Abstract
Chemical signals play an important role in spider sexual communication, yet the chemistry of spider sex pheromones remains poorly understood. Chemical identification of male-produced pheromone-mediating sexual behaviour in spiders has also, to our knowledge, not been reported before. This study aimed to examine whether chemically mediated strategies are used by males of the spider Pholcus beijingensis for increasing the probability of copulation. Based on data from gas chromatography–mass spectrometry analysis, electroantennography assay and a series of behavioural tests, we verified that (Z)-9-tricosene is a male-specific compound in the spider P. beijingensis. This compound acts as an aphrodisiac: it increases the likelihood that a female will mate. Mate-searching males release (Z)-9-tricosene to stimulate sexual behaviour of conspecific females. In the two-choice assay, however, sexually receptive females show no preference to the chambers containing (Z)-9-tricosene. This indicates that the male pheromone of P. beijingensis is not an attractant per se to the conspecific females. This is, to our knowledge, the first identification of a male-produced aphrodisiac pheromone in spiders.
Keywords: (Z)-9-tricosene, male pheromone, aphrodisiac, Araneae, Pholcidae
1. Introduction
Accurate mate identification is an important component in spider sexual communication. The exchange of chemical signals is probably the first type of communication in spiders that serves to bring males and females together (Weygoldt 1977). Although chemical communication has long been recognized in spiders, most of the early research concentrated on behavioural assays of spider sex pheromones (Prenter et al. 1994; Searcy et al. 1999; Anderson & Morse 2001; Kasumovic & Andrade 2004; Roberts & Uetz 2005; Leonard & Morse 2006; Stoltz et al. 2007). The chemistry of spider pheromones remains poorly understood (Gaskett 2007). Some studies have investigated the chemistry of spider pheromones, and a few sex pheromones of females have been identified to date (Schulz & Toft 1993; Prouvost et al. 1999; Papke et al. 2000, 2001; Trabalon et al. 2005; Xiao et al. 2009; Chinta et al. 2010; Jerhot et al. 2010). Although all the identified spider pheromones are released by females and received by males, there are known bioassay examples of male pheromones in spiders that mediate the courtship behaviour of conspecific males or females. For example, silk extracts from mature males of the wolf spider, Schizocosa ocreata, can affect the frequency of agonistic displays and inhibit courtship behaviour among conspecific males. This is indicative of male-produced pheromones bound to the silk acting as a male–male inhibitor (Rao Ayyagari & Tietjen 1987). In the funnel-web spider, Agelenopsis aperta, all females enter a quiescent state prior to the initiation of mating (Singer et al. 2000). The males can complete mating when the female is physiologically or behaviourally inactive. Becker et al. (2005) demonstrated that the courting males emit an airborne pheromone to induce female quiescence during courtship. Males of a small theridiid spider (Argyrodes sp.) have a protuberance on the front of their heads. This secretes an aphrodisiac that is sucked on by females during mating (Legendre & Lopez 1974; Becker et al. 2005). No one, to our knowledge, has reported, however, a chemical identification of male-produced pheromone-mediating sexual behaviours in spiders.
Pholcid spiders (Araneae, Pholcidae), known colloquially as ‘daddy-long-leg spiders’, are among the dominant web-building spiders distributed worldwide. They occupy a wide variety of habitats ranging from leaf litter to tree canopies; several species occur in caves and in close proximity to humans (Huber 2005). Sexual selection by female choice occurs in pholcids (Uhl 1998; Huber 1999; Uhl et al. 2005). Pholcus beijingensis is a common species found in various caves near Beijing in China. Spiders of this species usually construct untidy webs in dark and damp recesses of cave entrances (Chen & Li 2005). Pholcus beijingensis is polygamous and males and females have multiple mating partners. Males abandon their webs after their last moult to search for potential mates while the females wait on their webs for males (Chen & Li 2005). We have demonstrated that a combination of (E,E)-farnesyl acetate and hexadecyl acetate acts as a female-produced sex pheromone in this species (Xiao et al. 2009). The mate-searching males (MMs) can locate potential mates based on the sex pheromone associated with the female's silk. The approach of an MM, unlike a female or immature male, rarely triggers predatory or aggressive behaviour in the sexually receptive female (Chen & Li 2005; Xiao et al. 2009). Therefore, we hypothesized that MMs might emit scents acting at a close range as sexual attractants and/or stimulants for the conspecific mature females. To test the hypothesis that a male-produced pheromone exists in P. beijingensis, we isolated and identified chemical signals from the body extract of P. beijingensis and tested for chemoreceptor responses to the compound using electroantennography. We confirmed behavioural responses with female choice tests and behavioural assays.
2. Material and methods
(a). Spiders
Adult and subadult specimens of P. beijingensis were collected in March and April of 2008 at the entrance of a cave located to the southwest of Beijing (39°42.350′ N, 115°42.825′ E). Mean body weights of the adult males, adult females and subadult males (SMs) were 18.2 ± 3.3 mg (n = 10), 14.6 ± 2.8 mg (n = 10) and 9.6 ± 1.7 mg (n = 12), respectively. Each spider was kept in a glass cuvette (4 cm inner diameter (i.d.) 12 cm deep) with a small moistened wad of cotton on the bottom to provide humidity. The cuvettes were put in a climatic chamber (RXZ–268B, Ningbo Jiangnan Instrument Factory) under a 12 L : 12 D photoperiod regime at 25°C (day) and 23°C (night). About 10–15 fruitflies (Drosophila melanogaster) were provided to each spider for food once a week.
(b). Chemical procedures
As the site of pheromone production in the spider is unknown, we extracted the whole body for gas chromatography–mass spectrometry (GC–MS) analysis. Whole-body extraction was carried out as described (Budenberg et al. 1993; Leal et al. 1994). Sexually receptive females (RFs), MMs and SMs supplied the body extraction samples. In courtship sequences of P. beijingensis, the male unfolding its pedipalps towards the female is seen as a critical behavioural pattern because it implies courting success. At this point, the female turns from passive to active and approaches the male for mating (Xiao et al. 2009). To determine whether the adults were reproductively active, we paired each male with an adult female on her web and checked for courtship behaviour. We removed the male from the web when it unfolded its pedipalps towards the female. Ten spider pairs from the total 15 pairs were found to be sexually receptive in a 1 h observation period. These sexually RFs and males were chosen for the chemical analysis. Each spider was put into a 100 µl cuvette, which was inserted into a 1.5 ml screw-topped vial (Agilent Technologies, Santa Clara, CA, USA). Then, 40 µl dichloromethane (purity greater than 99.5%, Beijing Fine Chemical Company, Ltd, Beijing, China) was put into the cuvette to extract compounds from the spider body. Twenty-four hours later, we removed the spider from the cuvette and stored the remaining solution at −20°C until analysis by GC–MS.
