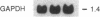Abstract
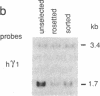
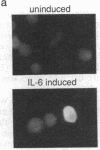
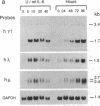
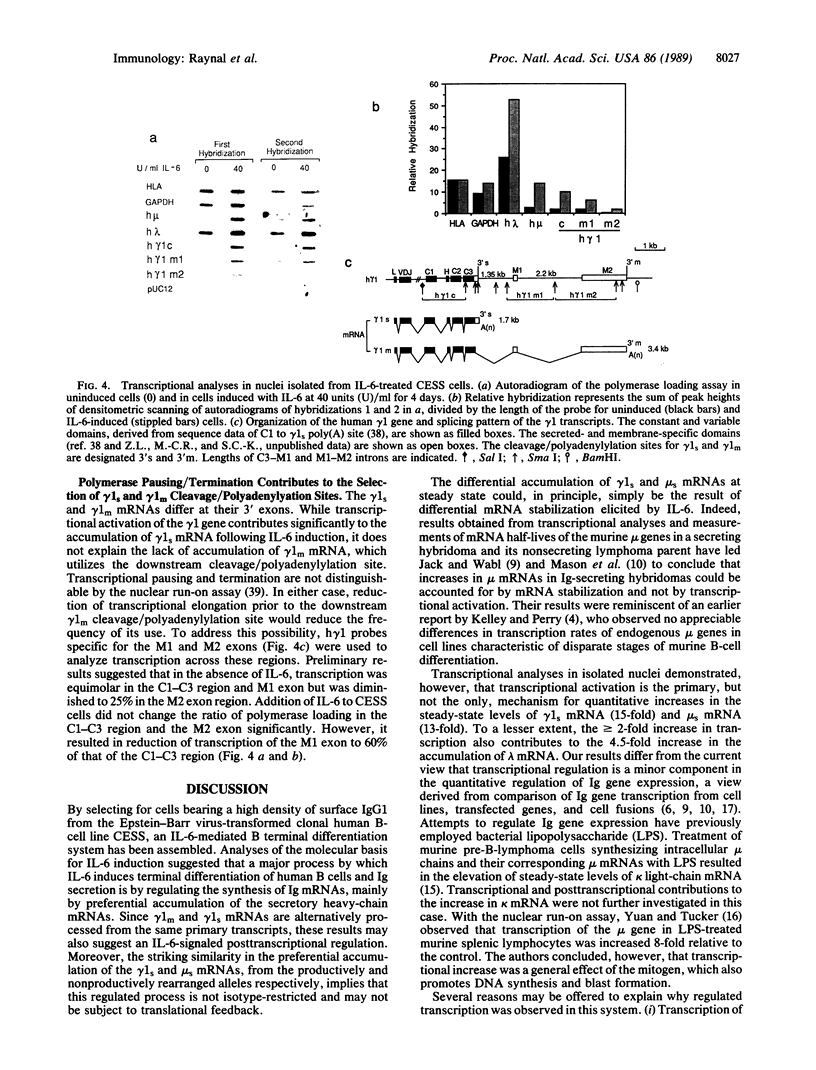
The molecular mechanism by which interleukin 6 (IL-6) induces terminal differentiation of B cells was investigated in a subpopulation of the clonal human B-lymphoblastoid cell line CESS selected for high density of cell surface IgG1. Induction of CESS cells with IL-6 resulted in a 15-fold preferential accumulation of secreted-specific gamma 1 (gamma 1s) mRNA but not of the alternatively processed membrane-specific gamma 1 (gamma 1m) mRNA. Similarly, microseconds mRNA but not the microns mRNA of the nonproductively rearranged mu heavy-chain allele was also increased. Accompanying the differential accumulation of gamma 1s mRNA was a 4.5-fold increase in lambda light-chain mRNA, leading to secretion of IgG1. Analyses of transcription in isolated nuclei demonstrated that transcriptional activation was the primary mechanism for quantitative increase of immunoglobulin mRNAs (5.5-fold for gamma 1 and mu and at least 2-fold for lambda). Since polymerase loading is diminished by 75% before reaching the downstream gamma 1m polyadenylylation site in CESS cells, irrespective of IL-6 induction, transcriptional pausing/termination appears intrinsic and contributes to the selection of gamma 1s and gamma 1m polyadenylylation sites in activated B cells. Furthermore, differential mRNA stabilization is likely to contribute to the alteration of the gamma 1s/gamma 1m mRNA ratio at IL-6 induction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Briskin M., Kuwabara M. D., Sigman D. S., Wall R. Induction of kappa transcription by interferon-gamma without activation of NF-kappa B. Science. 1988 Nov 18;242(4881):1036–1037. doi: 10.1126/science.3143155. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Connelly S., Manley J. L. A functional mRNA polyadenylation signal is required for transcription termination by RNA polymerase II. Genes Dev. 1988 Apr;2(4):440–452. doi: 10.1101/gad.2.4.440. [DOI] [PubMed] [Google Scholar]
- Danner D., Leder P. Role of an RNA cleavage/poly(A) addition site in the production of membrane-bound and secreted IgM mRNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8658–8662. doi: 10.1073/pnas.82.24.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Ellison J. W., Berson B. J., Hood L. E. The nucleotide sequence of a human immunoglobulin C gamma1 gene. Nucleic Acids Res. 1982 Jul 10;10(13):4071–4079. doi: 10.1093/nar/10.13.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Pedersen E., Logan J., Shenk T., Darnell J. E., Jr Transcription termination within the E1A gene of adenovirus induced by insertion of the mouse beta-major globin terminator element. Cell. 1985 Apr;40(4):897–905. doi: 10.1016/0092-8674(85)90349-6. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Kunkel H. G., Halper J. P., Harris S. R. Induction of in vitro differentiation and immunoglobulin synthesis of human leukemic B lymphocytes. J Exp Med. 1978 Dec 1;148(6):1570–1578. doi: 10.1084/jem.148.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Guise J. W., McDevitt M. A., Tucker P. W., Nevins J. R. Relative position and strengths of poly(A) sites as well as transcription termination are critical to membrane versus secreted mu-chain expression during B-cell development. Genes Dev. 1987 Jul;1(5):471–481. doi: 10.1101/gad.1.5.471. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Fu S. M., Yu D. T., Wang C. Y., Halper J. P., Kunkel H. G. The nature of the stimulatory cell in human allogeneic and autologous MLC reactions; role of isolated IgM-bearing B cells. J Immunol. 1979 Oct;123(4):1497–1503. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Guise J. W., Lim P. L., Yuan D., Tucker P. W. Alternative expression of secreted and membrane forms of immunoglobulin mu-chain is regulated by transcriptional termination in stable plasmacytoma transfectants. J Immunol. 1988 Jun 1;140(11):3988–3994. [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. Immunoglobulin mRNA stability varies during B lymphocyte differentiation. EMBO J. 1988 Apr;7(4):1041–1046. doi: 10.1002/j.1460-2075.1988.tb02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Perry R. P. Transcriptional and posttranscriptional control of immunoglobulin mRNA production during B lymphocyte development. Nucleic Acids Res. 1986 Jul 11;14(13):5431–5447. doi: 10.1093/nar/14.13.