Abstract
Modern whales are frequently described as an adaptive radiation spurred by either the evolution of various key innovations (such as baleen or echolocation) or ecological opportunity following the demise of archaic whales. Recent analyses of diversification rate shifts on molecular phylogenies raise doubts about this interpretation since they find no evidence of increased speciation rates during the early evolution of modern taxa. However, one of the central predictions of ecological adaptive radiation is rapid phenotypic diversification, and the tempo of phenotypic evolution has yet to be quantified in cetaceans. Using a time-calibrated molecular phylogeny of extant cetaceans and a morphological dataset on size, we find evidence that cetacean lineages partitioned size niches early in the evolutionary history of neocetes and that changes in cetacean size are consistent with shifts in dietary strategy. We conclude that the signature of adaptive radiations may be retained within morphological traits even after equilibrium diversity has been reached and high extinction or fluctuations in net diversification have erased any signature of an early burst of diversification in the structure of the phylogeny.
Keywords: adaptive radiation, body size, Cetacea, disparity, diversity
1. Introduction
With approximately 84 extant (Mead & Brownell 2005) and 594 named fossil species (Paleobiology Database online systematics archive Uhen 2010), cetaceans (whales, dolphins, porpoises) represent what is arguably the most successful invasion of the marine environment by a group of tetrapods. The cetacean fossil record spans roughly 53 Myr (Fordyce 2003; Berta et al. 2006), although modern whales and dolphins (Neoceti) first appear around 34 Ma. Despite an extensive fossil record, surprisingly little is known about the tempo of the neocete radiation. It has, however, been suggested to have been ‘explosive’, driven by the evolution of key characters relating to sociality and brain size (Marino et al. 2006), echolocation and baleen as key innovations in the odontocetes and mysticetes, respectively (Fordyce 1992), or radiation into vacant niches following the extinction of archaeocete lineages in the Early Oligocene (Fordyce & de Muizon 2001; Fordyce 2003; Clementz et al. 2006).
The near-simultaneous appearance of key innovations and ecological opportunity provides a rationale for hypothesizing that crown cetaceans might be the product of an Early Oligocene adaptive radiation. Despite varied definitions (Givnish & Sytsma 1997; Losos 2009; Olson & Arroyo-Santos 2009), a pervasive view of adaptive radiation is that it is the product of rapid divergence into new adaptive zones, resulting in a diverse, ecologically disparate clade (e.g. Simpson 1944; Schluter 2000; Harmon et al. 2003). Under such an adaptive radiation hypothesis, lineage diversification would be expected to have been rapid early in neocete history, along with simultaneous increases in ecological and/or functional disparity as new lineages diverged to fill new adaptive zones (Simpson 1944; Schluter 2000; Harmon et al. 2003; Rabosky & Lovette 2008a,b). Both lineage diversification and ecomorphological evolution in such cases should show signatures of density dependence (Rabosky & Lovette 2008a,b), transitioning from initially rapid rates to slow, equilibrium rates as niche space fills towards capacity (Freckleton & Harvey 2006).
The only quantitative test of the adaptive radiation hypothesis in neocetes is equivocal. A recent exploration of lineage diversification using a time-calibrated molecular phylogeny finds no support for rapid speciation early in the evolutionary history of extant cetaceans (Steeman et al. 2009). Rather, Steeman et al. (2009) suggest that patterns of ocean restructuring during the Oligocene and Miocene are better explanations for cetacean diversification. However, tests of the rapid-speciation component of adaptive radiation based on molecular phylogenies assume low and sometimes constant rates of species turnover (Rabosky & Lovette 2008a,b), an assumption that may rarely be met in older clades, and certainly seems to be violated by the Neogene cetacean fossil record (Uhen & Pyenson 2007). Furthermore, such tests assume that lineage diversification is correlated with the evolution of morphological or functional disparity, which may or may not be the case (Gavrilets & Losos 2009). A more rigorous approach would be to simultaneously test for signatures of adaptive radiation in patterns of lineage diversification and in the evolution of adaptive ecomorphological disparity. Unfortunately, such approaches are remarkably infrequent (but see Harmon et al. 2003) and have yet to be applied to crown group cetaceans.
We present a quantitative test of the adaptive radiation hypothesis in modern cetaceans that investigates the tempo of both lineage and ecomorphological evolution, using body length as a proxy for the many ecological, physiological and morphological traits that scale with size (see review by LaBarbara 1989). We construct a time scale for cetacean evolution incorporating most extant cetaceans species, based on mitochondrial sequence data and multiple fossil calibration points. Using this tree as a framework, we determine the patterns of lineage diversification and body size disparity, and test whether they are consistent with an early adaptive radiation. We then test whether dietary specialization may have driven differences in body size evolution in Cetacea, as is often the case for terrestrial mammals (e.g. Jarman 1974; Van Valkenburgh et al. 2004).