Analytical GC–MS was performed on an Agilent Technologies Network 6890N GC system coupled with a 5973 Mass Selective Detector using the NIST/EPA/NIH Mass Spectral Library (2002 version; Agilent Technologies). Chemstation software (Windows 2000) was used for data acquisition and processing. The GC was equipped with a 30 m HP5 − MS capillary column (0.25 mm i.d. × 0.25 µm film thickness; Agilent Technologies). Helium was used as the carrier gas at a flow rate of 1.0 ml min−1. The temperature of the injector was set at 280°C. Two microlitre aliquots of each sample were injected in the splitless mode. As dichloromethane is very volatile, we measured the volume of the solution remaining from the body extracts after injecting 2 µl into the GC–MS instrument so that we could calibrate the proportion of the extract injected for titre analysis. The oven temperature was programmed to increase from 100 to 300°C at 5°C min−1 and then held for 15 min. Electron-impact ionization used 70 eV and the scanning mass ranged from 30 to 450 amu. Compounds were identified tentatively by matching their gas chromatographic retention times and mass spectra with authentic analogues of the mass spectral library. Synthetic (Z)-9-tricosene (purity greater than 99.4%; Tokyo Chemical Industry Co., Ltd, Tokyo, Japan) and tricosane (purity greater than 99%, Alfa Aesar, a Johnson Matthey Co., MA, USA) were used to confirm the identification of natural products after separation on a non-polar column (HP5−MS, 0.25 mm × 30 m × 0.25 µm) and a polar column (DB − wax, 0.25 mm × 30 m × 0.25 µm, J&W Scientific, Folsom, CA, USA).
We determined the content of (Z)-9-tricosene from the body extract of one male P. beijingensis using an external standard. The synthetic compound (Z)-9-tricosene was diluted sequentially in dichloromethane to concentrations of 0.001, 0.01 and 0.1 µg µl−1. We injected 1 µl aliquots of each prepared solution into the GC–MS instrument. By comparing peak areas of the body extracts and those of the (Z)-9-tricosene solutions, we estimated that the peak areas of most male body extracts were larger than that of 0.001 µg (Z)-9-tricosene standard, but smaller than that of 0.01 µg. Therefore, we diluted (Z)-9-tricosene in dichloromethane to concentrations closely similar to the component of the male body extract for titre analysis. We injected 0.001, 0.002, 0.003, 0.004, 0.005, 0.006, 0.008 and 0.01 µg of the synthetic sample to obtain a calibration regression equation. The quantity of the male-produced (Z)-9-tricosene from one male body extract was calculated by comparing the peak area with that of the synthetic standard sample. We calculated the quantity of the male-produced pheromone from one male body extract according to the calibration regression equation and the volume of the extract solution.
(c). Electroantennogram (EAG) recording
The olfactory chemoreceptors for airborne, volatile pheromones have not yet been determined in spiders (Foelix 1996). Odours of conspecific females and of prey species, however, evoke electrical reactions in male pedipalps (Gemeno et al. 2000; Tichy et al. 2001). Putative chemoreceptors on female spider legs have been examined by electron microscopy (Ross & Smith 1979; Barth 2002). Electroantennogram (EAG) responses to the synthetic tricosane and (Z)-9-tricosene were recorded from the first leg tarsus of sexually RFs in our experiment. One leg of the tested spider was excised at the proximal base of the tarsus. The tip of the terminal tarsus segment was removed. Both the proximal end and distal ends of the tarsus were stuck on the electrode holder (PRG−2 probe; Syntech, Hilversum, The Netherlands) using electrically conductive gel. The electrode holder was connected to a high-impedance signal amplifier (Intelligent Data Acquisition Controller CS-55, Syntech). The amplified electrical potential signal from the antenna was recorded on a personal computer using the Syntech EAG software. An odour puff filtered through an active carbon filter was mixed with a humidified air-stream blowing continuously over the tarsus preparation at a rate of 250 ml min−1 through a silicone rubber pipe connected to a glass tube terminating 3 mm from the tarsus preparation. Both tricosane and (Z)-9-tricosene were dissolved in dichloromethane at 0.1 µg µl−1. Ten microlitre aliquots of each tested compound or dichloromethane alone (control) were absorbed on pieces of filter paper (5 × 30 mm). The filter paper was inserted into the glass pipette and exposed to the air stream 8 min later, by which time the solvent had evaporated at room temperature (25°C). The order of the odour stimuli was as follows: air (control), dichloromethane (solvent blank), tricosane, (Z)-9-tricosene, dichloromethane and air. The EAG responses were tested using five tarsus preparations each of a female P. beijingensis. All tests were repeated twice for each tarsus.
(d). Behavioural assays
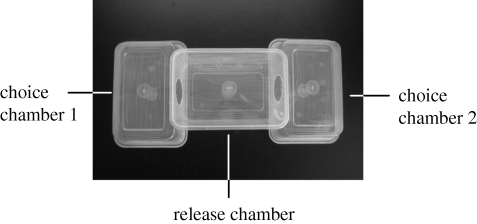
First, we tested the attractiveness of (Z)-9-tricosene to sexually RFs in a two-choice arena system used in previous behavioural assays of male responses to the female-produced sex pheromone of P. beijingensis (Xiao et al. 2009). The three chambers were upended and spliced with a hole on one or two sides (figure 1). The test female was released in the central chamber and could move freely to the left or right choice chamber. Sexually RFs were separated into three groups to test the attractiveness of (Z)-9-tricosene, tricosane, and a blend of (Z)-9-tricosene and tricosene, respectively. (Z)-9-tricosene, tricosane and the binary blend were dissolved in dichloromethane at concentrations of 0.001, 0.01 and 0.1 µg µl−1. The test females were introduced into the central release chamber and allowed to acclimatize to the surroundings for 1 h before the trials. A piece of filter paper containing 10 µl of the test solution was placed in one of the choice chambers; the other choice chamber contained a filter paper with an equal quantity of dichloromethane (solvent control). The choice chambers contained the chemical solution or the control solvent alternately between successive trials to eliminate any possible bias. As searching behaviour of this spider only occurs in the dark (Y.-H. Xiao, J.-X. Zhang & S.-Q. Li 2008, unpublished data), these tests were completed at night (usually from 20.00 to 22.00 h). We observed movement of the test females under infrared light and recorded which chamber it chose first during a 2 h observation period. If any test female was disturbed and escaped into a choice chamber as soon as the choice arena system was connected, we discarded the trial. Each test female was used twice in the two-choice behavioural assay. We rested each female for one week before the second test trial.
Figure 1.
Two-choice arena system used in the behavioural assays. The three chambers were upended to each other and spliced with a hole on one or two sides. The selecting spider was released in the central chamber and could move freely to the left or right choice chamber.
