Abstract
Steroid hormones have similar functions across vertebrates, but circulating concentrations can vary dramatically among species. We examined the hypothesis that variation in titres of corticosterone (Cort) and testosterone (T) is related to life-history traits of avian species. We predicted that Cort would reach higher levels under stress in species with higher annual adult survival rates since Cort is thought to promote physiological and behavioural responses that reduce risk to the individual. Conversely, we predicted that peak T during the breeding season would be higher in short-lived species with high mating effort as this hormone is known to promote male fecundity traits. We quantified circulating hormone concentrations and key life-history traits (annual adult survival rate, breeding season length, body mass) in males of free-living bird species during the breeding season at a temperate site (northern USA) and a tropical site (central Panama). We analysed our original data by themselves, and also combined with published data on passerine birds to enhance sample size. In both approaches, variation in baseline Cort (Cort0) among species was inversely related to breeding season length and body mass. Stress-induced corticosterone (MaxCort) also varied inversely with body mass and, as predicted, also varied positively with annual adult survival rates. Furthermore, species from drier and colder environments exhibited lower MaxCort than mesic and tropical species; T was lowest in species from tropical environments. These findings suggest that Cort0, MaxCort and T modulate key vertebrate life-history responses to the environment, with Cort0 supporting energetically demanding processes, MaxCort promoting survival and T being related to mating success.
Keywords: life-history trade-offs, steroid hormone, latitude, bird, reproduction, survival
1. Introduction
Major functions of many steroid hormones, including corticosterone and testosterone, are conserved among vertebrates, but circulating concentrations nonetheless vary widely across species. For example, among birds, baseline corticosterone concentrations (0.7–57 ng ml−1) and breeding season levels of testosterone (0.3–13 ng ml−1) range over almost two orders of magnitude (Garamszegi et al. 2005, 2008; Bókony et al. 2009). Stress-induced concentrations of corticosterone can vary by almost one order of magnitude (12–103 ng ml−1; Bókony et al. 2009). Previous comparative studies have identified several ecological factors that might account for this variation. Differences among species in stress-induced corticosterone levels during the breeding season have been related to variation in parental care and the reproductive value of a particular brood; testosterone variation has been related mainly to breeding season length, which depends primarily on latitude and altitude (Wingfield et al. 1995; Goymann et al. 2004; Garamszegi et al. 2008; Bókony et al. 2009). Less well understood is the degree to which observed hormone concentrations mediate life-history responses to environmental variation (Ricklefs & Wikelski 2002).
Life histories encompass suites of correlated traits such as longevity, fecundity, development rate and metabolic intensity (Stearns 1992; Charnov 1993). Vertebrate life histories have been aligned on a slow–fast continuum where species with ‘fast’ life-history strategies exhibit rapid development, intense metabolism, high reproductive rate and reduced longevity, while species with ‘slow’ life-history strategies show the opposite pattern, with slow development, low metabolism, low reproductive rate and increased longevity (Promislow & Harvey 1990; Ricklefs 2000; Jones et al. 2008; but see e.g. Lancaster et al. 2008). For example, American goldfinches (Carduelis tristis) from North America typically lay clutches of at least five eggs during a short breeding season of about two months, but have an annual adult survival rate of only 35 per cent (MAPS database; http://www.birdpop.org/nbii/NBIIHome.asp).
By contrast, spotted antbirds (Hylophylax n. naevioides) from a tropical lowland forest in Panama typically lay two eggs per clutch during an extended, five-month breeding season and have an annual adult survival rate of about 60 per cent (Willis 1972; Brawn et al. 1999). A slow–fast continuum suggests that various trade-offs constrain covariation between life-history traits across taxa such that, for example, fecundity and longevity cannot be maximized at the same time (Stearns 1992; Charnov 1993). Such trade-offs might result from the need to allocate limited resources such as energy, nutrients or time to competing processes, and/or from a genetic linkage of life-history traits (e.g. Roff & Fairbairn 2007).
Hormones are involved in life-history trade-offs as systemic signals that establish functional links among traits, produce pleiotropic effects, and regulate key behavioural and physiological transitions in organisms (Ketterson & Nolan 1992; Finch & Rose 1995; Sinervo & Svensson 1998; Zera & Harshman 2001; Hau 2007). Indeed, both corticosterone and testosterone regulate foundational processes in organisms. Glucocorticoid hormones such as corticosterone (the main glucocorticoid in birds) at baseline concentrations have primarily metabolic functions, such as regulating energy intake, storage and mobilization (e.g. Sapolsky et al. 2000; Landys et al. 2006). Additionally, when an individual experiences acute adverse conditions (for example, when being chased by a predator), glucocorticoid secretion can increase within 2–3 min, reaching peak concentrations within 30–60 min (Wingfield et al. 1998; Sapolsky et al. 2000). Such stress-induced increases in glucocorticoid concentrations redirect behaviour and physiology towards immediate survival functions and towards reducing risk to self (inducing an ‘emergency life-history stage’; Wingfield et al. 1998). These effects of acute stress-induced corticosterone include an increase in locomotor activity, inhibition of reproductive behaviour and enhancement of immune function (Dhabhar & McEwen 1997; Breuner et al. 1998; Sapolsky et al. 2000). Androgens, such as testosterone, generally support physiological and behavioural processes that enhance fecundity in male vertebrates. Circulating concentrations of testosterone in males of many vertebrate species are elevated during the breeding season (especially during the courtship and mating phase), when they promote the expression of secondary sexual characters and sperm maturation, as well as sexual (vocalization, courtship, copulation) and aggressive (mate-guarding, territorial) behaviours (Knobil & Neill 1998). Experimentally elevated testosterone concentrations typically increase reproductive output, home-range sizes and extra-pair fertilization rates, while interfering with parental care and depressing male survival rates (e.g. Marler & Moore 1988; Dufty 1989; Reed et al. 2006; McGlothlin & Ketterson 2008).
