Abstract
Batesian mimicry evolves when a palatable species (the ‘mimic’) co-opts a warning signal from a dangerous species (the ‘model’) and thereby deceives its potential predators. Longstanding theory predicts that this protection from predation should break down where the model is absent. Thus, mimics are expected to only co-occur with their model. Yet, many mimics violate this prediction and occur in areas where their model is absent. Here, we discuss the causes and consequences of such allopatric mimics. We also describe how these ‘rule-bending’ mimics provide critical insights into diverse topics ranging from how Batesian mimicry evolves to its possible role in speciation.
Keywords: mimicry, predation, speciation, gene flow, species ranges
1. Introduction
Batesian mimicry evolves when individuals of a palatable species gain the selective advantage of reduced predation because they resemble a toxic species that predators avoid (Ruxton et al. 2004). This idea traces to Bates (1862), who regarded convergent evolution between a palatable species (the ‘mimic’) and an unpalatable one (the ‘model’) as, ‘a most powerful proof of the theory of natural selection’ (Bates 1862, p. 511). Darwin and Wallace, the co-discoverers of natural selection, agreed. Indeed, Darwin wrote to Bates that his paper was, ‘one of the most remarkable and admirable papers I ever read’ (letter from Darwin to Bates, dated 20 November 1862; cited in Burkhardt et al. (2008)). Even today, Batesian mimicry is used in textbooks (e.g. Campbell & Reece 2005; Zimmer 2010) and popular books (e.g. Carroll 2009) as a prime example of natural selection's efficacy in promoting adaptation.
Although evolutionary biologists have long known about Batesian mimicry, many aspects of its evolution remain unclear (reviewed in Sherratt 2002; Brodie & Brodie 2004; Ruxton et al. 2004). One issue requiring clarification is whether and how a mimic can occur in the absence of its model (Pfennig et al. 2001; Ruxton et al. 2004; Joron 2008). As we describe in detail below, the occurrence of mimics outside the range of their model potentially undercuts the notion that selection to avoid predation drives convergence of models and their putative mimics. Consequently, it has long been postulated that mimics should only occur with their model (Ruxton et al. 2004). Indeed, as Wallace (1867, p. 8) put it, ‘The first law is, that in an overwhelming majority of cases of mimicry, the animals (or the groups) which resemble each other inhabit the same country, the same district, and in most cases are to be found together on the very same spot’.
Here we describe how, unexpectedly, many Batesian mimics occur in the absence of their model. We also consider the various factors that promote such paradoxical species distributions and explore the consequences of allopatric mimics. Finally, we highlight how the allopatric occurrence of Batesian mimics provides fertile testing ground for mimicry theory.
2. Mimics without models?
Classical Batesian mimicry theory predicts that mimics should occur only in sympatry with their model (reviewed in Ruxton et al. 2004). Specifically, this theory predicts that protection from predation should be inversely frequency dependent, such that its effectiveness decreases as the model becomes less abundant (in both a relative and absolute sense; Ruxton et al. 2004). Protection should break down completely when the model is entirely absent (as in allopatry), because predators that do not co-occur with the model will not be under selection to recognize it, or any other species that resemble it, as dangerous (Waldbauer & Sternburg 1987; Pfennig et al. 2001). Moreover, because mimics resemble models that are typically aposematic and, thus, conspicuous to potential predators (Ruxton et al. 2004), predation on these more apparent—but unprotected—allopatric mimics should be particularly intense.
Recent field experiments have substantiated these theoretical expectations. For example, free-ranging carnivore mammals attacked replicas of non-venomous scarlet kingsnakes (Lampropeltis elapsoides), which mimic eastern coral snakes (Micrurus fulvius), and Sonoran mountain kingsnakes (Lampropeltis pyromelana), which mimic Sonoran coral snakes (Micruroides euryxanthus), more frequently in allopatry than in sympatry with their model (Pfennig et al. 2001). Moreover, within allopatry, replicas exhibiting the mimetic pattern of L. elapsoides were attacked more frequently than replicas exhibiting a non-mimetic pattern, suggesting that allopatric mimics do indeed suffer enhanced predation (Pfennig et al. 2007). Thus, theoretical and empirical studies predict that the mimic's distribution should fall entirely within that of its model. Yet, do the geographical distributions of Batesian mimics support this prediction?
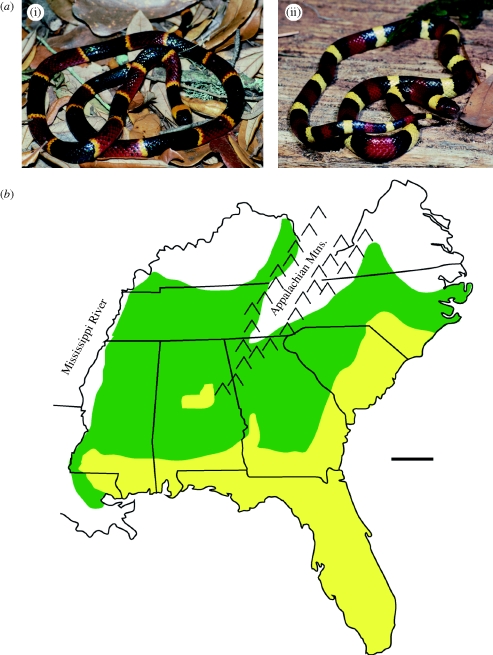
As expected, the geographical distributions of Batesian mimics and their models always overlap; there are no known situations in which a Batesian mimic occurs solely in allopatry (Ruxton et al. 2004). Contrary to expectation, however, the distributions of mimics often do not fall entirely within the distributions of their models. In particular, there are numerous, well-studied Batesian mimicry complexes in which mimics are found in both sympatry and allopatry with their model. Indeed, mimics can occur hundreds of kilometres outside the range of their model. For example, the coral snake mimics L. elapsoides (figure 1) and L. pyromelana (Stebbins 2003) extend 650 and 800 km, respectively, beyond the range of their coral snake models. Even more remarkable is the case of the (probably) coral snake mimic, L. zonata, which occurs 1500 km beyond the range of its model, M. euryanthus (Stebbins 2003). Moreover, a majority of a mimic's range may lie in allopatry. For example, for L. elapsoides, a remarkable 60 per cent of the total area of its range is in allopatry (area of allopatric range = 322 km2; area of sympatric range = 217 km2; figure 1).
