Abstract
Quorum sensing (QS) in bacteria is thought to enable populations of cells to coordinately and cooperatively regulate gene expression for traits that confer group benefits. While this view has strong empirical and theoretical support, it is increasingly appreciated that QS under natural conditions may be incapable of monitoring bacterial numbers and, furthermore, that QS is evolutionarily unstable owing to conflicts of interest among competing cells. An alternative hypothesis, termed diffusion sensing (DS), proposes that autoinducer secretion monitors the diffusive properties of the local environment, with benefits that are directly realized by individual cells rather than populations. Here, we test central predictions of this hypothesis using the competence signalling system of Streptococcus pneumoniae as our model, which regulates the induction of natural transformation by the secretion and detection of a small diffusible peptide, CSP (competence-stimulating peptide). By experimentally manipulating the diffusive properties of the growth medium, we found that there is no fixed quorum for competence induction. Instead, induction cell density scales with diffusivity. In agreement with QS and DS expectations, we show that the benefit of signal exploitation by mutant cells that can use but not secrete CSP is strongly frequency-dependent. However, we also find that the magnitude of this benefit declines significantly as diffusion is reduced, a result more consistent with the predictions of DS. Together, these data provide strong support for the DS hypothesis for autoinducer response systems. More specifically, our results imply that autonomous rather than group benefits should be sought in order to more completely understand the role and evolution of CSP signalling in pneumococci.
Keywords: quorum sensing, diffusion sensing, competence, Streptococcus pneumoniae
1. Introduction
Bacteria can produce, release, detect and respond to small hormone-like molecules or peptides, termed autoinducers, whose concentration increases in proportion to cell density. Regulation of behaviour by this process, termed quorum sensing (QS), allows bacteria to assess the density of conspecifics within their immediate vicinity in order to coordinate population-wide gene expression and behaviour (Waters & Bassler 2005; Bassler & Losick 2006) for traits that are presumably only of benefit when carried out by large numbers of cells. Although once considered restricted to isolated species and a handful of unique functions, it is now clear that bacterial QS is exceptionally widespread (Fuqua et al. 1996; Waters & Bassler 2005; Diggle et al. 2007a). QS is of considerable biomedical importance because it regulates the expression of a broad diversity of traits (e.g. the initiation of biofilm formation, the secretion of exoenzymes and virulence factors, and many others; Bassler & Losick 2006; Diggle et al. 2007b). It is also of significant evolutionary interest because its existence implies that bacteria communicate with one another using honest signals and, furthermore, that this communication is cooperative (Velicer 2003; Keller & Surette 2006; West et al. 2006, 2007).
While the interpretation that QS can act as a cooperative census taker is widely accepted and empirically supported (Waters & Bassler 2005; West et al. 2007), it is not without its detractors (Redfield 2002; Hense et al. 2007; Alberghini et al. 2009; Schertzer et al. 2009). Concerns have arisen from two sources. First, if QS occurs within spatially heterogeneous multi-species communities, where autoinducer concentrations can be either directly modified or diluted by diffusion or advection, or where groups of signalling cells are spatially clustered, the signal is likely to be an unreliable proxy for conspecific cell density (Hense et al. 2007). This difficulty simply implies that QS will fail to work in many natural situations. Second, because of conflicts of interest that may arise among competing individuals, QS-mediated regulation is susceptible to the invasion of cells that exploit others by either signalling less or by performing less of the cooperative act induced by QS (Redfield 2002; Velicer 2003; Keller & Surette 2006; West et al. 2006, 2007). This implies that if the effects of exploitation are not mitigated, QS will not be stably maintained.
In this paper, we test predictions derived from an alternative model for QS formulated by Redfield (2002) that overcomes these problems. This model predicts that QS did not evolve to assess cell density but rather to assess mass transfer properties of the local environment. Diffusion sensing (DS; Redfield 2002) posits that the direct role of autoinducer secretion is to monitor the rate at which molecules move away from the cell as a means of efficiently regulating the induced secretion of more costly molecules. By focusing on the response of individual cells as opposed to coordinated populations, DS can overcome the concerns about QS noted above. In so doing, it makes unique predictions about the response of cells to alterations in mass transfer properties of the environment and the effects of these changes on apparent exploiter cells that do not produce (or respond to) autoinducer. Specifically, DS predicts that there is no fixed quorum for the induction of autoinducer-mediated responses, but rather that the quorum is dependent upon mass transfer conditions. Indeed, with extreme diffusion limitation, the predicted quorum required to induce a response could be as low as a single cell, an outcome that has recently been experimentally verified (Shompole et al. 2003; Boedicker et al. 2009; Carnes et al. 2010). It also predicts that advantages accruing to signal exploiters will decline with reductions in local diffusivity, owing to decreased access to the secreted signal. Here, we test and find strong support for these two predictions using the peptide competence signalling system in S. pneumoniae.
S. pneumoniae (pneumococcus) is a gram-positive bacterium that typically resides as a commensal member of the nasopharyngeal flora (Bogaert et al. 2004). On occasion, this species can become pathogenic and cause diseases that range in severity from mild to fatal. A factor that contributes to pneumococcal virulence and increasingly to antibiotic treatment failure is the ability of these bacteria to take up and incorporate free DNA into their genomes by the process of natural transformation (Claverys et al. 2009; Johnsborg & Havarstein 2009). Transformation, which has been known since Griffith's classic experiments in the 1920s (Griffith 1928), was later found by Tomasz and colleagues (Tomasz & Hotchkiss 1964; Tomasz 1965, 1966; Tomasz & Mosser 1966) to be regulated by the secretion of a hormone-like extracellular peptide, called CSP, or competence-stimulating peptide. Since Tomasz's work, CSP-mediated induction of competence in S. pneumoniae has been interpreted as quorum-dependent (Havarstein et al. 1995, 1996; Claverys et al. 2009; Johnsborg & Havarstein 2009); indeed, pneumococcal competence was the first characterized QS system.