Experimental studies support the hypothesis that corticosterone and testosterone, through their actions on key behavioural, physiological and morphological processes, are involved in mediating life-history trade-offs (recent reviews: Oliveira 2004; Reed et al. 2006; Miles et al. 2007). Studies also suggest that steroid hormones regulate behavioural and physiological traits in a dose-dependent fashion (e.g. Casto et al. 2001; Buchanan et al. 2003; Husak et al. 2007), although there are exceptions (e.g. Balthazart & Hendrick 1979; Dallman et al. 1993; Breuner et al. 1998). This experimental evidence prompted us to ask whether variation in plasma concentrations of corticosterone and testosterone is associated with variation among species in life-history traits. We supposed that longer-lived species, which should invest more in the preservation of self (but see Ricklefs 2010), reach higher stress-induced corticosterone concentrations during acute stressful events than shorter-lived species (see also Wingfield et al. 1995; Ricklefs & Wikelski 2002). Conversely, we predicted that shorter-lived species, which should invest more heavily in reproduction than in survival, would exhibit higher testosterone concentrations during the courtship and mating phases of the breeding season compared with longer-lived species.
We assessed the generality of these predictions concerning the relationship between hormonal and life-history traits in a comparative analysis based on two datasets. First, in an original study using standardized methods, we determined circulating baseline corticosterone, stress-induced corticosterone and testosterone concentrations during the breeding season in males of a variety of free-living New World bird species from a north temperate and a near-equatorial region. At the same time, we also recorded original data (or compiled published data) on several life-history traits, including breeding season length (a measure of renesting potential and thus risk a parent will assume for a particular brood) and adult survival rate. Second, to confirm the patterns we identified with the analysis of our own data, we added published data, primarily from Bókony et al. (2009), Goymann et al. (2004) and Garamszegi et al. (2005, 2008), on hormones and life-history traits for passerine species, and repeated the analyses.
2. Material and methods
(a). Bird capture and blood sampling
We obtained blood samples from adult males of passerine species during the breeding season at the Kellogg Biological Station, Michigan, USA (latitude 42° N; end of June to end of July, 2003–2005) and in Soberania National Park, Panama (9° N; early March to early August, 2003 and 2004). Individuals were caught by passive mist netting and were immediately removed from the net. Individuals for which a sample (less than 50 µl blood) could be obtained within 3 min of capture were used to determine baseline corticosterone concentrations (Cort0). Those same individuals were then subjected to a standard capture–restraint protocol (Wingfield et al. 1982) during which they were held in opaque cloth bags and bled again at 30 and 60 min after capture (approx. 30 µl blood; MaxCort). Testosterone (T) samples were usually collected from a different set of individuals (owing to the small size of most species) within 10–30 min after capture (approx. 80–200 µl blood). Here we focus on peak breeding (or ‘seasonal maximum’) testosterone concentrations as the ‘highest average concentrations of testosterone that have been reported in free-living and actively breeding’ males of species (Goymann et al. 2004) and not the degree of social modulation in testosterone in response to stimulation from conspecifics. We were unable to determine the specific breeding stage of individuals, but focused our data collection on males in reproductive condition as evidenced, for example, by enlarged cloacal protuberances. After the initial blood sampling, birds were individually marked with a numbered aluminium ring and were weighed. Blood samples were kept on ice for less than 4 h until they were centrifuged at 500g for at least 4 min. The plasma supernatant was frozen at −20°C until analysis. Plasma samples were transported to Princeton University on dry ice under permits from Panamanian and US authorities. All samples were analysed in the laboratory of M.H. at Princeton University.
(b). Hormone analyses
Cort concentrations were determined in 21 sets of direct radioimmunoassays (e.g. Perfito et al. 2006). Multiple Cort samples from one individual (i.e. stress series) were included in the same assay to reduce variation within an individual; Cort samples of different individuals were randomly assigned to assays. Average recovery after extraction of samples with dichloromethane was (mean ± 1 s.e.m.) 74.5 ± 1.5 per cent. The lower limit of detection of our assays was at 2.35 ± 0.37 ng ml−1. Average intra-assay coefficient of variation was determined in two assays and was 13.8 per cent; average inter-assay coefficient of variation was 21.9 per cent. T concentrations were determined via column chromatography followed by radioimmunoassay (for assay details see Wikelski et al. 2003) in a total of four assays. Average recovery after extraction was 51.5 ± 7.3 per cent (lower than for corticosterone because of the preceding column chromatography). The lower limit of detection of our T assays was at 0.12 ± 0.03 ng ml−1. Average intra-assay coefficient of variation was not determined, but ranges between 2 and 16 per cent in our laboratory for this kind of assay (e.g. Gill et al. 2007). Average inter-assay coefficient of variation was 24.6 per cent. Inter-assay variation in both Cort and T assays was unusually high, probably because we conducted a large number of assays spread over different times of year and over multiple years. However, because individuals from different species were included in different assays at random, and only species averages were included in the statistical analyses, we believe effects of inter-assay variation to be minor.
Hormone samples below the limit of detection of our assays were set at the minimum detection limit for statistical analyses. T could not be analysed for all species in our original study because of small sample volumes, so we included additional published data from our own laboratory for some species (Wikelski et al. 2003), supplemented with values for a few species from the literature when authors had used radioimmunoassay techniques similar to ours (electronic supplementary material, table S1). Goymann et al. (2004) did not find any significant between-laboratory effects on published T data.
Table 1.
Results from AIC analyses for baseline corticosterone (Cort0), stress-induced corticosterone (MaxCort) and testosterone, using different datasets. The model with the highest weight (i.e. the most parsimonious model; high. model w.) for each hormonal trait and dataset is indicated, with contributing variables included in brackets, and the overall r2 for this model. Variable weights (var. weight, 0–1) are the sum of the importance values for each of the models including the independent variable (variables with high values are highlighted in bold). Raw regression coefficients (raw regr. coeff.) are given along with standardized regression coefficients (stand. regr. coeff.), the latter being normalized by the standard deviations of the observations for the dependent and independent variables.
