Abstract
Sexual reproduction relies on the recognition of conspecifics for breeding. Most experiments in birds have implicated a critical role for early social learning in directing subsequent courtship behaviours and mating decisions. This classical view of avian sexual imprinting is challenged, however, by studies of megapodes and obligate brood parasites, species in which reliable recognition is achieved despite the lack of early experience with conspecifics. By rearing males with either conspecific or heterospecific brood mates, we experimentally tested the effect of early social experience on the association preferences and courtship behaviours of two sympatrically breeding ducks. We predicted that redheads (Aythya americana), which are facultative interspecific brood parasites, would show a diminished effect of early social environment on subsequent courtship preferences when compared with their host and congener, the canvasback (Aythya valisineria). Contrary to expectations, cross-fostered males of both species courted heterospecific females and preferred them in spatial association tests, whereas control males courted and associated with conspecific females. These results imply that ontogenetic constraints on species recognition may be a general impediment to the initial evolution of interspecific brood parasitism in birds. Under more natural conditions, a variety of mechanisms may mitigate or counteract the effects of early imprinting for redheads reared in canvasback broods.
Keywords: brood parasitism, hybridization, sexual imprinting, life history, recognition systems
1. Introduction
Sexual reproduction requires the mixing of compatible genomes (Fisher 1958). In the face of costly hybridization, mating with conspecifics generally yields greater fitness, raising the question of how individuals recognize compatible partners for sexual reproduction (Salzen & Cornell 1968). Among birds, parental care is most often provided directly by the genetic parents, such that the phenotypic attributes of attending adults and siblings generally provide reliable information about the species-specific traits of suitable mates (Lorenz 1937; Irwin & Price 1999; ten Cate & Vos 1999; Slagsvold & Hansen 2001). In contrast, brush turkeys and their allies (Megapodidae) have no post-hatch parental care and obligate brood parasites are reared by other species (Hauber & Sherman 2001; Goth & Hauber 2004). As might be expected given their peculiar reproductive strategies, experimental work has demonstrated that the initial recognition of conspecifics is independent of early experience with social companions in young megapodes (Goth & Evans 2004) and in several obligate brood parasites (King & West 1977; Graham & Middleton 1989; Hauber et al. 2001; but see Soler & Soler 1999).
Experimental data also demonstrate, however, that there is no simple dichotomy of genetic versus experiential determination of species-recognition mechanisms (Bakker 1990; Goth & Hauber 2004). For example, the recognition of conspecifics and the development of effective courtship interactions may rely on both species-specific password-like cues and social learning in obligately parasitic brown-headed cowbirds (Molothrus ater; Freeberg et al. 1995; Hauber et al. 2001; Freed-Brown & White 2009) and indigobirds (Vidua spp.) (Payne et al. 2000). Likewise, a genetic predisposition towards conspecific communication signals may facilitate the preferential learning of species-specific cues in some non-parasitic songbirds (Dooling & Searcy 1982; Soha & Marler 2000; Woolley et al. 2010).
In comparison to the growing literature on species-recognition mechanisms in altricial brood parasitic birds, little is known about the role of early experience or the timing of sexual imprinting in precocial interspecific parasites (Weller 1968; Mattson & Evans 1974). Here, we examine the relative effects of social experience and species identity in two species of ducks (figure 1), the redhead (Aythya americana), which is a facultative interspecific brood parasite, and the canvasback (Aythya valisineria), which is the principal host of redhead parasitism (Sorenson 1991, 1997). The two species are broadly sympatric during the nesting season and redhead parasitism is a fundamental feature of both species' nesting biology, typically affecting more than 50 per cent of canvasback nests and accounting for the production of up to 50 per cent of redhead offspring (Sorenson 1998). We know of no other avian example in which a facultative brood parasite produces a significant portion of its offspring via interspecific parasitism. In contrast, canvasbacks sometimes parasitize each other but rarely parasitize other species (Weller 1959; Sorenson 1993). We completed a symmetrical cross-fostering experiment to test the hypothesis that the evolution of interspecific brood parasitism in redheads has been accompanied by changes in the ontogeny of species recognition as compared with the closely related and ecologically similar canvasback (Mattson & Evans 1974). We predicted that cross-fostered redhead males would recognize and court conspecific females irrespective of early social experience and would do so more reliably than experimentally ‘parasitic’ canvasback males reared with redheads.
