Abstract
Previous studies have suggested that the ability to inhabit harsh environments may be linked to advanced learning traits. However, it is not clear if individuals express such traits as a consequence of experiencing challenging environments or if these traits are inherited. To assess the influence of differential selection pressures on variation in aspects of cognition, we used a common garden approach to examine the response to novelty and problem-solving abilities of two populations of black-capped chickadees (Poecile atricapillus). These populations originated from the latitudinal extremes of the species's range, where we had previously demonstrated significant differences in memory and brain morphology in a multi-population study. We found that birds from the harsh northern population, where selection for cognitive abilities is expected to be high, significantly outperformed conspecifics from the mild southern population. Our results imply differences in cognitive abilities that may be inherited, as individuals from both populations were raised in and had experienced identical environmental conditions from 10 days of age. Although our data suggest an effect independent of experience, we cannot rule out maternal effects or experiences within the nest prior to day 10 with our design. Nevertheless, our results support the idea that environmental severity may be an important factor in shaping certain aspects of cognition.
Keywords: behavioural flexibility, environmental harshness, learning, neophobia, natural selection, problem-solving
1. Introduction
Animals living in energetically challenging (e.g. unpredictable and/or harsh) environments should benefit from advanced cognitive abilities (Dukas 1998; Shettleworth 1998, 2009). One aspect of advanced cognition often examined is behavioural flexibility or learning (Reader 2003), also termed plasticity or innovation (sensu Lefebvre et al. 1997). Rather than a fixed response to a given stimulus, behavioural flexibility allows for the expression of a variety of different behavioural outcomes under different contexts based on previous experiences (Dukas 1998; Reader 2003). Such flexibility seems to be adaptive and therefore has strong ecological and evolutionary relevance (Price et al. 2003; Biernaskie et al. 2009). For example, various aspects of learning or behavioural flexibility may play key roles in the success of biological invasions (e.g. Sol et al. 2002, 2005a; Martin & Fitzgerald 2005), the occupation of anthropogenic environments (e.g. Echeverria & Vassallo 2008), as well as in some of the basic ecological differences between populations and species (e.g. Greenberg 1983, 1984, 1990; Liker & Bokony 2009). However, the ultimate source of the production of these cognitive differences is poorly understood. Can all individuals of the species express advanced learning traits simply as a consequence of experiencing a challenging environment, or are these traits an inherited product of differential selection pressures in these environments?
Evidence for the relationship between increased learning capabilities and harsh environments has been observed in numerous taxa. For example, in an intraspecific comparison, Martin & Fitzgerald (2005) found that an actively invading population of house sparrows (Passer domesticus) had reduced levels of neophobia when compared with a long-established population. Moreover, several large-scale comparative analyses have shown positive relationships between living in anthropogenic habitats (i.e. those characterized by novelty and complexity) and feeding innovation in birds (Lefebvre et al. 1997; Sol et al. 2005a) as well as mammals (Lefebvre et al. 2004). Thus, taxa that more often show innovative foraging tactics appear to be those that are more successful at invading novel environments. These patterns have also been shown to correlate with brain size (or forebrain size), suggesting a morphological basis to the variance in cognition (Lefebvre et al. 1997; Sol et al. 2005a). Unfortunately, the comparative studies that make up the bulk of the large-scale evidence for the environmental complexity–cognition relationship (e.g. Lefebvre et al. 1997, 2004; Sol et al. 2002, 2005a) only present a ‘snapshot’ of traits influenced heavily by selection pressures in evolutionary history, which are generally unknown. These comparisons cannot be used to address the influence of specific experiences and population-level selection pressures. To address the effects of selection pressures on cognition, it may be more effective to examine differences in populations that are currently experiencing potentially different selection regimes.
The relationship between harsh environments and cognitive abilities is not limited to learning. Theory suggests that food-caching birds living in more harsh climates should cache more and have better spatial memory than those in more mild climates (Krebs et al. 1989; Sherry et al. 1989; Pravosudov & Grubb 1997; Pravosudov & Lucas 2001). Indeed, previous work supports the basis for this relationship between environmental harshness and memory, as birds from higher latitudes (i.e. more harsh climates) had better spatial memory (Pravosudov & Clayton 2002), probably owing to increased demands for accurate cache retrieval. Moreover, these behavioural differences seem to have a neurological basis. We have previously demonstrated a gradation of hippocampal attributes across populations, where hippocampal size and neuron number decrease with declining latitude (Pravosudov & Clayton 2002; Roth & Pravosudov 2009). Overall, then, there is strong theoretical and empirical evidence supporting a relationship between environmental severity and one aspect of cognition—spatial memory—along a latitudinal gradient in food-caching birds.