The molecular regulation of competence induction is similar to the positive feedback control of other well-characterized QS autoinducer response systems. Briefly, CSP is synthesized by comC, after which it is modified and secreted by a dedicated ABC-type transporter, ComAB. The detection of CSP occurs via a two-component signal transduction circuit, ComD and ComE, leading to the phosphorylation of the response regulator protein and regulation of gene expression of a series of downstream targets (Havarstein et al. 1995, 1996; Pestova et al. 1996; Claverys et al. 2009; Johnsborg & Havarstein 2009). While the term competence is often used as a shorthand for natural transformation, CSP actually regulates the transcription of more than 100 genes, only a fraction of which are required for transformation (Peterson et al. 2004). While some of these functions (for example bacteriocin secretion) may conceivably be most beneficial if coordinately expressed in high-density cultures (Gardner et al. 2004; Inglis et al. 2009), other group benefits for CSP-dependent traits are less obvious. Most prominent among this set of traits is transformation itself, a phenomenon for which the short- or long-term benefits to either individuals or populations remain obscure (Redfield 2001; Michod et al. 2008; Ragan & Beiko 2009; Vos 2009). Also unclear is the threshold density of cells required to induce transformation. In the laboratory, transformation is typically studied in batch culture that approximates a highly diffusive mass action environment. By contrast, pneumococci in vivo persist on or within cells of the mucosal surface within biofilms or as microcolonies (Hall-Stoodley et al. 2006; Allegrucci & Sauer 2007; McEllistrem et al. 2007; Reid et al. 2009), conditions that violate the assumptions of efficient mass transfer.
In this work we seek to determine, by using growth conditions that mimicked the biofilm growth of natural pneumococcal populations, the threshold density for spontaneously induced transformation and also the effect of these conditions on the benefit to signal mutants that may exploit and destabilize CSP signalling. Briefly, we show that: (i) the threshold cell density required for the CSP-mediated induction of transformation in S. pneumoniae is not fixed and is significantly reduced by decreasing environmental diffusivity; and (ii) signal exploitation by mutant cells able to respond to (but not produce) CSP can occur when mutants are at low frequencies, but this signal theft is significantly reduced with decreased diffusivity. Together, these results provide support for the DS model, and imply that autonomous rather than group benefits should be sought in order to more completely understand the role and evolution of CSP signalling in pneumococci.
2. Materials and methods
(a). Bacterial strains and growth conditions
Streptococcus pneumoniae strains used in this study are described in table 1. The cells were cultured at 37°C with 5 per cent CO2 in a static incubator. Non-competent cells were prepared by growing cultures to an OD600 of 0.3 in Complete Transformation Medium (CTM), corresponding to a cell density of approximately 3 × 108 ml−1, and then freezing in single-use 200 µl aliquots with 20 per cent glycerol at −80°C. CTM contains, per litre: 30 g tryptone soy broth, 1 g yeast extract, 10 ml of 0.1 M CaCl2 and 10 ml of 20 per cent BSA. CTM at pH 6.8 was used to suppress natural induction of competence, while pH 7.8 was used to promote natural induction. Isolation of genomic DNA from S. pneumoniae strains was performed using the GenElute Bacterial Genomic DNA Kit (Sigma) according to the manufacturer's protocol. For enumeration, cells were plated on TSY agar that contains, per litre: 30 g tryptic soy agar (LabM, UK), 5 g yeast extract and 3 per cent sheep blood. Antibiotics (Sigma in all cases) were used in the following concentrations: 5 µg ml−1 novobiocin, 3 µg ml−1 chloramphenicol and 100 µg ml−1 streptomycin.
Table 1.
List of Streptococcus pneumoniae strains used in this study. Cm, Chloramphenicol; Sm, Streptomycin; Nov, Novobiocin.
| strains | description | genetic markers | source |
|---|---|---|---|
| RX1 | non-encapsulated derivative of D39 | gift from F. Iannelli (Iannelli et al. 2005) | |
| DR93 | RX1, NovR | Nov-resistant | this study |
| FP5 | RX1, ΔcomC, CmR | Cm-resistant | gift from F. Iannelli (Iannelli et al. 2005) |
| R304 | CmR, SmR, NovR | Cm-, Nov-, Sm-resistant | gift from J.-P. Claverys |
| DR94 | RX1, spontaneous SmR | Sm-resistant | this study |
(b). Fitness of ΔcomC versus wild-type
Fitness differences between the ΔcomC strain, FP5, and the wild-type, RX1, were assessed with sixfold replication using competitive growth assays based on the protocol of Lenski et al. (1991). Briefly, aliquots of each genotype were removed from a −80°C freezer, thawed in CTM at pH = 7.8 and grown to an OD600 of 0.3. Cells were then diluted 10 000-fold and mixed 1 : 1 into new tubes of CTM, after which they were allowed to grow overnight. The relative densities of both competitors were estimated at the start of the competition and after overnight growth by plating appropriate dilutions onto TSY + blood agar with and without 3 µg ml−1 chloramphenicol. Fitness was estimated as the ratio of the Malthusian parameter of both strains, following Lenski et al. (1991).
(c). Threshold induction density and transformation rate of S. pneumoniae in different diffusive environments
In order to directly modify the rate of diffusion experienced by growing cells, the viscosity of the growth medium was increased by adding increasing concentrations of Pluronic F-127 (C5H14O4; Sigma). Pluronic F-127 is a non-toxic, di-block copolymer of polyoxyethlene and polyoxypropylene that exhibits thermoreversible gelling, forming a liquid at temperatures less than 15°C and a solid at temperatures greater than 15°C (Gilbert et al. 1998; Wirtanen et al. 1998). The degree of viscosity depends upon the concentration of Pluronic, and diffusion constants of various compounds, proteins and peptides are known to decline log-linearly as a function of the concentration of Pluronic (Gilbert et al. 1986; Suh & Jun 1996; Moore et al. 2000).