Figure 1.
An allopatric mimic. (a) (i) The highly venomous eastern coral snake, Micrurus fulvius, and (ii) its non-venomous mimic, Lampropeltis elapsoides. (b) Geographical distributions of model and mimic in the southeastern United States (green shade, allopatry (only mimic present); yellow shade, sympatry (model and mimic present). Scale bar, 200 km.
The existence of allopatric mimics poses a paradox for Batesian mimicry theory. Although some might contend that such a situation is atypical and requires no special explanation, allopatry in Batesian mimicry complexes may be more common than is often assumed. For example, two groups of organisms in which Batesian mimicry has been especially well studied are butterflies and snakes (e.g. Brower 1958; Wallace 1867; Wickler 1968; Greene & McDiarmid 1981; Pough 1988; Savage & Slowinski 1992; Brodie & Brodie 2004). We carried out an informal survey of representative butterfly and snake Batesian mimicry complexes in the United States and Canada and found that mimics occur in allopatry in a clear majority of cases (table 1). Thus, the true extent of allopatric occurrence of Batesian mimics may be greater than is generally supposed.
Table 1.
Representative butterfly and snake Batesian mimicry complexes in the United States and Canada, showing instances in which the mimic occurs entirely inside the geographical range of its model and instances in which the mimic occurs at least partly outside the geographical range of its model.
| mimicry complex (mimic species, model species) | does the mimic's range extend beyond its model's range? | reference |
|---|---|---|
| butterfly mimicry complexes | ||
| Limenitis arthemis astyananx, Battus philenor | yes | Ries & Mullen (2008) |
| Limenitis lorquini Adelpha bredowii | yes | Prudic et al. (2002) |
| Papilio glaucus, B. philenor | yes | Brower & Brower (1962) |
| Speyeria diana, B. philenor | no | Brower & Brower (1962) |
| Snake mimicry complexes | ||
| Lampropeltis elapsoides, Micrurus fulvius | yes | Pfennig et al. (2001) |
| Lampropeltis triangulum, M. fulvius | yes | Conant & Collins (1998) |
| Cemophora coccinea, M. fulvius | yes | Conant & Collins (1998) |
| Lampropeltis pyromelana, Micruroides euryxanthus | yes | Pfennig et al. (2001) |
| Lampropeltis triangulum, M. euryxanthus | yes | Stebbins (2003) |
| Lampropeltis zonata, M. euryxanthus | yes | Stebbins (2003) |
| Chionactis occipitalis, M. euryxanthus | yes | Stebbins (2003) |
| Chionactis palarostris, M. euryxanthus | no | Stebbins (2003) |
Regardless of the incidence of the phenomenon, clarifying the causes of allopatry in Batesian mimicry complexes is important for at least two reasons. First, the occurrence of mimics outside the range of their models has often been used to dispute whether such systems represent true Batesian mimicry complexes. For example, before recent field experiments confirmed the basic prediction that coral snake mimics are protected only in sympatry with their model (see above), the existence of these mimics in allopatry was used as prima facie evidence against the mimicry hypothesis (Brattstrom 1955; Grobman 1978). Second, as we describe below, the occurrence of mimics in allopatry with their model can have important evolutionary consequences. Before exploring these consequences, however, we first consider the important issue of why mimics occur outside the geographical range of their model.
3. Causes of allopatric mimics
Three non-mutually exclusive processes can explain the occurrence of allopatric mimics: selection, range contraction/expansion, and gene flow. Below, we discuss each process in turn.
(a). Selection
Selection might favour mimetic patterns even in the absence of the model through at least five major routes. Even when these routes do not actually favour a mimetic phenotype over an alternative non-mimetic phenotype, they could render the mimetic phenotype less costly in allopatry, thereby possibly either maintaining allopatric mimics in the presence of gene flow or maintaining them for longer periods of time following range contraction/expansion.
In the first route, both the model and the mimic are toxic, and what is regarded as a Batesian mimicry complex may actually be an unrecognized case of Müllerian mimicry. Each species would be protected, even in allopatry, because each is toxic and predators associate this noxiousness with a distinctive phenotype on which both species have converged independently. For instance, Viceroy butterflies (Limenitis archipppus) closely resemble several distasteful species of milkweed feeding butterflies in the genus Danaus. Once considered a classic example of defensive Batesian mimicry (Brower 1958), this relationship is now considered to represent quasi-Batesian (Speed 1999) or Müllerian mimicry, because subsequent research has demonstrated that L. archipppus sequester phenolic glycosides (Prudic et al. 2007), which are toxic to predators (Ritland 1991, 1995). Of course, if a putative Batesian mimic is found to be toxic, we no longer face a conundrum, even if it occurs in allopatry with its putative model: the putative mimic is protected from predation, even when it occurs alone. It is also possible that a species might be a Müllerian mimic only during certain times or places: a species' level of toxicity might vary temporally and spatially (especially in species that acquire their toxins from the environment; e.g. Saporito et al. 2007).