We extend this logic from memory for cache retrieval to learning. Because of the drastically lower temperatures, shorter winter day length and greater precipitation (snow cover) in the northern when compared with the southern parts of their range (see Roth & Pravosudov 2009), northern populations of food-caching birds must eat more to fulfil their daily energy requirements (Pravosudov & Grubb 1997; Pravosudov & Lucas 2001), but have less daylight in which to do it and encounter more obstacles (snow) that conceal food. Traits that increase food acquisition rates and increase the accuracy and speed of decision-making should be adaptive under these time-limited and energetically demanding conditions (Lefebvre et al. 1997; Pravosudov & Grubb 1997; McLean 2001; Pravosudov & Lucas 2001; Hills 2006; see also Sol et al. 2005b). It would be naive to presuppose that selection should work on a single behavioural/morphological trait. Instead, selection may result in the enhancement of numerous cognitive traits produced in various ways depending upon the selection pressures in the population. The ultimate effect would be that individuals with such traits might have a higher probability of recovering food, and hence of survival during the winter. Indeed, birds from the more harsh northern populations tend to have larger telencephalic regions relative to body mass (a trait associated with enhanced cognitive abilities; Lefebvre et al. 1997, 2004; Sol et al. 2002, 2005a) than those in the south. For example, based on our previous study, the telencephalon volume of chickadees from northern populations are larger than those from southern populations (ordered heterogeneity test: rspc = 0.774, p < 0.010; methods from Rice & Gaines 1994; based on data from Roth & Pravosudov 2009). Interestingly, body mass showed the opposite trend with birds from southern populations being significantly heavier than those from the north (ordered heterogeneity test: rspc = 0.899, p < 0.001; methods from Rice & Gaines 1994; based on mass data in Roth & Pravosudov 2009). Note that we do not intend to make causal statements about the overall or relative size of the brain and cognitive ability, but only point out their association. See Roth et al. (2010) for a detailed discussion of these topics.
The goal of this study was to examine the environmental complexity–cognition relationship using a common garden approach in a food-caching model system. To avoid the problems associated with comparing different species (see Macphail 1996), yet to assess two aspects of the cognitive abilities of different populations experiencing differential selection pressures, we examined the response to novel object and problem-solving tasks of hand-raised black-capped chickadees (Poecile atricapillus). We have chosen to work with the two populations at the latitudinal extremes of the species's range—Alaska (AK) and Kansas (KS)—as they showed the largest morphological differences (in both relative hippocampal volume and in telencephalon volume) along the previously demonstrated multi-population gradient of environmental harshness (Roth & Pravosudov 2009). Thus, it is from these most geographically and climatically distinct populations that we would expect to see the largest differences in learning, should they exist.
Our prediction was that birds from the climatically harsh northern population (AK), which had the larger brains in our previous work, would outperform conspecifics from the more mild southern population (KS), which had smaller brains. Given that these individuals were raised in the laboratory and had experienced identical environmental conditions (at least since day 10 after hatching), difference between these populations may suggest a component to learning that may potentially be the result of differential selection pressures within their respective populations.
2. Material and methods
(a). Collection sites
Black-capped chickadees (Poecile atricapillus) were collected during late May and early June 2009 from nests at the latitudinal extremes of their range (Anchorage, AK: 61°10′ N, 149°53′ W; Manhattan, KS: 39°08′ N, 96°37′ W). Two chicks were taken from each nest, with siblings used in the two different tests (see below). At both sites, we collected chicks from both natural nests and those in nest boxes (constructed from wood and PVC) in a wide range of habitats from anthropogenic to very ‘natural’. Average temperatures during the respective collection periods were as follows: AK: max = 18.3 ± 1.1°C, min = 7.2 ± 0.4°C; KS: max = 24.8 ± 1.4°C, min = 8.7 ± 1.1°C. The average day length during collection was 1149 min for AK and 856 min for KS.