2. Material and methods
(a). Time tree inference
We used BEAST v. 1.5.1 (Drummond & Rambaut 2007) to simultaneously infer cetacean phylogeny and divergence times for 84 cetacean taxa and two outgroups (hippopotamus and pig). Our sampling comprised all taxa for which cytochrome b (cytb) nucleotide sequences were available in GenBank. Following an extensive search of the comprehensive cetacean Paleobiology Database (Uhen 2010) and fossil cetacean literature, we identified seven fossils that could be unambiguously assigned to nodes within the phylogeny to serve as calibration points. These fossils spanned 55–10 Myr and were used to calibrate a relaxed molecular clock using log-normally distributed priors. Full details of the phylogenetic analyses conducted are provided in the electronic supplementary material accompanying this article.
(b). Lineage diversification
The summary statistic γ can be used to determine the extent to which branching events in a molecular phylogeny depart from those expected under a constant-rates process. We assessed γ for our cetacean phylogeny using the Monte Carlo constant-rates (MCCR) test (Pybus & Harvey 2000), as this approach allowed us to account for missing taxa. To further test for temporal slowdowns in diversification rate consistent with adaptive radiation, we compared the fit of a constant-rates or Yule model with exponential and logistic density-dependent decline models of lineage accumulation (DDX and DDL, respectively; Rabosky & Lovette 2008a) using Akaike information criterion (AIC) scores and Akaike weights (Burnham & Anderson 2002). MCCR tests and rate fitting were done using the LASER package (Rabosky 2006) for R.
To identify potential diversification rate shifts within crown cetaceans, we applied a recently developed method, MEDUSA (Alfaro et al. 2009), to a diversity tree of cetaceans. A diversity tree is a time-calibrated phylogenetic tree where each tip has been assigned a species richness value, based on taxonomic diversity. In previous studies (e.g. Alfaro et al. 2009), species richness values have been assigned to branches representing orders or other higher-level groupings of vertebrates. In our study, we assigned most tips a diversity of one, as we were working with a species-level tree. To account for missing species, we collapsed the genera Mesoplodon and Globicephala, and assigned them species richness values of 14 and 3, respectively. MEDUSA is a stepwise procedure that first fits a birth–death model to the diversity tree using a joint phylogenetic and taxonomic likelihood function developed by Rabosky et al. (2007). Next, the AIC score of this two-parameter model (one birth rate and one death rate) is compared with a five-parameter model where the birth and death rates are allowed to shift on the optimal branch of the phylogeny. If this five-parameter model (two birth rates, two death rates and a shift-location parameter) produces a substantial improvement in the AIC score, the five-parameter model is retained and compared with the best eight-parameter model. This process continues until the addition of rate parameters no longer improves the overall AIC score. For this study, we used an improvement in AIC score of 4 units or greater as the threshold for retaining rate shifts (Burnham & Anderson 2002).
(c). Body size disparity
We compiled data on average adult female body length and diet from the literature for each species in our phylogeny, where available (electronic supplementary material, table S3). Length estimates were preferred over mass as length can be more accurately estimated from photos and carcasses washed up on beaches (Whitehead & Mann 2000). Females were used as they tend to be the larger sex in cetaceans. Averages were preferred over maximum lengths so as to minimize the effects of outliers, geographical variants or misreported measurements. Body size data were natural log (ln)-transformed prior for all subsequent analyses. We defined three functionally different dietary categories: filter feeders, cephalopod specialists (greater than 70% squid in diet) and generalist fish eaters that can also include squid and crustaceans in their diet. The orca (Orcinus orca), which is the largest member of the dolphin family (Delphinidae), is unique among extant cetaceans as at least some populations specialize on large vertebrate prey such as pinnipeds and other cetaceans (‘transient type’; Bigg et al. 1990). The orca was therefore removed from our analyses, which looked for an association between size and diet.
To test whether cetacean body size evolution has slowed through time, we used the node-height test (Freckleton & Harvey 2006). We computed the absolute value of standardized independent contrasts (Felsenstein 1985) for body size on our tree and correlated them with the height of the node at which they are generated. Because independent contrasts are Brownian rate parameters for the branches over which they are calculated (McPeek 1995), a significant positive relationship between node age and absolute contrast value would indicate that rates of body size evolution have slowed through time, consistent with niche-filling (Freckleton & Harvey 2006). Following Harmon et al. (2003), we also calculated mean subclade disparity through time for body size. We compared observed body size disparity across our tree with that expected under a pure Brownian process by simulating body size evolution 10 000 times across our tree. The mean subclade disparity values for the observed and simulated data were plotted against node age and the morphological disparity index (MDI) calculated. MDI quantifies the overall difference in relative disparity of a clade compared with the expectation under the null Brownian motion model (Harmon et al. 2003). Negative MDI values indicate lower subclade disparity than expected under Brownian motion and are a common property of adaptively radiating clades. All analyses were conducted in R using the packages Ape (Paradis et al. 2004) and Geiger (Harmon et al. 2008).