Figure 1.
Comparison of head profiles of (a) female canvasback, (b) female redhead, (c) male canvasback, and (d) male redhead.
2. Material and methods
(a). Ontogenetic treatment of ducklings
Partially incubated eggs from the nests of captive redhead and canvasback females breeding in semi-natural enclosures at the Conservation and Research Center (CRC; Front Royal, Virginia, USA) and Patuxent Wildlife Research Center (Laurel, MD, USA), respectively, were collected and artificially incubated at CRC. The adults were first-generation captives hatched from redhead and canvasback eggs collected in Minnesota and North Dakota, respectively.
Upon hatching (28 May to 1 July 1991), ducklings were sexed by cloacal examination (Hochbaum 1942) and assigned to ‘broods’ comprising one male redhead, one male canvasback and two or three female redheads (redhead broods, n = 8), or two or three female canvasbacks (canvasback broods, n = 8). Thus, each brood included an ‘experimental’ male reared with heterospecific females and a ‘control’ male reared with females of his own species. It was not possible to form larger broods or rear ducklings with adult females owing to logistical constraints, but previous work has demonstrated that interaction with siblings contributes to both filial (Lickliter & Gottlieb 1986) and sexual (Schuetz 1965; Cheng et al. 1978) imprinting in Anas ducklings. Broods were reared in visual isolation in outdoor enclosures with ad libitum access to food, water and shelter. As they grew older, broods were moved to larger enclosures and were eventually combined into flocks (see below) in 12 × 18 m pens with grass and concrete ponds large enough for swimming and diving (4 × 4 ×0.5 m deep).
At approximately 137 days of age (range: 121–147), each brood was combined with another brood of the same majority (‘host’) species. In January, at approximately 213 days from hatch (range: 192–225), the broods were shuffled into four mixed-species flocks, each comprising four females of each species and two experimental males and two control males of each species. For each mixed-species flock, males and females were drawn from different broods, so that individual males had no familiarity with any of the individual females and vice versa.
(b). Spatial proximity trials
Association preferences of males were evaluated in a 4 × 4 m concrete pond with two 2 × 1 m compartments enclosed in chicken wire (figure 2a). After placing two female redheads in one compartment and two female canvasbacks in the other, the test male was released at the front centre of the pond. For 60 min, an observer in a blind 7 m in front of the pond used a custom computer program to continuously record the male's location and behavioural interactions with females. Each male was tested once in August or September (at an average age of 80 days, range: 68–90), and again in December (approx. 181 days, range: 160–195) and January (approx. 213 days, range: 192–225). Each male was tested for 30 min during a final set of trials in March (approx. 276 days, range: 254–294). Individual males were tested with a different set of four females in each trial and had no prior experience with any of the individual stimulus females. Each group of four females was used in two consecutive trials involving a control and experimental male of the same species. The species in each female compartment (left versus right) was alternated between pairs of trials and between successive trials for individual males. Only the March trials followed the formation of mixed-species flocks.
Figure 2.
(a) View from the observer's blind of the arena used for spatial proximity tests of male preferences for stimulus females. (b–d) Examples of courtship behaviours in experimental flocks. (b) Canvasback and redhead males simultaneously following and neck-stretching to a female redhead. (c) Mutual neck stretch by a canvasback pair. (d) Female redhead responding aggressively to a kink-necked call by a male canvasback.