Of particular relevance to this study, Pluronic has been used to simulate bacterial growth within biofilms by generating an artificial biofilm matrix and to simulate conditions that might prevail within the nasopharyngeal mucosa. Outer membrane protein expression patterns of Pluronic grown biofilm cells of many species are consistent with the induction of biofilm physiology (Gilbert et al. 1998; Clutterbuck et al. 2007) and are distinct from either planktonically grown cells or those grown upon an agar surface (Gilbert et al. 1998; Clutterbuck et al. 2007). Pneumococcal growth rates and those of other species (Gilbert et al. 1998; Wirtanen et al. 1998) are not changed when growing within a medium containing Pluronic when compared with Pluronic-free cells (mean Vm ± s.e.: 0%, 0.59 ± 0.02; 2%, 0.58 ± 0.03; 4%, 0.58 ± 0.01; 8%, 0.58 ± 0.01; 16%, 0.6 ± 0.02). At the concentrations of Pluronic used here—0, 2, 4, 8 and 16%—the medium never becomes fully solid but rather achieves very slightly increasing states of viscosity, with the maximum corresponding to the solidity of 0.4 per cent soft-agar. The thermoreversible properties of Pluronic enabled complete, efficient and very rapid recovery of S. pneumoniae cells, and also enabled precise modification of diffusivity.
In order to measure the maximal rate of spontaneous transformation and the threshold cell density for competence induction, we analysed a time course of natural transformation. Frozen aliquots of S. pneumoniae RX1 were gently thawed and grown in CTM at pH 6.8 to an OD600 of 0.3, after which they were inoculated via a 1/100-fold dilution into fresh CTM at pH 7.8 containing varying concentrations of Pluronic. Each tube was divided into a series of replicate 100 µl aliquots and then returned to the incubator. Because sampling during this assay is destructive, it is important to note that each time series is comprised of numerous independent aliquots. Every 30 min over a period of 4 h, one tube from each replicate time series was destructively sampled in order to estimate total cell density, as well as the proportion of the total CFU that were competent to become transformed. The initial cell density in each tube was approximately 1–3 × 106 ml−1. To determine the fraction of each population that was competent to become transformed at each time point, we added 5 µg ml−1 of gDNA of S. pneumoniae strain R304 to each sampled tube, vortexed gently to distribute the gDNA and then incubated these at 30°C for 30 min and 37°C for 1 h following standard protocols (Alloing et al. 1998). Transformation in these assays was estimated using the transfer of a single genetic marker; accordingly, our estimates should be interpreted as underestimates of the total transformation rates and competent cell fraction. After incubation, samples were cooled on ice to fully liquefy the Pluronic and uniformly distribute any potentially embedded or clumped cells, and then plated from various dilutions on TSY and on TSY/Strep to determine total CFU and the number of transformed cells, respectively. The fraction of transformed cells at each time point was calculated as the ratio of the number of transformed cells to the total CFU, both determined by plate counts. Between five and seven independent replicates were carried out for each Pluronic concentration, and data were analysed using GLM in SPSS.
(d). Co-culture of wild-type and ΔcomC strains
Co-culture experiments were carried out between DR93, a Nov-R derivative of wild-type RX1, and FP5, a ΔcomC strain unable to secrete CSP (Iannelli et al. 2005). Although this strain cannot produce CSP, it can be induced to competence by exogenous addition of CSP or by using the CSP produced by neighbouring cells. Experiments were carried out as for monoculture assays with RX1, but with the following difference. Cells were mixed at three initial frequencies from frozen aliquots, corresponding to 1 : 9, 1 : 1 and 9 : 1, before being divided into independent tubes for each point of the time course. Experiments were conducted in two Pluronic concentrations—0 per cent and 8 per cent—these points were found to clearly discriminate both the competence induction cell density and the peak-transformed fraction. Peak transformation rate of each strain was characterized as for monoculture by selective plating. Selection of S. pneumoniae DR93 and FP5 was carried out on 5 µg ml−1 novobiocin or 3 µg ml−1 chloramphenicol, respectively, while transformants of each strain were isolated on either antibiotic plus 100 µg ml−1 streptomycin. As a negative control, transformation of a ΔcomC strain was tested without CSP in the presence of 5 µg ml−1 of gDNA. No transformants were observed in these assays, consistent with the absolute requirement of CSP for competence.
As a further control, and in order to assess the role of CSP secretion in mediating differences between strains across Pluronic concentrations, we carried out assays, both in mono- and co-culture, using the same strains and conditions as above, where competence was artificially induced through the addition of 100 ng ml−1 of exogenous CSP rather than by allowing cells to become spontaneously competent. Because induction via exogenous CSP occurs and is dissipated rapidly, these time courses were carried out for only 1 h at 15 min intervals.
3. Results
(a). Competence induction with decreasing diffusivity
Natural competence in S. pneumoniae is induced when the concentration of the secreted peptide, CSP, exceeds a threshold level of approximately 10 ng ml−1 (Havarstein et al. 1995), which is reached at a cell density of approximately 108 cells ml−1 (although this varies across laboratories and media types (Chen & Morrison 1987). The competent state arises rapidly within the pneumococcal population and then declines. Consequently the fraction of competent cells through culture growth displays a peak, an observation that led to the recognition that induction is quorum-dependent (Tomasz & Hotchkiss 1964). To date, all estimates of the spontaneous induction density for transformation have been made in batch culture conditions that facilitate free diffusion of the competence peptide. We modified this critical environmental component, in order to more closely mimic conditions that would prevail during surface or tissue-associated biofilm growth, or growth within microcolonies or mucus, by growing cells in media in which diffusivity was decreased to varying degrees by the co-block polymer Pluronic F127, a solidifying agent for which concentration inversely scales with diffusivity (Gilbert et al. 1986; Suh & Jun 1996; Moore et al. 2000).
To control for the possible role of Pluronic itself on the induction of competence, we first examined the rates of transformation in conditions where competence was artificially induced with the exogenous addition of synthetic CSP. The CSP concentration in these experiments was 100 ng ml−1, tenfold higher than the concentration required to spontaneously induce competence, and was thus in considerable excess. The concentration of gDNA within each sampled aliquot, as for all experiments, was 5 µg ml−1. As anticipated if Pluronic does not cause a direct effect on the physiological competence of cells, we found no significant differences in the peak-transformed fraction of cells grown in 0 per cent and 8 per cent Pluronic (ANOVA, F1,8 = 1.94, p = 0.214).
