Abstract
Photoperiodic diapause is a crucial adaptation to seasonal environmental variation in a wide range of arthropods, but relatively little is known regarding the molecular basis of this important trait. In temperate populations of the mosquito Aedes albopictus, exposure to short-day (SD) lengths causes the female to produce diapause eggs. Tropical populations do not undergo a photoperiodic diapause. We identified a fatty acyl coA elongase transcript that is more abundant under SD versus long-day (LD) photoperiods in mature oocyte tissue of replicate temperate, but not tropical, A. albopictus populations. Fatty acyl CoA elongases are involved in the synthesis of long chain fatty acids (hydrocarbon precursors). Diapause eggs from a temperate population had one-third more surface hydrocarbons and one-half the water loss rates of non-diapause eggs. Eggs from a tropical population reared under SD and LD photoperiods did not differ in surface hydrocarbon abundance or water loss rates. In both a temperate and tropical population, composition of hydrocarbon chain lengths did not differ between eggs from SD versus LD conditions. These results implicate the expression of fatty acyl coA elongase and changes in quantity, but not composition, of egg surface hydrocarbons as important components of increased desiccation resistance during diapause in A. albopictus.
Keywords: invasive species, photoperiodic diapause, desiccation resistance, Aedes albopictus
1. Introduction
The Asian tiger mosquito, Aedes albopictus, is currently the most invasive mosquito species in the world (Benedict et al. 2007). Also of considerable public health concern, this aggressive daytime biting mosquito is capable of efficiently transmitting Chikungunya, dengue, West Nile and a variety of native North American arboviruses (Turell et al. 2001; Gratz 2004). In the last 30 years, A. albopictus has rapidly spread from its native Asian range across the world and is currently found in at least 28 countries on every continent except Australia and Antarctica (Benedict et al. 2007). This rapid spread has been accomplished by the worldwide transport of containers such as tyres and pots of ‘lucky bamboo’ that harbour eggs and/or larvae (Hawley et al. 1987; Scholte et al. 2008). Recent analyses indicate that there are few countries where A. albopictus could not exist and thus further spread and accompanying public health concern is likely (Benedict et al. 2007).
The first breeding population of A. albopictus in the US was discovered in Houston, TX in 1985 (Sprenger & Wuithiranyagool 1986), where it was probably introduced via a shipment of used automobile tyres from temperate Japan (Hawley et al. 1987). Within two years A. albopictus had spread rapidly throughout the US, extending as far north as Illinois and as far east and south as Jacksonville, FL (Moore 1999). This rapid range expansion was probably facilitated by the intact photoperiodic diapause response of the invading population (Hawley et al. 1987). In its native Asian range, A. albopictus occurs across an unusually broad latitudinal range, including temperate populations that undergo a photoperiodic diapause and tropical populations that do not enter a photoperiodic diapause (Hawley 1988). In temperate (diapausing) populations, exposure of pupal and adult females to short-day (SD) lengths induces a developmental arrest of pharate larvae inside the chorion of the egg (Wang 1966; Mori et al. 1981). It has been known for some time that diapause eggs of A. albopictus have increased survivorship under desiccating and cold-stress conditions relative to non-diapause eggs (Sota & Mogi 1992; Hanson & Craig 1994). However, the mechanistic basis of this stress resistance has not previously been determined.
Juliano & Lounibos (2005) showed that mosquitoes that produce desiccation-resistant eggs were more likely to become established in non-native habitats relative to mosquito species that produce desiccation-susceptible eggs, presumably because desiccation-resistant eggs are more likely to survive long-distance transport. Desiccation resistance in mosquitoes has primarily been studied by examining survival under a range of relative humidity conditions (Sota & Mogi 1992; Gray & Bradley 2005). However, in a more detailed mechanistic study, Benoit & Denlinger (2007) showed that diapausing adult females of Culex pipiens had substantially lower water loss rates relative to non-diapausing females owing to larger body size, decreased metabolism and an approximately two-fold higher accumulation of cuticular hydrocarbons. The molecular basis of increased desiccation resistance in diapausing C. pipiens has yet to be determined.
Herein we describe a fatty acyl coA elongase from A. albopictus that is upregulated under SD versus long-day (LD) photoperiods in mature (stage V) oocytes of temperate, but not tropical, populations. Fatty acyl coA elongases are involved in the formation of very long chain lipids which are known to affect water loss in insects (Blomquist et al. 1987). Using a comparative approach and based on well-established functional considerations, we link this expression pattern to an increase in hydrocarbon quantity and a decrease in water loss rate in diapause relative to non-diapause eggs. These results provide insight into the physiological processes contributing to the rapid global spread of this invasive mosquito.
2. Material and methods
(a). EST identification
We identified a 186 bp putative fatty acyl coA elongase expressed sequence tag (EST) as potentially upregulated under SD versus LD photoperiod treatments using a ‘SD minus LD’ suppressive subtractive hybridization (SSH) library of cDNAs isolated from mature (stage V) oocyte tissue. The SSH library was constructed using a laboratory F12 population from Berlin, NJ which was collected and reared as described below. A comprehensive description of the suppressive subtractive hybridization results is described in another paper (Urbanski et al. in press).
(b). 5′ and 3′ RACE
To determine the complete cDNA sequence of the putative fatty acyl coA elongase EST, rapid amplification of cDNA ends (RACE) was performed using the BD SMART RACE cDNA Amplification kit (BD Biosciences, San Jose, CA). Gene specific primers (Fatty1F: 5′-GAGGGTGTTTCGTAGCTGGAATGGTTTTCG-3′, Fatty2R: 5′-GGCCCAAGGCTTATGGAAAACCGAAAACC-3′) were used to PCR-amplify the 5′ and 3′ RACE products which were gel-purified using the Nucleo Trap Nucleic Acid Purification kit (Clontech, Mountainview, CA). The PCR products were then cloned using the TOPO TA Cloning kit with pCR2.1-TOPO Vector and One Shot Top10F′ chemically competent cells (Invitrogen, Carlsbad, CA). Plasmids were purified using the WizardPlus Miniprep DNA purification system (Promega, Madison, WI) and sequenced on an ABI 3100 Genetic Analyser using Big Dye chemistry and M13 primers modified for sequencing (M13LF: 5′-GTAAAACGACGGCCAGTGAATTGT-3′, M13LR: 5′-CAGGAAACAGCTATGACCATGATTAC-3′). Two additional PCR amplifications and sequencing reactions were required to determine the sequence of the 3′ end of the cDNA. Primers Fatty3F (5′-CGACTGCAACTACCCGAAAGCC-3′) and Fatty4F (5′-CTCCTTCACGCATACAAAGCGCAG-3′) were employed for these final steps. Contig assembly was performed with Sequencher v. 4.5 (Gene Codes Corporation, Ann Arbor, MI). The inferred amino acid sequence was deduced by using ORF Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/) and was used in a protein Blast search to identify putative orthologues.