(c). Behavioural observations
After the formation of mixed-species flocks, we observed the social behaviour and interactions of males during two kinds of observation sessions. Between late January and late February, we completed five 10 min focal animal observations per male, working through all 32 males in random order five times. Between late January and mid-March, we also observed each flock of 16 birds for 11 20 min sessions, scanning the flock and recording as many interactions and courtship displays as possible. During both types of observations, male courtship behaviours (follow, call, head throw, neck stretch; see Johnsgard 1965; Weller 1967) and the identity of the females to which they were directed were recorded (figure 2b–d). Evidence of pair formation, including consistent association with the same female during multiple observation sessions, mutual neck stretch displays and copulation solicitation (Johnsgard 1965), was also noted.
(d). Statistical analyses
Following prior work on the development of species-recognition systems in birds (Hauber 2003; Nelson & Marler 2005), we considered the individual males in our experiment, a few of which were siblings, as independent data points. The 16 males of each species originated from a total of 10 canvasback nests and eight redhead nests, with brothers generally assigned to different treatments. For choice tests, we used multivariate analysis of variance with month of trial, male species identity and fostering treatment (reared with redhead females versus reared with canvasback females) as main effects and the proportion of time spent in front of redhead females as the response variable. The initial model also included the three-way interaction term and all two-way interactions. Non-significant interactions and then non-significant main effects were removed from the model, retaining all main effects associated with a significant interaction term. Individual male identity was also retained as a random effect. We also used two-tailed, one-sample t-tests to determine whether the average association time for male treatment groups significantly differed from the random expectation of 50 per cent with each species. Our analysis of courtship behaviour in mixed flocks used the proportion of all displays directed towards redhead females as the response variable for each male. All proportional data were arcsine transformed (p′ = arcsin√p); raw values are used for illustrations.
3. Results
(a). Spatial proximity trials
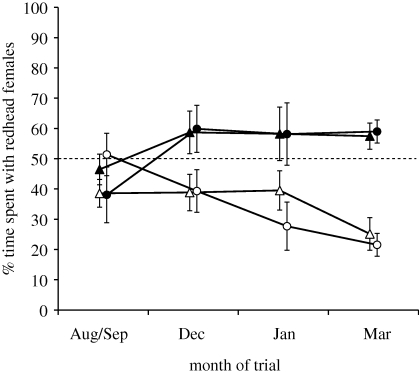
Ontogenetic treatment significantly and similarly affected male association preferences in both species (F1,30 = 43.435, p < 0.0001; figure 3). The proportion of time spent with redhead females in side-by-side choice tests was also affected by a significant interaction between trial date and ontogenetic treatment (F3,90 = 4.80, p = 0.0038; the main effects of trial date, F3,90 = 1.14, p = 0.34, and male species identity, F1,29 = 0.19, p = 0.67, were non-significant). Specifically, males reared in redhead broods spent more time with redhead females than did males reared in canvasback broods in the December (F1,117 = 8.87, p = 0.0035), January (F1,117 = 14.1, p = 0.0003) and March trials (F1,117 = 24.6, p < 0.00001; post hoc tests based on least-square means from the reduced model), whereas no significant treatment effect was observed in the first set of trials in August/September (F1,117 = 0.13, p = 0.71; figure 3). Based on individual male averages across the December, January and March trials, males of both species spent on average more than 50 per cent of their time with redhead females if reared in a redhead brood (redhead males: t = 2.52, p = 0.04; canvasback males: t = 4.69, p = 0.0022). Likewise, males of both species spent significantly more than 50 per cent of their time with canvasbacks if reared in a canvasback brood (redhead males: t = 4.56, p = 0.0026; canvasback males: t = 3.88, p = 0.0061).
Figure 3.
Outcome of spatial proximity trials (50% random expectation indicated by dashed line). Means (±s.e.) of raw values are presented for each treatment group (n = 8 males per group). Note that (% time with canvasbacks) = 100% − (% time spent with redheads). Lines with filled triangles, canvasbacks reared with redheads; lines with filled circles, redheads reared with redheads; lines with open triangles, canvasbacks reared with canvasbacks; lines with open circles, redheads reared with canvasbacks.