To test whether body size differences among cetacean clades may be explained by dietary specialization, we used a model-fitting approach to assess the null model of evolution under a pure Brownian process (Thomas et al. 2009). We tested three alternative scenarios against this null model: (i) the same rate but different means for dietary groups; (ii) different rates and different means for dietary groups; and (iii) different rates but the same mean. Internal branches were assigned to dietary groups based on ancestral state reconstructions conducted in Mesquite v. 2.7 (Maddison & Maddison 2009) using the MK1 likelihood model, which assumes a constant transition rate between dietary categories, as changes are rare. A difference of two negative log-likelihood units was taken as support for one state over the others. Where multiple states were equally likely, the state with the highest proportional likelihood was used. We assessed fit of the four models based on small-sample corrected AIC (AICc) scores and Akaike weights. We also repeated the dietary analysis using an Ornstein–Uhlenbeck (OU) model of trait evolution (Hansen 1997), which allowed size to evolve towards an adaptive optimum for each dietary category. For simplicity, throughout the rest of the paper we focus on the Brownian models, as the OU model fitted the data worse than the best-fitting Brownian model. Full details of the Brownian and OU model methods are given in the Methods section of the electronic supplementary material.
3. Results
(a). Time tree inference
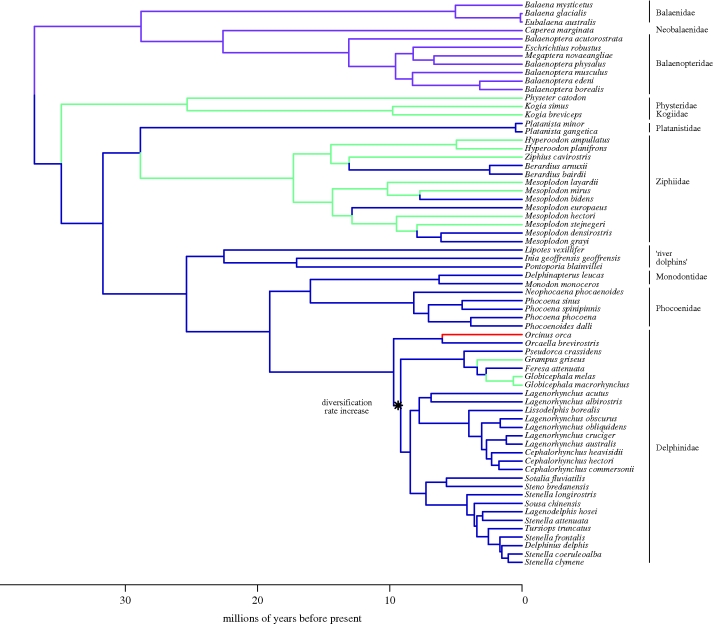
Our time tree (figure 1) is broadly congruent with other recently published studies of cetacean phylogeny (McGowen et al. 2009; Steeman et al. 2009). The split between hippopotamuses and cetaceans dates to 54.5 Ma (95% high posterior density, HPD: 54.1–55.1), and extant cetaceans (Neoceti) share a most recent common ancestor at 36.9 Ma (95% HPD: 34.4–39.9). Crown mysticetes originated at 28.8 Ma (95% HPD: 28–30.1). Crown odontocetes originated at 34.8 Ma (95% HPD: 30.9–38.7) and show a gradual pattern of lineage divergence. The sperm whales (Physeteroidea), beaked whales (Ziphiidae) and river dolphins (Platinistidae, Lipotidae, Iniidae and Pontoporidae) are old, originating prior to 20 Ma. The remaining odontocete families within the Delphinoidea are younger than 15 Myr old and the most speciose cetacean clade, Delphinidae, containing 36 of 84 species, is less than 10 Myr old.
Figure 1.
Time-calibrated phylogeny of Cetacea including all species for which both size and dietary data were available. Colours indicate diet: generalist fish (blue); filter feeder (purple); squid specialist (aquamarine); marine mammals (red). Diets assigned to internal nodes were reconstructed using maximum likelihood. The diversification rate increase in Delphinidae was detected using MEDUSA (see text).
(b). Lineage diversification
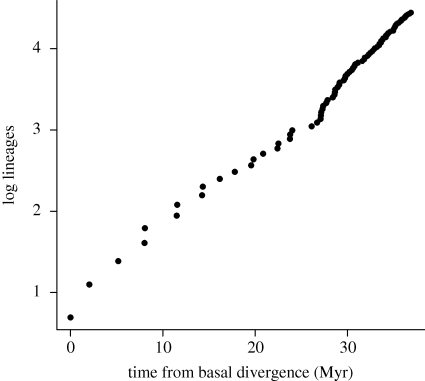
The MCCR test found no evidence for accelerated diversification in the early history of cetaceans (γ = 0.363, p = 0.72), a finding that is supported visually by a lineage-through-time plot (figure 2). However, when we compare the fit of the constant-rates model with the two density-dependent decline models of diversification for our tree, we are unable to find strong support for one model over the others (table 1). Therefore, we are unable to rule out a pattern of declining diversification rates even though γ is positive.
Figure 2.
Lineage-through-time plot derived from the complete, time-calibrated cetacean phylogeny. The upturn at approximately 26 Myr after basal divergence indicates an increased net rate of diversification over the past 10 Myr.
Table 1.