Second, we tested whether the sexually RFs could detect (Z)-9-tricosene as a cue of gender from the conspecific males and thus adjust their behaviour to mate with the males. In the laboratory experiments, males always held still on or under the female webs for a short time (usually from a few minutes to half an hour) after they had been introduced into the female webs. After a short interval for acclimation, the males started to move on the web to search for the female, which is the beginning of male courting sequences. As our former study mentioned (Xiao et al. 2009), male spiders of P. beijingensis display courting behaviour actively during most of the courtship sequence while females stay motionless on the web. When the male unfolds its pedipalps, the female turns from passive to active and approaches the male for mating. If females were not sexually receptive (e.g. subadult females or gravid adults), they would attack the intruders as soon as we introduced the male spiders on their webs. In a sense, keeping motionless (instead of showing aggressive behaviour) indicates the sexual acceptability of the females. Therefore, we compared male acclimation time, male courting time and mating time to illustrate differences between the treatment groups and the control group. Square boxes made of plastic-coated cardboard (22 × 22 cm; 8.5 cm deep) with transparent glass tops were used for observing spider courtship and copulation. All boxes were cleaned with 95 per cent ethanol and air dried before use. A glass dish with cotton soaked in distilled water was set in each box to supply humidity. Sexually RFs were randomly divided into four groups to observe the courting and mating behaviour of the males while exposed to (Z)-9-tricosene, tricosane, binary blends of (Z)-9-tricosene and tricosene, and dichloromethane (solvent control), respectively. Females were released into the box individually and were allowed to build their webs for 24 h prior to the trials. We placed a piece of filter paper with 10 µl of test solution at 0.1 µg µl−1 in each treated female box, while a filter paper with 10 µl dichloromethane alone was placed in each control female box. Twenty minutes later, MM spiders were introduced into all treated and control female boxes (one for each). For each trial, we recorded three parameters. (i) Male acclimating time, which is defined as the interval (min) between the male introduction into the female box and the male first moving on the web. During this period, the male holds still on or under the female web to acclimate to the surroundings. (ii) Male courting time, which is defined as the interval (min) between the male first moving on the web and mating initiation. (iii) Mating time, which is defined as the interval (min) between the male inserting his pedipalps into the female's genital pore until the mates disengaged. We observed 10 pairs in each treatment group and in the control group. Three pairs in the tricosane group and one pair in each other group failed to mate. Each female and male was used only once.
Third, we tested whether the sexually RFs could detect (Z)-9-tricosene as a cue for the presence of prey, and adjust their behaviour to take aggressive action against the intruders efficiently. All test females were kept individually in a glass cuvette (4 cm i.d. × 12 cm deep), in which they had lived for several days to acclimatize. No food was provided to the test spiders for 6 days before each trial. The females were divided into two groups: a treatment group (n = 17) and a control group (n = 17). We placed a filter paper with 10 µl of (Z)-9-tricosene at 0.1 µg µl−1 in each treated female cuvette, while a filter paper with 10 µl of dichloromethane solvent was placed in each control female cuvette. Twenty minutes later, 10 fruitflies were introduced into each treated and control female container. We observed predatory behaviour of the females for 2 h. The number of fruitflies caught by the females and the time that females spent on catching the first prey were recorded. Nine females in the test group and two females in the control group did not display predatory behaviour during the observation period. Each female was used only once.
(e). Statistical analyses
We measured the relative abundance of each compound by converting the peak area of a particular compound into a percentage of the summed peak areas from the 13 main GC peaks. First, we compared differences in relative abundance between the compounds extracted from MM and RF individuals; second, we compared quantitative differences between compounds from MM and SM spiders. These were analysed using either independent two-tailed Student's t-tests when the data were normally distributed or non-parametric Mann–Whitney U-tests when the data were not normally distributed.
Differences in EAG responses among various odour stimulus groups were analysed by one-way analysis of variance (ANOVA) with the least significant difference (LSD) test.
In the first behavioural assay, we used the χ2-test for goodness-of-fit to compare observed with expected counts for female choice data obtained from the two-choice experiment so that we could determine whether (Z)-9-tricosene or tricosane was attractive to the test females. In the second behavioural assay, the Kruskal–Wallis test was used when comparing differences in male acclimation and courting time among the four groups: the (Z)-9-tricosene exposure female group, the tricosane-exposure female group, the binary blend exposure female group and the control group, which did not have normally distributed raw data. Mann–Whitney U-tests were used for paired comparisons for courting time. Differences in the mating time among the four groups were analysed using one-way ANOVA as the raw data were normally distributed. In the third behavioural assay, differences in the time taken for catching the first prey between the (Z)-9-tricosene exposure female group and the solvent exposure female group were assessed using the Mann–Whitney U-test. Differences in the numbers of prey eaten between the treatment group and the control group were analysed using independent two-tailed Student's t-tests.
All statistical analyses were conducted using SPSS for Windows (v. 15.0; SPSS Inc., Chicago, IL, USA).
3. Results
(a). Chemical identification
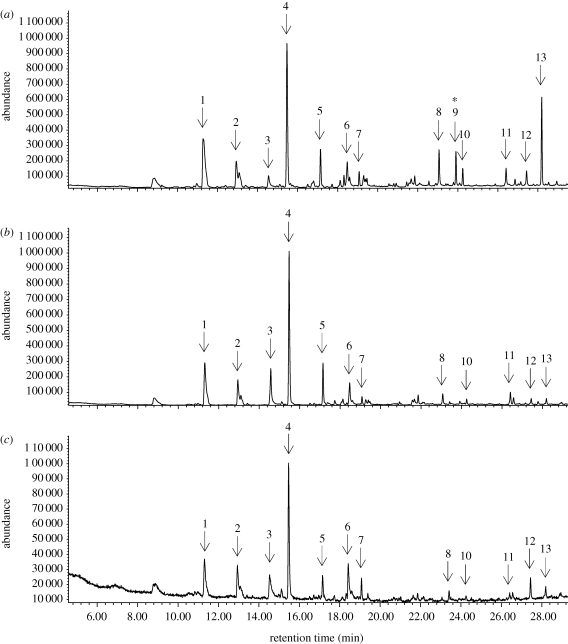
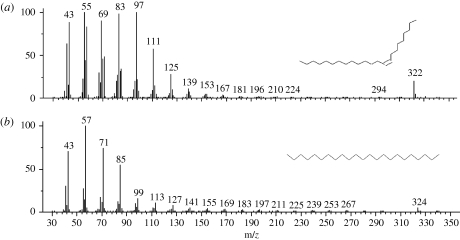
More than 20 different compounds in the whole-body extracts of P. beijingensis were detected by the GC–MS. Most were straight-chain aliphatic compounds, including aldehydes, ketones, acids, alkenes and alkanes. We tentatively identified 13 compounds that eluted in less than 30 min by matching GC retention times and mass spectra with analogues in the mass spectral library (figure 2). GC detection showed that compound 9 was present in body extracts of MM spiders while absent from body extracts of RFs and SMs (table 1). Except for compounds that were qualitatively different between the body extracts of the three spider groups, we also performed quantitative analyses on relative abundances of the relevant compounds obtained from the body extracts (table 1). Compound 10 showed a significantly greater proportion in body extracts of MM spiders than that of RFs or SMs. Compounds 9 and 10 were tentatively identified as (Z)-9-tricosene and tricosane, respectively, according to their retention times and mass spectra and later confirmed with the synthetic sample after separation on HP5–MS and a DB–wax column. The mass spectra of these natural products matched those of synthetic standards (figure 3).
Figure 2.
Total ion chromatograms of the crude extract from (a) a mate-searching male, (b) a sexually receptive female and (c) a subadult male. A non-polar column HP5–MS (0.25 mm × 30 m × 0.25 µm) was used. The numbers that label the GC peaks correspond to peak numbers in table 1. The asterisk indicates peak 9 is specific in the chromatogram of mate-searching male extract.
Table 1.