| hormonal trait | Cort0 |
MaxCort |
testosterone |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dataset | own (n = 24) | own (n = 20) | own (n = 9) | |||||||||||||||
| high. model w. | 0.23 (breeding season), r2 = 0.11 |
0.22 (mass and survival), r2 = 0.41 |
0.74 (breeding season), r2 = 0.79 |
|||||||||||||||
| variable | mass | surv. | breed. | envt. | mass | surv. | breed. | envt. | mass | surv. | breed. | envt. | ||||||
| var. weight | 0.27 | 0.28 | 0.48 | 0.44 | 0.79 | 0.53 | 0.42 | 0.38 | 0.08 | 0.08 | 0.97 | 0.11 | ||||||
| raw regr. coeff. | −0.07 | −0.29 | −0.06 | −0.18 | −0.46 | 1.12 | −0.06 | 0.17 | 0.13 | −0.74 | −0.43 | −0.28 | ||||||
| stand. regr. coeff. | −0.06 | −0.07 | −0.28 | −0.26 | −0.50 | 0.45 | −0.37 | 0.34 | 0.11 | −0.19 | −2.03 | −0.40 | ||||||
| dataset | own + published*, only mesic and tropical (n = 37) | own + published*, only mesic and tropical (n = 31) | own + published*, only mesic and tropical (n = 22) | |||||||||||||||
| high. model w. | 0.16 (mass and breeding season), r2 = 0.27 |
0.146 (mass and survival), r2 = 0.2 |
0.2 (envt), r2 = 0.47 |
|||||||||||||||
| var. weight | 0.52 | 0.24 | 0.69 | 0.58 | 0.60 | 0.45 | 0.36 | 0.46 | 0.26 | 0.27 | 0.19 | 0.99 | ||||||
| raw regr. coeff. | −0.31 | 0.04 | −0.08 | −0.26 | −0.26 | 0.64 | −0.03 | 0.14 | 0.26 | 0.96 | 0.001 | −0.69 | ||||||
| stand. regr. coeff. | −0.24 | 0.01 | −0.35 | −0.32 | −0.32 | 0.25 | −0.23 | 0.29 | 0.14 | 0.16 | 0.00 | −0.68 | ||||||
| dataset | own + published* incl. arid and cold (n = 41) | own + published* incl. arid and cold (n = 35) | own + published* incl. arid and cold (n = 26) | |||||||||||||||
| high. model w. | 0.11 (breeding season), r2 = 0.29 |
0.093 (mass and survival), r2 = 0.35 |
0.19 (envt.), r2 = 0.51 |
|||||||||||||||
| variable |
mass |
surv. |
breed. |
trop. |
arid |
cold |
mass |
surv. |
breed. |
trop. |
arid |
cold |
mass |
surv. |
breed. |
trop. |
arid |
cold |
| var. weight | 0.75 | 0.24 | 0.76 | 0.55 | 0.28 | 0.25 | 0.47 | 0.31 | 0.35 | 0.47 | 0.91 | 0.22 | 0.22 | 0.25 | 0.2 | 0.99 | 0.21 | 0.48 |
| raw regr. coeff. | −0.31 | −0.15 | −0.08 | −0.24 | −0.18 | 0.46 | −0.27 | 0.76 | −0.03 | 0.15 | −0.46 | −0.05 | 0.14 | 0.77 | −0.01 | −0.7 | −0.11 | 0.44 |
| stand. regr. coeff. | −0.24 | −0.04 | −0.36 | −0.29 | −0.11 | 0.28 | −0.28 | 0.35 | −0.23 | 0.27 | −0.55 | −0.06 | 0.08 | 0.13 | −0.04 | −0.65 | −0.06 | 0.26 |
*Goymann et al. (2004); Garamszegi et al. (2005, 2008) and Bókony et al. (2009).
(c). Life-history data
Apparent annual survival rate (‘survival rate’ hereafter) in Panama was estimated using mark–recapture data and models for open populations. Individuals were captured using mist nets, marked with individually numbered leg bands and released. Mist net sampling was conducted for 3 days each, in two to three locations per site (typically around 600 net hours) in March and July (i.e. during the dry and wet season, respectively) between 1979 and 2006 for forest species (Karr et al. 1990; Brawn et al. 1999) and between 2003 and 2006 for species in more open second-growth habitats. We estimated the probability of annual survival using models that accounted for the biases introduced by ‘transient birds’ that should not be considered as members of the sampled resident populations (Pradel et al. 1997). For the questions asked here, we considered survival rate (after the second recapture interval) and recapture probability to be constant over time. Where necessary, we calculated the appropriate correction factor for overdispersion inherent with binomial sampling. All analyses were conducted with the program MARK v. 4.3 (White & Burnham 1999). Estimated survival rate data for temperate species were obtained from the MAPS database, which presents estimates of survival rate using similar sampling and modelling protocols. These long-term data come from a number of North American banding stations, analysed by geographical region. Data on average breeding season lengths were compiled from published species accounts (Birds of North America online, Cornell Laboratory of Ornithology, http://bna.birds.cornell.edu), supplemented by original unpublished data collected by W. D. and T. R. Robinson. Data on body mass were obtained from our own measurements, supplemented by the above species accounts (electronic supplementary material, table S1).
(d). Adding published data
To test whether the findings obtained from our study would be supported in a larger-scale comparative analysis, we added published data for passerine species on Cort0 and MaxCort (from Bókony et al. 2009), and on breeding season T (Goymann et al. 2004; Garamszegi et al. 2005, 2008; electronic supplementary material, table S1). We also included body mass and breeding season length primarily from those publications. We added estimates of annual survival from MAPS data and other sources (electronic supplementary material, table S1). We excluded non-passerine birds from our analyses to ensure common evolutionary history and homogeneity in general morphology, physiology, development and behaviour.
(e). Statistics
We only included species in our analyses for which we had data for all three key life-history traits (body mass, breeding season length and survival rate). To quantify the corticosterone ‘stress response’, we determined MaxCort for each individual by taking its highest Cort value, obtained after either 30 or 60 min of the capture–restraint protocol. We based our analyses on species medians (single values when n = 1) of Cort0 and MaxCort to discount outliers because Cort can vary in concentration within minutes; we used species means for T (single values when n = 1) because T is less labile, and this follows the practice used for the published data included in our study. Initial exploratory analyses on species for which we had at least n = 3 individuals gave qualitatively similar findings to when we included species for which sample size was n = 1 individual (n = 1 for 8/24 species for Cort0, 12/20 for MaxCort and 0/3 for T; see electronic supplementary material, table S1). In our original dataset, MaxCort was highly correlated with two other measures of the corticosterone stress response: the area under the curve (Spearman's ρ = 0.875, p < 0.0005) and the difference between Cort0 and MaxCort (Spearman's ρ = 0.955, p < 0.0005). All dependent variables, as well as body mass, were log10-transformed prior to analyses to test for differences between species independently of original measurement units, and reflecting the lognormal distribution of the variables. Survival rate was arcsin/square-root transformed (as raw data were proportions) and environment was entered as a binary variable (mesic/tropical). For breeding season length, we used untransformed data because data were normally distributed.