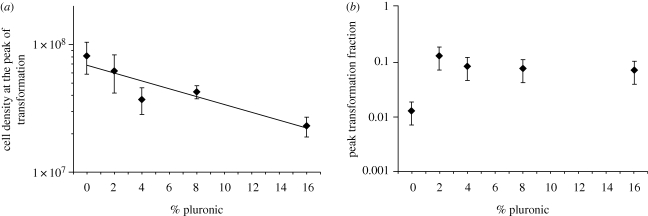
We next allowed competence to develop spontaneously by endogenous CSP production across a range of Pluronic concentrations designed to directly modify the diffusivity of CSP. We predicted that decreasing diffusion of CSP with increasing concentrations of Pluronic would cause cells to detect higher local concentrations of the peptide, which would have two consequences: (i) a Pluronic concentration-dependent decline in induction density; and (ii) an increase in the peak fraction of transformed cells as a function of Pluronic concentration. Consistent with the first prediction, we found a significant negative relationship between the concentration of Pluronic and the threshold induction density for competence (linear regression: R2 = 0.36, F1,22 = 12.03, p = 0.002; figure 1a). Under planktonic conditions (0% Pluronic) the induction density was approximately 8.2 × 107 cells ml−1, while in 16 per cent Pluronic, the maximum concentration explored in this study, this value was approximately 2.3 × 107 cells ml−1, a reduction of more than 3.5-fold. This result contrasts with the prediction of a fixed threshold density anticipated under a strictly QS model. In support of the second prediction, and in contrast to the case when competence is artificially induced with synthetic CSP, we found a significant effect of Pluronic on the peak-transformed cell fraction (ANOVA: F4,22 = 7.24, p = 0.001). Interestingly, while there was an approximately tenfold increase in transformation at 2 per cent Pluronic, no further increases were observed at higher concentrations (figure 1b). Together these data provide strong evidence that the secretion and response to endogenously produced CSP can be significantly modified by growth in an environment where the diffusivity, and hence the availability, of this peptide is changed. The consequences of altering the local availability of CSP are significant both in terms of the density of cells required to initiate this classically quorum-dependent response as well as the response of cells to increased local concentrations of endogenous signal.
Figure 1.
(a) Cell density at the peak of transformation and (b) peak-transformed fraction of populations of wild-type S. pneumoniae RX1 grown in increasing concentrations of Pluronic F127 ranging from 0 to 16 per cent. Values represent the mean ± s.e. In (a), the line indicates the line of best-fit from a linear regression (statistical details are provided in the text).
(b). Signal non-producers and their mitigation
Like many so-called public goods products that are secreted by bacteria, CSP produced by one cell can potentially be bound by a non-producer in order to induce competence. If CSP-mediated responses are beneficial, but CSP production specifically and/or competence induction more generally are costly, non-producers may benefit at the expense of wild-type producers. In order to determine if there are costs associated with competence, we estimated the relative fitness of wild-type cells when competed against an isogenic mutant that does not produce CSP, hereafter called ΔcomC. Consistent with the idea that there are significant costs associated with competence, we found that the relative fitness of the ΔcomC strain is significantly greater than that of the wild-type cells (mean fitness of FP5 ± s.e. = 1.102 ± 0.014, t-test: t5 = 7.42, p = 0.001). In addition, we also found that when competence is artificially induced with excess synthetic CSP, the ΔcomC strain was transformed at a significantly higher rate than its wild-type parent (mean transformation rate ± s.e.: wild-type, 0.044 ± 0.005; ΔcomC strain, 0.079 ± 0.011; t-test: t14 = −2.96, p = 0.01). This difference in transformation rate is similar to what has been previously seen in a comA mutant (Havarstein et al. 1995), and corresponds to an approximately 1.66-fold increase in transformability. The results of these assays indicate that there are significant costs associated with the induction or expression of competence that could potentially be exploited by non-producing CSP cells.
Given the apparent benefits of signal non-production, we hypothesized that the ΔcomC strain would exploit the CSP secretion of wild-type strains during growth in mixed culture. Additionally, we hypothesized that the benefits to the ΔcomC strain would vary as a function of the relative frequencies of the wild-type and ΔcomC cells, and as a function of the diffusivity of the medium. More specifically, when the wild-type was rare, we anticipated that the overall concentration of CSP would be lower, thus reducing transformation in the ΔcomC strain. Secondly, we anticipated that these frequency-dependent effects would be modified by the diffusivity of the environment, further limiting CSP access and decreasing transformation in the ΔcomC strain. To test these predictions, we examined differences in transformability between these two strains when grown from varying initial frequencies in both 0 per cent and 8 per cent Pluronic.
As in the control assay outlined above, the co-culture experiment was first carried out by inducing competence with the addition of excess synthetic CSP rather than allowing competence to arise spontaneously. Under these conditions, where exploitation of wild-type CSP production by the ΔcomC strain is prevented, we anticipated that there would be neither an effect of strain frequency nor Pluronic concentration on the relative transformability of either strain. This expectation was realized for both predictions (frequency: F2,24 = 0.37, p = 0.69; Pluronic concentration: F1,24 = 1.62, p = 0.22).