Results from fitting diversification-process models to the complete ultrametric cetacean tree and a tree truncated at 10 Ma. Models are ranked from best to worst, according to AIC scores and Akaike weights (wtAIC). dAIC scores indicate the difference between the candidate model and the best-fitting model. Also given is the log likelihood (Lk) for each model.
| model | Lk | AIC | dAIC | wtAIC |
|---|---|---|---|---|
|
complete ultrametric cetacean tree | ||||
| Yule | 22.521 | −43.042 | 0 | 0.569 |
| DDX | 22.582 | −41.164 | 1.878 | 0.222 |
| DDL | 22.521 | −41.042 | 2.000 | 0.209 |
|
tree truncated at 10 Ma | ||||
| Yule | −25.354 | 52.709 | 0 | 0.399 |
| DDX | −24.580 | 53.161 | 0.452 | 0.318 |
| DDL | −24.701 | 53.402 | 0.693 | 0.282 |
MEDUSA revealed strong support for a rate shift at the node uniting crown Delphinidae, excluding Orca and Orcaella (ΔAIC = 15.07; figure 1). The net diversification rate for this clade (0.2772 lineages Myr−1) was over three times higher than the background rate of diversification in other cetaceans (0.086 lineages Myr−1). In order to account for potential biases in the MCCR test or diversification models that might result from the explosive delphinid radiation, we truncated our tree at 10 Ma, just prior to the delphinid radiation, and recomputed γ and AIC scores for the fit of the three diversification process models. The MCCR test still fails to provide a significantly negative γ (γ = −0.750; p = 0.22), although support for the two density-dependent decline models increases slightly, relative to the constant-rates model (table 1).
(c). Body size disparity
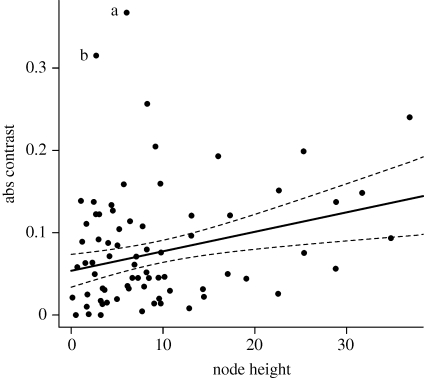
The node-height test resulted in a positive but non-significant relationship between the absolute values of standardized length contrasts and node age (r = 0.212, d.f. = 71, p = 0.064). A scatter plot of contrast values against node age (figure 3) reveals that two young contrasts, corresponding to those between O. orca and Orcaella brevirostris, and between Feresa attenuata and Globicephala species, are extreme outliers, with large rate values. Repetition of the node-height test with these outliers removed results in a significant positive relationship between absolute standardized contrasts and node age (r = 0.218, d.f. = 69, p = 0.006), indicating that body size evolution has slowed through time, which is consistent with the niche-filling hypothesis (Freckleton & Harvey 2006).
Figure 3.
Plot of absolute body size (abs) contrasts against node height for the node-height test. The two outliers are contrasts between (a) O. orca and O. brevirostris, and (b) Globicephala spp. and F. attenuata. The regression line and 95% confidence intervals are computed after removal of the outliers.
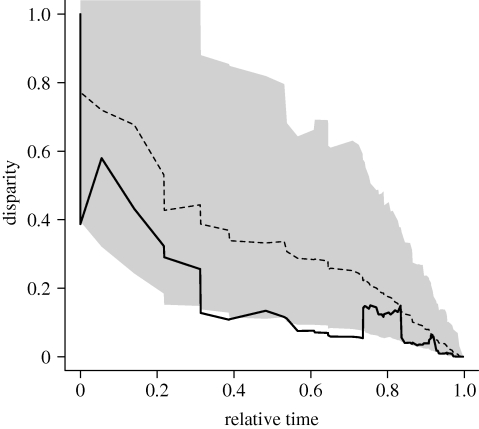
Subclade disparity through time is lower than expected under a Brownian motion model of body size evolution (figure 4). This is confirmed quantitatively by an MDI of −0.17. Despite an overall pattern of decrease through time, subclade disparity shows two increases: one occurring shortly after the origination of neocetes and another between approximately 11 and 6 Ma (approx. 0.7–0.825 relative time), coincident with the radiations of several extant families, such as Delphinidae, Phocoenidae and Balaenopteridae. We assessed the probability of obtaining a negative MDI when the underlying evolutionary process follows Brownian motion by computing the MDI between each of the 10 000 simulated datasets and our cetacean data, and determining the proportion of cases in which an MDI greater than or equal to zero is obtained. The resulting proportion of 0.0542 suggests that our result is unlikely to be the result of morphological evolution under a simple, one-rate Brownian motion process.
Figure 4.
Mean subclade disparity through time (DTT) for cetacean body size (lower solid line). The upper dashed line indicates the median subclade DTT based on 10 000 simulations of character evolution on the cetacean phylogeny under Brownian motion. The grey shaded area indicates the 95% DTT range for the simulated data.