In an initial set of analyses, which we do not report here because results were highly congruent with Akaike's information criteria (AIC) analyses below, we used multiple regression models to ask to what extent the independent variables explained interspecific variation in each of the hormone traits. We then repeated those models including phylogenetic information using the phylogenetic generalized least-squares approach (Martins & Hansen 1997; for a phylogenetic topology see electronic supplementary material, figure S1). Conventional and phylogenetically informed analyses gave similar results, indicating that shared phylogenetic history does not account for much of the interspecific variation in hormone concentrations among passerines observed in this study. Similar results were also obtained when including taxonomy as a variable in the AIC models below.
We next used AIC modified for small sample size (AICc) to weight linear regression models based on each possible combination of one to all of the independent variables. Parameter importance values (0–1, termed ‘variable weights’ in table 1) were calculated for each of the independent variables as the sum of the importance values for each of the models that included the independent variable (each predictor variable appeared in the same number of candidate models). Weighted regression coefficients were calculated from the model weights in the same manner. Standardized regression coefficients were the weighted coefficients multiplied by the ratio of the standard deviations of the independent and dependent variables. Rather than assigning probability values to variables in a particular model, we estimated the relative weights of alternative models including the independent variables and provide estimates of the regression coefficients that account for uncertainty (Burnham & Anderson 1998). We first analysed our original dataset by itself, then included published data that were obtained in mesic and wet tropical environments, as in our study, and repeated the model selection process. Finally, because latitudinal effects were found in previous comparative analyses, we conducted specific hypothesis tests for latitudinal effects by adding data for species from cold and arid higher-latitude environments, and repeated the analysis. In the latter analyses, environment was coded as three variables: tropical, arid and cold, which were scored as 1 or 0. Mesic environments were coded as 0, 0, 0 for each of these environment variables. Because the full dataset included only two arid- and two cold-environment species, we used one-way ANOVA followed by Tukey's HSD post hoc test to identify effects of environment on hormone levels, using four additional arid-environment species and four cold-environment species for which Cort0 and MaxCort data were available from our own or published datasets, but which lacked a complete set of life-history traits.
3. Results
(a). Baseline corticosterone (Cort0)
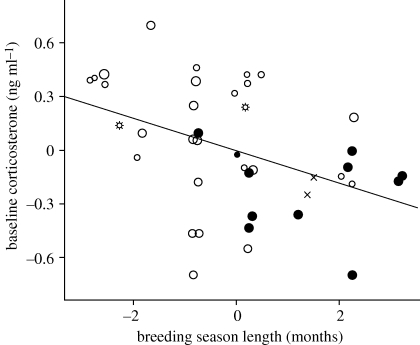
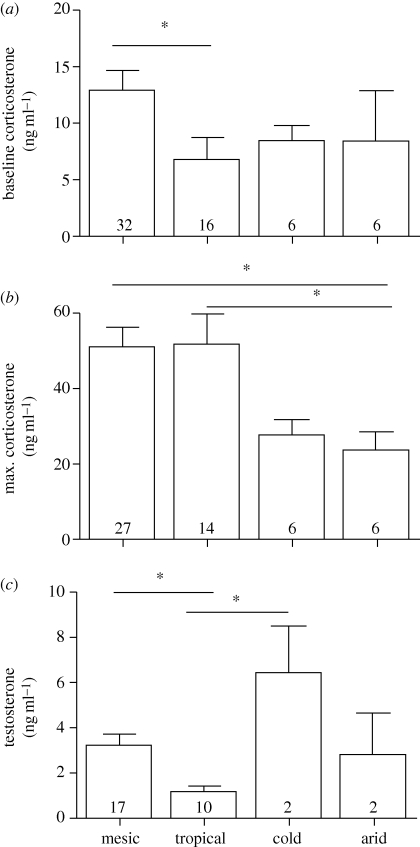
In our own original dataset, the AICc model with the highest weight (0.23, r2 = 0.11) included only breeding season length (table 1). However, variable importance values suggested that environment (mesic, tropical) was almost equally influential, such that species with long breeding seasons and species from the tropics had lower Cort0 than species with short breeding seasons and from mesic habitats (table 1; see also figure 1). The published data strengthened the explanatory values of the model (r2 = 0.27) and identified body mass as an important influence, with larger species having lower Cort0 than smaller species (electronic supplementary material, figure S2a). Environment remained an important variable in the larger dataset, with tropical species exhibiting lower Cort0 concentrations (table 1). A separate analysis showed a strong effect of environment on Cort0, with tropical species, but not cold- or arid-environment species, having lower Cort0 than mesic species (F3,55 = 3.54, p = 0.02; for post hoc results, see figure 2a).
Figure 1.
Partial regression plot showing inverse relationship between baseline corticosterone concentrations (Cort0; ng ml−1) and breeding season length (months). Data are displayed as unstandardized residuals after controlling for body mass in a linear regression model. The figure shows the largest dataset used in this study. Larger symbols denote own original dataset, smaller symbols refer to published data. Environments are coded as: open circles, mesic; filled circles, tropical; crosses, arid; stars, cold (arctic or alpine).
Figure 2.
Interspecific variations in concentrations (mean ± s.e.; ng ml−1) of (a) baseline corticosterone, (b) stress-induced corticosterone and (c) testosterone, in males from species breeding in different environments. Sample sizes are given in bars; significant differences between environments as derived from Tukey's HSD post hoc tests are indicated by horizontal lines and asterisks.