Comparison of relative abundance of compounds in body extracts of the spider P. beijingensis (mean ± s.d.). (MM, mate-searching males; RF, sexually receptive females; SM, subadult males.)
| peak no. | retention time (min) | compounds | relative abundance (%) |
statistical significance (p) |
|||
|---|---|---|---|---|---|---|---|
| MM (n = 8) | RF (n = 9) | SM (n = 9) | MM versus RF | MM versus SM | |||
| 1 | 11.28 | tetradecanal | 19.09 ± 3.09 | 22.97 ± 3.77 | 10.36 ± 5.27 | 0.036 | 0.001 |
| 2 | 12.94 | heptadecene | 4.89 ± 1.20 | 6.44 ± 1.35 | 5.31 ± 2.27 | 0.025 | 0.645 |
| 3 | 14.54 | tetradecanoic acid | 4.08 ± 4.05 | 6.62 ± 5.61 | 10.29 ± 4.57 | 0.386a | 0.009a |
| 4 | 15.46 | hexadecanal | 26.76 ± 5.02 | 32.89 ± 2.32 | 26.28 ± 7.59 | 0.005 | 0.884 |
| 5 | 17.14 | 2-heptadecanone | 6.18 ± 1.62 | 6.60 ± 1.30 | 3.05 ± 1.47 | 0.560 | 0.003 a |
| 6 | 18.48 | hexadecanoic acid | 4.24 ± 3.33 | 4.07 ± 2.17 | 12.43 ± 4.69 | 0.904 | 0.001 |
| 7 | 19.08 | tricosene | 2.43 ± 0.47 | 1.91 ± 0.32 | 3.84 ± 3.63 | 0.016 | 0.630a |
| 8b | 23.06 | silaceous compound | 3.98 ± 1.34 | 3.64 ± 1.40 | 4.30 ± 2.40 | 0.615 | 0.744 |
| 9 c,d | 23.91 | (Z)-9-tricosene | 4.53 ± 2.08 | ||||
| 10d,e | 24.24 | tricosane | 4.56 ± 2.87 | 1.09 ± 0.59 | 2.00 ± 0.97 | 0.011 | 0.041 |
| 11b | 26.41 | silaceous compound | 2.91 ± 0.47 | 3.01 ± 0.80 | 4.13 ± 1.46 | 0.847a | 0.124a |
| 12 | 27.44 | pentacosane | 8.31 ± 6.75 | 1.79 ± 1.15 | 9.60 ± 5.59 | 0.029 | 0.673 |
| 13f | 28.20 | diisooctyl phthalate | 8.04 ± 3.34 | 8.98 ± 6.14 | 8.40 ± 6.70 | 0.697 | 0.891 |
ap-values were tested by using the Mann–Whitney U-test; others were tested by using the independent t-test.
bThe silaceous compound were not considered as compounds from the spider body extracts but contaminants from the GC column.
cThe compound (Z)-9-tricosene is specific in the body extract of mate-searching males.
dThe compound was verified with synthetic standard samples after separation with a non-polar column (HP5−MS) and a polar column (DB−wax); other compounds were identified by comparison with spectra listed in the NIST Mass Spectral Library (Agilent Technologies 2002).
eRelative abundance of the compound tricosane in the body extract of mate-searching males was significantly more than that in body extract of sexually receptive females or subadult males.
fThis compound is a plasticizer contaminant and probably came from the box that was used to contain the spider to build their web.
Figure 3.
Mass spectra of (a) peak 9 and (b) peak 10 in the body extract of mate-searching males. They were identified as (Z)-9-tricosene and tricosane by comparing retention time and mass spectra with analogue in the mass spectra library (NIST 2002) and confirmed after separating the authentic standards on a non-polar column (HP5–MS) and a polar column (DB–wax).
(b). EAG responses
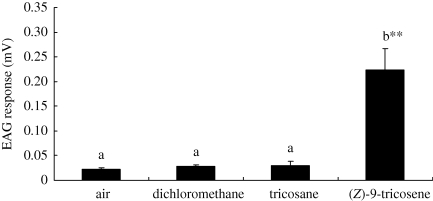
EAG responses of first leg tarsi of RF spiders to the putative pheromone components (Z)-9-tricosene and tricosane are shown in figure 4. The tarsus preparations showed strong responses to (Z)-9-tricosene. The mean EAG amplitude response to (Z)-9-tricosene (0.223 mV) was significantly higher (ANOVA with LSD test, p < 0.01) than responses to the air blank (0.022 mV), solvent control (0.028 mV) or tricosane (0.030 mV). Nevertheless, differences in EAG responses among the tricosane-stimulating group, the air-stimulation group and the chloromethane-stimulating group are not statistically significant (LSD test, p > 0.05). These results clearly demonstrate that (Z)-9-tricosene evoked significant EAG responses from the tarsi preparations of the sexually RFs, while tricosane seemed to be inactive.
Figure 4.
EAG responses (mean ± s.e.) of first leg tarsi (n = 5) of sexually receptive female P. beijingensis to putative pheromone compounds. The order of the odour stimuli was as follows: air (control), dichloromethane (solvent blank), tricosane and (Z)-9-tricosene. **p < 0.01 (one-way ANOVA with LSD test).
(c). Titre analyses
We determined the quantity of (Z)-9-tricosene from the body extract of one male by comparing the peak GC–MS area detected from the extract of an MM spider with that detected from the synthetic standard. The calibration regression equation is y = 0.302x–0.259 (r2 = 0.918, S = 0.311, p < 0.001). We calculated the quantity of the male-produced pheromone from one male body extract according to the calibration regression equation and the volume of the extract solution. There were large individual variations in pheromone titre from MM spiders. The amount of (Z)-9-tricosene varied from 0.033 to 0.342 µg, with a mean value of 0.114 ± 0.036 µg (± s.e.).
(d). Behavioural responses of females
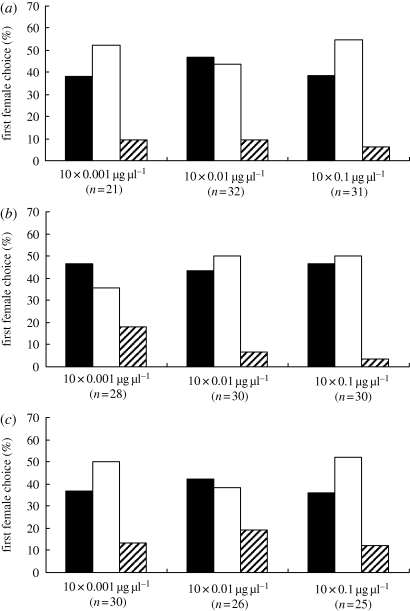
In the two-choice arena trials, sexually RFs displayed no significant preference between the treated chamber and the solvent chamber in the trials of three chemical dosages: (Z)-9-tricosene: 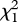 (0.01 µg) = 0.474, p = 0.491;
(0.01 µg) = 0.474, p = 0.491; 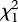 (0.1 µg) = 0.034, p = 0.853;
(0.1 µg) = 0.034, p = 0.853;  (1 µg) = 0.862, p = 0.353; tricosane:
(1 µg) = 0.862, p = 0.353; tricosane: 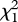 (0.01 µg) = 0.391, p = 0.532;
(0.01 µg) = 0.391, p = 0.532; 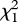 (0.1 µg) = 0.143, p = 0.705;
(0.1 µg) = 0.143, p = 0.705; 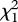 (1 µg) = 0.034, p = 0.853; and the binary blend:
(1 µg) = 0.034, p = 0.853; and the binary blend:  (0.01 µg) = 0.615, p = 0.433;
(0.01 µg) = 0.615, p = 0.433; 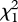 (0.1 µg) = 0.048, p = 0.827;
(0.1 µg) = 0.048, p = 0.827; 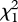 (1 µg) = 0.727, p = 0.394; figure 5). These negative results of the two-choice assay show that neither (Z)-9-tricosene nor tricosane is an attractant released by the male P. beijingensis for conspecific females.