(c). Quantitative reverse transcriptase-PCR
Five laboratory colonies of A. albopictus were established for qRT-PCR experiments. For three temperate populations (Burlington, NJ (NJ1); Salem, NJ (NJ2); Manassas, VA (VA)), we collected at least 300 individual larvae and pupae from at least 15 tyres within a site. The mosquitoes were transferred to the laboratory where they were reared for at least three generations under near-optimal conditions at 21°C and approximately 80 per cent RH as described in Armbruster & Conn (2006). We also established laboratory colonies for two tropical populations (Honolulu, HI (HI); Kuala Lumpur, Malaysia (KL)) using at least 1,000 eggs collected from oviposition traps and kindly provided by Mr Pingjun Yan, Department of Health, Honolulu, HI, and Dr Indra Vythilingam, Institute of Medical Research, Kuala Lumpur, Malaysia, respectively. A. albopictus is native to Kuala Lumpur and is thought to have invaded Hawaii during the 1890s (Joyce 1961), most probably from a location in the Indian Ocean (Mousson et al. 2005). Tropical populations, which do not undergo a photoperiodic diapause, were included to confirm that expression differences in response to LD versus SD conditions were directly attributable to diapause and not to ancillary effects of differential photoperiod. The fact that we have used two geographically disparate tropical populations that do not undergo photoperiodic diapause and that have presumably evolved independently over the last at least 100 years represents a conservative test for parallel underlying patterns of gene expression.
We reared two replicate cages (i.e. biological replicates) for each of the three temperate populations and each of the two tropical populations under both SD and LD photoperiods. For each biological replicate, approximately 300 male and female pupae from each temperate population (F3–F6 laboratory generation) and from each tropical population (F6–F10 laboratory generation) were divided with half exposed to SD photoperiods and half exposed to LD photoperiods. Ten to 20-day-old adult females were blood fed to repletion on a human host, and four days post-bloodmeal females were collected four hours after ‘lights on’ and stored at −80°C. Mature (stage V) oocytes, identified by the appearance of a visible exochorion surface pattern, were dissected directly into RNAlater (Ambion, Beverly, MA). Although we included females from a 10-day range of chronological age, this variation is unlikely to have a large effect on the abundance of mature oocyte transcripts since ovarian development is more strongly influenced by time since blood meal than chronological age (Clements 1992).
RNA was extracted from mature oocyte tissue using TRI Reagent (Sigma Aldrich, St Louis, MO) followed by an isopropanol precipitation. DNase treatment was then performed for each sample by adding 1 μl DNase per 5 μg total RNA in 1× DNase reaction buffer to a volume of 100 μl. The mixture was incubated at 37°C for 10 min, followed by the addition of 1 μl of EDTA and a second incubation at 75°C for 10 min. DNase treatment was followed by a phenol–chloroform cleanup and RNA precipitation. The resulting RNA was then used to perform qRT-PCR, comparing fatty acyl coA elongase expression of SD photoperiod versus LD photoperiod treatments with a Brilliant II SYBR Green 1-Step qRT-PCR Kit (Stratagene, La Jolla, CA) on an Mx3000P qPCR machine (Stratagene, La Jolla, CA). Ribosomal protein L34 (NCBI accession no. AF144549) was used as an endogenous control in all qRT-PCR reactions. The primer sequences are as follows: Fatty5F (5′-CCCCGGACAAAGGATTGGC-3′), Fatty5R (5′-GGGTGTTTCGTAGCTGGAATGG-3′), L34F (5′-AGAAGCTCAGCGGAATCAAG-3′), L34R (5′-GGGCTCGTCTACCACGTTTA-3′). Three replicate reactions (technical replicates) were performed for each RNA sample. Each reaction consisted of 1 μl of 50 ng μl−1 RNA as template and 3 μl each of the forward and reverse primers at a concentration of 1mM. Each reaction consisted of a 30 min reverse transcription step at 50°C and 10 min at 95°C, followed by 45 cycles of 30 s at 95°C, 1 min at 63°C and 30 s at 72°C. A final cycle consisting of 1 min at 95°C, 30 s at 55°C, and 30 s at 95°C was performed to determine PCR specificity from the dissociation curve. Cycle threshold (CT) values were averaged across triplicate reactions and used to determine fold change differences between SD and LD treatments for each population replicate using the 2(−ΔΔC(t)) method (Livak & Schmittgen 2001).
To confirm that SD photoperiods resulted in the production of diapause eggs in temperate but not tropical populations, a separate cage of mosquitoes was reared in parallel under LD or SD conditions as described above for each biological replicate. Eggs were collected and stimulated to hatch as previously described (Armbruster & Conn 2006). Per cent hatch was recorded for each replicate.
(d). Hydrocarbon quantity
For analysis of hydrocarbon quantity the VA and KL populations were reared under SD and LD conditions as described above. Because of the large number of eggs required for these assays, five paired-replicate samples of eggs (SD and LD) were obtained over the course of three laboratory generations for both populations. For each population, paired samples of SD and LD eggs were handled in parallel, treated identically, and were 2–3 weeks old at the time of analysis. However, the paired-replicate samples from the temperate population were not all collected and analysed simultaneously with the paired replicate samples from the tropical population. Lipids distributed on the surface of eggs were extracted by adding approximately 25 mg of eggs from SD or LD conditions to 1 ml of hexane. The mixture was gently agitated for 5 min and then filtered through a glass pre-filter (Millipore). This process was repeated twice before the filter was examined under a dissecting microscope to verify that no eggs had been broken. The resulting 2 ml of surface lipid extract was evaporated using nitrogen gas and stored at −70°C. This extraction procedure produces a sample that is over 99 per cent hydrocarbon as determined by gas chromatography/mass-spectroscopy (GC/MS, data not shown). Also, a fourth extraction performed on both SD and LD eggs did not produce any detectable lipids. Surface lipid extracts (hydrocarbons) were quantified using a vanillin assay as described by Van Handel (1985). The dried lipid extract was heated to 100°C with 200 μl concentrated sulphuric acid for 10 min, and then 4.8 ml of vanillin solution (600 mg/500 ml 68% phosphoric acid) was added. The final solution was mixed in a vortex for 10 s and allowed to sit for 5 min until the solution turned red. The optical density of the solution at 525 nm was measured in a spectrophotometer and compared with a standard curve to determine the total mass of lipid in the sample. Total lipid mass was then converted to a μg lipid mg−1 wet egg mass to normalize differences in sample weight.