(b). Behavioural observations
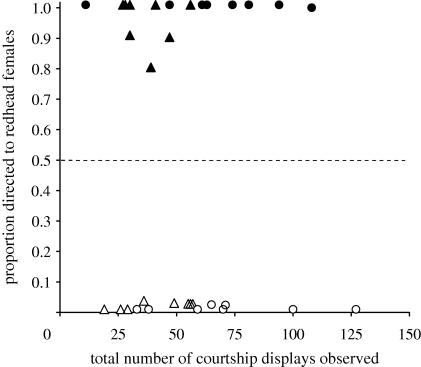
Individual males directed all or nearly all of their courtship behaviour towards females of one species, such that the mate preference of each male was unambiguous (figure 2b–d). Ontogenetic treatment strongly affected the proportion of male courtship behaviour directed towards redheads (F1,28 = 1333, p < 0.0001); males of both species courted almost exclusively redhead females if they were reared in redhead broods, and canvasback females if they were reared in canvasbacks broods (figure 4). In addition to this strong main effect, we detected a significant interaction between male species identity and ontogenetic treatment (F1,28 = 5.66, p = 0.024), reflecting slightly greater fidelity to a single preferred species in the courtship behaviour of redhead males (figure 4). If anything, this subtle species by treatment interaction runs counter to our a priori prediction that cross-fostered redheads would be more likely to court their own species than cross-fostered canvasbacks.
Figure 4.
Male courtship preferences as indicated by the proportion of all observed courtship displays directed towards redhead females during focal animal observations and flock watches (50% random expectation indicated by dashed line). Note that (% displays directed to canvasbacks) = 100% − (% displays directed to redheads). Filled triangles, canvasbacks reared with redheads; filled circles, redheads reared with redheads; open triangles, canvasbacks reared with canvasbacks; open circles, redheads reared with canvasbacks.
As evidenced by consistent association and mutual behaviours, most males paired with an individual female before the end of the experiment. Patterns of pair formation paralleled the results of our association tests and courtship data; all 25 males that paired successfully paired with a female of the same species as their original female brood mates (binomial test: p << 0.0001). Although pairing success did not differ significantly (χ2 = 4.9, d.f. = 3, p > 0.17), only four of eight canvasback males reared with redheads paired successfully, whereas seven of eight males in each of the other three treatment groups formed pairs. Females in at least six interspecific pairs were observed to solicit copulations (three pairs with male redheads and three pairs with male canvasbacks). Latency to pair formation, as measured by the earliest date that a female clearly engaged in mutual behaviour with each male, was significantly greater for cross-fostered males (mean = 26 February) than for males reared with females of their own species (mean = 6 February; Mann–Whitney U = 134.5, p < 0.002).
4. Discussion
Consistent with a critical role for early social experience in the development of species recognition in birds (Lorenz 1937), our experiment shows that the species identity of brood mates can determine subsequent spatial association and courtship behaviours in male redheads and canvasbacks. Contrary to our initial prediction, the reproductive strategy of facultative interspecific brood parasitism in redheads has not lead to the evolution of any detectable differences between redheads and canvasbacks in the development or mechanisms of species recognition, at least as measured under the conditions of this experiment. Males of both species spent more time with and courted almost exclusively females of the species with which they were reared, demonstrating that misdirected courtship behaviour is a potential consequence of cross-fostering in redheads, which are frequent interspecific parasites, and also in canvasbacks, which are rarely, if ever, reared by other species.