(1 µg) = 0.727, p = 0.394; figure 5). These negative results of the two-choice assay show that neither (Z)-9-tricosene nor tricosane is an attractant released by the male P. beijingensis for conspecific females.
Figure 5.
Results of the attractiveness for female Pholcus beijingensis to (a) (Z)-9-tricosene, (b) tricosane and the binary blends of (Z)-9-tricosene and (c) tricosane. Trials were completed in the two-choice arena system. (a) Black bars, (Z)-9-trocosene chamber; white bars, solvent chamber; striped bars, release chamber; (b) black bars, ticosane chamber; white bars, solvent chamber; striped bars, release chamber; (c) black bars, binary blends chamber; white bars, solvent chamber; striped bars, release chamber. ‘Release chamber’ refers to females that within the 2 h observation period failed to leave the central arena into which they had been introduced 1 h prior to the start of the trial.
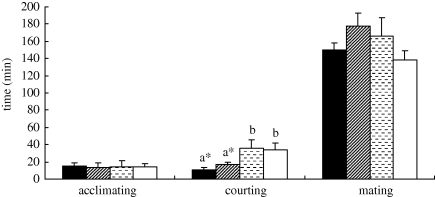
In the second behavioural assay, there was no significant difference in male acclimation time among the binary blend group, the (Z)-9-tricosene group, the tricosane group and the control group (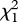 = 1.473, d.f. = 3, p = 0.689, Kruskal–Wallis test; figure 6). In all successful mating pairs, average mating times of the mates in the three treatment groups were a little longer than those of the mates in the control group but there was no significant difference between them (F = 1.632, d.f.1 = 3, d.f.2 = 30, p = 0.203 by one-way ANOVA; figure 6). Males in the binary blend group and the (Z)-9-tricosene group, however, spent much less time courting females than did males in the tricosane group and the control group (
= 1.473, d.f. = 3, p = 0.689, Kruskal–Wallis test; figure 6). In all successful mating pairs, average mating times of the mates in the three treatment groups were a little longer than those of the mates in the control group but there was no significant difference between them (F = 1.632, d.f.1 = 3, d.f.2 = 30, p = 0.203 by one-way ANOVA; figure 6). Males in the binary blend group and the (Z)-9-tricosene group, however, spent much less time courting females than did males in the tricosane group and the control group (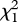 = 8.407, d.f. = 3, p = 0.038, Kruskal–Wallis test; figure 6). The difference of male courting time between the binary blend group and the (Z)-9-tricosene group was not significant (U = 28.500, p = 0.288, Mann–Whitney U-test). This indicates that tricosane is not a vital component for stimulating sexual behaviour in females.
= 8.407, d.f. = 3, p = 0.038, Kruskal–Wallis test; figure 6). The difference of male courting time between the binary blend group and the (Z)-9-tricosene group was not significant (U = 28.500, p = 0.288, Mann–Whitney U-test). This indicates that tricosane is not a vital component for stimulating sexual behaviour in females.
Figure 6.
Influences of (Z)-9-tricosene, tricosane and the binary blend of both (Z)-9-tricosene and tricosane on courtship and mating behaviour of P. beijingensis. Black bars, binary blend; striped bars, (Z)-9-tricosene; dashed bars, tricosane; white bars, solvent control. Acclimating time is defined as the interval (min) between the male introduction into the female box and the male first moving on the web. In this period, the males keep still on the box side or bottom. Male courting time is defined as the interval (min) between the male first moving on the web and mating initiation. During this period, the male court the female until mating. Mating time is defined as the interval between the male inserting his pedipalps into the female's genital pore until the mates disengaged. Data are mean ± s.e. *p < 0.05.
In the third behavioural assay, females exposed to (Z)-9-tricosene spent a mean of 22.65 min in catching the first fruitfly, while females exposed to the solvent dichloromethane spent a mean of 25.93 min. There was no significant difference between these times (p = 0.673 by Mann–Whitney U-test; table 2). This result indicates that females in the treatment group did not initiate predatory behaviour more quickly than did females in the control group. On the contrary, 88 per cent of females in the control group caught the fruitflies for food while just 47 per cent of females in the treatment group caught their prey during the 2 h observation period. Females in the control group caught a mean of 2.35 fruitflies for food during the 2 h period, while females in the test group caught a mean of only 1.0. This difference was statistically significant (t = 2.380, d.f. = 32, p = 0.023, by Student's t-test; table 2).
Table 2.
Predatory behaviour of females exposed to (Z)-9-tricosene in comparison with that of females exposed to solvent only. Females exposed to (Z)-9-tricosene were in the test group and females exposed to solvent only (dichloromethane) were in the control group.
| test group | predatory amount | time on catching the first fruitflies | control group | predatory amount | time on catching the first fruitflies |
|---|---|---|---|---|---|
| 1 | 2 | 20 | 1 | 1 | 50 |
| 2 | 1 | 50 | 2 | 2 | 2 |
| 3 | 0 | — | 3 | 2 | 26 |
| 4 | 0 | — | 4 | 3 | 35 |
| 5 | 0 | — | 5 | 8 | 2 |
| 6 | 0 | — | 6 | 2 | 37 |
| 7 | 2 | 2 | 7 | 4 | 39 |
| 8 | 3 | 30 | 8 | 3 | 1 |
| 9 | 0 | — | 9 | 2 | 47 |
| 10 | 0 | — | 10 | 5 | 10 |
| 11 | 1 | 57 | 11 | 1 | 20 |
| 12 | 4 | 20 | 12 | 1 | 1 |
| 13 | 0 | — | 13 | 0 | — |
| 14 | 0 | — | 14 | 2 | 70 |
| 15 | 2 | 1 | 15 | 3 | 3 |
| 16 | 2 | 1 | 16 | 0 | — |
| 17 | 0 | — | 17 | 1 | 46 |
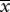 |
1.00 | 22.65 | 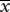 |
2.35a | 25.93 |
aSignificant difference between the average number of fruitflies caught by females in the control group and those caught by females in the test group.