In the second route, selection might maintain mimetic phenotypes in allopatry if predators possess an unlearned, generalized avoidance of certain phenotypes, such as those with bright, contrasting colours (Lindstrom 1999). For example, experiments have demonstrated that two species of birds, turquoise-browed motmots (Eumomota superciliosa) and great kiskadees (Pitangus sulphuratus), show unlearned avoidance responses towards dowels painted with red-yellow-black rings (Smith 1975, 1977), which are diagnostic of coral snakes and their mimics (Greene & McDiarmid 1981). Such unlearned avoidance responses might even be expressed in allopatry (but see Rubinoff & Kropach 1970). For instance, naive striated herons (Butorides striatus) exhibit unlearned avoidance responses towards highly venomous yellow-bellied sea snakes (Pelamis platurus), but not towards harmless snakes or eels, even when these herons are derived from populations where sea snakes do not occur (Caldwell & Rubinoff 1983). Other experiments have demonstrated that naive avian predators avoid black and yellow stripes (Schuler & Hesse 1985; Lindstrom 1999), bright red (Roper 1990), and red and yellow prey items (Mastrota & Mench 1995). More generally, many predators appear to harbour unlearned avoidance of novel stimuli (Coppinger 1970). Thus, many predators might possess an unlearned, generalized avoidance of aposematic signals (Exnerova et al. 2007). Even a slight tendency towards avoiding such phenotypes would reduce the costs of expressing a mimetic phenotype in allopatry.
In the third route, a noxious species and its presumed mimic might have converged independently on a similar phenotype that deters predation, but for different selective reasons (Gadow 1911). A phenotype that acts as a warning signal might, for instance, in some environmental contexts actually enhance crypsis. For example, some researchers have suggested that the brightly coloured, ringed patterns that characterize coral snakes and their mimics (see figure 1) might be cryptic on certain backgrounds or in certain contexts. In particular, on rough backgrounds, such patterns might break up the form of an elongate body, thereby generating a disruptive effect (Brattstrom 1955). Moreover, to some visual systems, these patterns might cause a moving snake to blur into a solid colour and make the snake appear motionless, thereby aiding its escape from predators (Pough 1976). Thus, depending upon the environmental context, a phenotype might function both as a warning and cryptic signal, thereby selectively maintaining it in both sympatry and allopatry.
In each of the above three routes, multiple species might have independently converged on the same phenotype that predators avoid. However, a fourth route by which mimetic phenotypes are selectively maintained in allopatry occurs when the model and mimic have independently converged on a phenotype that is favoured for selective reasons other than deterring predation (Malcolm 1990). For example, if competitors (or prey) are distracted by conspicuous signals, or if they harbour an unlearned, generalized fear of them (see above), then mimetic individuals might gain an advantage over non-mimetic individuals in resource competition, even in allopatry (Rashed & Sherratt 2007; Cheney 2010). Moreover, although mimetic phenotypes might be opposed by natural selection in allopatry, sexual selection might maintain them in such regions (e.g. Summers et al. 1999; Maan & Cummings 2008). Additionally, conspicuous phenotypes might possess non-signalling functions, such as aiding thermoregulation (Lindstedt et al. 2009). Thus, phenotypic similarity between a model and mimic might reflect convergent evolution in response to similar selective pressures other than predation. Moreover, the mimetic phenotype might have originally evolved because of the benefits of mimicry, and only later acquired additional functions (or vice versa). Regardless of how such phenotypes evolved, a mimetic phenotype might provide its bearer with multiple selective benefits, some of which might maintain such phenotypes in allopatry.
Finally, a fifth route by which mimetic phenotypes might be selectively maintained in allopatry occurs when predators have large home ranges or when they migrate between areas where models are present to those where models are absent. This idea, first proposed by Poulton (1909), is plausible if predators with either innate (Smith 1975, 1977) or learned avoidance, owing to co-occurrence with a toxic model, subsequently avoid aposematic signals when they encounter a palatable look-alike in areas where the model does not occur. For instance, it has been suggested that certain coral snake mimics occur in allopatry (see above), in part because predatory birds migrate seasonally from sympatry (where they are at risk of encountering coral snakes) to allopatry (Grobman 1978).
In the fifth route described above, expansion of the predator's geographical range maintains mimics in allopatry. In §3b,c, we describe how changes in the model's or mimic's geographical range could maintain mimics in allopatry.
(b). Range contraction
A second major cause of allopatric mimics is the differential extinction of model populations. Specifically, the mimetic phenotype might persist in allopatry if the model (but not the mimic) has undergone a range contraction. In such situations, predators might continue to avoid mimics in the newly created allopatric region if they had previously learned or evolved innate tendencies to avoid the dangerous model in what was formerly sympatry. Even if predators fail to avoid mimics in allopatry, there may not have been sufficient time for the mimic to undergo a similar range contraction or to evolve a non-mimetic pattern (see below).
Such range contraction might have contributed to allopatric mimics in a coral snake mimicry complex. In the eastern United States, non-venomous scarlet kingsnakes (L. elapsoides) and milksnakes (Lampropeltis triangulum) closely resemble highly venomous coral snakes (M. fulvius), but they range well outside the latter's current distribution (Conant & Collins 1998). Micrurus fulvius are now restricted to the southeastern corner of the United States. However, fossils from the Middle Miocene (11.5–16 Myr ago) of Nebraska reveal that they may have formerly occurred much farther north and west of their present-day distribution (Holman 2000); i.e. in areas where mimics currently occur in allopatry. The model, therefore, might have experienced a much greater range contraction than did their mimics, which might have contributed to the occurrence of allopatric mimics in this system.
(c). Range expansion and gene flow
Range expansion and/or gene flow by mimics from sympatry into allopatry might maintain mimetic phenotypes in allopatry. Range expansion might create allopatric mimics if models do not undergo a similar range expansion. For example, Batesian mimicry involving herbivorous insects (e.g. butterflies) may be strongly influenced by ecological limitations on the ranges of their respective host plants. If a mimic's host plant expands its range more rapidly in response to changing environmental conditions than its model's host, then dispersal and colonization by the mimetic herbivore could lead to persistence of the mimetic phenotype, even in areas where the model is locally rare or absent. Similarly, recent evidence suggest that the coral snake mimic, L. elapsoides (figure 1), underwent a range expansion into allopatry within the past 10 000 years (Harper & Pfennig 2008). The lack of a similar range expansion by its model (which actually appears to have undergone an earlier range contraction; see above) might explain the occurrence of allopatric mimics in this system.