It is important to note, however, that the rearing environment ducklings experienced in our study differed from typical conditions in nature in several ways. Perhaps most importantly, males were exposed to females of one species for an extended period of time, from hatch (28 May–1 July) through the subsequent January. In nature, ducklings begin flying at approximately 60 days of age (Mowbray 2002; Woodin & Michot 2002), after which there is considerable movement and mixing of birds in advance of autumn migration. The design of this experiment was intentionally extreme, as we sought in this initial study to produce conditions under which early social experience was most likely to influence mate preferences. Had a clear difference between the two species been found, with cross-fostered redheads showing a preference for their own species, it would have been strong evidence that sexual imprinting mechanisms had been modified in redheads to cope with the predicament of being reared by another species. Additional experiments are needed to test whether redheads are similar to some altricial interspecific brood parasites, including Molothrus cowbirds (Freeberg et al. 1995) and Vidua finches (Payne et al. 1998), in which sensitive periods for learning critical conspecific traits are ontogenetically delayed or more malleable than in non-parasitic species (e.g. Hansen et al. 2008).
While it is not known whether early social experience influences migration in redheads, the spatial overlap between redhead and canvasback populations in winter is substantially less than during the breeding season. Redheads reach their highest density along the Gulf Coast, particularly the Laguna Madre of southern Texas and northern Mexico, whereas canvasbacks are more numerous on the Atlantic and Pacific coasts (Weller 1964; Mowbray 2002). If migration direction has a strong genetic component in ducks, as in some other birds (Berthold & Helbig 2008), or if inherited differences in habitat preference (e.g. Grosch 2004) mediate the composition of flocks prior to migration in late summer/early autumn, parasitic redheads may join wintering flocks comprised primarily of other redheads. Experience with redheads during autumn and winter might reverse initial preferences, as observed in some obligate brood parasites (Goth & Hauber 2004).
An additional factor that might mitigate the effects of cross-fostering for parasitic redheads is the variable species composition of natural canvasback broods. Parasitized canvasback nests typically receive an average of three or more redhead eggs (e.g. Sorenson 1991), such that canvasback broods often have two or more redhead ducklings. Multiple parasitic ducklings might provide relevant and more salient imprinting stimuli for each other (Kruijt et al. 1983), particularly if there is an innate predisposition towards imprinting on own species (Dooling & Searcy 1982; Soha & Marler 2000; Woolley et al. 2010). Also in contrast to natural conditions, however, our experimental broods were not cared for by an adult female. While the relative importance of siblings versus brood hen as sources of sexual imprinting stimuli is unknown and might differ between redheads and canvasbacks, an adult female canvasback present from hatch may provide a more effective imprinting stimulus than brood mates, which only gradually attain adult form.
Our experiment suggests that social ontogeny has the potential to constrain species-recognition abilities and might reduce the lifetime fitness of parasitic male redhead ducklings through misdirected mating effort. This would in turn alter the relative fitness benefits of alternative female reproductive tactics (i.e. parasitic egg laying, nesting and mixed tactics) (Sorenson 1991; McRae 1998). While there are a few observations of male redheads courting female canvasbacks in nature (Hochbaum 1944; Weller 1967; M.D.S. 1991, 1992, unpublished data), hybridization between the two species is rare; Haramis (1982) encountered only two hybrid males in the course of handling over 13 000 male canvasbacks in Chesapeake Bay. Thus, under natural conditions, most male redheads hatched from parasitic eggs apparently develop a preference for conspecific females.
Female mate choice also may help to explain the rarity of hybridization between these species in natural populations, as canvasback females, essentially all of which are reared in canvasback broods, would be expected to reject the courtship advances of malimprinted male redheads. In our captive study, latency to pair formation was greater for cross-fostered males, but interspecific pairs eventually formed and females of both species solicited copulations from heterospecific mates. With an even sex ratio and only half of the males in our experimental flocks courting their own species, however, females in our experiment had limited options. This is in marked contrast to the strongly male-biased adult populations observed in both redheads and canvasbacks (Mowbray 2002; Woodin & Michot 2002). Females in our experiment also experienced an equal number of male redheads and canvasbacks throughout their post-hatch development, perhaps broadening their template for an acceptable mate (Reeve 1989). Recent studies provide examples of both learned (e.g. Witte & Caspers 2006; Freed-Brown & White 2009) and genetically determined (Saether et al. 2007) mate preferences in female birds. Owing to limitations on the number of subjects available, our study focused on male behaviour only; the development of mate preferences in female waterfowl requires additional study (ten Cate 1985).