Chicks were approximately 10 days old at the time of collection and were hand-raised indoors on site until they were approximately 18 days old. To retain consistency in the hand-raising environment between the two sites, T.C.R., assisted by the same technician, worked at both locations. In addition, the indoor environment was as similar as possible. Temperature was maintained between 21 and 23°C and lighting conditions were similar and on the same schedule (15 : 9, L : D), beginning the first day of collection. Chicks were transported to the University of Nevada, Reno, via ground (from KS) and air (from AK), in the same containers (wood nesting boxes; see below). We attempted to transport the chicks as rapidly as possible and control the environment as much as possible during transportation.
(b). Hand-rearing and housing
All chicks were fed a diet of: Orlux Handmix formula (Versele-Laga, Deinze, Belgium); wax worms (Pyralidae sp.); meal worms (Tenebrio molitor); phoenix worms (Hermetia illucens); crickets (Acheta domesticus); a slurry consisting of dog food (Canidae, San Luis Obispo, CA), cat food (Natura EVO, Santa Clara, CA), Orlux Insect Patee Premium and Orlux Handmix; and nut powder pellets consisting of pulverized pine nuts (Pinus koraiensis), peanuts (Arachis hypogaea), sunflower seeds (Helianthus sp.) and Insect Patee. Food types were systematically cycled throughout the day and were offered every 20 min during daylight hours, so the chicks from both locations ate at approximately the same frequency and for the same time period during the day. Food (same diet as above less Handmix, all in whole form, plus Roudybush Crumbles and Purina Game Starter) and water were provided ad libitum after birds reached independence (approx. 30–35 days after hatch).
During hand-rearing, chicks were housed in groups of four to six individuals in 17 × 17 × 24 cm wooden boxes filled with sawdust to simulate nest cavities. At the fledgling stage (approx. 18–20 days after hatch), chicks were housed as sibling pairs in 120 × 42 × 60 cm wire cages. At the dispersal stage (approx. 60 days after hatching), all birds were moved into a solitary, permanent arrangement in 60 × 42 × 60 cm wire cages. Sex was estimated via wing chord measurements. To reduce aggression between males, birds were placed in an M/F/F/M arrangement within a row of four cages (all within visual contact). The populations were systematically partitioned as AK/KS/AK/KS within these rows, with siblings located in different rooms.
Beginning in early August and until mid-October, the light cycle gradually shifted (approx. 0.5 h per week) to 9 : 15 (L : D). All tests occurred on this light cycle. Tests began when birds were approximately five months old. However, because of the asynchronous breeding of the two populations, the AK birds were on average slightly less than three weeks younger than the KS individuals (average age at testing: AK, 20.8 weeks; KS, 23.7 weeks).
(c). Learning tests
We focused on two aspects of learning: problem-solving and the response to novelty. Innovative problem-solving is a goal-oriented form of learning, whereby an animal encounters a novel problem with a known goal or reward (often food) and must perform a series of novel steps to achieve the goal (Dukas 1998; e.g. Webster & Lefebvre 2001; Keagy et al. 2009). The speed required to solve the task is frequently used as an indication of the animal's ability to learn (e.g. Carlier & Lefebvre 1996). Habituation to a non-threatening novel object can also be viewed as a form of this type of learning. Although a novel object may initially be perceived as risky, through the process of examination the animal learns that the object is not a threat. As learning is inherent to both of these processes, the performance on these tasks probably reflects selection on the ability to learn (sensu Dukas 1998).
(i). Problem-solving test
Problem-solving tests were conducted approximately 2 h after lights-on from 4–9 November 2009. This test was performed with 24 birds (12 AK, 12 KS) from different nests. The problem-solving test involved removing galvanized steel washers (3.5 cm diameter, 1.5 cm diameter hole; roughly equal to the mass of the birds, approx. 15 g) covered with clear 3M acetate from a 3 × 5 grid of 1.5 cm wells drilled into a wooden board (40 × 18 cm) containing wax worms. All birds had been fully habituated to the boards (total duration of prior exposure > 30 h) in their home cages. Birds were habituated to the washers for 8 h the day prior to the test. During this habituation, washers were secured to the boards (adjacent to, but not covering, the wells) with double-sided tape so that they could be touched, but not moved. Wax worms were offered in 8 of the 15 wells, and habituation was considered successful if birds took all wax worms (which occurred in all cases).