Comparison of models of body size evolution in relation to differences in diet results in preferential selection of the model with the same rate but different phylogenetic means for the three dietary groups (tables 2 and 3). Substantially more weight falls on this model than any other. The next best models are the Brownian motion model with different rates and different phylogenetic means for dietary groups, and the OU model with different optimal sizes for each group. ΔAICc for both of these models are greater than 4 (table 2), indicating that they fit relatively poorly in comparison to the best-fit model (Burnham & Anderson 2002). The other two Brownian models performed extremely poorly relative to the best model (table 2).
Table 2.
Comparison of the fit of four Brownian motion (BM) models and one Ornstein–Uhlenbeck (OU) model of body size evolution based on diet type in cetaceans. Models are ranked from best to worst, using the small-sample corrected Akaike's information criterion (AICc) and Akaike weights (wtAICc). dAICc scores indicate the difference between the candidate model and the best-fitting model. Also given are the number of parameters for each model (K) and the log likelihood (Lk).
| model | K | Lk | AICc | dAICc | wtAICc |
|---|---|---|---|---|---|
| BM: same rate/different means | 4 | −11.121 | 30.898 | 0 | 0.96 |
| BM: different rates/different means | 6 | −11.025 | 35.473 | 4.575 | 0.01 |
| OU: same rate/multiple optima | 5 | −12.303 | 35.606 | 4.708 | 0.01 |
| BM: same rate/same mean | 2 | −16.522 | 37.235 | 6.337 | 0.02 |
| BM: different rates/same mean | 4 | −16.189 | 41.034 | 10.135 | 0.01 |
Table 3.
Comparison of parameter estimates for the four Brownian motion (BM) models and one Ornstein–Uhlenbeck (OU) model of body size evolution based on diet type in cetaceans. Models are ranked from best to worst based on AICc scores, as in table 2. Body size values are phylogenetic means for Brownian models and optimal trait values for OU models. The ‘strength of selection’ parameter, alpha, is estimated in the OU model only.
| body size |
rates |
||||||
|---|---|---|---|---|---|---|---|
| model | fish | squid | filter | fish | squid | filter | alpha |
| BM: same rate/different means | 1.171 | 0.270 | 1.373 | 0.378–2.41 | 0.29–2.42 | 0.51–3.96 | — |
| BM: different rates/different means | 1.171 | 0.270 | 1.373 | 0.378–2.42 | 0.29–2.43 | 0.51–3.97 | — |
| OU: same rate/multiple optima | 0.936 | 2.469 | 4.119 | 0.42 | 0.42 | 0.42 | 0.71539 |
| BM: same rate/same mean | 1.700 | 1.700 | 1.700 | 0.378–2.41 | 0.29–2.42 | 0.51–3.96 | — |
| BM: different rates/same mean | 1.700 | 1.700 | 1.700 | 0.378–2.42 | 0.29–2.43 | 0.51–3.97 | — |
4. Discussion
A central prediction of the adaptive radiation hypothesis sensu Schluter (2000) is that phenotypes diversify early in clade history along ecological axes. Our study reveals clear evidence that body size niches were partitioned early in the evolutionary history of modern Cetacea and that these broadly correspond to different dietary strategies. However, the case for a neocete adaptive radiation is complicated by the lack of evidence for rapid initial lineage diversification in the group. Like Steeman et al. (2009), we found no evidence for an early burst of diversification in extant neocetes based on the γ statistic (Pybus & Harvey 2000). Although we did identify a phylogenetically restricted shift in diversification rate in the Delphinidae, analysis of the tempo of neocete diversification before this radiation still shows a pattern that is not significantly different from a constant-rates process.
(a). Lineage diversification and the possibility of adaptive radiation of modern cetaceans
It is well understood that extinction can mask the signal of rapid initial diversification in molecular phylogenies (Pybus & Harvey 2000; Rabosky & Lovette 2008a,b; Quental & Marshall 2009). Both γ and the MCCR test are conservative with respect to extinction, which can lead to increased type II errors (Pybus & Harvey 2000; Rabosky & Lovette 2008a,b). Furthermore, even if diversification dynamics are density-dependent and extinction rates are low, γ values will be less negative than the critical value of −1.645 if a clade has yet to reach its equilibrium diversity, or if it has reached equilibrium and a constant-rates pattern of diversification has eroded the signal of density-dependent declines in rate (Quental & Marshall 2009). Pybus & Harvey (2000) warned that a non-significant γ did not allow acceptance of a null, constant-rates process. Our analyses using the model-fitting approach of Rabosky & Lovette (2008a,b) validate this. The two variants on a density-dependent speciation process (Rabosky & Lovette 2008a) together receive about the same weight as the constant-rates diversification processes for our cetacean phylogeny. Therefore, one possible explanation for not finding an early burst of diversification in modern neocetes is that sufficient extinction has occurred within the group to erase the signature of a rapidly radiating clade. Evidence in support of this explanation comes from the cetacean fossil record; multiple radiations that have left no living descendants, including large predacious forms (Physteroidea) and open-ocean piscivores (Kentriodontidae), or clades that were formally more speciose but have subsequently waned and been ecologically replaced (archaic river dolphin clades: Lipotidae, Iniidae, Pontoporidae and Platinistoidea). Uhen & Pyenson (2007) also found evidence of increased rates of extinction for neocetes in the Late Miocene. Taken together, these lines of evidence suggest that high rates of turnover have affected cetaceans during the Neogene, but whether it actually eroded the signal of an early burst of diversification cannot be determined from our dataset.