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H., Taga T., Akira S., Kishi H., Miki Y., Saiki O., Yamamura Y., Kishimoto T. Effect of B cell differentiation factor (BCDF) on biosynthesis and secretion of immunoglobulin molecules in human B cell lines. J Immunol. 1985 Feb;134(2):990–995. [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Kobrin B. J., Milcarek C., Morrison S. L. Sequences near the 3' secretion-specific polyadenylation site influence levels of secretion-specific and membrane-specific IgG2b mRNA in myeloma cells. Mol Cell Biol. 1986 May;6(5):1687–1697. doi: 10.1128/mcb.6.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Falck-Pedersen E., Darnell J. E., Jr, Shenk T. A poly(A) addition site and a downstream termination region are required for efficient cessation of transcription by RNA polymerase II in the mouse beta maj-globin gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8306–8310. doi: 10.1073/pnas.84.23.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderious A., Chen-Kiang S. Pausing and premature termination of human RNA polymerase II during transcription of adenovirus in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5931–5935. doi: 10.1073/pnas.81.19.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. O., Williams G. T., Neuberger M. S. The half-life of immunoglobulin mRNA increases during B-cell differentiation: a possible role for targeting to membrane-bound polysomes. Genes Dev. 1988 Aug;2(8):1003–1011. doi: 10.1101/gad.2.8.1003. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Nelson K. J., Haimovich J., Perry R. P. Mode of regulation of immunoglobulin mu- and delta-chain expression varies during B-lymphocyte maturation. Cell. 1984 Feb;36(2):329–338. doi: 10.1016/0092-8674(84)90226-5. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Hall B. Cell-specific expression of secreted versus membrane forms of immunoglobulin gamma 2b mRNA involves selective use of alternate polyadenylation sites. Mol Cell Biol. 1985 Oct;5(10):2514–2520. doi: 10.1128/mcb.5.10.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kishimoto T., Miki Y., Kuritani T., Kaieda T., Yoshizaki K., Yamamura Y. T cell-replacing factor- (TRF) induced IgG secretion in a human B blastoid cell line and demonstration of acceptors for TRF. J Immunol. 1981 Aug;127(2):412–416. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. Regulated production of mu m and mu s mRNA requires linkage of the poly(A) addition sites and is dependent on the length of the mu s-mu m intron. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8883–8887. doi: 10.1073/pnas.83.23.8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. The regulated production of mu m and mu s mRNA is dependent on the relative efficiencies of mu s poly(A) site usage and the c mu 4-to-M1 splice. Mol Cell Biol. 1989 Feb;9(2):726–738. doi: 10.1128/mcb.9.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Milstein C. P. Human immunoglobulin heavy chain genes: evolutionary comparisons of C mu, C delta and C gamma genes and associated switch sequences. Nucleic Acids Res. 1981 Sep 25;9(18):4509–4524. doi: 10.1093/nar/9.18.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Ruether J. E., Maderious A., Lavery D., Logan J., Fu S. M., Chen-Kiang S. Cell-type-specific synthesis of murine immunoglobulin mu RNA from an adenovirus vector. Mol Cell Biol. 1986 Jan;6(1):123–133. doi: 10.1128/mcb.6.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Noma T., Honjo T. Rearranged immunoglobulin heavy chain variable region (VH) pseudogene that deletes the second complementarity-determining region. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5194–5198. doi: 10.1073/pnas.81.16.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Ueda S., Obata M., Nikaido T., Nakai S., Honjo T. Structure of human immunoglobulin gamma genes: implications for evolution of a gene family. Cell. 1982 Jun;29(2):671–679. doi: 10.1016/0092-8674(82)90183-0. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Proudfoot N. Alpha-thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3' end processing in the human alpha 2 globin gene. EMBO J. 1986 Nov;5(11):2915–2922. doi: 10.1002/j.1460-2075.1986.tb04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Transcriptional regulation of the mu-delta heavy chain locus in normal murine B lymphocytes. J Exp Med. 1984 Aug 1;160(2):564–583. doi: 10.1084/jem.160.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]