Gene flow might also promote the maintenance of allopatric mimics. In particular, allopatric mimics may arise as a consequence of gene flow between mimetic individuals, sympatric with the model, and non-mimetic conspecifics that occur outside the model's range. Hybridization between mimetic and non-mimetic subspecies of the polytypic butterfly Limenitis arthemis astyanax complex may represent an example of this phenomenon (Mullen et al. 2008), and recent work suggests that the position of this hybrid zone is maintained by frequency dependent selection on wing pattern (Ries & Mullen 2008). Ongoing gene flow between sympatry and allopatry may also account for the occurrence of allopatric mimics in the L. elapsoides-M. fulvius coral snake mimicry complex (Harper & Pfennig 2008).
In some cases, range expansions by mimics into allopatry might have an anthropogenic cause. For example, many species of hoverflies (Diptera: Syrphidae) are Batesian mimics of bees and wasps (reviewed in Gilbert 2005). Yet, in Europe, some mimetic species frequently occur in areas where their models are absent (Howarth & Edmunds 2000). Azmeh et al. (1998) suggested that human-induced changes to the habitat might explain why these mimics occur in allopatry. They suggested that the shift in present-day allopatry from ancient forest to agricultural or urban habitat might have greatly increased the abundance of the hoverfly's aphid prey, which could account for the mimetic hoverflies' expansion into allopatry.
To summarize, three processes—selection, range contraction/expansion and gene flow—can promote the allopatric occurrence of Batesian mimics. Although these different processes need not act mutually exclusively, each could, in theory, act alone and thereby account for allopatric mimics.
4. Apostatic predation and the maintenance of allopatric mimics
Once one or more of the above factors (see §3) promotes the allopatric occurrence of Batesian mimics, even if selection normally disfavours allopatric mimics (Pfennig et al. 2007), it might not act strongly against such mimics if they are rare. Indeed, in a polymorphic population consisting of mimetic and non-mimetic phenotypes, selection acting in allopatry might actually favour rare mimetic phenotypes over more common non-mimetic phenotypes, if predators engage in frequency-dependent (i.e. apostatic) predation (Punzalan et al. 2005; Merilaita & Ruxton 2009). With apostatic predation, predation on common prey phenotypes is higher than expected (i.e. based on their actual abundance within the population), if predators develop through learning (or evolve) preferences for those prey phenotypes that they encounter frequently (search image formation is a common explanation offered for apostatic predation; reviewed in Endler 1991). By contrast, predation on rare phenotypes is lower than expected. Although not all predators engage in an apostatic fashion (indeed, some are antiapostatic; see Lindstrom et al. 2001; Endler & Mappes 2004), a recent empirical test suggests that predation on allopatric coral snake mimics in the eastern United States might be apostatic (Pfennig et al. 2007). With apostatic predation, mimics might persist in allopatry as long as they are rare, because predator-mediated selection against rare mimetic phenotypes is weak or non-existent. Consistent with this hypothesis, mimetic phenotypes are much rarer than non-mimetic phenotypes is allopatric populations of coral snake mimics in the eastern United States (Williams 1978).
5. Consequences of allopatric mimics
Evaluating which of the factor(s) listed in §§3 and 4 contribute to allopatric distributions of mimics will require more research. These studies are important, not only to clarify why Batesian mimics occur in allopatry, but also because the existence of such allopatric mimics can have important evolutionary ramifications. Below, we describe three such consequences of allopatric mimics.
(a). Variable selection for mimicry
The sympatry/allopatry dichotomy that we have presented up to now oversimplifies the true nature of the distributions and abundances of models and mimics in many natural systems. In most cases, the abundance of the model probably declines gradually as one approaches the sympatry/allopatry boundary. Consequently, the fitness benefits of mimicry may attenuate long before reaching this boundary. Thus, selection for mimicry should vary spatially, even within sympatry.
To see how variation in model abundance can lead to variation in selection for mimicry (Endler & Mappes 2004) consider that in areas where the probability of encountering the model is likely to be high, such as in deep sympatry, predators would be under strong selection to avoid any species that remotely resembles the model, especially if the model is highly noxious (Getty 1985). Therefore, in such areas, even imprecise mimics may be protected from predation (Sherratt 2002). By contrast, in areas where the probability of encountering the model is likely to be low, such as on the sympatry–allopatry boundary, selection to avoid the model (and any look-alikes) should be weak (Huheey 1964; Oaten et al. 1975; Getty 1985). In such situations, only those mimics that most closely resemble the model should receive any protection, and natural selection should favour only the best mimics; i.e. those that are the most precise replicas of their model. Indeed, if the model becomes very rare near the boundary, even perfect mimicry might not be favoured (especially if the model is not particularly noxious). In these cases, an alternative (e.g. cryptic) phenotype might be favoured over a phenotype that perfectly matches a (rare) model.
Recent studies of geographical variation in model–mimic resemblances in a coral snake mimicry complex have substantiated these predictions (Harper & Pfennig 2007). Thus, counter intuitively, the best mimics might occur on the sympatry–allopatry boundary rather than in deep sympatry. Consequently, during gene flow, alleles for the best mimics would not have to travel far to reach allopatry.
In summary, when the range of a Batesian mimic extends beyond that of its model, predators in different populations will necessarily vary in their likelihood of encountering the model. Consequently, selection for mimicry will also vary spatially.