(e). Hydrocarbon composition
To determine the chain length composition of hydrocarbons on the surface of VA and KL eggs, three replicate batches of eggs from SD and LD conditions were collected from both populations and surface lipids were extracted in hexane as described above except that approximately 10 mg of eggs were used for each replicate sample. The hexane-dissolved hydrocarbons in each sample were dried under a gentle stream of nitrogen, re-suspended in 100 μl of high-purity chloroform, and placed in sampling vials with glass inserts and stored at −70°C until analysed by GC/MS.
For GC/MS analysis the hydrocarbon samples were hand-injected (2 μl each) into a Thermo-Finnigan Trace GC/MS instrument with a Restek 30 m fused silica column (I.D. 25 mm, 95% dimethyl siloxane, 5% diphenyl). The injector temperature was set to 220°C and the oven was programmed to heat from 160°C to 300°C, increasing 8°C min−1 and holding the 300°C temperature for an additional 5 min. Helium gas was used as the carrier at a rate of 50 ml min−1. The detector was set to detect mass units from 50 amu to 450 amu, and peak areas were quantified using Xcalibur software that also drove the GC/MS unit (Thermo, Inc.). This chromatographic programme allowed sufficient chromatographic resolution to separate all hydrocarbon peaks for quantification. Hydrocarbon peaks were identified according to their spectral patterns (identified by general chromatographic signature as well as comparison with the NIST and Wiley Chemical libraries) and chain length was established by comparison with authentic standards of heptacosane and hexacosane loaded into a separate sample.
(f). Water loss rates
Three replicates of 15 eggs per replicate were collected on successive days from VA and KL populations reared under SD and LD conditions. All eggs were 10–14 days old at the time of analysis. In insects, exposure to 0 per cent RH permits the net transpiration rates (integumental plus respiratory water loss) to be determined since under these conditions no water can be gained from the atmosphere (Wharton 1985). This allows water loss to be measured according to Wharton (1985):
| 2.1 |
where m0 is the initial water mass, mt is the water mass at any time t and k represents the rate of water loss (Wharton 1985). Thus, the slope of a plot of ln (mt/m0) versus time is the net transpiration rate (water loss rate) expressed as per cent per day.
Therefore, to measure water loss, the three-paired replicate batches of 15 LD and 15 SD eggs from both the VA and KL populations were initially weighed using an electrobalance (CAHN 25, Ventron Co., Cerritos, CA), and then moved into a sealed glass desiccator maintained at 20–22°C and 0 per cent RH, generated by solid CaSO4 and verified with a hygrometer (Thomas Scientific, Philadelphia, PA). Eggs were weighed individually every other day without enclosure and returned to the above conditions within 2 min. Eggs were dried until the mass was constant for five consecutive days. The amount of water available for exchange (water mass) was measured as the difference between the initial mass and the dry mass.
(g). Statistical analyses
To analyse qRT-PCR gene expression data, we compared fold change values (Livak & Schmittgen 2001) of temperate (diapausing) populations to fold change values of tropical (non-diapausing) populations using a two-tailed two-sample Wilcoxon rank sum test. We note that employing a two-tailed test is a conservative approach, because the transcript we tested using qRT-PCR was isolated from a ‘SD minus LD’ SSH cNDA library, thus providing a priori evidence that the transcript is differentially expressed as a component of the diapause response. Although a one-tailed test could therefore be justified in these analyses of gene expression, we have chosen to report conservative probability values based on a two-tailed test. Using a Wilcoxon rank sum test, we also (i) compared the fold changes of the two tropical populations (KL versus HI) and (ii) tested whether the fold changes in the temperate populations or the tropical populations were significantly different from 1 (i.e. no differential expression in response to SD versus LD photoperiod). Surface hydrocarbon quantities of paired replicate samples of SD and LD eggs from each population were compared for each population using paired t-tests. As noted above, the paired replicate samples from the temperate population were not all collected and analysed simultaneously with the paired replicate samples from the tropical population, so it is not statistically valid to directly compare hydrocarbon quantities between the temperate and tropical eggs. To compare the composition of hydrocarbons from eggs produced by females reared under SD and LD conditions from both temperate and tropical populations, peak areas from GC/MS output were quantified and converted to proportions and analysed using NMDS (Non-Metric Multidimensional Scaling), a robust ordination technique (Minchin 1987). We used the proportion of each hydrocarbon to create a dissimilarity matrix among the following treatments using the Bray–Curtis dissimilarity coefficient (Faith et al. 1987): (i) LD versus SD samples from the temperate population, (ii) LD versus SD samples from the tropical population, and (iii) LD and SD samples from the temperate population versus LD and SD samples from the tropical population. We then tested for differences between treatments using ANOSIM (Analysis of Similarity, Warwick et al. 1990) with 1000 permutations. We used analysis of variance (ANOVA) to test for the effect of population, photoperiod and population-by-photoperiod interaction on water loss rates, all with d.f. = 1,175. The ANOVA was followed by an a posteriori comparison of treatment means with Bonferroni correction to control for experiment-wise error (p < 0.05).
3. Results
(a). Race
The full-length A. albopictus fatty acyl coA elongase cDNA sequence (NCBI accession no. GQ168593) consists of a 372 bp 5′UTR, a 1080 bp (359 amino acid) open reading frame and a 495 bp 3′UTR including the poly-A tail. Amino acid residues 4–259 correspond to the highly conserved ELO superfamily, which is involved in long chain fatty acid elongation systems. A protein Blast indicated 96 per cent inferred amino acid identity with a fatty acyl coA elongase in Aedes aegypti (Ribeiro et al. 2007), 86 per cent identity with AGAP004373-PA in Anopheles gambiae, 61 per cent identity with elongation of very long chain fatty acids protein 1 in C. pipiens quinquefasciatus and 66 per cent with CG31522 isoform B in Drosophila melanogaster (see the electronic supplementary material, S1).