The results of our experiment suggest that sexual imprinting mechanisms may represent an important general constraint on the evolution of both facultative and obligate interspecific brood parasitism in birds (Slagsvold & Hansen 2001). The rarity of interspecific courtship and hybridization in natural redhead and canvasback populations, however, suggests that a variety of factors may help to mitigate the strong effect of rearing environment that we observed here. Additional experiments simulating a more natural progression of changing social environments through autumn and winter and exploring the timing and reversibility of the sexual imprinting process might reveal differences between the two species that are associated with the evolution of interspecific parasitism in redheads.
Acknowledgements
Animal protocols were approved by the scientific and veterinary staff of the National Zoological Park and followed guidelines of the Animal Behaviour Society and Ornithological Council.
We are indebted to Michael Haramis, Joe Kotok, the Patuxent Wildlife Research Center and Agassiz National Wildlife Refuge for crucial logistical support of this work. Carolyn Emerick, Chris Kirkpatrick, Steve Leathery, Warren Lynch, Rick Mazza, Dan Sprague and Lisa Ware assisted with care and observations of the ducks. Jeff DaCosta, Darren Irwin and two anonymous reviewers provided valuable comments on an earlier version of the manuscript. Funding was provided to M.D.S. and S.R.D. by the Smithsonian Institution and the Friends of the National Zoo. M.E.H. was supported by awards from Yale University.
References
- Bakker T. C. M.1990Genetic variation in female mating preference. Neth. J. Zool. 40, 617–642 (doi:10.1163/156854290X00136) [Google Scholar]
- Berthold P., Helbig A. J.2008The genetics of bird migration: stimulus, timing, and direction. Ibis 134, 35–40 (doi:10.1111/j.1474-919X.1992.tb04731.x) [Google Scholar]
- Cheng K. M., Shoffner R. N., Phillips R. E., Lee F. B.1978Mate preference in wild and domesticated (game-farm) mallards (Anas platyrhynchos): I. Initial preference. Anim. Behav 26, 996–1003 (doi:10.1016/0003-3472(78)90088-X) [Google Scholar]
- Dooling R. J., Searcy M. H.1982Early perceptual selectivity in the swamp sparrow. Dev. Psychobiol. 13, 499–506 (doi:10.1002/dev.420130508) [DOI] [PubMed] [Google Scholar]
- Fisher R. A.1958The genetical theory of natural selection. New York, NY: Dover [Google Scholar]
- Freeberg T. M., King A. P., West M. J.1995Social malleability in cowbirds (Molothrus ater artemisiae): species and mate recognition in the first 2 years of life. J. Comp. Psych. 109, 357–367 [Google Scholar]
- Freed-Brown G., White D. J.2009Acoustic mate copying: female cowbirds attend to other females' vocalizations to modify their song preferences. Proc. R. Soc. B 276, 3319–3325 (doi:10.1098/rspb.2009.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth A., Evans C. S.2004Social responses without early experience: Australian brush-turkey chicks use specific visual cues to aggregate with conspecifics. J. Exp. Biol. 207, 2199–2208 (doi:10.1242/jeb.01008) [DOI] [PubMed] [Google Scholar]
- Goth A., Hauber M. E.2004Ecological approaches to species recognition in birds through studies of model and non-model species. Ann. Zool. Fennici 41, 823–842 [Google Scholar]
- Graham D. S., Middleton A. L. A.1989Conspecific recognition by juvenile brown-headed cowbirds. Bird Behav. 8, 14–22 [Google Scholar]