(b). Degradation of the mimetic phenotype
A possible ramification of allopatry in Batesian mimicry complexes is the breakdown of the mimetic phenotype by selection. Although mimics are generally protected from predation in sympatry (Pfennig et al. 2001), they might often experience higher than random predation in allopatry (Pfennig et al. 2007). Accordingly, allopatric mimics might often either go extinct or evolve a non-mimetic phenotype (Harper & Pfennig 2008; Joron 2008).
Selection to break down maladaptive Batesian mimicry has been demonstrated in butterflies (Ries & Mullen 2008) and snakes (Harper & Pfennig 2008). In butterflies, selection acts against the introgression of mimetic alleles into a previously non-mimetic population (Limenitis a. arthemis) that has secondarily come into contact with a mimetic subspecies (L. a. astyanax; Mullen et al. 2008; Savage & Mullen 2009). In snakes, range expansion by the coral snake mimic L. elapsoides has lead to selection against mimicry in allopatry and erosion of the mimetic phenotype despite ongoing gene flow from sympatry (Harper & Pfennig 2008). Thus, in the absence of an alternative form of selection favouring the mimetic phenotype (see §§3a and 4), allopatric mimics are unlikely to persist.
(c). Speciation
Selection should always favour the maintenance, and even enhancement, of the mimetic phenotype in sympatry. By contrast, selection might often favour the breakdown of this phenotype in allopatry (Pfennig et al. 2007; Harper & Pfennig 2008; Joron 2008). In such situations, allopatric and sympatric populations would experience a divergent pattern of natural selection. Indeed, the transition between these two selective environments might be abrupt. If mimics are selected against in allopatry, but if the best mimics occur on the sympatry–allopatry boundary (see §5a), then there will be a sharp transition between these two contrasting selective environments.
As a consequence of this divergent selection, individuals that select mates from the opposite selective environment would produce offspring that are poorly adapted for either selective environment. These offspring would presumably be intermediate in phenotype and therefore neither mimetic (favoured in the sympatric selective environment) nor cryptic (favoured in the allopatric selective environment). Consequently, selection should favour individuals that mate within their own selective environment. Such assortative mating could, over time, lead to reduced gene flow between selective environments, thereby possibly resulting in the evolution of reproductive isolation between sympatric and allopatric populations.
Presently, there are no known examples in which allopatric mimics have lead to speciation. However, populations in sympatry and allopatry should experience strong divergent selection, and speciation is a possible outcome of such divergent selection (Jiggins et al. 2001, 2004; Naisbit et al. 2001; Servedio 2004).
6. Allopatric mimics as testing ground for mimicry theory
The above discussion rests on the assumption that the system of interest is actually a Batesian mimicry complex. Allopatric mimics are valuable because they enable tests of such fundamental assumptions. Indeed, the occurrence of mimics in allopatry allows for tests of the critical expectation that protection from predators should break down where the unpalatable form is not present. Consequently, such tests can be used to establish whether or not a putative mimic is, in fact, a true Batesian mimic (Pfennig et al. 2001).
More generally, allopatric mimics provide an ideal setting for testing mimicry theory, as illustrated by three additional examples. First, allopatric mimics enable tests of how Batesian mimicry evolves in the first place. Evolutionary biologists have long debated how such resemblances can arise gradually (Charlesworth & Charlesworth 1975). Indeed, it is unclear how a population can transition from a cryptic ancestral phenotype to a derived mimetic one if the population must pass through a phase in which it expresses a phenotype intermediate between these two extremes. Generally, such intermediates should be disfavoured because they would be neither cryptic nor mimetic (Charlesworth & Charlesworth 1975). However, intermediate phenotypes might be favoured in areas where models are abundant, such as in deep sympatry (see §5a). Essentially, high model abundance may facilitate the gradual evolution of Batesian mimicry. A recent empirical test, using a Batesian mimicry complex in which mimics occur in both sympatry and allopatry, supports this hypothesis (Kikuchi & Pfennig 2010).
Second, such systems can be used to explore mimic–model coevolution. Because models may suffer increased predation as mimics become more numerous, it has long been postulated that selection should cause models to evolve away from their mimics (Gavrilets & Hastings 1998; Speed & Turner 1999; Rowland et al. 2007). However, this ‘chase-away’ selection should be strong only in areas where models are rare relative to mimics, such as at the sympatry–allopatry boundary. Moreover, such selection might be overcome by gene flow from models in deep sympatry, where any mimicry ‘burden’ should be weak (because models are more abundant, many mimics in deep sympatry are likely to be imprecise replicas of the model; Harper & Pfennig 2007; see also §5a). Tests of this hypothesis, using systems in which mimics occur in both sympatry and allopatry, could help resolve this issue.
Finally, the occurrence within the same species of both good mimics in sympatry and poor mimics in allopatry (see §5b) presents an ideal opportunity for investigating the genetic basis of mimicry. Specifically, by evaluating the phenotypes produced by the offspring of crosses between good and poor mimics, one can gain insights into the genetic architecture of the mimetic pattern. For example, one could use such data to evaluate whether mimicry involves just a few mutations of large effect, as has been postulated (Joron 2008).
7. Concluding remarks
Longstanding theory predicts that the geographical ranges of Batesian mimics should always fit entirely within that of their model (Wallace 1867; Ruxton et al. 2004). Yet, in direct opposition to this longstanding theoretical expectation, mimics often occur outside the range of their model (figure 1 and table 1). Clarifying the processes that account for allopatric mimics (see §§3 and 4) is imperative, because the allopatric occurrence of Batesian mimics can have important evolutionary consequences (see §5). Moreover, the occurrence of Batesian mimics in allopatry offers an ideal setting in which to test mimicry theory (see §6).
