (doi:10.1093/beheco/8.6.583) [Google Scholar]
- Enquist M., Leimar O.1987Evolution of fighting behaviour: the effect of variation in resource value. J. Theor. Biol. 127, 187–205 (doi:10.1016/S0022-5193(87)80130-3) [Google Scholar]
- Fischer K., Perlick J., Galetz T.2008Residual reproductive value and male mating success: older males do better. Proc. R. Soc. B 275, 1517–1524 (doi:10.1098/rspb.2007.1455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M. I. M., Benson W. W.1998Small male advantage in the territorial tropical butterfly Heliconius sara (Nymphalidae): a paradoxical strategy? Anim. Behav. 56, 533–540 (doi:10.1006/anbe.1998.0840) [DOI] [PubMed] [Google Scholar]
- Hofmann H. A., Schildberger K.2001Assessment of strength and willingness to fight during aggressive encounters in crickets. Anim. Behav. 62, 337–348 (doi:10.1006/anbe.2001.1746) [Google Scholar]
- Hofmann H. A., Stevenson P. A.2000Flight restores fight in crickets. Nature 403, 613 (doi:10.1038/35001137) [DOI] [PubMed] [Google Scholar]
- Huntingford F. A., Turner A. K.1987Animal conflict. London, UK: Chapman & Hall [Google Scholar]
- Iwasaki M., Delago A., Nishino H., Aonuma H.2006Effects of previous experience on the agonistic behaviour of male crickets, Gryllus bimaculatus. Zool. Sci. 23, 863–872 [DOI] [PubMed] [Google Scholar]
- Jones M. J., Lace L. A., Harrison E. C., Stevens-Wood B.1998Territorial behavior in the speckled wood butterflies Pararge xiphia and P. aegeria of Madeira: a mechanism for interspecific competition. Ecography 21, 297–305 [Google Scholar]
- Kemp D. J.2000Contest behavior in territorial male butterflies: does size matter? Behav. Ecol. 11, 591–596 (doi:10.1093/beheco/11.6.591) [Google Scholar]
- Kemp D. J.2002Sexual selection constrained by life history in a butterfly. Proc. R. Soc. Lond. B 269, 1341–1345 (doi:10.1098/rspb.2002.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J.2003Twilight fighting in the evening brown butterfly, Melanitis leda (L.) (Nymphalidae): age and residency effects. Behav. Ecol. Sociobiol. 54, 7–13 (doi:10.1007/s00265-003-0602-7) [Google Scholar]
- Kemp D. J., Wiklund C.2001Fighting without weaponry: a review of male–male contest competition in butterflies. Behav. Ecol. Sociobiol. 49, 429–442 (doi:10.1007/s002650100318) [Google Scholar]
- Kemp D. J., Wiklund C.2004Residency effects in animal contest. Proc. R. Soc. Lond. B 271, 1707–1711 (doi:10.1098/rspb.2004.2775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Wiklund C., Gotthard K.2006aLife history effects upon contest behaviour: age as a predictor of territorial contest dynamics in two populations of the speckled wood butterfly, Pararge aegeria L. Ethology 112, 471–477 (doi:10.1111/j.1439-0310.2005.01173.x) [Google Scholar]
- Kemp D. J., Wiklund C., Van Dyck H.2006bContest behaviour in the speckled wood butterfly (Pararge aegeria): seasonal phenotypic plasticity and the functional significance of flight performance. Behav. Ecol. Sociobiol. 59, 403–411 (doi:10.1007/s00265-005-0064-1) [Google Scholar]
- Killian K. A., Allen J. R.2008Mating resets male cricket aggression. J. Insect Behav. 21, 535–548 (doi:10.1007/s10905-008-9148-x) [Google Scholar]
- Knapton R. W.1985Lek structure and territoriality in the chryxus arctic butterfly, Oeneis chryxus (Satyridae). Behav. Ecol. Sociobiol. 17, 389–395 (doi:10.1007/BF00293218) [Google Scholar]
- Lederhouse R. C.1982Territorial defence and lek behavior of the black swallowtail butterfly, Papilio polyxenes. Behav. Ecol. Sociobiol. 10, 109–118 (doi:10.1007/BF00300170) [Google Scholar]