(b). Quantitative reverse transcriptase-PCR
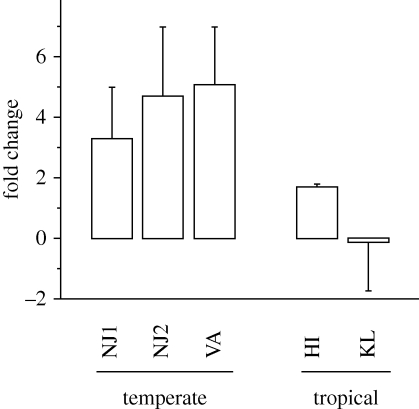
Fatty acyl coA elongase was upregulated under SD photoperiod relative to LD photoperiod treatments in temperate but not tropical populations (figure 1). The fold change values for temperate populations comparing SD relative to LD expression ranged from 1.6 to 7 and differed significantly (Z = 2.1, p = 0.036) from 1 (i.e. no difference in expression in response to photoperiod). The fold change values for the tropical populations ranged from 0.56 to 1.7 and did not differ significantly from 1 (Z = 1.29, p = 0.20). Fold changes of temperate (diapausing) populations were significantly different from fold changes in tropical (non-diapausing) populations (Wilcoxon signed-rank test, Z = 2.04, p = 0.04). The fold changes of the two tropical populations were not significantly different (Z = 1.22, p = 0.22).
Figure 1.
Mean (± s.e.) fold change of fatty acyl coA elongase in mature oocytes of female A. albopictus exposed to short-day relative to long-day photoperiods. Fold change values differ significantly between temperate (NJ1, NJ2, VA) and tropical (HI, KL) populations (Wilcoxon signed-rank test, Z = 2.038, p = 0.04).
(c). Hydrocarbon quantity
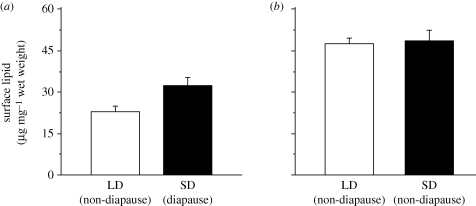
In the temperate population, there were 29 per cent more surface hydrocarbons on diapause versus non-diapause eggs (figure 2a; diapause mean = 32.29 ± 4.26 μg mg−1 wet weight; non-diapause mean = 22.93 ± 4 μg mg−1 wet weight; paired t-test, t = 2.9, p = 0.04). In the tropical population, there was no difference in egg surface hydrocarbon quantity between LD and SD photoperiod treatments (figure 2b; SD mean = 48.53 ± 6.1 μg mg−1 wet weight; LD mean = 47.5 ± 3.46 μg mg−1 wet weight; paired t-test, t = −0.3, p = 0.78).
Figure 2.
Mean (± s.e.) egg surface lipid (= hydrocarbon) quantities for a (a) temperate (VA) and (b) tropical (KL) population of A. albopictus. Long-day (open bar) and short-day (filled bar) treatments differ significantly for the temperate (paired t-test, t = 2.90, p = 0.04) but not tropical population (paired t-test, t = −0.3, p = 0.78).
(d). Hydrocarbon composition
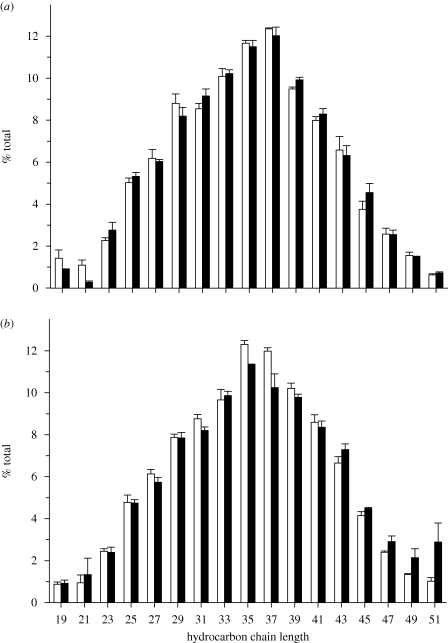
The surface lipids of A. albopictus eggs featured 17 detectable major unsaturated hydrocarbons ranging in odd numbers from 19 to 51 carbons in length (figure 3). Minor hydrocarbons were a very small proportion of the overall signal (less than 2%), and were therefore omitted. Non-metric multidimensional scaling indicated no significant difference in hydrocarbon composition between: (i) diapause versus non-diapause eggs from the temperate population (ANOSIM R = −0.0741, p = 0.49), (ii) non-diapause eggs produced under SD versus LD photoperiods from the tropical population (ANOSIM R = 0.0370, p = 0.50), and (iii) diapause and non-diapause eggs from the temperate population versus SD and LD eggs from the tropical population (ANOSIM R = −0.0074, p = 0.49).
Figure 3.
Composition of egg surface hydrocarbons from (a) temperate (VA) and (b) tropical (KL) populations of A. albopictus produced under long-day (open bar) and short-day (filled bar) treatments. Bars represent mean (± s.e.). Non-metric multidimensional scaling indicated no significant difference in hydrocarbon composition between: (i) diapause versus non-diapause eggs from the temperate population (ANOSIM R = −0.0741, p = 0.49), (ii) non-diapause eggs produced under LD versus SD photoperiods from the tropical population (ANOSIM R = 0.0370, p = 0.50) and (iii) diapause and non-diapuase eggs from the temperate population versus SD and LD eggs from the tropical population (ANOSIM R = −0.0074, p = 0.49).