A pre-trial control occurred approximately 1 h before lights-off the day prior to the test. During this control, one wax worm was placed on each board, and we recorded the latency to remove the worm (300 s max). The boards were then removed. The following morning, birds were allowed to feed for 1 h after lights-on, and then deprived of food for 1 h before the problem-solving trial. The boards were introduced into the cages with one wax worm in each of the same eight wells as during the habituation period, but now washers covered all 15 wells. The birds could see the worms, but could only retrieve a worm by moving the washer. The birds could not puncture the acetate.
The trials occurred in the home cages, with birds in the cage row (i.e. two AK, two KS) tested simultaneously. All trials were observed remotely with a live video feed to another room and recorded using Sony DCR-SR300 and DCR-SR47 digital video cameras on tripods. We recorded the latency (in seconds) to land on the board and the latency to take the first worm (3600 s max). We considered the problem solved when the bird had taken a worm. To control for motivation, a post-trial control was performed. After 3600 s, one wax worm was placed on top of the board and we recorded the latency to take the worm (300 s max).
(ii). Novelty test
Novelty tests were conducted 0.5 h after lights-on from 18–21 October 2009. This test was performed with 25 birds (12 AK, 13 KS) from different nests (siblings of the birds in the problem-solving test). All birds were deprived of food 0.5 h prior to lights-off the evening before testing and until after the test the following day (approx. 2 h after lights-on). Birds were recorded using video as in the previous test. We recorded the cumulative latency to approach and remove food (a single wax worm) from a control (usual type 300 ml circular stainless steel) and novel feeder (usual type feeder modified with paint and protruding bolts) in an A : B : A (control : treatment : control) design. The feeders were placed in the centre of the home cage floor and the birds were recorded for 300 s (during the controls) and 1800 s (during the treatment). We recorded the latency (in seconds) to touch the feeder, to sit on the feeder and to take the worm from the feeder for both control and treatment trials.
(d). Statistical analyses
Repeated-measures analysis of variance tests were used to test the overall models for population (AK and KS) and within-subject effects (controls and treatment). In addition, we used planned comparisons to confirm that controls were not significantly different within populations. We also compared the habituation time in the novelty test (treatment minus pre-control times) and the time to solve the problem (latency to take the worm minus latency to land on the board) with t-tests. Data were log-transformed for all analyses; raw data are presented in figures for clarity (means ± s.e. are reported; α = 0.05).
3. Results
(a). Problem-solving
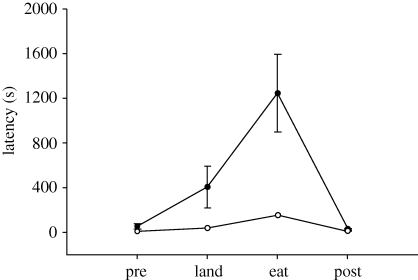
Problem-solving was assessed by the time required to remove a transparent, weighted cover from a well containing a food item. The motivation to land on the testing apparatus was assessed with pre- and post-treatment trials of a single wax worm placed on the board. The individuals from AK landed on the testing board, uncovered the well and removed the wax worm significantly faster than those from KS (population: F1,22 = 32.888, p < 0.001; within-subject: F2,46 = 181.825, p < 0.001; figure 1). There was no effect of motivation or habituation to the experimental set-up, as there were no differences between pre- and post-treatment times in either population (AK: p = 0.092; KS: p = 0.320). The time to solve the task (latency to eat minus latency to land) was significantly longer for the KS population (t22 = −5.340, p < 0.001).
Figure 1.
Latency to the completion of a problem-solving task (removing a weighted, transparent cover from a well containing a wax worm) in black-capped chickadees. Pre- and post-treatment exposure of a wax worm on the testing apparatus controlled for motivation, the tendency to feed on the floor of the cage and habituation to the testing set-up. Filled circles, Kansas; open circles, Alaska.