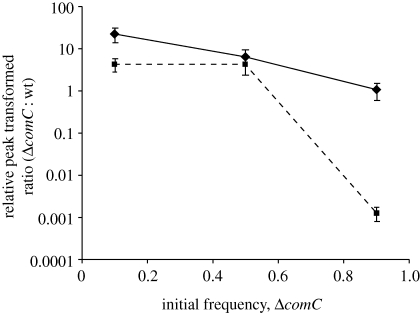
This result contrasts with the case where competence in co-culture was allowed to develop spontaneously through the endogenous production of CSP. We emphasize again that the only source of CSP in this assay was from wild-type cells. Results that are consistent with our central predictions are shown in figure 2. We found that the ability of the ΔcomC strain to exploit the endogenous CSP production of wild-type cells declines as wild-type cells become rarer (ANOVA, F2,20 = 42.92, p < 0.001) and as the concentration of Pluronic increases (ANOVA, F1,20 = 35.08, p < 0.001). Additionally, we find a significant interaction between the frequency of wild-type cells and the concentration of Pluronic (ANOVA, F1,20 = 10.65, p = 0.002), an effect that is driven largely by the fact that ΔcomC strains in 8 per cent Pluronic are severely limited in their ability to become transformed. The causes of these results become clearer when we focus on the response of each strain alone. We find that the transformation rate of the wild-type strain grown in co-culture with the ΔcomC strain is significantly higher when it is rare than when it is common (mean percentage transformation ± s.e.: 10%, 0.58 ± 0.19; 50%, 0.07 ± 0.0002; 90%, 0.14 ± 0.03; ANOVA, F2,19 = 14.63, p < 0.001), and also that the overall rate of transformation of this strain is higher in 8 per cent than in 0 per cent Pluronic (mean percentage transformation ± s.e. in 8%: 10%, 2.28 ± 0.68; 50%, 0.04 ± 0.02; 90%, 0.20 ± 0.06; ANOVA, F1,19 = 12.99, p = 0.003), a result consistent with the data shown in figure 1. This indicates that an increasing fraction of wild-type CSP is used by the mutant in co-culture as the wild-type becomes more common, and also that diffusion of CSP is decreased (and is thus sequestered by the wild-type producers) in 8 per cent Pluronic. At the same time, the ΔcomC strain is transformed less as it becomes more common owing to decreased availability of CSP from the declining wild-type cells (mean percentage transformation ± s.e.: 10%, 2.54 ± 0.35; 50%, 0.44 ± 0.21; 90%, 0.55 ± 0.31; ANOVA, F2,21 = 34.57, p < 0.001), a result that becomes more pronounced in 8 per cent Pluronic (mean percentage transformation ± s.e.: 10%, 0.75 ± 0.19; 50%, 0.72 ± 0.21; 90%, 0.002 ± 0.0005; ANOVA, F1,21 = 30.68, p < 0.001).
Figure 2.
Relative peak-transformed ratio of ΔcomC∶wild-type cells as a function of both the initial frequency of ΔcomC and the concentration of Pluronic F127 in the medium. Values represent mean ± s.e. Diamonds and continuous line, 0 per cent; squares and broken line, 8 per cent.
4. Discussion
Regulation of bacterial traits by QS is considered to represent one of the clearest examples of cooperative or social behaviour in microbes (Keller & Surette 2006; Diggle et al. 2007b; West et al. 2006, 2007). Yet under a broad range of natural conditions, this method of quorum monitoring may be highly inaccurate, because secreted inducers would be degraded or diluted in multi-species communities, or concentrated within microcolonies, and evolutionarily unstable, because such systems would be susceptible to exploitation by cells that do not produce the quorum-dependent signal (Redfield 2002; Diggle et al. 2007b; Hense et al. 2007; West et al. 2007). Redfield (2002) and Hense et al. (2007) recognized that reduced diffusive loss of secreted signals provided a partial solution to both problems, because signals would remain in close proximity to the cells that produced them, thus providing information about the local (diffusive) environment, and also limiting the availability of secreted signals to non-producers. Here, we tested central predictions of these ideas, namely that: (i) the size/cell density of the quorum for induction not fixed but is rather a function of the local diffusive conditions; and that (ii) benefits accruing to cells that do not contribute to the pool of secreted signal will decline with decreasing environmental diffusivity.
Using competence signalling in S. pneumoniae as our model, we find strong support for both predictions. First, we show (figure 1a,b) that the quorum required to induce spontaneous competence declines with increasing concentrations of Pluronic. Second, we show (figure 2) that cells that can use the secreted signals produced by neighbours but are unable to produce it themselves benefit through signal theft in mass action environments but less so at low diffusivity. From these results, we draw two conclusions. First, there is no fixed cell density at which competence is induced in pneumococcal competence signalling. These data are consistent with recent experimental results in other systems (Dulla & Lindow 2008; Parent et al. 2008; Boedicker et al. 2009). Second, despite significant costs associated with competence signalling, both in terms of fitness and transformability, signal non-producers in poorly diffusive environments will be limited in their ability to realize benefits of signal theft, especially when competence-associated functions are required for cell fitness.
A qualitative explanation for this first result is that the role of increasing concentrations of Pluronic is to steepen the gradient of CSP surrounding each producing cell. Mechanistically, this is caused by the physical structure of the environment, which obstructs transfer of the peptide, with the consequence that each producer experiences a higher local CSP concentration. As the steepness of the CSP gradient increases, the cell density required for induction declines. For the same reason, the peak-transformed fraction of cells should increase. In our results, it is unclear why this fraction increased nearly tenfold at 2 per cent but did not show any further increases at higher Pluronic concentrations. However, a number of potentially interdependent factors may have contributed to this outcome: (i) the number and spatial clustering of CSP producers; (ii) the quantity of CSP synthesized by each cell (or heterogeneity of same); (iii) the responsiveness of cells to varying concentrations of CSP; and (iv) the rate at which CSP diffuses away from producing cells. At present, these values are unknown in this system, and are only beginning to be quantified in other systems using single-cell analysis. We intend in future work to focus on these mechanistic explanations more fully. However, in light of our evidence from control experiments that there are no differences in the peak-transformed cell fraction when synthetic CSP is added to cells grown in 0 and 8 per cent Pluronic, it is clear that the overall local concentration, and its modification by diffusion, of CSP are of paramount importance.