(b). Stress-induced corticosterone concentrations (MaxCort)
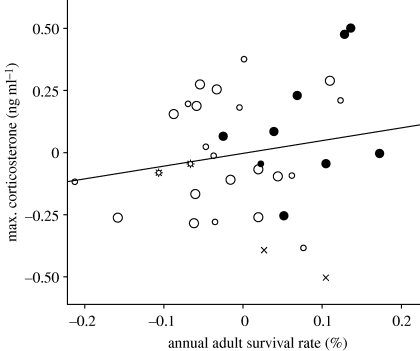
Body mass and survival rate best explained interspecific variations in MaxCort concentrations, both in our original dataset (r2 = 0.41) and in the larger dataset including published data (r2 = 0.20 and 0.35, respectively; table 1). Body mass (importance = 0.79) was a stronger effect than survival rate (0.53) in our original dataset; smaller species and species with higher adult survival rates reached higher MaxCort concentrations during a standardized capture–restraint protocol (table 1; electronic supplementary material, figure S2b). Regression slopes for both body mass and survival rate were higher when both variables were included in the regression, suggesting that the two variables make independent and opposite contributions to MaxCort (table 1). Furthermore, in the larger dataset, an environmental effect became apparent, with two arid-environment species exhibiting particularly low MaxCort concentrations. A separate ANOVA confirmed that arid-environment species reach significantly lower MaxCort concentrations than mesic species (F3,49 = 4.87, p = 0.005; figure 2b).
MaxCort concentrations were not correlated with baseline corticosterone concentrations (own dataset: Pearson's correlation coefficient = 0.005, p > 0.9, n = 20; large dataset: Pearson's correlation coefficient = 0.35, p > 0.3, n = 35).
(c). Testosterone (T)
In our original dataset, AICc analyses identified breeding season length (variable weight 0.97, r2 = 0.79) to be the main predictor of interspecific variation in T, species with longer breeding seasons having lower T. Analysis of the large dataset did not support this finding but suggested a primary influence of environment (variable weight 0.99, r2 = 0.47), with species from tropical latitudes having lower T concentrations and species from cold environments potentially having higher T concentrations (table 1). A separate analysis confirmed these environmental effects (F3,27 = 3.97, p < 0.02): tropical species had lower T than mesic and cold-environment species (figure 2c).
Testosterone concentrations were not correlated with either Cort0 (own dataset: Pearson's correlation coefficient = 0.28, p > 0.4, n = 9; large dataset: Pearson's correlation coefficient = 0.38, p > 0.05, n = 26) or MaxCort (own dataset: Pearson's correlation coefficient = −0.58, p > 0.09, n = 9; large dataset: Pearson's correlation coefficient = −0.16, p > 0.4, n = 24).
4. Discussion
Circulating concentrations of baseline corticosterone (Cort0) and stress-induced corticosterone (MaxCort) in males varied with key life-history traits among species of passerine birds, both in a standardized original study on New World temperate and tropical species and in a re-analysis of published data encompassing species from other environments. These findings support the hypothesis that corticosterone may be part of the physiological machinery that underlies life-history strategies in vertebrates. Although testosterone concentrations (T) varied considerably among environments, association of T with species' other traits was not evident. Interestingly, the three hormonal measurements were uncorrelated, suggesting that they vary independently among species in response to environmental and other life-history traits. Finally, as in other studies (e.g. Garamszegi et al. 2008; Bókony et al. 2009), we could not detect a phylogenetic signal in our analyses, indicating that within the passerine species studied here, shared evolutionary history accounts for little of the observed interspecific variation in steroid hormone concentrations.
(a). Baseline corticosterone and life-history traits
The factor that most consistently explained interspecific variations in Cort0 concentrations was breeding season length: Cort0 was lowest in species with long breeding seasons (table 1 and figure 1). Cort0 was also lower in tropical species (which also have longer breeding seasons) and in larger species (table 1). At baseline concentrations, glucocorticoid hormones are thought to have mainly metabolic functions, preparing and adjusting organismal functions to predictable variations in energetic demands over the course of the day and between seasons (‘permissive effects’; Sapolsky et al. 2000; see also Romero 2002; Landys et al. 2006). The relationships we identified above of Cort0 with breeding season length, body mass and environment are consistent with its primary role as a metabolic hormone. Species with shorter breeding seasons probably sustain greater reproductive intensity, requiring mobilization of more energy reserves on a daily basis to sustain a higher workload. Individuals of smaller species have higher mass-specific metabolic rates (Calder 1996), consistent with an inverse relationship between Cort0 and body mass (see also Bókony et al. 2009). Finally, lowland tropical species experience benign temperatures and have lower mass-specific metabolic rates than species from other environments (e.g. Wiersma et al. 2007), thus requiring less Cort0 to promote metabolic processes. Thus, variation in Cort0 concentrations appears to be consistent with a role in supporting the ‘metabolic’ plane of a species (i.e. Cort0 is related to predictable variation among species in metabolic requirements).
A recent large-scale comparative analysis (from which data on passerine species were extracted for the larger dataset in this study) related Cort0 to ‘brood value’, a composite variable directly related to clutch size and inversely related to number of broods per year and average reproductive lifespan (Bókony et al. 2009). Our results suggest that breeding season length might drive much of this correlation. The same study did not find an effect of latitude on Cort0, but our finding that tropical species have lower Cort0 than mesic temperate species might have been obscured by including arid- and cold-environment species among the temperate and high-latitude birds.
(b). Stress-induced corticosterone and life-history traits
Body mass was most strongly associated with interspecific variation in MaxCort concentrations following a standardized capture–restraint protocol, with smaller species reaching higher concentrations (table 1). Smaller species presumably live ‘closer to the edge’ when conditions are challenging because they have higher mass-specific metabolic rates and lower energy reserves, which together result in shorter fasting survival times than in larger species (Calder 1996). Hence, during challenging environmental conditions, higher MaxCort concentrations might benefit individuals of smaller species by ensuring the allocation of resources to the greatest extent to processes that promote survival.
Survival was consistently included together with body mass in the best AIC model explaining MaxCort variation, both in our original and in the larger dataset (table 1). Furthermore, the relationship between MaxCort and survival was in the direction predicted by life-history theory: species with higher survival rates reached higher MaxCort concentrations (figure 3). The positive relationship of survival rate with MaxCort is consistent with the notion that higher concentrations of this hormonal trait may facilitate processes that reduce risk to the individual—for example, energy-intensive escape responses—thereby protecting survival. Bókony et al.'s (2009) recent analyses suggested that latitude primarily explained variation among species in peak corticosterone concentrations, but they did not include species traits other than ‘brood value’ in their analysis. Life-history traits typically exhibit strong latitudinal clines. Our analysis (including passerine species from Bókony et al. 2009) suggests that their latitudinal effect on MaxCort is associated with a higher survival rate among the larger tropical species, although the independent influence of tropical environment per se remained relatively strong.