A number of critical issues remain to be explored. Here, we highlight five topics that are promising. First, is allopatry more prevalent among certain taxa or when mimicking certain types of models? For example, allopatric mimics may be associated with models that are highly toxic more often than with those that are less toxic. Second, are there any examples of the reverse—models occurring where mimics are absent—and, if so, do allopatric and sympatric models tend to differ? If selection generally favours models that evolve away from their mimics (see §6), such selection might act differentially on allopatric and sympatric models, causing allopatric and sympatric models to diverge (as might occur between allopatric and sympatric mimics; see §5c). Third, in explaining the occurrence of allopatric mimics, what is the relative importance of non-selective processes, such as gene flow and range contraction/expansion (see §3b,c), as opposed to selective processes, such as mate choice (see §3a) or apostatic predation (see §4)? Fourth, can allopatric mimics compensate for expressing an inappropriate ‘fixed’ pattern or shape by altering their behaviour? For example, are there behavioral differences between allopatric and sympatric mimics, such that the former are less bold (in the same way that imprecise mimics of noxious butterflies tend to fly faster than more precise mimics; reviewed in Gilbert 2005)? Fifth, can divergent selection acting on different populations of mimics in sympatry and allopatry actually promote the formation of new species (see §5c)?
In short, studies into the causes and consequences of ‘rule-bending’ allopatric mimics promise to offer fundamental insights into the evolution of mimicry and thereby continue to provide, ‘a most powerful proof of the theory of natural selection’.
Acknowledgements
We thank K. Pfennig, D. Kikuchi, J. Santos, and two anonymous referees for helpful comments on the paper.
References
- Azmeh S., Owen J., Sorensen K., Grewcock D., Gilbert F.1998Mimicry profiles are affected by human-induced habitat changes. Proc. R. Soc. Lond. B 265, 2285–2290 (doi:10.1098/rspb.1998.0572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates H. W.1862Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 23, 495–566 (doi:10.1111/j.1096-3642.1860.tb00146.x) [Google Scholar]
- Brattstrom B. H.1955The coral snake ‘mimic’ problem and protective coloration. Evolution 9, 217–219 (doi:10.2307/2405591) [Google Scholar]
- Brodie E. D., III, Brodie E. D., Jr2004Venomous snake mimicry. In The venomous reptiles of the western hemisphere, vol. II (eds Campbell J. A., Lamar W. W.), pp. 617–633 Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- Brower J. V.1958Experimental studies of mimicry in some North American butterflies. 1. The monarch, Danaus plexippus, and Viceroy, Limenitis archippus archippus. Evolution 12, 32–47 [Google Scholar]
- Brower L. P., Brower J. V.1962The relative abundance of model and mimic butterflies in natural populations of the Battus philenor mimicry complex. Ecology 43, 154–158 [Google Scholar]
- Burkhardt F., Evans S., Pearn A. M.2008Evolution: selected letters of Charles Darwin 1860–1870. Cambridge, UK: Cambridge University Press [Google Scholar]
- Caldwell G. S., Rubinoff R. W.1983Avoidance of venomous sea snakes by naive herons and egrets. Auk 100, 195–198 [Google Scholar]
- Campbell N. A., Reece J. B.2005Biology. San Francisco, CA: Pearson Benjamin Cummings [Google Scholar]
- Carroll S. B.2009Remarkable creatures: epic adventures in the search for the origins of species. Boston, MA: Houghton Mifflin Harcourt [Google Scholar]
- Charlesworth D., Charlesworth B.1975Theoretical genetics of Batesian mimicry. I. Single-locus models. J. Theor. Biol. 55, 283–303 (doi:10.1016/S0022-5193(75)80081-6) [DOI] [PubMed] [Google Scholar]
- Cheney K. L.2010Multiple selective pressures apply to a coral reef fish mimic: a case of Batesian–aggressive mimicry. Proc. R. Soc. B 277, 1849–1855 (doi:10.1098/rspb.2009.2218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant R., Collins J. T.1998A field guide to reptiles and amphibians of eastern and central North America. Boston, MA: Houghton Mifflin Company [Google Scholar]
- Coppinger R. P.1970Effects of experience and novelty on avian feeding behavior with reference to evolution of warning coloration in butterflies. 2. Reactions of naive birds to novel insects. Am. Nat. 104, 323–335 [Google Scholar]
- Endler J. A.1991Interactions between predators and prey. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 169–196 London, UK: Blackwell [Google Scholar]
- Endler J. A., Mappes J.2004Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547 [DOI] [PubMed] [Google Scholar]
- Exnerova A., Stys P., Fuckiova E., Vesela S., Svadova K., Prokopova M., Jarosik V., Fuchs R., Landova E.2007Avoidance of aposematic prey in European tits (Paridae): learned or innate? Behav. Ecol. 18, 148–156 (doi:10.1093/beheco/arl061) [Google Scholar]
- Gadow H.1911Isotely and coral snakes. Zoologische Jahrbücher für Systematik, Ökologie und Geographie der Tiere 31, 1–24 [Google Scholar]
- Gavrilets S., Hastings A.1998Coevolutionary chase in two-species systems with applications to mimicry. J. Theor. Biol. 191, 415–427 (doi:10.1006/jtbi.1997.0615) [DOI] [PubMed] [Google Scholar]