(e). Water loss rates
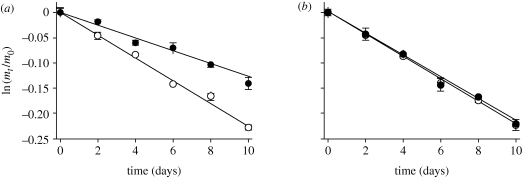
The net transpiration (= water loss) rates for isolated eggs were extremely low (figure 4). Diapause eggs from a temperate (VA) population had water loss rates nearly half those of non-diapause eggs (figure 4; diapause = 1.32 ± 0.18% d−1; non-diapause = 2.25 ± 0.2% d−1). Results of ANOVA indicated that water loss rates were affected by population (F1,175 = 147.94, p < 0.001), photoperiod (F1,175 = 134.24, p < 0.001) and population-by-photoperiod interaction (F1,175 = 126.95, p < 0.001). A posteriori comparison of mean water loss rates indicated that the temperate (VA) diapause eggs had significantly lower water rates (p < 0.05) than non-diapause eggs from the temperate (VA) population and SD and LD non-diapausing eggs from the tropical (KL) population, all of which did not differ significantly (p > 0.05). Both diapause and non-diapause eggs could tolerate loss of 25–27% of their water content, and thus, owing to their differences in water loss rates, diapause eggs are able to survive much longer. These survival rates were similar to those found by Sota & Mogi (1992). Experiments indicate that water vapour uptake is not used as a source to replenish egg water stores, and thus eggs probably rely on contact with free water to increase their water content (data not shown).
Figure 4.
Water loss rate (slope of (ln (mt/m0)) versus time) of A. albopictus eggs from (a) temperate (VA) and (b) tropical (KL) population produced under long-day (open circle) and short-day (filled circle) photoperiods. Water loss rates were affected by a significant population-by-photoperiod interaction (F1,175 = 126.95, p < 0.001). Mean water loss rates of temperate (VA) diapause eggs were lower (a posteriori contrast with Bonferroni correction, p < 0.05) than non-diapause eggs from the temperate (VA) population and SD and LD non-diapause eggs from the tropical (KL) population, which did not differ significantly (a posteriori contrast with Bonferroni correction, p > 0.05).
4. Discussion
The recurring arrival of the harsh winter conditions in temperate zone habitats represents a fundamental challenge to the survival and reproduction of a wide variety of insects. Many insects surmount this challenge by means of photoperiodic diapause, the ability to assess day length (photoperiod) as a token cue for initiating seasonally appropriate developmental arrest (Tauber et al. 1986). Photoperiodic diapause thus provides an adaptive mechanism for the temporal coordination of growth, development and dormancy in a seasonal environment. At the same time, it has become increasingly clear that diapause does not represent a simple physiological shutdown, but rather is a physiologically dynamic state with unique patterns of gene expression at specific points along the trajectory from initiation to maintenance and termination of diapause (reviewed by Denlinger 2002). Processes related to stress tolerance, such as cold and desiccation resistance, appear to be particularly important physiological components of the diapause response (Yoder & Denlinger 1991a; Benoit & Denlinger 2007; Rinehart et al. 2007).
The fatty acyl coA elongase we describe was isolated from a ‘SD minus LD’ SSH library constructed using mature (stage V) oocyte tissue of a temperate (diapausing) population. Full details of the SSH library are described in another paper (Urbanski et al. in press). We found that fatty acyl coA elongase transcripts were more abundant in mature oocyte tissue under diapause inducing SD conditions relative to diapause averting LD conditions in replicate temperate (diapausing) but not tropical (non-diapausing) populations (figure 1). Because fatty acyl coA elongases encode proteins involved in the synthesis of very long chain surface lipids that are known to mediate desiccation resistance in a diverse group of insects (Blomquist et al. 1987; Vaz et al. 1988; Juárez 1994, 2004; Yoder et al. 1995; Benoit & Denlinger 2007; Juárez & Fernández 2007), we hypothesized that the increased transcript abundance of this gene might be related to the previously documented increased survival of diapause relative to non-diapause eggs under desiccating conditions (Sota & Mogi 1992). Consistent with this hypothesis, we found that in a temperate population, diapause eggs had approximately 30 per cent more surface lipids (more than 99% hydrocarbon) than non-diapause eggs, but that eggs from a tropical population reared under SD and LD conditions did not differ in surface lipid quantities (figure 2). We also found that diapause eggs from a temperate population had approximately one-half the water loss rate of non-diapause eggs, but that eggs from tropical females reared under SD and LD conditions did not differ in water loss rates (figure 4).
The vast majority of studies investigating the molecular physiology of diapause consider a single population reared on diapause-averting and diapause-inducing conditions. Our experimental design leverages the rare opportunity to compare the molecular physiology of temperate and tropical populations from within the same species that do and do not undergo a photoperiodic diapause response (Hawley et al. 1987; Hawley 1988). This comparative approach provides a particularly strong basis for establishing that the physiological changes we describe in the temperate (diapausing) populations are causal components of the diapause response, rather than more general responses to photoperiod per se. While a number of studies have compared molecular aspects of photoperiodic diapause between diapausing populations and non-diapausing mutant or selected strains isolated in the laboratory (Pavelka et al. 2003; Syrova et al. 2003; Goto et al. 2006), we know of no other molecular physiology studies that have explicitly compared naturally occurring diapausing and non-diapausing populations. Furthermore, because we used qRT-PCR to examine fatty acyl coA elongase transcript abundance in replicate temperate and tropical populations (figure 1), our results further control for potential intraspecific variation in gene expression (Whitehead & Crawford 2005) unrelated to the diapause response of A. albopictus.
The fatty acyl coA elongase we describe in A. albopictus contains the highly conserved ELO superfamily domain, and the inferred amino acid sequence exhibits 96 per cent identity to an A. aegypti fatty acyl coA elongase, identified from salivary gland transcripts annotated by Ribeiro et al. (2007). Fatty acid elongation in insects has been studied in most detail in Musca domestica, Blatella germanica and Triatoma infestans (Vaz et al. 1988; Juárez 2004; Juárez & Fernández 2007). The consensus view from these studies is that fatty acyl coA elongases encode proteins which are involved in the formation of long chain fatty acids (Vaz et al. 1988; Juárez 2004; Juárez & Fernández 2007), which can then be converted by decarboxylation into hydrocarbon chains (Major & Blomquist 1978).