Figure 3.
Partial regression plot showing positive relationship between maximal corticosterone concentrations (MaxCort; ng ml−1) and survival rate (%). Data are displayed as unstandardized residuals after controlling for body mass in a linear regression model. The figure shows the largest dataset used in this study. Larger symbols denote own original dataset, smaller symbols refer to published data. Environments are coded as: open circles, mesic; filled circles, tropical; crosses, arid; stars, cold (arctic or alpine).
Higher MaxCort concentrations may reduce risk to individuals during acute stressful episodes in various ways (Wingfield et al. 1995; Sapolsky et al. 2000; Wingfield & Sapolsky 2003). First, MaxCort can promote behaviours that allow the individual to escape adverse conditions (e.g. by relocating to a different habitat). Second, MaxCort can lead to a rapid inhibition of costly activities that are not acutely essential to survival, such as reproduction. Third, MaxCort can re-establish bodily homeostasis by increasing feeding rates and energy storage, thereby also readying an individual for potential subsequent stressful events (e.g. the return of a predator). Finally, MaxCort also functions to shut down the initial stress response in a timely manner to prevent damage to the organism.
Analysis of our large dataset, which included species from arid and cold environments as well as mesic and tropical environments, indicated that (breeding) environment contributes to interspecific variations in MaxCort. Species from arid environments, and probably also from cold environments, reach lower MaxCort in response to a standardized acute stressor compared with species in mesic and tropical environments (figure 2b). Reduced stress responses in climatically unpredictable arid and cold environments could reduce brood desertion where suitable conditions and sufficient time for re-nesting are uncertain (Wingfield & Sapolsky 2003). Our results of a multi-layered relationship of both Cort0 and MaxCort with life-history traits and environmental variables support recent suggestions that life-history trade-offs are multi-dimensional and hierarchical, with allocation decisions at one level probably influencing processes at subsequent levels (e.g. Lancaster et al. 2008).
(c). Testosterone and life-history traits
In our original dataset, breeding season length best explained variation in T concentrations of male birds. This finding suggests that T might promote mating effort (e.g. investment into courtship and territorial defence), since species with shorter (and presumably more intense) breeding seasons have higher peak breeding T than species with long breeding seasons. However, in the large dataset, the effects of breeding season length were subsumed by environment; specifically, T was lower in tropical (longer breeding seasons) compared with mesic temperate species, and higher in cold-environment species (shorter breeding seasons; figure 2c). Two previous studies reported that breeding season length was a strong predictor of interspecific variations in T; however, one was conducted on an exclusively tropical dataset (Goymann et al. 2004) and the second, while including species from other latitudes, found latitude to be a stronger predictor than breeding season length (Garamszegi et al. 2008).
Although breeding season length did not explain T variation in our large dataset, higher peak breeding T in species breeding in cold environments suggests that T is tied to mating effort as species in such extreme environments are expected to prioritize reproduction whenever it is possible. It is also important to point out that our study, like other published studies addressing ‘peak breeding T’, may have missed peaks in T during the mating or courtship phase in certain species. Furthermore, studies in which T is determined in a more standardized way—for example, after a simulated territorial intrusion or after an injection of gonadotropin-releasing hormone (to induce maximal release of T)—may be required to better detect relationships between T and life-history traits (e.g. McGlothlin et al. 2010). Finally, more data on T in a larger set of species, along with ecological and life-history traits, will be necessary to test the relative importance of other factors such as mating system and parental care (Wingfield et al. 1990; Hirschenhauser & Oliveira 2006), altitude and territoriality (Goymann et al. 2004), breeding synchrony and migration (Garamszegi et al. 2008), population density, extended paternal care and cooperative breeding (summarized in Hau et al. 2008).
(d). Relationships between hormonal traits
Cort0 and MaxCort were not correlated among species in any of our datasets. This might be surprising at first, since corticosterone at both baseline and elevated concentrations originates from the adrenal cortex (Nelson 2005). However, cellular pathways of corticosterone action are thought to differ depending on circulating concentrations—Cort0 mainly exerting biological effects by binding to mineralocorticoid receptors, and MaxCort mainly acting via glucocorticoid receptors (Sapolsky et al. 2000). Hence, the action of a single hormone, when secreted at different concentrations, may be dissociated via receptor dynamics (among other processes), thereby diversifying its effects on the phenotype. Such divergent effects of different concentrations of corticosterone could be shaped by different selection pressures, leading to the observed lack of correlation. Likewise, neither corticosterone trait was correlated with testosterone concentrations, suggesting that the three hormonal traits function, and possibly also evolve, independently.
5. Conclusions
The current data support the hypothesis that steroid hormones are involved in shaping interspecific variation in life-history strategies in male birds. These findings are exciting for the emerging field of evolutionary endocrinology because they reveal associations between circulating hormone concentrations (i.e. the signalling part of an endocrine pathway) and life-history traits.
While the results from our standardized original study and those of the larger dataset were mostly congruent, it is important to point out the limitations of comparative studies using large, heterogeneous datasets. Some comparative studies have reported differences between laboratories in hormone concentrations (Bókony et al. 2009), while others have not found a significant laboratory effect (Goymann et al. 2004). Furthermore, populations of the same species can differ in hormone concentrations and life-history traits in different environments, indicating that hormone and life-history data should be obtained from individuals of the same population to uncover meaningful patterns. Finally, comparative analyses like the present study do not allow one to infer causation. Experimental manipulations are needed to determine whether, and in which way, interspecific variations in hormone concentrations are related to differences in life-history strategies (e.g. Reed et al. 2006; Lancaster et al. 2008). The biological actions of hormones are determined by a large number of down-stream processes, such as the presence and dynamics of steroid-binding globulins in the plasma and, of cellular steroid receptors, and many other factors in the signalling pathways (Nelson 2005; Hau 2007) that will need to be included in more detailed interspecific comparisons in the future. Subsequent studies should also determine whether the relationships detected here for males hold for females, and for vertebrate taxa other than passerine birds, as well.