(b). The evolution of ecomorphological disparity in cetaceans
Using the model-fitting approach of Thomas et al. (2006, 2009), we provide evidence for a correlation between body size and diet, supporting the hypothesis that there is an ecological basis for early size diversification in Cetacea, which is indicative of an adaptive radiation (sensu Schluter 2000). We found strong preference for a model of body size evolution with different phylogenetic means among dietary strategies but the same rate of size evolution across Cetacea. The largest phylogenetic mean body size was recovered for the Mysticeti, which includes the largest animal to have ever lived, the blue whale (Balaenoptera musculus). Extant mysticetes are specialist filter feeders, using their baleen to filter small marine animals (mainly zooplankton) from the water. The acquisition of large body sizes by mysticetes, which occurred in the Miocene (Fordyce & Barnes 1994), is commonly ascribed to the abundance and ease of capture of their highly nutritious prey.
Odontocetes differ from mysticetes in exhibiting a general decrease in body size though time. Extant odontocetes are typically fish or squid feeders, with fish feeding being the ancestral, and more widely distributed, dietary strategy. The phylogenetic mean body size for fish feeders was larger than that found for squid feeders (table 3). However, extant squid specialists are generally much larger than their small piscivorous relatives. Squid feeders form a polyphyletic assemblage nested within the generally piscivorous odontocetes. The small phylogenetic mean of squid feeders indicates that this guild attained their large body sizes from smaller ancestors. In fact, all squid feeders are larger than the phylogenetic mean of the group and all but one (Kogia breviceps) are larger than the phylogenetic mean of fish feeders. Although our models show that rates of body size evolution have been similar among dietary groups, distinct patterns of selection appear to have been present in cetacean guilds. While fish-feeding odontocetes declined in size over time, large body size appears to have been selected for in squid-feeding taxa. Existing research demonstrates the functional utility of this condition for these taxa. Extant squid specialists dive to great depths to hunt and capture prey. Most species make routine dives of 800–1000 m (Heide-Jørgensen et al. 2002 and references therein), and the deepest diver, the sperm whale (Physeter catodon), dives to depths of 2035 m (Watkins et al. 1993) for durations of at least 138 min (Watkins et al. 1985). Larger animals have increased absolute oxygen capacities and decreased mass-specific metabolic rates, providing significant physiological advantages when diving to great depths. Indeed, size scales positively allometrically with odontocete dive capacity (Noren & Williams 2000). Our disparity-through-time analysis shows that size variation, and therefore possibly dietary strategy, was partitioned among subclades relatively early in cetacean history. This is in broad agreement with the fossil record, which indicates that the teutophagous dietary strategy has been present throughout most of the evolutionary history of several odontocete families (Fordyce & Barnes 1994).
Although our disparity-through-time analysis shows low levels of subclade disparity, there are some periods of cetacean history that show elevated levels of subclade disparity that are discordant with the hypothesis of adaptive radiation. This is particularly true during the period between 11 and 6 Ma (figure 4). This may be a case of ‘the exception proves the rule’ as cephalopod specialization mostly occurs within clades that are old relative to most odontocetes (Physeteridae, Kogiidae and Ziphiidae), but recent transitions to squid specialization have occurred within the past 10 Myr in the primarily piscivorous Delphinidae (figure 1). The recent dietary shift to squid feeding in Grampus and, in particular, Globicephala (figure 3) explains the rapid increase in body size of these taxa compared with their delphinid relatives. Another delphinid, O. orca, also exhibits a recent rapid increase in size at this time, which may be explained by a different dietary transition. Orcas are unique among extant cetaceans as they prey on large marine mammals, including pinnipeds, sperm whales (P. catodon) and grey whales (Eschrichtius robustus). The rapid evolution of large body size in orcas presumably reflects a response to this specific predatory strategy, rather than an adaptation to deep diving and squid feeding.
5. Conclusion
Our analyses point to the importance of considering both diversity and disparity when testing for predictions of adaptive radiation. It is particularly notable that patterns of morphological evolution can retain a signature of early niche-filling, despite some evidence for secondary radiations into areas of niche space not previously occupied by the subclade in question (e.g. Globicephala). It may be that the signature of adaptive radiation is retained in patterns of ecomorphological disparity even after equilibrium species diversity has been reached and high extinction or fluctuations in net diversification have erased any signature in the structure of the phylogeny. This would be expected if early lineage diversification also results in ecological niche-filling, leaving limited opportunity for subsequent switches in ecology by younger members of extant subclades (Harmon et al. 2003). Alternatively, speciation and ecomorphological diversity in neocetes may not be linked (i.e. if there was no concomitant increase in lineage diversity when size and dietary niches were partitioned). The rich cetacean fossil record provides a valuable resource for testing these ideas, once inclusive phylogenetic analyses become available.