- Getty T.1985Discrimininability and the sigmoid functional response: how optimal foragers could stabilize model–mimic complexes. Am. Nat. 125, 239–256 [Google Scholar]
- Gilbert F.2005The evolution of imperfect mimicry. In Insect evolutionary ecology (eds Fellowes M. D. E., Holloway G. J., Rolff J.), pp. 231–288 Wallingford, UK: CABI [Google Scholar]
- Greene H. W., McDiarmid R. Y.1981Coral snake mimicry: does it occur? Science 213, 1207–1212 (doi:10.1126/science.213.4513.1207) [DOI] [PubMed] [Google Scholar]
- Grobman A. B.1978An alternative to the coral snake mimic problem (Reptilia, Serpentes, Elapidae). J. Herpetol. 12, 1–11 (doi:10.2307/1563495) [Google Scholar]
- Harper G. R., Jr, Pfennig D. W.2007Mimicry on the edge: why do mimics vary in resemblance to their model in different parts of their geographical range? Proc. R. Soc. B 274, 1955–1961 (doi:10.1098/rspb.2007.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper G. R., Pfennig D. W.2008Selection overrides gene flow to break down maladaptive mimicry. Nature 451, 1103–1106 (doi:10.1038/nature06532) [DOI] [PubMed] [Google Scholar]
- Holman J. A.2000Fossil snakes of North America: origin, evolution, distribution, paleoecology. Bloomington, IN: Indiana University Press [Google Scholar]
- Howarth B., Edmunds M.2000The phenology of Syrphidae (Diptera): are they Batesian mimics of Hymenoptera? Biol. J. Linn. Soc. 71, 437–457 (doi:10.1111/j.1095-8312.2000.tb01268.x) [Google Scholar]
- Huheey J. E.1964Studies in warning coloration and mimicry. IV. A mathematical model of model–mimic frequencies. Ecology 45, 185–188 (doi:10.2307/1937125) [Google Scholar]
- Jiggins C. D., Naisbit R. E., Coe R. L., Mallet J.2001Reproductive isolation caused by colour pattern mimicry. Nature 411, 302–305 (doi:10.1038/35077075) [DOI] [PubMed] [Google Scholar]
- Jiggins C. D., Estrada C., Rodrigues A.2004Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J. Evol. Biol. 17, 680–691 (doi:10.1111/j.1420-9101.2004.00675.x) [DOI] [PubMed] [Google Scholar]
- Joron M.2008Batesian mimicry: can a leopard change its spots—and get them back? Curr. Biol. 18, R476–R479 (doi:10.1016/j.cub.2008.04.009) [DOI] [PubMed] [Google Scholar]
- Kikuchi D. W., Pfennig D. W.2010High model abundance may permit the gradual evolution of Batesian mimicry: an experimental test. Proc. R. Soc. B 277, 1041–1048 (doi:10.1098/rspb.2009.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt C., Lindstrom L., Mappes J.2009Thermoregulation constrains effective warning signal expression. Evolution 63, 469–478 (doi:10.1111/j.1558-5646.2008.00561.x) [DOI] [PubMed] [Google Scholar]
- Lindstrom L.1999Experimental approaches to studying the initial evolution of conspicuous aposematic signalling. Evol. Ecol. 13, 605–618 (doi:10.1023/A:1011004129607) [Google Scholar]
- Lindstrom L., Alatalo R. V., Lyytinen A., Mappes J.2001Strong antiapostatic selection against novel rare aposematic prey. Proc. Natl Acad. Sci. USA 98, 9181–9184 (doi:10.1073/pnas.161071598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan M. E., Cummings M. E.2008Female preferences for aposematic signal components in a polymorphic poison frog. Evolution 62, 2334–2345 (doi:10.1111/j.1558-5646.2008.00454.x) [DOI] [PubMed] [Google Scholar]
- Malcolm S. B.1990Mimicry: status of a classical evolutionary paradigm. Trends Ecol. Evol. 5, 57–62 (doi:10.1016/0169-5347(90)90049-J) [DOI] [PubMed] [Google Scholar]
- Mastrota F. N., Mench J. A.1995Color avoidance in Northern bobwhites: effects of age, sex, and previous experience. Anim. Behav. 50, 519–526 (doi:10.1006/anbe.1995.0266) [Google Scholar]
- Merilaita S., Ruxton G. D.2009Optimal apostatic selection: how should predators adjust to variation in prey frequencies? Anim. Behav. 77, 239–245 (doi:10.1016/j.anbehav.2008.09.032) [Google Scholar]
- Mullen S. P., Dopman E. B., Harrison R. G.2008Hybrid zone origins, species boundaries, and the evolution of wing-pattern diversity in a polytypic species complex of North American admiral butterflies (Nymphalidae: Limenitis). Evolution 62, 1400–1417 (doi:10.1111/j.1558-5646.2008.00366.x) [DOI] [PubMed] [Google Scholar]
- Naisbit R. E., Jiggins C. D., Mallet J.2001Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and Heliconius melpomene. Proc. R. Soc. B 268, 1849–1854 (doi:10.1098/rspb.2001.1753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaten A., Pearce C. E., Smyth M. E.1975Batesian mimicry and signal detection theory. Bull. Math. Biol. 37, 367–387 [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Harcombe W. R., Pfennig K. S.2001Frequency-dependent Batesian mimicry. Nature 410, 323 (doi:10.1038/35066628) [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Harper G. R., Jr, Brumo A. F., Harcombe W. R., Pfennig K. S.2007Population differences in predation on Batesian mimics in allopatry with their model: selection against mimics is strongest when they are common. Behav. Ecol. Sociobiol. 61, 505–511 (doi:10.1007/s00265-006-0278-x) [Google Scholar]
- Pough F. H.1976Multiple cryptic effects of crossbanded and ringed patterns of snakes. Copeia 1976, 335–365 [Google Scholar]
- Pough F. H.1988Mimicry of vertebrates: are the rules different? Am. Nat. 131, S67–S102 [Google Scholar]
- Poulton E. B.1909Mimicry in the butterflies of North America. Ann. Entomol. Soc. Am. 2, 203–242 [Google Scholar]