A steep gradient of CSP surrounding producing cells will ensure that these cells have access to the peptide at concentrations similar to the level at which it is produced, while also causing neighbouring cells, regardless of their production status, to see less of it. This limitation is particularly relevant for non-producers, who, for public goods products, have been called both cheats and exploiters because they profit at the expense of others by not secreting costly products (more typically the response to induction rather than the signal itself). The success of exploiters and the conditions favouring their invasion and maintenance has been extensively studied from both a theoretical and empirical perspective (West et al. 2006, 2007). Social evolution theory predicts, and numerous experiments have shown, that the success of public goods cheats will be negatively frequency-dependent, because when they are rare the concentration of the public good produced by the common wild-type will be highest and vice versa. Consistent with this simple prediction, we have found significant frequency-dependent effects on transformability of a ΔcomC strain that can use the CSP secreted by wild-type cells but not produce CSP itself. When this strain is rare, its transformation rate in liquid is more than tenfold that of the wild-type value; however, when common, this advantage is eliminated. Of greater interest here, and with respect to the distribution of public goods and the efficacy of public goods signalling under natural conditions, is the response when rare of the ΔcomC strain when diffusion is limited. Here, as in liquid medium, the advantage to this strain is negatively frequency-dependent, but the overall benefit is reduced. More important, when this strain is common, its transformation is less than 1/1000-fold of the wild-type, a difference that underlies the interaction between the concentration of Pluronic and the frequency of the ΔcomC strain (figure 2). Overall, this outcome is anticipated if the role of decreased diffusion is to sharpen the CSP gradient around producing cells. This increases the local availability of CSP, to the advantage of the wild-type producer, while decreasing it to other cells, ultimately to their detriment. Because they have not been explicitly sought among natural isolates, CSP null strains are not known; however, our results suggest that they would be constrained under conditions of diffusion limitation.
There are a number of caveats to these results that are worth noting. First, although we have sought to directly manipulate diffusion, cells in our treatments are initially well distributed. That is, there is no spatial structure of genotypes. However, once cells initiate growth, it may be the case that cells grown in Pluronic-containing media disperse less readily and thus become more densely packed into microcolonies, which could also serve to locally increase CSP concentrations. To examine this possibility, we carried out microscopic examination of cells grown in 0 and 8 per cent, but these show no indication of differential clumping (see electronic supplementary material), suggesting that this is not a cause of our results. Recently, the role and importance of cell clustering on apparently quorum-dependent behaviours was considered by Hense et al. (2007), who introduced the idea of efficiency sensing. This hypothesis is similar to DS in that it posits an individual benefit to signalling cells, but efficiency sensing does allow group benefits to colonies of related cells that are spatially clustered. Although clumping does not appear to have occurred in these experiments, we intend in future to explicitly incorporate this potentially important factor into this system. Second, pneumococcal signalling is mediated by secreted peptides that are known to be more metabolically costly to produce than gram-negative autoinducers such as AI2 (Keller & Surette 2006). For this reason, the generality of our results might be limited to gram-positive cell signalling. However, we suspect that the same general result would be found in gram-negative signalling, but more in terms of the response to the signal rather than the signal itself. Third, transformation and competent cell fraction in these assays was estimated using the transfer of a single genetic marker; accordingly, our estimates are almost certainly underestimates of both these values. Incorporation of both additional markers and a luminescent reporter construct for competence induction will in future ensure that our estimates better reflect total transformation rates and competent cell fraction of these populations. Finally, our focus in this work is on pneumococcal transformation, which is one among many functions of CSP signalling (Peterson et al. 2004). The consequences of reduced diffusion measurably and significantly alter transformation, but may have important consequences for other CSP-mediated traits that we have not measured. A central aim of future work will be to better understand these functions under a range of environmentally relevant conditions in order to better understand the role and evolution of CSP signalling more generally.
There are a number of important implications of these results, both specific to pneumococcal signalling and more generally with respect to the broader understanding of QS. CSP-mediated induction of competence in S. pneumoniae has long been interpreted as quorum-dependent. However, for two reasons it has been unclear whether the in vitro quorum induction density was the same as required for competence induction in vivo. First, in vivo densities may be considerably lower than the putative in vitro quorum threshold. Second, cells in vivo persist in conditions that deviate markedly from the assumptions of a mass action environment. Rather, growth is more likely to be found on mucosal surfaces as biofilms, within highly viscous mucous itself or within cells of the mucosal lining than in a highly diffusive liquid (Hall-Stoodley et al. 2006; Allegrucci & Sauer 2007; McEllistrem et al. 2007; Reid et al. 2009). These differences, together with our results, imply that even at low densities there may be opportunities for competence induction at rates that will have been underestimated from laboratory studies. Population genetic estimates indicate that recombination via transformation in S. pneumoniae, both within this species and with congeners, is rife, serving as an important source of novel virulence and antibiotic resistance determinants (Hanage et al. 2009). Our data reveal that this recombination need not require large populations, but rather could potentially occur among small numbers of cells, as long as growth is taking place in a minimally diffusive environment or within microcolonies. In addition to transformation, there are numerous other traits that are induced by competence (Peterson et al. 2004), including biofilm formation and biocide production (Suntharalingam & Cvitkovitch 2005; Johnsborg et al. 2008), two traits that are known to be quorum-dependent in a variety of species. Our results indicate that the expression of these traits should be considered in a variety of inducing conditions. In particular, during episodes of disease that result in increased mucus production, the induction of competence, and any benefits derived from it, may be similarly increased. In addition, these data suggest that putative benefits of competence should be sought at the level of individual pneumococci rather than for populations of cells. More generally, our results suggest that the interpretation of competence, as well as some other public goods traits with apparently quorum-dependent expression, social may need to be re-evaluated, or at the very least considered under a broader regime of inducing conditions. If, under natural conditions, the induction of quorum-dependent responses is independent of cell number, this would imply that, at least for some species, QS is neither cooperative nor social, an interpretation more consistent with the role proposed by Redfield (2002) and Hense et al. (2007).
Acknowledgements
We are grateful to F. Iannelli and J.-P. Claverys, who generously provided some of the strains used in this study, and to A. McBain for help with microscopy. We appreciate the constructive comments of Bruce R. Levin and two anonymous reviewers who improved the clarity of this manuscript. In addition, we thank Tim Cooper, Vaughn Cooper, Andrew McBain, Chris Knight and Arjan de Visser for their helpful comments on an earlier version of this manuscript. Support was provided by a BBSRC grant (BBF0020681) to D.E.R.