Acknowledgements
We thank Luke Harmon, Shauna Price, Gavin Thomas and two anonymous reviewers, as well as Associate Editor Trevor Price for helpful comments on the manuscript. Thanks also to Justine Jackson-Rickets, an undergraduate work-study student at Duke University, for collecting the majority of the dietary data. Work was supported by National Science Foundation grants DEB 0918748 to M.E.A., DEB 0842397 to M.E.A. and F.S., DEB 0709792 to G.J.S. and a NESCent postdoctoral fellowship to S.A.P. (NESCent NSF no. EF-0423641). MEDUSA development was partially supported by an R hackathon and Short Term Visitors Award from NESCent.
References
- Alfaro M. E., Santini F., Brock C., Alamillo H., Dornburg A., Rabosky D. L., Carnevale G., Harmon L. J.2009Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc. Natl Acad. Sci. USA 106, 13 410–13 414 (doi:10.1073/pnas.0811087106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta A., Sumich J. L., Kovacs K. M.2006Marine mammals: evolutionary biology, 2nd edn.San Diego, CA: Associated Press [Google Scholar]
- Bigg M. A., Olesiuk P. F., Ellis G. M., Ford J. K. B., Balcomb K. C.1990Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State. Rep. Int. Whal. Comm. 12, 383–405 [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- Clementz M., Goswami A., Gingerich P., Koch P.2006Isotopic records from early whales and sea cows: contrasting patterns of ecological transition. J. Vertebr. Paleontol. 26, 355–370 (doi:10.1671/0272-4634(2006)26[355:IRFEWA]2.0.CO;2) [Google Scholar]
- Drummond A. J., Rambaut A.2007BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J.1985Phylogenies and the comparative method. Am. Nat. 126, 1–25 [Google Scholar]
- Fordyce R. E.1992Cetacean evolution and Eocene/Oligocene environments. In Eocene–Oligocene climatic and biotic evolution (eds Prothero D. R., Berggren W.). Princeton, NJ: Princeton University Press [Google Scholar]
- Fordyce R. E.2003Cetacean evolution and Eocene–Oligocene oceans revisited. In From greenhouse to icehouse: the marine Eocene–Oligocene transition (eds Prothero D. R., Ivany L. C., Nesbitt E. R.), pp. 154–170 New York, NY: Columbia University Press [Google Scholar]
- Fordyce R. E., Barnes L. G.1994The evolutionary history of whales and dolphins. Annu. Rev. Earth Planet Sci. 22, 419–455 (doi:10.1146/annurev.ea.22.050194.002223) [Google Scholar]
- Fordyce R. E., de Muizon C.2001Evolutionary history of whales: a review. In Secondary adaptation of tetrapods to life in water. Proc. Int. Meeting, Poitiers 1996 (eds Mazin J.-M., de Buffrenil V.), pp. 169–234 Munich, Germany: Verlag Dr Friedriech Pfeil [Google Scholar]
- Freckleton R. P., Harvey P. H.2006Detecting non-Brownian trait evolution in adaptive radiations. PLoS Biol. 4, e373 (doi:10.1371/journal.pbio.0040373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S., Losos J. B.2009Adaptive radiation: contrasting theory with data. Science 323, 732–737 (doi:10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- Givnish T., Sytsma K.1997Molecular evolution and adaptive radiation. New York, NY: Cambridge University Press [Google Scholar]
- Hansen T. F.1997Stabilizing selection and the comparative analysis of adaptation. Evolution 51, 1341–1351 (doi:10.2307/2411186) [DOI] [PubMed] [Google Scholar]
- Harmon L., Schulte J., Larson A., Losos J.2003Tempo and mode of evolutionary radiation in iguanian lizards. Science 301, 961–964 (doi:10.1126/science.1084786) [DOI] [PubMed] [Google Scholar]
- Harmon L., Weir J., Brock C., Glor R., Challenger W.2008Geiger: investigating evolutionary radiations. Bioinformatics 24, 129–131 (doi:10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- Heide-Jørgensen M. P., Bloch D., Stefansson E., Mikkelsen B., Ofstad L. H., Dietz R.2002Diving behaviour of long-finned pilot whales Globicephala melas around the Faroe Islands. Wildl. Biol. 8, 307–313 [Google Scholar]
- Jarman P. J.1974The social organisation of antelope in relation to their ecology. Behaviour 48, 215–267 (doi:10.1163/156853974X00345) [Google Scholar]
- LaBarbara M.1989Analyzing body size as a factor in ecology and evolution. Annu. Rev. Ecol. Syst. 20, 97–117 (doi:10.1146/annurev.es.20.110189.000525) [Google Scholar]
- Losos J. B.2009Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Berkeley, CA: University of California Press [Google Scholar]
- Maddison W. P., Maddison D. R.2009Mesquite: a modular system for evolutionary analysis, v. 2.72 See http://mesquiteproject.org [Google Scholar]
- Marino L., Sol D., Toren K., Lefebvre L.2006Does diving limit brain size in cetaceans? Mar. Mammal. Sci. 22, 413–425 (doi:10.1111/j.1748-7692.2006.00042.x) [Google Scholar]