- Prudic K. L., Shapiro A. M., Clayton N. S.2002Evaluating a putative mimetic relationship between two butterflies, Adelpha bredowii and Limenitis lorquini. Ecol. Entomol. 27, 68–75 (doi:10.1046/j.0307-6946.2001.00384.x) [Google Scholar]
- Prudic K. L., Khera S., Solyom A., Timmermann B. N.2007Isolation, identification, and quantification of potential defensive compounds in the viceroy butterfly and its larval host-plant, Carolina willow. J. Chem. Ecol. 33, 1149–1159 (doi:10.1007/s10886-007-9282-5) [DOI] [PubMed] [Google Scholar]
- Punzalan D., Rodd F. H., Hughes K. A.2005Perceptual processes and the maintenance of polymorphism through frequency-dependent predation. Evol. Ecol. 19, 303–320 (doi:10.1007/s10682-005-2777-z) [Google Scholar]
- Rashed A., Sherratt T. N.2007Mimicry in hoverflies (Diptera: Syrphidae): a field test of the competitive mimicry hypothesis. Behav. Ecol. 18, 337–344 (doi:10.1093/beheco/arl089) [Google Scholar]
- Ries L., Mullen S. P.2008A rare model limits the distribution of its more common mimic: a twist on frequency-dependent Batesian mimicry. Evolution 62, 1798–1803 (doi:10.1111/j.1558-5646.2008.00401.x) [DOI] [PubMed] [Google Scholar]
- Ritland D. B.1991Revising a classic butterfly mimicry scenario: demonstration of Müllerian mimicry between Florida viceroys (Limenitis archippus floridensis) and Queens (Danaus gilippus berenice). Evolution 45, 918–934 (doi:10.2307/2409699) [DOI] [PubMed] [Google Scholar]
- Ritland D. B.1995Comparative unpalatability of mimetic viceroy butterflies (Limenitis archippus) from four south-eastern United States populations. Oecologia 103, 327–336 (doi:10.1007/BF00328621) [DOI] [PubMed] [Google Scholar]
- Roper T. J.1990Responses of domestic chicks to artificially colored insect prey: effects of previous experience and background color. Anim. Behav. 39, 466–473 (doi:10.1016/S0003-3472(05)80410-5) [Google Scholar]
- Rowland H. M., Ihalainen E., Lindström L., Mappes J., Speed M. P.2007Co-mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67 (doi:10.1038/nature05899) [DOI] [PubMed] [Google Scholar]
- Rubinoff I., Kropach C.1970Differential reactions of Atlantic and Pacific predators to sea snakes. Nature 228, 1288–1290 (doi:10.1038/2281288a0) [DOI] [PubMed] [Google Scholar]
- Ruxton G. D., Sherratt T. N., Speed M. P.2004Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press [Google Scholar]
- Saporito R. A., Donnelly M. A., Jain P., Garraffo H. M., Spande T. F., Daly J. W.2007Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon 50, 757–778 (doi:10.1016/j.toxicon.2007.06.022) [DOI] [PubMed] [Google Scholar]
- Savage J. M., Slowinski J. B.1992The colouration of the venomous coral snakes (family Elapidae) and their mimics (families Aniliidae and Colubridae). Biol. J. Linn. Soc. 45, 235–254 (doi:10.1111/j.1095-8312.1992.tb00642.x) [Google Scholar]
- Savage W. K., Mullen S. P.2009A single origin of Batesian mimicry among hybridizing populations of admiral butterflies (Limenitis arthemis) rejects an evolutionary reversion to the ancestral phenotype. Proc. R. Soc. B 276, 2557–2565 (doi:10.1098/rspb.2009.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Hesse E.1985On the function of warning coloration: a black and yellow pattern inhibits prey-attack by naive domestic chicks. Behav. Ecol. Sociobiol. 16, 249–255 (doi:10.1007/BF00310988) [Google Scholar]
- Servedio M. R.2004The evolution of premating isolation: local adaptation and natural and sexual selection against hybrids. Evolution 58, 913–924 [DOI] [PubMed] [Google Scholar]
- Sherratt T. N.2002The evolution of imperfect mimicry. Behav. Ecol. 13, 821–826 (doi:10.1093/beheco/13.6.821) [Google Scholar]
- Smith S. M.1975Innate recognition of coral snake pattern by a possible avian predator. Science 187, 759–760 (doi:10.1126/science.187.4178.759) [DOI] [PubMed] [Google Scholar]
- Smith S. M.1977Coral-snake pattern recognition and stimulus generalisation by naive great kiskadees (Aves: Tyrannidae). Nature 265, 535–536 (doi:10.1038/265535a0) [Google Scholar]
- Speed M. P.1999Batesian, quasi-Batesian or Mullerian mimicry? Theory and data in mimicry research. Evol. Ecol. 13, 755–776 (doi:10.1023/A:1010871106763) [Google Scholar]
- Speed M. P., Turner J. R. G.1999Learning and memory in mimicry. II. Do we understand the mimicry spectrum? Biol. J. Linn. Soc. 67, 281–312 (doi:10.1111/j.1095-8312.1999.tb01935.x) [Google Scholar]
- Stebbins R. C.2003Western reptiles and amphibians. Boston, MA: Houghton Mifflin Company [Google Scholar]
- Summers K., Symula R., Clough M., Cronin T.1999Visual mate choice in poison frogs. Proc. R. Soc. B 266, 2141–2145 (doi:10.1098/rspb.1999.0900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbauer G. P., Sternburg J. G.1987Experimental field demonstration that two aposematic butterfly color patterns do not confer protection against birds in Northern Michigan. Am. Midland Nat. 118, 145–152 (doi:10.2307/2425637) [Google Scholar]
- Wallace A. R.1867Mimicry and other protective resemblances among animals. Westminster Foreign Q. Rev. 32, 1–43 [Google Scholar]
- Wickler W.1968Mimicry in plants and animals. New York, NY: McGraw-Hill [Google Scholar]
- Williams K. L.1978Systematics and natural history of the American milk snake, Lampropeltis triangulum.. Milwaukee Public Museum Publications in Biology and Geology 2, 1–258 [Google Scholar]
- Zimmer C.2010The tangled bank: an introduction to evolution. Greenwood Village, CO: Roberts and Company [Google Scholar]