In conclusion, the results of this study contribute to our understanding of the physiological mechanisms that underlie life-history strategies (Ricklefs & Wikelski 2002) by suggesting a link to endocrine mechanisms. An important next step will be to integrate the current findings on hormones with the recently documented covariation of life history with other major physiological systems such as immune function (e.g. Lee et al. 2008) and metabolism (e.g. Wiersma et al. 2007).
Acknowledgements
M. Pless, C. Zawilsky, M. Evans and K. Klasing helped in field and laboratory. J. Touchton, W. D. and T. R. Robinson shared unpublished data. M. Romero, W. Goymann, J. Ouyang, T. Greives, three reviewers and the Associate Editor provided valuable comments on previous versions of the manuscript. This study was funded by an Integrated Research Challenge Grant from the National Science Foundation (IBN 0212587). R.E.R. was supported in part by a Research Award from the Alexander von Humboldt Foundation. All procedures were approved by the Princeton University Animal Care and Use Committee.
References
- Balthazart J., Hendrick J. C.1979Relationships between the daily variations of social behavior and of plasma FSH, LH and testosterone levels in the domestic duck Anas platyrhynchos L. Behav. Proc. 4, 107–128 (doi:10.1016/0376-6357(79)90027-5) [DOI] [PubMed] [Google Scholar]
- Bókony V., Lendvai A. Z., Liker A., Angelier F., Wingfield J. C., Chastel O.2009Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598 (doi:10.1086/597610) [DOI] [PubMed] [Google Scholar]
- Brawn J. D., Karr J. R., Nichols J. D., Robinson W. D.1999Demography of tropical forest birds in Panama: how do transients affect estimates of survival rates? Proc. Int. Ornithol. Conf. 22, 297–305 [Google Scholar]
- Breuner C. W., Greenberg A. L., Wingfield J. C.1998Noninvasive corticosterone treatment rapidly increases activity in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii). Gen. Comp. Endocrinol. 111, 386–394 (doi:10.1006/gcen.1998.7128) [DOI] [PubMed] [Google Scholar]
- Buchanan K. L., Evans M. R., Goldsmith A. R.2003Testosterone, dominance signalling and immunosuppression in the house sparrow, Passer domesticus. Behav. Ecol. Sociobiol. 55, 50–59 (doi:10.1007/s00265-003-0682-4) [Google Scholar]
- Burnham K. P., Anderson D. R.1998Model selection and inference: a practical information-theoretic approach. New York, NY: Springer-Verlag [Google Scholar]
- Calder W. A., III1996Size, function, and life history, 2nd edn.Cambridge, MA: Harvard University Press [Google Scholar]
- Casto J. M., Nolan V., Ketterson E. D.2001Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis). Am. Nat. 157, 408–420 (doi:10.1086/319318) [DOI] [PubMed] [Google Scholar]
- Charnov E. L.1993Life history invariants. Oxford, UK: Oxford University Press [Google Scholar]
- Dallman M., Strack A., Akana S., Bradbury M. J., Hanson E., Scribner K., Smith M.1993Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Frontiers Neuroendocrinol. 14, 303–347 (doi:10.1006/frne.1993.1010) [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S., McEwen B. S.1997Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 11, 286–306 (doi:10.1006/brbi.1997.0508) [DOI] [PubMed] [Google Scholar]
- Dufty A. M.1989Testosterone and survival. Horm. Behav. 23, 185–193 (doi:10.1016/0018-506X(89)90059-7) [DOI] [PubMed] [Google Scholar]
- Finch C. E., Rose M. R.1995Hormones and the physiological architecture of life history evolution. Quart. Rev. Biol. 70, 1–52 [DOI] [PubMed] [Google Scholar]
- Garamszegi L. Z., Eens M., Hurtrez-Bousses S., Moller A. P.2005Testosterone, testes size, and mating success in birds: a comparative study. Horm. Behav. 47, 389–409 (doi:10.1016/j.yhbeh.2004.11.008) [DOI] [PubMed] [Google Scholar]
- Garamszegi L. Z., Hirschenhauser K., Bokony V., Eens M., Hurtrez-Bousses S., Moller A. P., Oliveira R. F., Wingfield J. C.2008Latitudinal distribution, migration, and testosterone levels in birds. Am. Nat. 172, 533–546 (doi:10.1086/590955) [DOI] [PubMed] [Google Scholar]
- Gill S. A., Alfson E. D., Hau M.2007Context matters: female aggression and testosterone in a year-round territorial neotropical songbird (Thryothorus leucotis). Proc. R. Soc. B 274, 2187–2194 (doi:10.1098/rspb.2007.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W., Moore I. T., Scheuerlein A., Hirschenhauser K., Grafen A., Wingfield J. C.2004Testosterone in tropical birds: effects of environmental and social factors. Am. Nat. 164, 327–334 (doi:10.1086/422856) [DOI] [PubMed] [Google Scholar]
- Hau M.2007Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays 29, 133–144 (doi:10.1002/bies.20524) [DOI] [PubMed] [Google Scholar]
- Hau M., Gill S. A., Goymann W.2008Tropical field endocrinology: ecology and evolution of testosterone concentrations in male birds. Gen. Comp. Endocrinol. 157, 241–248 (doi:10.1016/j.ygcen.2008.05.008) [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K., Oliveira R. F.2006Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 71, 265–277 (doi:10.1016/j.anbehav.2005.04.014) [Google Scholar]
- Husak J. F., Irschick D. J., Meyers J. J., Lailvaux S. P., Moore I. T.2007Hormones, sexual signals, and performance of green anole lizards (Anolis carolinensis). Horm. Behav. 52, 360–367 (doi:10.1016/j.yhbeh.2007.05.014) [DOI] [PubMed] [Google Scholar]
- Jones O. R., et al. 2008Senescence rates are determined by ranking on the fast–slow life-history continuum. Ecol. Lett. 11, 664–673 (doi:10.1111/j.1461-0248.2008.01187.x) [DOI] [PubMed] [Google Scholar]
- Karr J. R., Nichols J. D., Klimkiewicz M. K., Brawn J. D.1990Survival rates of birds of tropical and temperate forests: will the dogma survive? Am. Nat. 136, 277–291 (doi:10.1086/285098) [Google Scholar]
- Ketterson E. D., Nolan V.1992Hormones and life histories: an integrative approach. Am. Nat. 140, S33–S62 (doi:10.1086/285396) [DOI] [PubMed] [Google Scholar]