- McGowen M. R., Spaulding M., Gatesy J.2009Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Mol. Phylogenet. Evol. 53, 891–906 (doi:10.1016/j.ympev.2009.08.018) [DOI] [PubMed] [Google Scholar]
- McPeek M. A.1995Testing hypotheses about evolutionary change on single branches of a phylogeny using evolutionary contrasts. Am. Nat. 45, 686–703 [Google Scholar]
- Mead J. G., Brownell R. L., Jr2005Order Cetacea. In Mammal species of the world: a taxonomic and geographic reference (eds Wilson D. E., Reeder D. M.), 3rd edn, pp. 723–743 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Noren S., Williams T.2000Body size and skeletal muscle myoglobin of cetaceans: adaptations for maximizing dive duration. Comp. Biochem. Phys. A 126, 181–191 [DOI] [PubMed] [Google Scholar]
- Olson M. E., Arroyo-Santos A.2009Thinking in continua: beyond the ‘adaptive radiation’ metaphor. BioEssays 31, 1337–1346 (doi:10.1002/bies.200900102) [DOI] [PubMed] [Google Scholar]
- Paradis E., Claude J., Strimmer K.2004APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- Pybus O., Harvey P.2000Testing macro-evolutionary models using incomplete molecular phylogenies. Proc. R. Soc. Lond. B 267, 2267–2272 (doi:10.1098/rspb.2000.1278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quental T. B., Marshall C. R.2009Extinction during evolutionary radiations: reconciling the fossil record with molecular phylogenies. Evolution 63, 3158–3167 (doi:10.1111/j.1558-5646.2009.00794.x) [DOI] [PubMed] [Google Scholar]
- Rabosky D.2006Laser: a maximum likelihood toolkit for detecting temporal shifts in diversification rates from molecular phylogenies. Evol. Bioinform. 2, 247–250 [PMC free article] [PubMed] [Google Scholar]
- Rabosky D., Lovette I.2008aDensity-dependent diversification in North American wood warblers. Proc. R. Soc. B 275, 2363–2371 (doi:10.1098/rspb.2008.0630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky D., Lovette I.2008bExplosive evolutionary radiations: decreasing speciation or increasing extinction through time? Evolution 62, 1866–1875 (doi:10.1111/j.1558-5646.2008.00409.x) [DOI] [PubMed] [Google Scholar]
- Rabosky D. L., Donnellan S. C., Talaba A. L., Lovette I. J.2007Exceptional among-lineage variation in diversification rates during the radiation of Australia's most diverse vertebrate clade. Proc. R. Soc. B 274, 2915–2923 (doi:10.1098/rspb.2007.0924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D.2000The ecology of adaptive radiation. Oxford, UK: Oxford University Press [Google Scholar]
- Simpson G. G.1944The tempo and mode in evolution. New York, NY: Columbia University Press [Google Scholar]
- Steeman M. E., et al. 2009Radiation of extant cetaceans driven by restructuring of the oceans. Syst. Biol. 58, 573–585 (doi:10.1093/sysbio/syp060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. H., Freckleton R. P., Székely T.2006Comparative analyses of the influence of developmental mode on phenotypic diversification rates in shorebirds. Proc. R. Soc. B 273, 1619–1624 (doi:10.1098/rspb.2006.3488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. H., Meiri S., Phillimore A. B.2009Body size diversification in Anolis: novel environment and island effects. Evolution 63, 2017–2030 (doi:10.1111/j.1558-5646.2009.00694.x) [DOI] [PubMed] [Google Scholar]
- Uhen M. D.2010Cetacea online systematics archive 11. See The paleobiology database, http://paleodb.org/cgi-bin/bridge.pl?user=Guest&action=displayPage&page=OSA_Cetacea [Google Scholar]
- Uhen M. D., Pyenson N. D.2007Diversity estimates, biases, and historiographic effects: resolving cetacean diversity in the Tertiary. Palaeontol. Electron. 10, 1–22 [Google Scholar]
- Van Valkenburgh B., Wang X., Damuth J.2004Cope's rule, hypercarnivory, and extinction in North American canids. Science 306, 101–104 (doi:10.1126/science.1102417) [DOI] [PubMed] [Google Scholar]
- Watkins W. A., Moore K. E., Tyack P.1985Investigations of sperm whale acoustic behaviors in the southeast Caribbean. Cetology 49, 1–15 [Google Scholar]
- Watkins W. A., Daher M. A., Fristrup K. M., Howald T. J., Notarbartolo di Sciara G.1993Sperm whales tagged with transponders and tracked underwater by sonar. Mar. Mam. Sci. 9, 55–67 (doi:10.1111/j.1748-7692.1993.tb00426.x) [Google Scholar]
- Whitehead H., Mann J.2000Female reproductive strategies in cetaceans. In Cetacean societies: field studies of dolphins and whales (eds Mann J., Connor R. C., Tyack P. L., Whitehead P. L. H.), pp. 219–246 Chicago, IL: University of Chicago Press [Google Scholar]