- Grosch K.2004Hybridization between redstart Phoenicurus phoenicurus and black redstart P. ochruros, and the effect on habitat exploitation. J. Avian Biol. 35, 217–223 (doi:10.1111/j.0908-8857.2004.03128.x) [Google Scholar]
- Hansen B. T., Johannessen L. E., Slagsvold T.2008Imprinted species recognition lasts for life in free-living great tits and blue tits. Anim. Behav. 75, 921–927 (doi:10.1016/j.anbehav.2007.07.023) [Google Scholar]
- Haramis G. M.1982Records of redhead × canvasback hybrids. Wilson Bull. 94, 599–602 [Google Scholar]
- Hauber M. E.2003Lower begging responsiveness of host versus parasitic brown-headed cowbird (Molothrus ater) nestlings is related to species identity but not to early social experience. J. Comp. Psych. 117, 24–30 [DOI] [PubMed] [Google Scholar]
- Hauber M. E., Sherman P. W.2001Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci. 10, 609–616 (doi:10.1016/S0166-2236(00)01916-0) [DOI] [PubMed] [Google Scholar]
- Hauber M. E., Russo S. A., Sherman P. W.2001A password for species recognition in a brood parasitic bird. Proc. R. Soc. Lond. B 268, 1041–1048 (doi:10.1098/rspb.2001.1617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochbaum H. A.1942Sex and age determination of waterfowl by cloacal examination. Trans. N. Am. Wildl. Nat. Resour. Conf. 7, 299–307 [Google Scholar]
- Hochbaum H. A.1944Canvasback on a prairie marsh. Washington, DC: American Wildlife Institute [Google Scholar]
- Irwin D. E., Price T.1999Sexual imprinting, learning and speciation. Heredity 82, 347–354 (doi:10.1038/sj.hdy.6885270) [DOI] [PubMed] [Google Scholar]
- Johnsgard P. A.1965Handbook of waterfowl behavior. Ithaca, NY: Cornell University Press [Google Scholar]
- King A. P., West M. J.1977Species identification in the North American cowbird: appropriate responses to abnormal song. Science 195, 1002–1004 (doi:10.1126/science.841321) [DOI] [PubMed] [Google Scholar]
- Kruijt J. P., ten Cate C. J., Meeuwissen G. B.1983The influence of siblings on the development of sexual preferences of male zebra finches. Dev. Psychobiol. 16, 233–239 (doi:10.1002/dev.420160309) [DOI] [PubMed] [Google Scholar]
- Lickliter R., Gottlieb G.1986Visually imprinted maternal preference in ducklings is redirected by social-interaction with siblings. Dev. Psychobiol. 19, 265–277 (doi:10.1002/dev.420190312) [DOI] [PubMed] [Google Scholar]
- Lorenz K.1937The companion in the bird's world. Auk. 54, 245–273 [Google Scholar]
- Mattson M. E., Evans R. M.1974Visual imprinting and auditory-discrimination learning in young of canvasback and semiparasitic redhead (Anatidae). Can. J. Zool. 52, 421–427 (doi:10.1139/z74-051) [Google Scholar]
- McRae S. B.1998Relative reproductive success of female moorhens using conditional strategies of brood parasitism and parental care. Behav. Ecol. 9, 93–100 [Google Scholar]
- Mowbray T. B.2002Canvasback (Aythya valisineria). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; (doi:10.2173/bna.659). [Google Scholar]
- Nelson D. A., Marler P.2005Do bird nestmates learn the same songs? Anim. Behav. 69, 1007–1010 (doi:10.1016/j.anbehav.2005.01.001) [Google Scholar]
- Payne R. B., Payne L. L., Woods J. L.1998Song learning in brood parasitic indigobirds Vidua chalybeata: song mimicry of their host species. Anim. Behav. 55, 1537–1553 (doi:10.1006/anbe.1997.0701) [DOI] [PubMed] [Google Scholar]