Surface lipids have been associated with desiccation resistance in a wide variety of insects (Blomquist et al. 1987; Gibbs 1998) by functioning to decrease water loss rates (Armold & Regnier 1975; Yoder & Denlinger 1991b; Yoder et al. 1992, 1995; Benoit & Denlinger 2007). Furthermore, previous studies have also found increased surface hydrocarbons (Bell et al. 1975; Coudron & Nelson 1981; Kaneko & Katagiri 2004) and in some cases also decreased water loss rates (Yoder & Denlinger 1991b; Benoit & Denlinger 2007) specifically associated with diapause. Increased production of epicuticular lipids thus appears to be a common component of both diapause and aestivation (dry season diapause) in insects (Tauber et al. 1986). Previous studies on A. gambiae (Goltsev et al. 2009) and several Aedes (Telford 1957; Beckel 1958; Rezende et al. 2008) have implicated the serosal cuticle to be important in determining desiccation resistance of eggs. While we cannot rule out the possibility that the hexane extraction procedure we used may have removed some lipids from the serosal cuticle inside the chorion, our results emphasize that surface lipids on the outside of the chorion can play an important role in determining the egg desiccation resistance. A previous study has documented de novo hydrocarbon synthesis in insect eggs prior to oviposition (Juárez 1994), supporting a direct role for the fatty acyl coA elongase transcript we describe in mediating egg desiccation resistance. However, hydrocarbons may also be transported through the hemolymph to the oocytes before oviposition and/or be synthesized during embryological development (Juárez 1994). Future studies will use RNA interference (RNAi) to ‘knock down’ transcript levels at multiple developmental stages in order to more precisely elucidate the mechanisms linking increased fatty acyl coA elongase transcript abundance in mature (stage V) oocytes to increased surface hydrocarbon abundance of embryonated eggs.
Differences in the composition of surface hydrocarbons have also been documented as a component of the diapause programme in some insects (Jurenka et al. 1998; Kaneko & Katagiri 2004). However, our results indicate the diapause programme of A. albopictus involves quantitative (figure 2) but not compositional (figure 3a) changes in surface hydrocarbons. This conclusion is similar to results in several other insect systems (Yoder et al. 1995; Kaneko & Katagiri 2004). The range of hydrocarbon chain lengths detected from the surface of both temperate and tropical A. albopictus eggs (19–51 carbons, figure 3) is greater than the range of cuticular hydrocarbons found in D. melanogaster (approx. 21–33 carbons, Foley et al. 2007), but consistent with the diversity found in other insects (15–55 carbons, Nelson & Blomquist 1995).
It is important to note that the association between surface lipid levels and water-loss rates appears to differ between temperate versus tropical eggs. The water loss rate of the non-diapause eggs from the temperate population does not differ from the water loss rates of the eggs from the tropical population (figure 4). However, although a direct statistical comparison is not valid because paired replicate samples of tropical and temperate eggs were not all collected at the same time for the quantitative hydrocarbon analysis (see §2), the tropical eggs appear to have higher surface lipid levels than the temperate eggs (figure 2). As noted above, differences in the composition of hydrocarbons could in principle contribute to the differences between surface hydrocarbon abundance and water loss rates in temperate versus tropical eggs. However, the compositional hydrocarbon profiles of temperate and tropical populations do not differ significantly and in fact are remarkably similar (figure 3). These results emphasize that in addition to surface hydrocarbons, there are a variety of potential metabolic and structural differences between temperate and tropical eggs that could affect water loss rates. For example, higher metabolic rates of tropical embryos (Hadley 1994), differences in osmolite concentration (Benoit et al. 2009), and size (Benoit & Denlinger 2007) or structural properties (Woods 2005) could all explain why eggs from tropical populations appear to have higher quantities of surface lipids (figure 2) but equivalent water loss rates (figure 4) relative to non-diapause temperate eggs. We are currently investigating a number of these factors.
Despite the caveats noted above, our results implicate fatty acyl coA elongase transcript abundance and hydrocarbon synthesis as important physiological components of stress resistance during diapause in A. albopictus. Based on the strong association between surface lipid production and desiccation resistance in other insects (Armold & Regnier 1975; Blomquist et al. 1987; Yoder & Denlinger 1991b; Yoder et al. 1992, 1995; Benoit & Denlinger 2007), we believe the fatty acyl coA elongase we have characterized may be involved in mediating the desiccation resistance of non-diapause eggs in A. albopictus. Furthermore, because the inferred FATTY ACYL COA ELONGASE amino acid sequence is highly conserved (see the electronic supplementary material, S1) we hypothesize that our results may be pertinent to elucidating the physiological basis of desiccation resistance of the eggs of other mosquito vectors. For example, we hypothesize that a similar pathway may mediate the desiccation resistance of eggs in A. aegypti, even though A. aegypti does not undergo a photoperiodic diapause.
In mosquitoes, egg desiccation resistance is a trait of fundamental ecological importance that has been shown to play a role in mediating species interactions (Juliano et al. 2002) as well as contributing to the ability to become established in non-native habitats (Juliano & Lounibos 2005). Our current results emphasize that because stress response physiology is a critical component of the diapause response, studying the physiology of diapause is likely to uncover fundamental pathways of stress response physiology relevant to a wide variety of ecological phenomena. Ultimately, elucidating the underlying physiological basis of stress response traits such as egg desiccation resistance may help to develop novel approaches to pest control by genetically or chemically disrupting important stress resistance pathways.
Acknowledgements
We thank Mr Pingjun Yan and Dr Indra Vythilingam for providing tropical eggs of A. albopictus and Gina Wimp for assistance with statistical analysis. We also thank two anonymous reviewers for helpful comments on previous versions of this manuscript. This work was supported by funds from Georgetown University to P. Armbruster, the Cosmos Club Foundation of Washington, D.C. to J. Urbanski and NIH R01 AI1058279 to D. L. Denlinger.