(b). Response to novelty
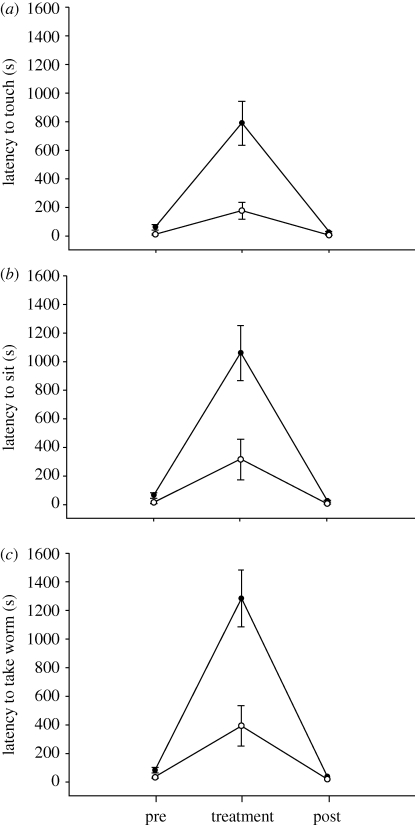
The response to novelty was assessed by the latency to approach, sit on and finally take a food item from a novel feeder. Motivation was controlled with both pre- and post-treatment exposures of the same food item in a familiar feeder. There was a large and significant difference between the two populations in the latency to approach (population: F1,23 = 13.691, p = 0.001; within-subject: F2,46 = 92.724, p < 0.001), sit on (population: F1,23 = 12.359, p = 0.002; within-subject: F2,46 = 104.262, p < 0.001) and take the food item from (population: F1,23 = 11.528, p = 0.002; within-subject: F2,46 = 142.876, p < 0.001; figure 2) the novel feeder . The individuals from the KS population took significantly longer than those from the AK population to approach (t23 = −3.349, p = 0.003), sit on (t23 = −2.972, p = 0.007) and take the wax worm from (t23 = −3.159, p = 0.004) the novel feeder relative to the pre-treatment control. Comparisons of the pre- and post-treatment controls showed that motivation, the tendency to feed on the floor of the cage and/or the testing set-up did not play a substantive role in the results (touch: AK, p = 0.181; KS, p = 0.019; sit: AK, p = 0.146; KS, p = 0.014; take: AK, p = 0.027; KS, p = 0.005; figure 2). Although we did see small, yet significant, differences in some pre/post comparisons, the latencies were lower in the post-trials in all cases. This suggests some habituation in both populations within the study, but these differences are very minor relative to the overall treatment effect (figure 2).
Figure 2.
The response to novelty of black-capped chickadees as assessed by the latency to (a) approach, (b) sit on and (c) take a wax worm from a novel feeder. Pre- and post-treatment exposure of a wax worm in a familiar feeder controlled for motivation and the tendency to feed on the floor of the cage. Filled circles, Kansas; open circles, Alaska.
4. Discussion
We found significant differences in problem-solving and neophobia between two populations originating from drastically different environmental conditions. Our results suggest that selection has produced variance in the ability to learn (sensu Dukas 1998), as the chickadee population from the more harsh environment (AK) were faster in problem-solving and less neophobic relative to their southern conspecifics (KS) despite being raised in identical environments since age 10 days post-hatch. Thus, there seems to be the possibility of an inherited component (genetic and/or maternal effects) to the speed of problem-solving and habituation to novelty within this species, although we could not rule out any experiential or environmental effects taking place prior to day 10, when blind chicks were in a dark nest cavity. As both of these traits are aspects of learning (Dukas 1998; Reader 2003; Lefebvre et al. 2004), our results suggest that selection may favour enhanced learning abilities in more extreme climates, at least in this species.
We found differences in both of our measures of learning, suggesting that there may be a difference in the selection pressures for these aspects of learning between these populations from extremely different climates. This does not imply, however, that all aspects of learning will necessarily be different or that all aspects of cognition will necessarily be superior in the more northern populations. One very broad interpretation of our results could be that selection might enhance all types of cognitive qualities in more harsh climates. However, Pravosudov & Clayton (2002) reported differences in spatial memory but not colour memory in a two-population comparison in this same species. This may suggest that the selection on cognition is quite complex, and differing selection pressures in different populations must be considered thoroughly (Dukas 1998; Shettleworth 1998). On the other hand, the test for colour memory by Pravosudov & Clayton (2002) was purposefully simplistic (a single colour) as the goal of that study was to test for motivation to perform a spatial task and not to test for differences in memory for colour per se. Still, it is not to say that selection should enhance all cognition. Rather, selection should enhance cognitive abilities that may affect fitness under specific environmental conditions. So, in the case of the food-caching chickadee, selection for spatial memory (which is important for successful cache recovery) has been shown to be particularly important for the northern population, but presumably under less selection in the southern population (Pravosudov & Clayton 2002). Selection for colour memory, however, may not be a function of climate in this system as both populations seem to use it similarly (Pravosudov & Clayton 2002). According to the adaptive specialization hypothesis, specific differences observed between the populations should be a function of the specific selection regimes experienced by those populations.