4. Discussion
Our study revealed that females of P. beijingensis exposed to (Z)-9-tricosene initiated mating with the males far more quickly than did females without exposure. MM individuals thus release (Z)-9-tricosene acting as a sexual stimulant for conspecific females. Males of many spider species have long been known to use chemical tactics that increase the probability of mating. For example, males release silk-bound semiochemicals for attracting females or inhibiting the courtship behaviours of conspecific males (Ross & Smith 1979; Yoshida & Suzuki 1981; Roland 1984; Rao Ayyagari & Tietjen 1987; Suter et al. 1987; Becker et al. 2005). (Z)-9-tricosene is, however, to our knowledge, the first identified male pheromone found in spiders. Based on the results of the behavioural assay, this pheromone acts like an aphrodisiac in that it increases the likelihood that a female will mate (figure 6). The MM individuals appear to release (Z)-9-tricosene to stimulate the sexual behaviour (copulation) of RF spiders so that they can mate more quickly (figure 6). It seems that, however, this aphrodisiac pheromone is not attractive to the females because the females showed no preference to the chambers containing (Z)-9-tricosene in the two-choice assay (figure 5). In nature, most male spiders leave their retreats or webs at maturity and start wandering around or spin their own nests right next to the potential mates (Foelix 1996). The MMs in many spider species are apparently attracted by female sex pheromones (Gaskett 2007). In contrast to males, female spiders usually apply the sit-and-wait tactic for mating. Female P. beijingensis remain motionless during most of the male courtship. Only after the males unfold their palps at the last phase of the courting procedure will the females turn from passive to active, which leads directly to copulation (Xiao et al. 2009).
(Z)-9-tricosene has also been identified as a sex pheromone released by the female housefly Musca domestica (Carlson et al. 1971), which is a prey species of P. beijingensis. Later, some other compounds such as (Z)-9,10-epoxytricosane, (Z)-14-tricosene-10-one and some methyl alkanes were found to enhance the male housefly's sexual activity in combination with (Z)-9-tricosene (Uebel et al. 1976; Rogoff et al. 1980). A number of animal species use chemical camouflage to lure prey or access hosts (Dettner & Liepert 1994; Akino et al. 1999; Geiselhardt et al. 2006). A good example for chemical mimicry in the spiders is found among the bolas spiders. They usually feed exclusively on males of a restricted number of moth species (Yeargan 1994). Adult female bolas spiders attract male moth prey by combinations of aggressive chemical mimicry with a specialized weapon (the bolas) and behaviour (Eberhard 1977; Stowe et al. 1987; Gemeno et al. 2000; Haynes et al. 2002). Cuticular hydrocarbons have also been reported as mimic chemicals in spiders. Thus, cuticular lipids of the spider Gamasomorpha maschwitzi, which lives in colonies of the Southeast Asian army ant Leptogenys distinguenda, and its host ant were virtually identical (Schulz 2004). This predatory spider acquires colony-specific cuticular hydrocarbons from their ant prey (Elgar & Allan 2004). The salticid spider Cosmophasis bitaeniata, which preys on the larvae of the green tree ant, Oecophylla smaragdina, mimics the cuticular hydrocarbon pattern of its host to avoid detection by major worker ants (Allan et al. 2002). In the study of Nentwig (1983), Pholcus showed high consumption rates of Coleoptera, Heteroptera, Hymenoptera Parasitica, Formicidae, Lepidoptera, Nuroptera, Orthoptera and Dermaptera. In our laboratory experiments, P. beijingensis accepted almost all offered prey species including fruitflies, springtails, mosquitoes, houseflies, ants and even conspecific spiderlings. In field investigations, several insect species and other small arthropods including mosquitoes (Chironomidae, Tipulidae, Cecidomyiidae), moths (Gelechiidae), ants (Formicidae), bedbugs (Coreidae), houseflies (Muscidae) and pillbugs (Porcellionidae) were captured by P. beijingensis (Y.-H. Xiao, J.-X. Zhang & S.-Q. Li 2009, unpublished data). Whether the male P. beijingensis mimics the sex pheromones of their prey needs further investigation and is beyond the scope of this paper.
Hydrocarbons serve many functions in insects. They comprise a significant portion of the cuticular lipids that prevent desiccation, and are important in chemical communication (Howard & Blomquist 1982). (Z)-9-tricosene is a very common compound of cuticular hydrocarbons of insects in general. As well as in the housefly, (Z)-9-tricosene was also found in another fly species (Haematobia irritans) and males contained more than females (Macldey 1977). Intriguingly, (Z)-9-tricosene has been found in several other insects as a biologically active component. Zhang et al. (2003) identified five monounsaturated compounds including (Z)-9-tricosene from the Asian long-horned beetle, Anoplophora glabripennis and demonstrated that these compounds stimulate copulatory behaviour in males. In the social wasp, Vespa crabro, cuticular hydrocarbons including (Z)-9-tricosene are involved in the phenomenon of nest-mate recognition (Ruther et al. 2002). Thom et al. (2007) reported that (Z)-9-tricosene along with three other hydrocarbons could be isolated from the scent of waggle-dancing foragers of the honeybee (Apis mellifera). These compounds are semiochemicals, inducing worker recruitment to the food source.
It is not unique that the male spider of P. beijingensis shares its semiochemical with an insect species. Our previous study on the silk-bound pheromone of the spider P. beijingensis showed that the female sex pheromone components, (E,E)-farnesyl acetate and hexadecyl acetate, are found not only in some invertebrate species such as insects but also in mammals such as voles (Xiao et al. 2009). The spider pheromone 8-methyl-2-nonanone, isolated from the orb-web spider A. aperta (Papke et al. 2001), resembles a known pheromone component of the caddisfly, Hesperophylax occidentalis (Bjostad et al. 1996) and of the Asia palm weevil, Rhynchophorus ferrugineus (Hallett et al. 1993). The contact sex pheromone of Tegenaria atrica consists of a complex mixture of methyl esters and their fatty acids, which are semiochemicals of several insects such as the mosquito (Aedes aegypti) and the honeybee (Breed 1998; Ganesan et al. 2006). Nevertheless, two other identified spider sex pheromones, (R)-HBA and its dimer from the sheet-web spider Linyphia triangularis (Schulz & Toft 1993) and (S)-1,1′-dimethyl citrate from the wandering spider Cupiennius salei (Papke et al. 2000), seem to be structurally unique pheromone compounds in spiders.
Acknowledgements
The manuscript benefited greatly from comments by Robert W. Murphy (University of Toronto, Canada). This study was supported by the National Natural Sciences Foundation of China (NSFC-30870271) and by the National Science Fund for Fostering Talents in Basic Research (Special Subjects in Animal Taxonomy, NSFC-J0630964/J0109).