References
- Armbruster P., Conn J. E.2006Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 99, 1234–1243 (doi:10.1603/0013-8746(2006)99[1234:GVOLGI]2.0.CO;2) [Google Scholar]
- Armold M. T., Regnier F. E.1975Developmental study of cuticular hydrocarbons of Sarcophaga bullata. J. Insect Physiol. 21, 1827–1833 (doi:10.1016/0022-1910(75)90249-8) [DOI] [PubMed] [Google Scholar]
- Beckel W. E.1958Investigations of impermeability, diapause, and hatching in the eggs of the mosquito Aedes hexodontus. Can. J. Zool. 36, 541–554 (doi:10.1139/z58-050) [Google Scholar]
- Bell R. A., Nelson P. R., Borg T. K., Caldwell D. L.1975Wax secretion in non-diapausing and diapausing pupae of the tobacco hornworm Manduca sexta. J. Insect Physiol. 21, 1725–1799 (doi:10.1016/0022-1910(75)90186-9) [Google Scholar]
- Benedict M. Q., Levine R. S., Hawley W. A., Lounibos L. P.2007Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector-borne Zoonotic Dis. 7, 76–85 (doi:10.1089/vbz.2006.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J. B., Denlinger D. L.2007Suppression of water loss during adult diapause in the northern house mosquito Culex pipiens. J. Exp. Biol. 210, 217–226 (doi:10.1242/jeb.02630) [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Lopez-Martinez G., Elnitsky M. A., Lee R. E., Jr, Denlinger D. L.2009Dehydration-induced cross tolerance of Belgica antarctica larvae to cold and heat is facilitated by trehalose accumulation. Comp. Biochem. Physiol. A 152, 518–523 (doi:10.1016/j.cbpa.2008.12.009) [DOI] [PubMed] [Google Scholar]
- Blomquist G. J., Nelson D. R., de Renobales M.1987Chemistry, biochemistry and physiology of insect cuticular lipids. Arch. Insect. Biochem. Physiol. 6, 227–265 (doi:10.1002/arch.940060404) [Google Scholar]
- Clements A. N.1992The biology of mosquitoes: development, nutrition and reproduction. London, UK: Chapman and Hall [Google Scholar]
- Coudron T. A., Nelson D. R.1981Characterization and distribution of the hydrocarbons found in diapausing pupae tissues of the tobacco hornworm, Manduca sexta L. J. Lipid Res. 22, 103–112 [PubMed] [Google Scholar]
- Denlinger D. L.2002Regulation of diapause. Annu. Rev. Entomol. 47, 93–122 (doi:10.1146/annurev.ento.47.091201.145137) [DOI] [PubMed] [Google Scholar]
- Faith D. P., Minchin P. R., Belbin L.1987Compositional dissimilarity as a robust measure of ecological distance. Vegetation 69, 57–68 (doi:10.1007/BF00038687) [Google Scholar]
- Foley B., Chenoweth S. F., Nuzhdin S. V., Blows M. W.2007Natural genetic variation in cuticular hydrocarbon expression in male and female Drosophila melanogaster. Genetics 175, 1465–1477 (doi:10.1534/genetics.106.065771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs A. G.1998Water-proofing properties of cuticular lipids. Am. Zool. 38, 471–482 (doi:10.1093/icb/38.3.471) [Google Scholar]
- Goltsev Y., Rezende G. L., Vranizan K., Lanzaro G., Valle D., Levine M.2009Developmental and evolutionary basis for drought tolerance of the Anopheles gambiae embryo. Dev. Biol. 330, 462–470 (doi:10.1016/j.ydbio.2009.02.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S. G., Han B., Denlinger D. L.2006A nondiapausing variant of the flesh fly, Sarcophaga bullata, that shows arrhythmic adult eclosion and elevated expression of two circadian clock genes, period and timeless. J. Insect Physiol. 52, 1213–1218 (doi:10.1016/j.jinsphys.2006.09.003) [DOI] [PubMed] [Google Scholar]
- Gratz N. G.2004Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. 18, 215–227 (doi:10.1111/j.0269-283X.2004.00513.x) [DOI] [PubMed] [Google Scholar]
- Gray E. M., Bradley T. J.2005Physiology of desiccation resistance in Anopheles gambiae and Anopheles arabiensis. Am. J. Trop. Med. Hyg. 73, 553–559 [PubMed] [Google Scholar]
- Hadley N. F.1994Water relations of terrestrial arthropods. San Diego, CA: Academic Press [Google Scholar]
- Hanson S. M., Craig G. B.1994Cold acclimation, diapause, and geographic origin affect cold hardiness in eggs of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 31, 192–201 [DOI] [PubMed] [Google Scholar]
- Hawley W. A.1988The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. 4, 1–39 [PubMed] [Google Scholar]
- Hawley W. A., Reiter P., Copeland R. S., Pumpuni C. B., Craig G. B.1987Aedes albopictus in North America—probable introduction in used tires from northern Asia. Science 236, 1114–1116 (doi:10.1126/science.3576225) [DOI] [PubMed] [Google Scholar]
- Joyce C. R.1961Potentialities for accidental establishment of exotic mosquitoes in Hawaii. Proc. Hawaii Entomol. Soc. 17, 407–413 [Google Scholar]
- Juárez P.1994Hydrocarbon biosynthesis in Triatoma infestans. Arch. Insect Biochem. Physiol. 25, 193–206 (doi:10.1002/arch.940250303) [DOI] [PubMed] [Google Scholar]
- Juárez M. P.2004Fatty acyl-CoA elongation in Blatella germanica integumental microsomes. Arch. Insect Biochem. Physiol. 56, 170–178 (doi:10.1002/arch.20007) [DOI] [PubMed] [Google Scholar]
- Juárez M. P., Fernández G. C.2007Cuticular hydrocarbons of triatomines. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 147, 711–730 (doi:10.1016/j.cbpa.2006.08.031) [DOI] [PubMed] [Google Scholar]
- Juliano S. A., Lounibos L. P.2005Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 8, 558–574 (doi:10.1111/j.1461-0248.2005.00755.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano S. A., O'Meara G. F., Morrill J. R., Cutwa M. M.2002Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 130, 458–469 (doi:10.1007/s004420100811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenka R. A., Holland D., Krafsur E. S.1998Hydrocarbon profiles of diapausing and reproductive adult face flies (Musca autumnalis). Arch. Insect Biochem. Physiol. 37, 206–214 (doi:10.1002/(SICI)1520-6327(1998)37:3<206::AID-ARCH3>3.0.CO;2-Q) [Google Scholar]
- Kaneko J., Katagiri C.2004Epicuticular wax of large and small white butterflies, Pieris brassicae and Prapae crucivora: qualitative and quantitative comparison between diapause and non-diapause pupae. Naturwissenschaften 91, 320–323 (doi:10.1007/s00114-004-0535-7) [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D.2001Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (doi:10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- Major M. A., Blomquist G. J.1978Biosynthesis of hydrocarbons in insects. Decarboxylation of long chain acid to n-alkanes in Periplaneta. Lipids 13, 323–328 (doi:10.1007/BF02533722) [Google Scholar]