Given that we have very specific predictions based on our previous study of multiple populations along a latitudinal gradient (Roth & Pravosudov 2009), we emphasize that these results are not likely to be due to chance alone. Our selection of the two populations in this study was based on our previous multi-population studies of the relationship between environmental harshness, memory and brain morphology, as well as theoretical differences (e.g. Pravosudov & Grubb 1997; Pravosudov & Lucas 2001; Pravosudov & Clayton 2002; Roth & Pravosudov 2009). As we had already shown the large-scale pattern between the environment and the brain, our next objective was to examine the relevance of individual experiences by comparing the differences in learning capabilities between populations with maximal differences in brain morphology. Although the inclusion of additional populations would have increased the scope of our comparison, we were limited by logistical and ethical constraints.
Our data suggest the possibility of an inherited effect on learning; however, there are two important caveats to this interpretation. First, owing to the logistical difficulty of hand-raising very small birds, we collected chicks from the nest at approximately 10 days of age. Thus, it is possible that experiences during early development (from hatching to day 10) could have produced the observed results. We think that this is unlikely as it is around day 10 that black-capped chickadees' eyes begin to open, and any experiences would have occurred in a dark nesting cavity. Thus, the visual conditions that the two populations experienced were probably very similar. However, we cannot rule out the possibility that thermal differences or differences in parental feeding had an effect on our results. Second, we cannot rule out the possibility of maternal effects. It is possible that our observed differences could have been due to physiological decisions made by the mother prior to egg laying. For example, stressed mothers may deposit increased levels of corticosterone into their eggs to ‘prepare’ the young for a challenging environment (Chin et al. 2009). This possibility, in particular, may explain the differences in response to novelty. It is possible that exposure to corticosterone during development may produce a response in specific brain regions such as the amygdala, which may affect neophobia responses (Burns et al. 1996). However, we argue that these maternal effects, should they exist, are likely to be the result of selection as well. Thus, it is still not the individual chicks' experiences that produce such effects. Moreover, differences in corticosterone would not clearly explain the differences in problem-solving abilities between the populations. Still, as a consequence of these caveats, we interpret our results as evidence of a possible inherited effect, since maternal effects are an aspect of inheritance, but suggest that future studies consider breeding experiments to fully dissociate these factors.
Although one possible explanation of our results is a heritable component to cognition, we acknowledge that complex behaviours are probably the result of both inheritance and experience. For example, Greenberg (1983, 1984) supports an experiential explanation for specific responses such as neophobia. These studies suggest that differences in foraging niches themselves may be the product of ontogenetic experiences produced in part by neophobia. Experiences may still be important and may produce variation in addition to that generated through inheritance. It is possible that the ultimate differences in foraging niches created by experience as realized in Greenberg's (1984) study may be the result of genetic differences in response to novelty between different species. In other words, using Greenberg's approach, some of the ecological differences between generalists and specialists may be due to genetic difference in response to novelty. Ontogenetic experience may then be the mechanism by which a particular species is ‘introduced’ to (and maintained in) its habitat. This will require further study.
Overall, our data suggest a large difference in some aspects of the cognitive abilities of black-capped chickadees that may be due in part to differential selection pressures within different environments. Based on theory and our previous work comparing brains of chickadees from multiple populations across a gradient of environmental harshness, we suggest that these results are probably due to the climatic severity of the environments. A complementary explanation is that the observed differences are not the result of climatic harshness per se, but of range expansion. Several studies suggest an important role of behavioural flexibility in the success of biological invasions (Martin & Fitzgerald 2005). It is possible that the AK population has more recently (on an evolutionary scale) been involved in range expansion, at least since the retreat of the glaciers during the last Ice Age (Harrap & Quinn 1995). Thus, rather than an effect of current climatic conditions, the AK population may possess faster learning skills owing to their ancestors' recent range expansion. It is important to point out that the range expansion and environmental harshness hypotheses are not mutually exclusive; both could be relevant to our study system. Both of these hypotheses, nevertheless, imply a selective component to the differences between the populations, suggesting that these cognitive traits are important, adaptive and probably the product of natural selection rather than individual experiences alone.