- Knobil E., Neill J. D.1998The physiology of reproduction, vol. 1, 2 New York, NY: Raven Press [Google Scholar]
- Lancaster L. T., Hazard L. C., Clobert J., Sinervo B. R.2008Corticosterone manipulation reveals differences in hierarchical organization of multidimensional reproductive trade-offs in r-strategist and K-strategist females. J. Evol. Biol. 21, 556–565 (doi:10.1111/j.1420-9101.2007.01478.x) [DOI] [PubMed] [Google Scholar]
- Landys M. M., Ramenofsky M., Wingfield J. C.2006Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149 (doi:10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- Lee K. A., Wikelski M., Robinson W. D., Robinson T. R., Klasing K. C.2008Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363 (doi:10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- Marler C. A., Moore M. C.1988Evolutionary costs of aggression revealed by testosterone manipulations in free-living male lizards. Behav. Ecol. Sociobiol. 23, 21–26 (doi:10.1007/BF00303053) [Google Scholar]
- Martins E. P., Hansen T. F.1997Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 (doi:10.1086/286013) [Google Scholar]
- McGlothlin J. W., Ketterson E. D.2008Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlothlin J. W., Whittaker D. J., Schrock S. E., Gerlach N. M., Jawor J. M., Snajdr E. A., Ketterson E. D.2010Natural selection on testosterone production in a wild songbird population. Am. Nat. 175, 687–701 (doi:10.1086/652469) [DOI] [PubMed] [Google Scholar]
- Miles D. B., Sinervo B., Hazard L. C., Svensson E. I., Costa D.2007Relating endocrinology, physiology and behaviour using species with alternative mating strategies. Funct. Ecol. 21, 653–665 (doi:10.1111/j.1365-2435.2007.01304.x) [Google Scholar]
- Nelson R. J.2005An introduction to behavioral endocrinology, 3rd edn.Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- Oliveira R. F.2004Social modulation of androgens in vertebrates: mechanisms and function. Adv. Study Behav. 34, 165–239 (doi:10.1016/S0065-3454(04)34005-2) [Google Scholar]
- Perfito N., Bentley G., Hau M.2006Tonic activation of brain GnRH immunoreactivity despite reduction of peripheral reproductive parameters in opportunistically breeding zebra finches. Brain Behav. Evol. 67, 123–134 (doi:10.1159/000090977) [DOI] [PubMed] [Google Scholar]
- Pradel R., Hines J., Lebreton J.-D., Nichols J. D.1997Capture–recapture survival models taking account of transients. Biometrics 53, 60–72 (doi:10.2307/2533097) [Google Scholar]
- Promislow D. E. L., Harvey P. H.1990Living fast and dying young—a comparative analysis of life-history variation among mammals. J. Zool. 220, 417–437 (doi:10.1111/j.1469-7998.1990.tb04316.x) [Google Scholar]
- Reed W. L., Clark M. E., Parker P. G., Raouf S. A., Arguedas N., Monk D. S., Snajdr E., Nolan V., Jr, Ketterson E. D.2006Physiological effects on demography: a long-term experimental study of testosterone's effects on fitness. Am. Nat. 167, 667–683 (doi:10.1086/503054) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E.2000Density dependence, evolutionary optimization, and the diversification of avian life histories. Condor 102, 9–22 (doi:10.1650/0010-5422(2000)102[0009:DDEOAT]2.0.CO;2) [Google Scholar]
- Ricklefs R. E.2010Parental investment and avian reproductive rate: Williams's principle reconsidered. Am. Nat. 175, 350–361 (doi:10.1086/650371) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E., Wikelski M.2002The physiology/life history nexus. Trends Ecol. Evol. 17, 462–468 (doi:10.1016/S0169-5347(02)02578-8) [Google Scholar]
- Roff D. A., Fairbairn D. J.2007The evolution of trade-offs: where are we? J. Evol. Biol. 20, 433–447 (doi:10.1111/j.1420-9101.2006.01255.x) [DOI] [PubMed] [Google Scholar]
- Romero L. M.2002Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U.2000How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- Sinervo B., Svensson E.1998Mechanistic and selective causes of life history trade-offs and plasticity. Oikos 83, 432–442 (doi:10.2307/3546671) [Google Scholar]
- Stearns S. C.1992The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- White G. C., Burnham K. P.1999Program MARK: survival estimation from populations of marked animals. Bird Study 46, 120–138 (doi:10.1080/00063659909477239) [Google Scholar]
- Wiersma P., Munoz-Garcia A., Walker A., Williams J. B.2007Tropical birds have a slow pace of life. Proc. Natl Acad. Sci. USA 104, 9340–9345 (doi:10.1073/pnas.0702212104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M., Hau M., Robinson W. D., Wingfield J. C.2003Reproductive seasonality of seven neotropical passerine species. Condor 105, 683–695 (doi:10.1650/7251) [Google Scholar]
- Willis E. O.1972The behaviour of spotted antbirds. Ornithol. Monogr. 10, 1–157 [Google Scholar]
- Wingfield J. C., Sapolsky R. M.2003Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 15, 711–724 [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Smith J. P., Farner D. S.1982Endocrine responses of white-crowned sparrows to environmental stress. Condor 84, 399–409 (doi:10.2307/1367443) [Google Scholar]
- Wingfield J. C., Hegner R. E., Dufty A. M., Jr, Ball G. F.1990The ‘challenge-hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 (doi:10.1086/285134) [Google Scholar]
- Wingfield J. C., O'Reilly K. M., Astheimer L. B.1995Modulation of the adrenocortical responses to acute stress in arctic birds: a possible ecological basis. Am. Zool. 35, 285–294 [Google Scholar]
- Wingfield J. C., Maney D. L., Breuner C. W., Jacobs J. D., Lynn S., Ramenofsky M., Richardson R. D.1998Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- Zera A. J., Harshman L. G.2001The physiology of life history trade-offs in animals. Ann. Rev. Ecol. Syst. 32, 95–126 (doi:10.1146/annurev.ecolsys.32.081501.114006) [Google Scholar]