References
- Alberghini S., Polone E., Corich V., Carlot M., Seno F., Trovato A., Squartini A.2009Consequences of relative cellular positioning on quorum sensing and bacterial cell-to-cell communication. FEMS Microbiol. Lett. 292, 149–161 (doi:10.1111/j.1574-6968.2008.01478.x) [DOI] [PubMed] [Google Scholar]
- Allegrucci M., Sauer K.2007Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 189, 2030–2038 (doi:10.1128/JB.01369-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloing G., Martin B., Granadel C., Claverys J. P.1998Development of competence in Streptococcus pneumoniae: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol. 29, 75–83 (doi:10.1046/j.1365-2958.1998.00904.x) [DOI] [PubMed] [Google Scholar]
- Bassler B. L., Losick R.2006Bacterially speaking. Cell 125, 237–246 (doi:10.1016/j.cell.2006.04.001) [DOI] [PubMed] [Google Scholar]
- Boedicker J. Q., Vincent M. E., Ismagilov R. F.2009Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew. Chem.-Int. Ed. 48, 5908–5911 (doi:10.1002/anie.200901550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert D., de Groot R., Hermans P. W. M.2004Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4, 144–154 (doi:10.1016/S1473-3099(04)00938-7) [DOI] [PubMed] [Google Scholar]
- Carnes E. C., Lopez D. M., Donegan N. P., Cheung A., Gresham H., Timmins G. S., Brinker C. J.2010Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat. Chem. Biol. 6, 41–45 (doi:10.1038/nchembio.264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Morrison D. A.1987Modulation of competence for genetic transformation in Streptococcus pneumoniae. J. Gen. Microbiol. 133, 1959–1967 [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Martin B., Polard P.2009The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiol. Rev. 33, 643–656 (doi:10.1111/j.1574-6976.2009.00164.x) [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. L., Cochrane C. A., Dolman J., Percival S. L.2007Evaluating antibiotics for use in medicine using a poloxamer biofilm model. Ann. Clin. Microbiol. Antimicrob. 6, (doi:10.1186/1476-0711-6-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle S. P., Crusz S. A., Camara M.2007aQuorum sensing. Curr. Biol. 17, R907–R910 (doi:10.1016/j.cub.2007.08.045) [DOI] [PubMed] [Google Scholar]
- Diggle S. P., Griffin A. S., Campbell G. S., West S. A.2007bCooperation and conflict in quorum-sensing bacterial populations. Nature 450, 411–417 (doi:10.1038/nature06279) [DOI] [PubMed] [Google Scholar]
- Dulla G., Lindow S. E.2008Quorum size of Pseudomonas syringae is small and dictated by water availability on the leaf surface. Proc. Natl Acad. Sci. USA 105, 3082–3087 (doi:10.1073/pnas.0711723105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C., Winans S. C., Greenberg E. P.1996Census and consensus in bacterial ecosystems: the LuxR–LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50, 727–751 (doi:10.1146/annurev.micro.50.1.727) [DOI] [PubMed] [Google Scholar]
- Gardner A., West S. A., Buckling A.2004Bacteriocins, spite and virulence. Proc. R. Soc. Lond. B 271, 1529–1535 (doi:10.1098/rspb.2004.2756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. C., Hadgraft J., Bye A., Brookes L. G.1986Drug release from Pluronic F-127 gels. Int. J. Pharm. 32, 223–228 (doi:10.1016/0378-5173(86)90182-1) [Google Scholar]
- Gilbert P., Jones M. V., Allison D. G., Heys S., Maira T., Wood P.1998The use of poloxamer hydrogels for the assessment of biofilm susceptibility towards biocide treatments. J. Appl. Microbiol. 85, 985–990 [DOI] [PubMed] [Google Scholar]
- Griffith F.1928The significance of pneumococcal types. J. Hyg. (Lond). 27, 113–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L., et al. 2006Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. J. Am. Med. Assoc. 296, 202–211 (doi:10.1001/jama.296.2.202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanage W. P., Fraser C., Tang J., Connor T. R., Corander J.2009Hyper-recombination, diversity, and antibiotic resistance in pneumococcus. Science 324, 1454–1457 (doi:10.1126/science.1171908) [DOI] [PubMed] [Google Scholar]
- Havarstein L. S., Coomaraswamy G., Morrison D. A.1995An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl Acad. Sci. USA 92, 11 140–11 144 (doi:10.1073/pnas.92.24.11140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havarstein L. S., Gaustad P., Nes I. F., Morrison D. A.1996Identification of the streptococcal competence-pheromone receptor. Mol. Microbiol. 21, 863–869 (doi:10.1046/j.1365-2958.1996.521416.x) [DOI] [PubMed] [Google Scholar]
- Hense B. A., Kuttler C., Mueller J., Rothballer M., Hartmann A., Kreft J. U.2007Opinion: does efficiency sensing unify diffusion and quorum sensing? Nat. Rev. Microbiol. 5, 230–239 (doi:10.1038/nrmicro1600) [DOI] [PubMed] [Google Scholar]
- Iannelli F., Oggioni M. R., Pozzi G.2005Sensor domain of histidine kinase ComD confers competence pherotype specificity in Streptoccoccus pneumoniae. FEMS Microbiol. Lett. 252, 321–326 (doi:10.1016/j.femsle.2005.09.008) [DOI] [PubMed] [Google Scholar]
- Inglis R. F., Gardner A., Cornelis P., Buckling A.2009Spite and virulence in the bacterium Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 106, 5703–5707 (doi:10.1073/pnas.0810850106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsborg O., Havarstein L. S.2009Regulation of natural genetic transformation and acquisition of transforming DNA in Streptococcus pneumoniae. FEMS Microbiol. Rev. 33, 627–642 (doi:10.1111/j.1574-6976.2009.00167.x) [DOI] [PubMed] [Google Scholar]