Acknowledgements
We are grateful to E. Horne, B. Van Slyke, K. Hampton and Kansas State University's Konza Prairie Biological Station for their assistance at our Kansas site. We are also indebted to C. Handel, V. Jorgensen, M. Pajot and the United States Geological Survey's Alaska Science Center for their assistance at our Alaska site. C. Freas and J. Ream assisted in nestling collection. C. Freas and G. Hanson assisted in animal care and maintenance. We are also grateful to K. Otter for logistical advice, and to our many colleagues (too numerous to mention) for advice on hand-rearing chickadees. This research was funded in part by grants from the National Science Foundation (IOB-0615021) and the National Institutes of Health (MH079892 and MH076797). Birds were collected under United States Fish and Wildlife (MB022532), Alaska (09-020), Kansas (SC-039-2009) and Nevada (S30942) permits. This research was supervised by the University of Nevada, Reno, IACUC (protocol no. A05/06-35), and followed all federal and local guidelines for the use of animals in research.
References
- Biernaskie J. M., Walker S. C., Gegear R. J.2009Bumblebees learn to forage like Bayesians. Am. Nat. 174, 413–423 (doi:10.1086/603629) [DOI] [PubMed] [Google Scholar]
- Burns L. H., Annett L., Kelley A. E., Everitt B. J., Robbins T. W.1996Effects of lesions to amygdale, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behav. Neurosci. 110, 60–73 (doi:10.1037/0735-7044.110.1.60) [DOI] [PubMed] [Google Scholar]
- Carlier P., Lefebvre L.1996Differences in individual learning between group-foraging and territorial Zenaida doves. Behaviour 133, 1197–1207 (doi:10.1163/156853996X00369) [Google Scholar]
- Chin E. H., Love O. P., Verspoor J. J., Williams T. C., Rowley K., Burness G.2009Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proc. R. Soc. B 276, 499–505 (doi:10.1098/rspb.2008.1294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukas R.1998Evolutionary ecology of learning. In Cognitive ecology (ed. Dukas R.), pp. 129–174 Chicago, IL: University of Chicago Press [Google Scholar]
- Echeverria A. I., Vassallo A. I.2008Novelty responses in a bird assemblage inhabiting an urban area. Ethology 114, 616–624 (doi:10.1111/j.1439-0310.2008.01512.x) [Google Scholar]
- Greenberg G. R.1983The role of neophobia in determining the degree of foraging specialization in some migrant warblers. Am. Nat. 122, 444–453 (doi:10.1086/284148) [Google Scholar]
- Greenberg G. R.1984Neophobia in the foraging site selection of a neotropical migrant bird—an experimental study. Proc. Natl Acad. Sci. USA 81, 3778–3780 (doi:10.1073/pnas.81.12.3778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg G. R.1990Feeding neophobia and ecological plasticity—a test of the hypothesis with captive sparrows. Anim. Behav. 39, 375–379 (doi:10.1016/S0003-3472(05)80884-X) [Google Scholar]
- Harrap S., Quinn D.1995Chickadees, tits, and treecreepers. Princeton, NJ: Princeton University Press [Google Scholar]
- Hills T. T.2006Animal foraging and the evolution of goal-directed cognition. Cogn. Sci. 30, 3–41 [DOI] [PubMed] [Google Scholar]
- Keagy J., Savard J.-F., Borgia G.2009Male satin bowerbird problem-solving ability predicts mating success. Anim. Behav. 78, 809–817 (doi:10.1016/j.anbehav.2009.07.011) [Google Scholar]
- Krebs J. R., Sherry D. F., Healy S. D., Perry V. H., Vaccarino A. L.1989Hippocampal specialization of food-storing birds. Proc. Natl Acad. Sci. USA 86, 1388–1392 (doi:10.1073/pnas.86.4.1388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L., Whittle P., Lascaris E., Finkelstein A.1997Feeding innovations and forebrain size in birds. Anim. Behav. 53, 549–560 (doi:10.1006/anbe.1996.0330) [Google Scholar]