- Martínez-Lendech N., Córdoba-Aguilar A., Serrano-Meneses M. A.2007Body size and fat reserves as possible predictors of male territorial status and contest outcome in the butterfly Eumaeus toxea Gadart (Lepidoptera: Lycaenidae). J. Ethol. 25, 195–199 (doi:10.1007/s10164-007-0040-5) [Google Scholar]
- Maynard Smith J., Parker G. A.1976The logic of asymmetric contests. Anim. Behav. 24, 159–175 [Google Scholar]
- Otronen M.1988The effect of prior experience on the outcome of fights in the burying beetle, Nicrophorus humator. Anim. Behav. 40, 980–982 (doi:10.1016/S0003-3472(05)81000-0) [Google Scholar]
- Parker G. A.1974Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223–243 (doi:10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- Peixoto P. E. C., Benson W. W.2008Body mass and not wing length predicts territorial success in a tropical satyrine butterfly. Ethology 114, 1069–1077 (doi:10.1111/j.1439-0310.2008.01555.x) [Google Scholar]
- Rosenberg R. H., Enquist M.1991Contest behaviour in Weidemeyer's admiral butterfly Limenitis weidemeyerii (Nymphalidae): the effect of size and residency. Anim. Behav. 42, 805–811 (doi:10.1016/S0003-3472(05)80124-1) [Google Scholar]
- Rutowski R. L.1992Male mate-locating behavior in the common eggfly, Hypolimnas bolina (Nymphalidae). J. Lepid. Soc. 46, 24–38 [Google Scholar]
- Rutte C., Taborsky M., Brinkhof M. W. G.2006What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21 (doi:10.1016/j.tree.2005.10.014) [DOI] [PubMed] [Google Scholar]
- Shreeve T. G.1987The mate location behaviour of the male speckled wood butterfly, Pararge aegeria, and the effect of phenotypic differences in hind-wing spotting. Anim. Behav. 35, 682–690 (doi:10.1016/S0003-3472(87)80104-5) [Google Scholar]
- Shreeve T. G., Smith A. G.1992The role of weather-related habitat use on the impact of the European speckled wood Pararge aegeria on the endemic Pararge xiphia on the island of Madeira. Biol. J. Linn. Soc. 46, 59–75 [Google Scholar]
- Stutt A. D., Willmer P.1998Territorial defence in speckled wood butterflies: do the hottest males always win? Anim. Behav. 55, 1341–1347 (doi:10.1006/anbe.1998.0728) [DOI] [PubMed] [Google Scholar]
- Takeuchi T.2006aMatter of size or matter of residency experience? Territorial contest in a green hairstreak, Chrysozepharus smaragdinus (Lepidoptera: Lycaenidae). Ethology 112, 293–299 (doi:10.1111/j.1439-0310.2006.01140.x) [Google Scholar]
- Takeuchi T.2006bThe effect of morphology and physiology on butterfly territoriality. Behaviour 143, 393–403 (doi:10.1163/156853906775897879) [Google Scholar]
- Takeuchi T., Honda K.2009Early comers become owners: effect of residency experience on territorial contest dynamics in a lycaenid butterfly. Ethology 115, 767–773 (doi:10.1111/j.1439-0310.2009.01665.x) [Google Scholar]
- Temeles E. J.1994The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim. Behav. 47, 339–350 (doi:10.1006/anbe.1994.1047) [Google Scholar]
- Van Dyck H., Matthysen E., Dhondt A. A.1997The effect of wing colour on male behavioural strategies in the speckled wood butterfly. Anim. Behav. 53, 39–51 (doi:10.1006/anbe.1996.0276) [Google Scholar]
- Whitehouse M. E. A.1997Experience influences male-male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae). Anim. Behav. 53, 913–923 (doi:10.1006/anbe.1996.0313) [Google Scholar]
- Wickman P.-O., Wiklund C.1983Territorial defence and its seasonal decline in the speckled wood butterfly (Pararge aegeria). Anim. Behav. 31, 1206–1216 (doi:10.1016/S0003-3472(83)80027-X) [Google Scholar]