- Payne R. B., Payne L. L., Woods J. L., Sorenson M. D.2000Imprinting and the origin of parasite–host species associations in brood-parasitic indigobirds Vidua chalybeata. Anim. Behav. 59, 69–81 (doi:10.1006/anbe.1999.1283) [DOI] [PubMed] [Google Scholar]
- Reeve H. K.1989The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435 [Google Scholar]
- Saether S. A., et al. 2007Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95–97 (doi:10.1126/science.1141506) [DOI] [PubMed] [Google Scholar]
- Salzen E. A., Cornell J. M.1968Self-perception and species recognition in birds. Behaviour 30, 44–65 [DOI] [PubMed] [Google Scholar]
- Schuetz F.1965Sexuelle Pragung bei Anatiden. [Sexual imprinting among the Anatidae]. Z. Tierpsychol. 22, 50–103 [PubMed] [Google Scholar]
- Slagsvold T., Hansen B. T.2001Sexual imprinting and the origin of obligate brood parasitism in birds. Am. Nat. 158, 354–367 (doi:10.1086/321994) [DOI] [PubMed] [Google Scholar]
- Soha J. A., Marler P.2000A species-specific acoustic cue for selective song learning in the white-crowned sparrow. Anim. Behav. 60, 297–306 (doi:10.1006/anbe.2000.1499) [DOI] [PubMed] [Google Scholar]
- Soler M., Soler J. J.1999Innate versus learned recognition of conspecifics in great spotted cuckoos Clamator glandarius. Anim. Cogn. 2, 97–102 (doi:10.1007/s100710050029) [Google Scholar]
- Sorenson M. D.1991The functional significance of parasitic egg laying and typical nesting in redhead ducks: an analysis of individual behaviour. Anim. Behav. 42, 771–796 (doi:10.1016/S0003_3472(05)80122-8) [Google Scholar]
- Sorenson M. D.1993Parasitic egg-laying in canvasbacks: frequency, success, and individual behavior. Auk 110, 57–69 [Google Scholar]
- Sorenson M. D.1997Effects of intra- and interspecific brood parasitism on a precocial host, the canvasback Aythya valisineria. Behav. Ecol. 8, 153–161 [Google Scholar]
- Sorenson M. D.1998Patterns of parasitic egg laying and typical nesting in redhead and canvasback ducks. In Parasitic birds and their hosts (eds Rothstein S. I., Robinson S. K.), pp. 357–375 New York, NY: Oxford University Press [Google Scholar]
- ten Cate C.1985On sex differences in sexual imprinting. Anim. Behav. 33, 1310–1317 [Google Scholar]
- ten Cate C., Vos D. R.1999Sexual imprinting and evolutionary processes in birds: a reassessment. Adv. Study Behav. 28, 1–31 [Google Scholar]
- Weller M. W.1959Parasitic egg laying in the redhead (Aythya americana) and other North American Anatidae. Ecol. Monogr. 29, 333–365 [Google Scholar]
- Weller M. W.1964Distribution and migration of the redhead. J. Wildl. Manage. 28, 64–103 [Google Scholar]
- Weller M. W.1967Courtship of the redhead (Aythya americana). Auk. 84, 544–559 [Google Scholar]
- Weller M. W.1968The breeding biology of the parasitic black-headed duck. Living Bird 7, 169–208 [Google Scholar]
- Witte K., Caspers B.2006Sexual imprinting on a novel blue ornament in zebra finches. Behaviour 143, 969–991 [Google Scholar]
- Woodin M. C., Michot T. C.2002Redhead (Aythya americana). In The birds of North America online (ed. Poole A.), Ithaca, NY: Cornell Lab of Ornithology; (doi:10.2173/bna.695). [Google Scholar]
- Woolley S. M. N., Hauber M. E., Theunissen F. E.2010Developmental experience alters information coding in auditory midbrain and forebrain neurons. Dev. Neurobiol. 7, 235–252 (doi:10.1002/dneu.20783) [DOI] [PMC free article] [PubMed] [Google Scholar]