- Lefebvre L., Reader S. M., Sol D.2004Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233–246 (doi:10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- Liker A., Bokony V.2009Larger groups are more successful in innovative problem solving in house sparrows. Proc. Natl Acad. Sci. USA 106, 7893–7898 (doi:10.1073/pnas.0900042106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphail E. M.1996Cognitive function in mammals: the evolutionary perspective. Cogn. Brain Res. 3, 279–290 (doi:10.1016/0926-6410(96)00013-4) [DOI] [PubMed] [Google Scholar]
- Martin L. B., Fitzgerald L. A.2005A taste for novelty in invading house sparrows Passer domesticus. Behav. Ecol. 16, 702–707 (doi:10.1093/beheco/ari044) [Google Scholar]
- McLean A. N.2001Cognitive abilities—the result of selective pressures on food acquisition? Appl. Anim. Behav. Sci. 71, 241–258 (doi:10.1016/S0168-1591(00)00181-7) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V., Clayton N. S.2002A test of the adaptive specialization hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav. Neurosci. 116, 515–522 (doi:10.1037/0735-7044.116.4.515) [PubMed] [Google Scholar]
- Pravosudov V. V., Grubb T. C.1997Management of fat reserves and food caches in tufted titmice (Parus bicolor) in relation to unpredictable food supply. Behav. Ecol. 8, 332–339 (doi:10.1093/beheco/8.3.332) [Google Scholar]
- Pravosudov V. V., Lucas J. R.2001A dynamic model of short-term energy management in small food-caching and non-caching birds. Behav. Ecol. 12, 207–218 (doi:10.1093/beheco/12.2.207) [Google Scholar]
- Price T. D., Quarnstrom A., Irwin D. E.2003The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440 (doi:10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader S. M.2003Innovation and social learning: individual variation and brain evolution. Anim. Biol. 53, 147–158 (doi:10.1163/157075603769700340) [Google Scholar]
- Rice W. R., Gaines S. D.1994The ordered-heterogeneity family of tests. Biometrics 50, 746–752 (doi:10.2307/2532788) [Google Scholar]
- Roth T. C., Pravosudov V. V.2009Hippocampal volume and neuron numbers increase along a gradient of environmental harshness: a large-scale comparison. Proc. R. Soc. B 276, 401–405 (doi:10.1098/rspb.2008.1184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T. C., Brodin A., Smulders T. V., LaDage L. D., Pravosudov V. V.2010Is bigger always better? A critical appraisal of the use of volumetric analysis in the study of the hippocampus. Phil. Trans. R. Soc. B 365, 915–931 (doi:10.1098/rstb.2009.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry D. F., Vaccarino A. L., Buckenham K., Herz R. S.1989The hippocampal complex of food-storing birds. Brain Behav. Evol. 34, 308–317 (doi:10.1159/000116516) [DOI] [PubMed] [Google Scholar]
- Shettleworth S. J.1998Cognition, evolution, and behaviour. Oxford, UK: Oxford University Press [Google Scholar]
- Shettleworth S. J.2009The evolution of comparative cognition: is the snark still a boojum? Behav. Process. 80, 210–217 (doi:10.1016/j.beproc.2008.09.001) [DOI] [PubMed] [Google Scholar]
- Sol D., Timmermans S., Lefebvre L.2002Behavioural flexibility and invasion success in birds. Anim. Behav. 63, 495–502 (doi:10.1006/anbe.2001.1953) [Google Scholar]
- Sol D., Duncan R. P., Blackburn T. M., Cassey P., Lefebvre L.2005aBig brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465 (doi:10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol D., Stirling D. G., Lefebvre L.2005bBehavioral drive or behavioral inhibition in evolution: subspecific diversification in holarctic passerines. Evolution 59, 2677–2699 [PubMed] [Google Scholar]
- Webster S. J., Lefebvre L.2001Problem solving and neophobia in a columbiform-passeriform assemblage in Barbados. Anim. Behav. 62, 23–32 (doi:10.1006/anbe.2000.1725) [Google Scholar]