- Minchin P. R.1987Simulation of multidimensional community patterns: towards a comprehensive model. Vegetation 71, 145–156 [Google Scholar]
- Moore C. G.1999Aedes albopictus in the United States: current status and prospects for further spread. J. Am. Mosq. Control Assoc. 15, 221–227 [PubMed] [Google Scholar]
- Mori A., Oda T., Wada Y.1981Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Trop. Med. 23, 79–90 [Google Scholar]
- Mousson L., Dauga C., Garrigues T., Schaffner F., Vazeille M., Failoux A. B.2005Phylogeography of Aedes (Stegomyia) aegypti (L.) and Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) based on mitochondrial DNA variations. Genet. Res. 86, 1–11 (doi:10.1017/S0016672305007627) [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Blomquist R. J.1995Insect waxes. In Waxes: chemistry and molecular biology and functions (ed. Hamilton R. J.), pp. 1–90 Dundee, UK: The Oily Press [Google Scholar]
- Pavelka J., Shimada K., Kostal V.2003TIMELESS: a link between fly's circadian and photoperiodic clocks? Eur. J. Entomol. 100, 255–265 [Google Scholar]
- Rezende G. L., Martins A. J., Gentile C., Farnesi L. C., Pelajo-Machado M., Peixoto A. A., Valle D.2008Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Dev. Biol. 8, 82 (doi:10.1186/1471-213X-8-82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. M. C., Arca B., Lombardo F., Calvo E., Phan V. M., Chandra P. K., Wikel S. K.2007An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics 8, (doi:10.1186/1471-2164-8-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J. P., Li A. L., Yocum G. D., Robich R. M., Hayward S. A. L., Denlinger D. L.2007Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc. Natl Acad. Sci. USA 104, 11 130–11 137 (doi:10.1073/pnas.0703538104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte E. J., Dijkstra E., Blok H., De Vries A., Takken W., Hofhuis A., Koopmans M., De Boer A., Reusken C. B. E. M.2008Accidental importation of the mosquito Aedes albopictus into the Netherlands: a survey of mosquito distribution and the presence of dengue virus. Med. Vet. Entomol. 22, 352–358 (doi:10.1111/j.1365-2915.2008.00763.x) [DOI] [PubMed] [Google Scholar]
- Sota T., Mogi M.1992Survival time and resistance to desiccation of diapause and nondiapause eggs of temperate Aedes (Stegomyia) mosquitoes. Entomol. Exp. Appl. 63, 155–161 (doi:10.1007/BF00343575) [Google Scholar]
- Sprenger D., Wuithiranyagool T.1986The discovery and distribution of Aedes albopictus in Harris County, Texas. J. Am. Mosq. Control Assoc. 2, 217–219 [PubMed] [Google Scholar]
- Syrova Z., Dolezel D., Saumann I., Hodkova M.2003Photoperiodic regulation of diapause in linden bugs: are period and clock genes involved? Cell. Mol. Life Sci. 60, 2510–2515 (doi:10.1007/s00018-003-3227-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber M. J., Tauber C. A., Masaki S.1986Seasonal adaptations of insects. New York, NY: Oxford University Press [Google Scholar]
- Telford A. D.1957The pasture Aedes of central and northern California. The egg stage: gross embryology and resistance to desiccation. Ann. Entomol. Soc. Am. 56, 537–543 [Google Scholar]
- Turell M. J., O'Guinn M. L., Dohm D. J., Jones J. W.2001Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J. Med. Entomol. 38, 130–134 (doi:10.1603/0022-2585-38.2.130) [DOI] [PubMed] [Google Scholar]
- Urbanski J., Aruda A., Armbruster P.In press A transcriptional element of the diapause program in the Asian tiger mosquito, Aedes albopictus, identified by suppressive subtractive hybridization. J. Insect Physiol. (doi:10.1016/j.jinsphys.2010.03.008) [DOI] [PubMed] [Google Scholar]
- Van Handel E.1985Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1, 302–304 [PubMed] [Google Scholar]
- Vaz A. H., Blomquist G. J., Reitz R. C.1988Characterization of the fatty acyl elongation reactions involved in hydrocarbon biosynthesis in the housefly, Musca domestica L. Insect Biochem. 18, 177–184 (doi:10.1016/0020-1790(88)90022-4) [Google Scholar]
- Wang R. L.1966Observations on the influence of photoperiod on egg diapause in Aedes albopictus Skuse. Acta Entomol. Sinica 15, 75–77 [Google Scholar]
- Warwick R. M., Clarke K. R., Suharnso1990A statistical analysis of coral community responses to the 1982–1983 El Niño in the Thousand Islands, Indonesia. Coral Reefs 8, 171–179 (doi:10.1007/BF00265008) [Google Scholar]
- Wharton G. W.1985Water balance of insects. In Comprehensive insect physiology, biochemistry, and pharmacology, vol. 4 (eds Kerkut G. A., Gilbert L. I.), pp. 565–603 Oxford, UK: Pergamon Press [Google Scholar]
- Whitehead A., Crawford D. L.2005Variation in tissue-specific gene expression among natural populations. Genome Biol. 6, R13 (doi:10.1186/gb-2005-6-2-r13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. A.2005Oxygen and water flux across eggshells of Manduca sexta. J. Exp. Biol. 208, 1297–1308 (doi:10.1242/jeb.01525) [DOI] [PubMed] [Google Scholar]
- Yoder J. A., Denlinger D. L.1991aWater balance in flesh fly pupae and water vapour absorption associated with diapause. J. Exp. Biol. 157, 273–286 [Google Scholar]
- Yoder J. A., Denlinger D. L.1991bA comparison of the water balance characteristics of temperate and tropical fly pupae. Physiol. Entomol. 16, 375–380 (doi:10.1111/j.1365-3032.1991.tb00575.x) [Google Scholar]
- Yoder J. A., Denlinger D. L., Dennis M. W., Kolattukudy P. E.1992Enhancement of diapausing flesh fly puparia with additional hydrocarbons and evidence for alkane biosynthesis by a decarbonylation mechanism. Insect Biochem. Mol. Biol. 22, 237–243 (doi:10.1016/0965-1748(92)90060-R) [Google Scholar]
- Yoder J. A., Blomquist G. J., Denlinger D. L.1995Hydrocarbon profiles from puparia of diapausing and nondiapausing flesh flies (Sarcophaga crassipalpis) reflect quantitative rather than qualitative differences. Arch. Insect Biochem. Physiol. 28, 377–385 (doi:10.1002/arch.940280407) [Google Scholar]