References
- Akino T., Knapp J. J., Thomas J. A., Elmes G. W.1999Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 266, 1419–1426 (doi:10.1098/rspb.1999.0796) [Google Scholar]
- Allan R. A., Capon R. J., Brown W. V., Elgar M. A.2002Mimicry of host cuticular hydrocarbons by salticid spider Cosmophasis bitaeniata that preys on larvae of tree ants Oecophylla smaragdina. J. Chem. Ecol. 28, 835–848 (doi:10.1023/A:1015249012493) [DOI] [PubMed] [Google Scholar]
- Anderson J. T., Morse D. H.2001Pick-up lines: cues used by male crab spiders to find reproductive females. Behav. Ecol. 12, 360–366 (doi:10.1093/beheco/12.3.360) [Google Scholar]
- Barth F. G.2002A spider's world: senses and behaviour. New York, NY: Springer [Google Scholar]
- Becker E., Riechert S., Singer F.2005Male induction of female quiescence/catalepsis during courtship in the spider, Agelenopsis aperta. Behaviour 142, 57–70 (doi:10.1163/1568539053627767) [Google Scholar]
- Bjostad L. B., Jewett D. K., Brigham D. L.1996Sex pheromone of caddisfly Hesperophylax occidentalis (Banks) (Trichoptera: Limnephilidae). J. Chem. Ecol. 22, 103–121 (doi:10.1007/BF02040203) [DOI] [PubMed] [Google Scholar]
- Breed M. D.1998Recognition pheromones of the honey bee. Bioscience 48, 463–470 (doi:10.2307/1313244) [Google Scholar]
- Budenberg W. J., Ndiege I. O., Karago F. W.1993Evidence for volatile male-produced pheromone in banana weevil Cosmopolites sordidus. J. Chem. Ecol. 19, 1905–1916 (doi:10.1007/BF00983795) [DOI] [PubMed] [Google Scholar]
- Carlson D. A., Mayer M. S., Silhacek D. L., James J. D., Beroza M., Bierl B. A.1971Sex attractant pheromone of the house fly: isolation, identification and synthesis. Science 174, 76–78 (doi:10.1126/science.174.4004.76) [DOI] [PubMed] [Google Scholar]
- Chen H. F., Li S. Q.2005The reproductive behaviour of Pholcus beijingens (in Chinese). Chin. J. Zool. 40, 14–20 [Google Scholar]
- Chinta S. P., Goller S., Lux J., Funke S., Uhl G., Schulz S.2010The sex pheromone of the wasp spider Argiope bruennichi. Angew. Chem. Int. Ed. 49, 2033–2036 [DOI] [PubMed] [Google Scholar]
- Dettner K., Liepert C.1994Chemical mimicry and camouflage. Annu. Rev. Entomol. 39, 129–154 (doi:10.1146/annurev.en.39.010194.001021) [Google Scholar]
- Eberhard W. G.1977Aggressive chemical mimicry by a bolas spider. Science 198, 1173–1175 (doi:10.1126/science.198.4322.1173) [DOI] [PubMed] [Google Scholar]
- Elgar M. A., Allan R. A.2004Predatory spider mimics acquire colony-specific cuticular hydrocarbons from their ant model prey. Naturwissenschaften 91, 143–147 (doi:10.1007/s00114-004-0507-y) [DOI] [PubMed] [Google Scholar]
- Foelix R. F.1996Biology of spiders, 2nd edn New York, NY: Oxford University Press [Google Scholar]
- Ganesan K., Mendki M. J., Suryanarayana M. V. S., Prakash S., Malhotra R. C.2006Studies of Aedes aegypti (Diptera: Culicidae) ovipositional responses to newly identified semiochemicals from conspecific eggs. Aust. J. Entomol. 45, 75–80 (doi:10.1111/j.1440-6055.2006.00513.x) [Google Scholar]
- Gaskett A. C.2007Spider sex pheromones: emission, reception, structures, and functions. Biol. Rev. 82, 26–48 [DOI] [PubMed] [Google Scholar]
- Geiselhardt S. F., Geiselhardt S., Peschke K.2006Chemical mimicry of cuticular hydrocarbons: how does Eremostibes opacus gain access to breeding burrows of its host Parastizopus armaticeps (Coleoptera, Tenebrionidae)? Chemoecology 16, 59–68 (doi:10.1007/s00049-005-0330-8) [Google Scholar]
- Gemeno C., Yeargan K. V., Haynes K. F.2000Aggressive chemical mimicry by the bolas spider Mastophora hutchinsoni: identification and quantification of a major prey's sex pheromone components in the spider's volatile emissions. J. Chem. Ecol. 26, 1235–1243 (doi:10.1023/A:1005488128468) [Google Scholar]
- Hallett R. H., Gries G., Gries R., Borden J. H., Czyzewska E., Oehlschlager A. C., Pierce H. D., Angerilli N. P. D., Rauf A.1993Aggregation pheromones of two Asian palm weevils, Rhynchophorus ferrugineus and R. vulneratus. Naturwissenschaften 80, 328–331 (doi:10.1007/BF01141908) [Google Scholar]
- Haynes K. F., Gemeno C., Yeargan K. V., Millar J. G., Johnson K. M.2002Aggressive chemical mimicry of moth pheromones by a bolas spider: how does this specialist predator attract more than one species of prey? Chemoecology 12, 99–105 (doi:10.1007/s00049-002-8332-2) [Google Scholar]
- Howard R., Blomquist G.1982Chemical ecology and biochemistry of insect hydrocarbons. Ann. Rev. Entomol. 27, 149–172 (doi:10.1146/annurev.en.27.010182.001053) [DOI] [PubMed] [Google Scholar]
- Huber B. A.1999Sexual selection in pholcid spiders (Araneae, Pholcidae): artful chelicerae and forceful genitalia. J. Arachnol. 27, 135–141 [Google Scholar]
- Huber B. A.2005The Pholcid spiders of Africa (Araneae: Pholcidae): state of knowledge and directions for future research. In African biodiversity: molecules, organisms, ecosystems (eds Huber B. A., Sinclair B. J., Heinz L. K.), pp. 181–186 Berlin, Germany: Springer [Google Scholar]
- Jerhot E., Stoltz J. A., Andrade M. C. B., Schulz S.2010Acylated serine derivatives: a unique class of arthropod pheromones of the Australian redback spider, Latrodectus hasselti. Angew. Chem. Int. Ed. 49, 2037–2040 [DOI] [PubMed] [Google Scholar]
- Kasumovic M. M., Andrade M. C. B.2004Discrimination of airborne pheromones by mate-searching male western black widow spiders (Latrodectus hesperus): species- and population-specific responses. Can. J. Zool. 82, 1027–1034 (doi:10.1139/z04-081) [Google Scholar]
- Leal W. S., Panizzi A. R., Niva C. C.1994Alarm pheromone system of leaf-footed bug Leptoglossus zonatus (Heteroptera: Coreidae). J. Chem. Ecol. 20, 1209–1216 [DOI] [PubMed] [Google Scholar]
- Legendre R., Lopez A.1974Étude histologique de quelques formations glandulaires chez les Araignées du genre Argyrodes (Theridiidae) et description d'un nouveau type de glande: la glande clypéale des màles. Bull. Soc. Zool. France 99, 453–460 [Google Scholar]
- Leonard A. S., Morse D. H.2006Line-following preferences of male crab spiders, Misumena vatia. Anim. Behav. 71, 717–724 (doi:10.1016/j.anbehav.2005.08.004) [Google Scholar]
- Macldey J. W.1977Attractants and body hydrocarbon constituents of the horn fly. In Haematobia irritans (L.) PhD thesis, University Florida, Gainesville, USA [Google Scholar]