- Johnsborg O., Eldholm V., Bjornstad M. L., Havarstein L. S.2008A predatory mechanism dramatically increases the efficiency of lateral gene transfer in Streptococcus pneumoniae and related commensal species. Mol. Microbiol. 69, 245–253 (doi:10.1111/j.1365-2958.2008.06288.x) [DOI] [PubMed] [Google Scholar]
- Keller L., Surette M. G.2006Communication in bacteria: an ecological and evolutionary perspective. Nat. Rev. Microbiol. 4, 249–258 (doi:10.1038/nrmicro1383) [DOI] [PubMed] [Google Scholar]
- Lenski R. E., Rose M. R., Simpson S. C., Tadler S. C.1991Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2000 generations. Am. Nat. 138, 1315–1341 (doi:10.1086/285289) [Google Scholar]
- McEllistrem M. C., Ransford J. V., Khan S. A.2007Characterization of in vitro biofilm-associated pneumococcal phase variants of a clinically relevant serotype 3 clone. J. Clin. Microbiol. 45, 97–101 (doi:10.1128/JCM.01658-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michod R. E., Bernstein H., Nedelcu A. M.2008Adaptive value of sex in microbial pathogens. Infect. Genet. Evol. 8, 267–285 (doi:10.1016/j.meegid.2008.01.002) [DOI] [PubMed] [Google Scholar]
- Moore T., Croy S., Mallapragada S., Pandit N.2000Experimental investigation and mathematical modeling of Pluronic (R) F127 gel dissolution: drug release in stirred systems. J. Control. Rel. 67, 191–202 (doi:10.1016/S0168-3659(00)00215-7) [DOI] [PubMed] [Google Scholar]
- Parent M. E., Snyder C. E., Kopp N. D., Velegol D.2008Localized quorum sensing in Vibrio fischeri. Colloids Surf. B-Biointerf. 62, 180–187 (doi:10.1016/j.colsurfb.2007.09.031) [DOI] [PubMed] [Google Scholar]
- Pestova E. V., Havarstein L. S., Morrison D. A.1996Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol. Microbiol. 21, 853–862 (doi:10.1046/j.1365-2958.1996.501417.x) [DOI] [PubMed] [Google Scholar]
- Peterson S. N., et al. 2004Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51, 1051–1070 (doi:10.1046/j.1365-2958.2003.03907.x) [DOI] [PubMed] [Google Scholar]
- Ragan M. A., Beiko R. G.2009Lateral genetic transfer: open issues. Phil. Trans. R. Soc. B 364, 2241–2251 (doi:10.1098/rstb.2009.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield R. J.2001Do bacteria have sex? Nat. Rev. Genet. 2, 634–639 (doi:10.1038/35084593) [DOI] [PubMed] [Google Scholar]
- Redfield R. J.2002Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10, 365–370 (doi:10.1016/S0966-842X(02)02400-9) [DOI] [PubMed] [Google Scholar]
- Reid S. D., Hong W. Z., Dew K. E., Winn D. R., Pang B., Watt J., Glover D. T., Hollingshead S. K., Swords W. E.2009Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J. Infect. Dis. 199, 786–794 (doi:10.1086/597042) [DOI] [PubMed] [Google Scholar]
- Schertzer J. W., Boulette M. L., Whiteley M.2009More than a signal: non-signaling properties of quorum sensing molecules. Trends Microbiol. 17, 189–195 (doi:10.1016/j.tim.2009.02.001) [DOI] [PubMed] [Google Scholar]
- Shompole S., Henon K. T., Liou L. E., Dziewanowska K., Bohach G. A., Bayles K. W.2003Biphasic intracellular expression of Staphylococcus aureus virulence factors and evidence for Agr-mediated diffusion sensing. Mol. Microbiol. 49, 919–927 (doi:10.1046/j.1365-2958.2003.03618.x) [DOI] [PubMed] [Google Scholar]
- Suh H., Jun H. W.1996Physicochemical and release studies of naproxen in poloxamer gels. Int. J. Pharm. 129, 13–20 (doi:10.1016/0378-5173(95)04210-5) [Google Scholar]
- Suntharalingam P., Cvitkovitch D. G.2005Quorum sensing in streptococcal biofilm formation. Trends Microbiol. 13, 3–6 (doi:10.1016/j.tim.2004.11.009) [DOI] [PubMed] [Google Scholar]
- Tomasz A.1965Control of competent state in pneumococcus by a hormone-like cell product—an example for a new type of regulatory mechanism in bacteria. Nature 208, 155–159. (doi:10.1038/208155a0) [DOI] [PubMed] [Google Scholar]
- Tomasz A.1966Model for the mechanism controlling the expression of competent state in pneumococcus cultures. J. Bacteriol. 91, 1050–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Hotchkiss R. D.1964Regulation of transformability of pneumococcal cultures by macromolecular cell products. Proc. Natl Acad. Sci. USA 51, 480–487. (doi:10.1073/pnas.51.3.480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Mosser J. L.1966On nature of pneumococcal activator substance. Proc. Natl Acad. Sci. USA 55, 58–66. (doi:10.1073/pnas.55.1.58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer G. J.2003Social strife in the microbial world. Trends Microbiol. 11, 330–337 (doi:10.1016/S0966-842X(03)00152-5) [DOI] [PubMed] [Google Scholar]
- Vos M.2009Why do bacteria engage in homologous recombination? Trends Microbiol. 17, 226–232 (doi:10.1016/j.tim.2009.03.001) [DOI] [PubMed] [Google Scholar]
- Waters C. M., Bassler B. L.2005Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell. Dev. Biol. 21, 319–346 (doi:10.1146/annurev.cellbio.21.012704.131001) [DOI] [PubMed] [Google Scholar]
- West S. A., Griffin A. S., Gardner A., Diggle S. P.2006Social evolution theory for microorganisms. Nat. Rev. Microbiol. 4, 597–607 (doi:10.1038/nrmicro1461) [DOI] [PubMed] [Google Scholar]
- West S. A., Diggle S. P., Buckling A., Gardner A., Griffins A. S.2007The social lives of microbes. Ann. Rev. Ecol. Evol. Syst. 38, 53–77 (doi:10.1146/annurev.ecolsys.38.091206.095740) [Google Scholar]
- Wirtanen G., Salo S., Allison D. G., Mattila-Sandholm T., Gilbert P.1998Performance evaluation of disinfectant formulations using poloxamer-hydrogel biofilm-constructs. J. Appl. Microbiol. 85, 965–971 [DOI] [PubMed] [Google Scholar]