- Nentwig W.1983The prey of web-building spiders compared with feeding experiments (Araneae: Araneidae, Linyphiidae, Pholcidae, Agelenidae). Oecologia 56, 132–139 (doi:10.1007/BF00378229) [DOI] [PubMed] [Google Scholar]
- Papke M., Schulz S., Tichy H., Gingl E., Ehn R.2000Identification of a new sex pheromone from the silk dragline of the tropical wandering spider Cupiennius salei. Angew. Chem. Int. Ed. 39, 4339–4341 (doi:10.1002/1521-3773(20001201)39:23<4339::AID-ANIE4339>3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- Papke M. D., Riechert S. E., Schulz S.2001An airborne female pheromone associated with male attraction and courtship in a desert spider. Anim. Behav. 61, 877–886 (doi:10.1006/anbe.2000.1675) [Google Scholar]
- Prenter J., Elwood R. W., Montgomery W. I.1994Assessments and decisions in Metellina segmentata (Araneae: Metidae): evidence of a pheromone involved in mate guarding. Behav. Ecol. Sociobiol. 35, 39–43 (doi:10.1007/BF00167058) [Google Scholar]
- Prouvost O., Trabalon M., Papke M., Schulz S.1999Contact sex signals on web and cuticle of Tegenaria atrica (Araneae, Agelenidae). Arch. Insect Biochem. Physiol. 40, 194–202 (doi:10.1002/(SICI)1520-6327(1999)40:4<194::AID-ARCH4>3.0.CO;2-P) [Google Scholar]
- Rao Ayyagari L., Tietjen W. J.1987Preliminary isolation of male-inhibitory pheromone of the spider Schizocosa ocreata (Araneae, Lycosidae). J. Chem. Ecol. 13, 237–244 (doi:10.1007/BF01025884) [DOI] [PubMed] [Google Scholar]
- Roberts J. A., Uetz G. W.2005Information content of female chemical signals in the wolf spider, Schizocosa ocreata: male discrimination of reproductive state and receptivity. Anim. Behav. 70, 217–223 (doi:10.1016/j.anbehav.2004.09.026) [Google Scholar]
- Rogoff W. M., Gretz G. H., Sonnet P. F., Schwarz M.1980Responses of male house flies to muscalure and to combinations of hydrocarbons with and without muscalure. Environ. Entomol. 9, 605–606 [Google Scholar]
- Roland C.1984Chemical signals bound to the silk in spider communication (Arachnida, Araneae). J. Arachnol. 11, 309–314 [Google Scholar]
- Ross K., Smith R. L.1979Aspects of the courtship behavior of the black widow spider, Latrodectus hesperus (Araneae: Theridiidae), with evidence for the existence of a contact sex pheromone. J. Arachnol. 7, 69–77 [Google Scholar]
- Ruther J., Sieben S., Schricker B.2002Nestmate recognition in social wasps: manipulation of hydrocarbon profiles induces aggression in the European hornet. Naturwissenschaften 89, 111–114 (doi:10.1007/s00114-001-0292-9) [DOI] [PubMed] [Google Scholar]
- Schulz S.2004Semiochemistry of spiders. In Advances of chemical ecology (eds Cardé R. T., Millar J. G.), pp. 110–150 Cambridge, UK: Cambridge University Press [Google Scholar]
- Schulz S., Toft S.1993Identification of a sex-pheromone from a spider. Science 260, 1635–1637 (doi:10.1126/science.260.5114.1635) [DOI] [PubMed] [Google Scholar]
- Searcy L. E., Rypstra A. L., Persons M. H.1999Airborne chemical communication in the wolf spider Pardosa milvina. J. Chem. Ecol. 25, 2527–2533 (doi:10.1023/A:1020878225553) [Google Scholar]
- Singer F., Riechert S. E., Xu H., Morris A. W., Becker E., Hale J. A., Noureddine M. A.2000Analysis of courtship success in the funnel-web spider Agelenopsis aperta. Behaviour 137, 93–117 (doi:10.1163/156853900501890) [Google Scholar]
- Stoltz J. A., McNeil J. N., Andrade M. C. B.2007Males assess chemical signals to discriminate just-mated females from virgins in redback spiders. Anim. Behav. 74, 1669–1674 (doi:10.1016/j.anbehav.2007.03.011) [Google Scholar]
- Stowe M. K., Tumlinson J. H., Heath R. R.1987Chemical mimicry: bolas spiders emit components of moth prey species sex pheromones. Science 236, 964–967 (doi:10.1126/science.236.4804.964) [DOI] [PubMed] [Google Scholar]
- Suter R. B., Shane C. M., Hirscheimer A. J.1987Communication by cuticular pheromones in a linyphiid spider. J. Arachnol. 15, 157–162 [Google Scholar]
- Thom C., Gilley D. C., Hooper J., Esch H. E.2007The scent of the waggle dance. PLoS Biol. 5, e228 (doi:10.1371/journal.pbio.0050228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy H., Gingl E., Ehn R., Papke M., Schulz S.2001Female sex pheromone of a wandering spider (Cupiennius salei): identification and sensory reception. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 187, 75–78 [DOI] [PubMed] [Google Scholar]
- Trabalon M., Niogret J., Legrand-Frossi C.2005Effect of 20-hydroxyecdysone on cannibalism, sexual behavior, and contact sex pheromone in the solitary female spider, Tegenaria atrica. Gen. Comp. Endocrinol. 144, 60–66 (doi:10.1016/j.ygcen.2005.04.011) [DOI] [PubMed] [Google Scholar]
- Uebel E. C., Sonnet P. E., Miller R. W.1976House fly sex pheromone: enhancement of mating strike activity by combination of (Z)-9-tricosene with branched saturated hydrocarbons. Environ. Entomol. 5, 905–908 [Google Scholar]
- Uhl G.1998Mating behaviour in the cellar spider, Pholcus phalangioides, indicates sperm mixing. Anim. Behav. 56, 1155–1159 (doi:10.1006/anbe.1998.0854) [DOI] [PubMed] [Google Scholar]
- Uhl G., Schmitt S., Schafer M.2005Fitness benefits of multiple mating versus female mate choice in the cellar spider (Pholcus phalangioides). Behav. Ecol. Sociobiol. 59, 69–76 (doi:10.1007/s00265-005-0010-2) [Google Scholar]
- Weygoldt P.1977Communication in crustaceans and arachnids. In How animals communicate (ed. Sebeok T. A.), pp. 303 Bloomington, IN: Indiana University Press [Google Scholar]
- Xiao Y. H., Zhang J. X., Li S. Q.2009A two-component female-produced pheromone of the spider Pholcus beijingensis. J. Chem. Ecol. 35, 769–778 (doi:10.1007/s10886-009-9660-2) [DOI] [PubMed] [Google Scholar]
- Yeargan K. V.1994Biology of bolas spiders. Annu. Rev. Entomol. 39, 81–99 (doi:10.1146/annurev.en.39.010194.000501) [Google Scholar]
- Yoshida H., Suzuki Y.1981Silk as a cue for mate location in the jumping spider, Carrhotus xanthogramma (Latreille) (Araneae: Salticidae). Appl. Entomol. Zool. 16, 315–317 [Google Scholar]
- Zhang A., Oliver J. E., Chauhan K., Zhao B., Xia L., Xu Z.2003Evidence for contact sex recognition pheromone of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Naturwissenschaften 90, 410–413 (doi:10.1007/s00114-003-0452-1) [DOI] [PubMed] [Google Scholar]