Abstract
Although glucocorticoid hormones are considered important physiological regulators for surviving adverse environmental stimuli (stressors), evidence for such a role is sparse and usually extrapolated from glucocorticoid effects under laboratory, short-term and/or non-emergency conditions. Galápagos marine iguanas (Amblyrhynchus cristatus) provide an excellent model for determining the ultimate function of a glucocorticoid response because susceptibility to starvation induced by El Niño conditions is essentially their only major natural stressor. In a prospective study, we captured 98 adult male marine iguanas and assessed four major components of their glucocorticoid response: baseline corticosterone titres; corticosterone responses to acute stressors (capture and handling); the maximal capacity to secrete corticosterone (via adrenocorticotropin injection); and the ability to terminate corticosterone responses (negative feedback). Several months after collecting initial measurements, weak El Niño conditions affected the Galápagos and 23 iguanas died. The dead iguanas were typified by a reduced efficacy of negative feedback (i.e. poorer post-stress suppression of corticosterone release) compared with surviving iguanas. We found no prior differences between dead and alive iguanas in baseline corticosterone concentrations, responses to acute stressors, nor in capacity to respond. These data suggest that a greater ability to terminate a stress response conferred a survival advantage during starvation.
Keywords: stress, dexamethasone, adrenocorticotropin
1. Introduction
Using the Galápagos islands as a natural laboratory, we previously demonstrated that corticosterone concentrations, the species-typical glucocorticoid in reptiles (Greenberg & Wingfield 1987), were highly correlated with survivorship of marine iguanas (Amblyrhynchus cristatus) during an El Niño-induced famine (Romero & Wikelski 2001). Galápagos marine iguanas feed exclusively on the rich marine algae growing in the intertidal and subtidal seas surrounding the islands (Wikelski & Trillmich 1994; Wikelski et al. 1997). Every few years, recurrent global climate events (El Niño) decrease the nutrient-rich upwelling and as a result the amount of algae is greatly reduced, leading to wide-spread starvation (Wikelski et al. 1997). Both baseline and stress-induced corticosterone concentrations were inversely correlated with survival in iguana populations (Romero & Wikelski 2001).
This natural experiment of El Niño-induced starvation provides several major advantages for using marine iguanas as a model for examining the role of corticosterone in surviving natural stressors. First, reliance on one food source subjects marine iguanas to a fairly regular cycle of unintentional fasting, and potential starvation, every few years (Laurie 1989; Wikelski 2005). Second, these iguanas have few natural predators (Kruuk & Snell 1981), leaving the El Niño-induced starvation as the major stressor for adult iguanas. Third, even though they can live several decades, marine iguanas are highly sedentary and rarely move from a several hundred metre area of coastline (Wikelski & Trillmich 1994). Strong site fidelity allows us to monitor individual animals and to estimate annual survival rates (Wikelski & Trillmich 1997). Fourth, owing to their unfamiliarity with humans, marine iguanas are easy to capture repeatedly (Rubenstein & Wikelski 2005).
We used the natural year-to-year variation in climate in the Galápagos archipelago to let nature conduct an experiment for us. During an El Niño, marine iguanas are food-stressed and many individuals die of starvation. We hypothesized that corticosterone secretion helps individuals survive, as predicted by ecological theories of stress and biomedical theories of corticosterone physiology. To test this hypothesis, we initially characterized the sensitivity and robustness of an individual's corticosterone response prior to an El Niño. We then determined the fate of these individuals during and after an El Niño. This pre-emptive physiological characterization of individuals in combination with subsequent monitoring allowed us to determine whether and to what degree individual differences in the hormonal stress response predisposed animals to survive in the wild.
Corticosterone is one of the hallmarks of the vertebrate stress response (Sapolsky et al. 2000). Its release culminates from activation of the hypothalamic–pituitary–inter-renal (HPI) axis. The final steps consist of adrenocorticotropin (ACTH) being released from the pituitary and stimulating secretion of corticosterone from the iguana inter-renal gland. Negative feedback then serves to turn off the response. Background information on HPI axis function in each marine iguana is necessary to fully characterize an individual's ability to mount a corticosterone response. We tested four aspects of the HPI axis. First, we determined initial unstressed corticosterone titres, believed to be important in basic energy metabolism (Romero 2004). Second, we determined each individual's response to a standardized stressor, capture and handling (Wingfield & Romero 2001). Third, we tested the inter-renal's maximal ability to secrete corticosterone by injecting exogenous ACTH (Cyr & Romero 2009). Fourth, we tested the ability of the animal to regulate the termination of the corticosterone response via negative feedback (Romero 2004). Understanding the individual variation in these regulatory pathways allowed us to determine whether different responses provided an advantage when coping with the emergency conditions of an El Niño.
Although corticosterone is believed to be vital in helping protect an animal from adverse environmental conditions (Wingfield & Romero 2001), how it does so is not well understood (Sapolsky et al. 2000). Corticosterone can provide short-term behavioural (Wingfield & Romero 2001) and physiological (Sapolsky et al. 2000) benefits in coping with stressors, including stressors consisting of worsening environmental conditions (e.g. Romero et al. 2000). A few studies have directly correlated either baseline or stress-induced corticosterone titres with survival (Romero & Wikelski 2001; Brown et al. 2005; Blas et al. 2007; Cabezas et al. 2007), but none to date have explored which pathways of the corticosterone physiology might be most beneficial for survival.
2. Material and methods
We captured 98 iguanas on Santa Fe island (90°2′ W, 0°50′ S) in early December 2002 (breeding season) during benign conditions. Selection was not random. Because previous data indicated that the largest animals in the population were most likely to die during an El Niño (Wikelski et al. 1997), we actively targeted the largest individuals to provide the greatest chance of seeing a result. Furthermore, in order to control for potential differential survival by sex, we selected only males.
Immediately upon capture, each iguana was placed head-first into an opaque cloth bag and a blood sample collected from the caudal vein on the underside of the tail. Blood was collected into heparinized microhaematocrit tubes using a hypodermic needle or into 2 ml heparinized vacutainer tubes. These initial baseline samples were obtained in less than 2 min for all but 12 animals, where samples were collected 2–3 min after capture. Previous work indicated that this is well before corticosterone concentrations begin to rise in response to the stress of capture and handling in this species (Romero & Reed 2005).
After collecting the initial sample, iguanas were maintained in the opaque bags until all samples were collected. At 30 min after capture, all iguanas were bled a second time to determine each individual's response to the acute stressor of capture and handling. Corticosterone is known to increase in response to capture and handling in marine iguanas (Romero & Wikelski 2001). Following this second blood sample, iguanas were divided into three groups. The first (n = 8) was maintained in cloth bags with subsequent samples collected at 60, 120 and 240 min post-capture. This group served as the control for the dexamethasone (DEX) group (see below).
A second group (n = 51) was immediately injected intraperitoneally with 50 IU kg−1 ACTH (porcine). Earlier work indicated that this dose is effective at stimulating corticosterone release in marine iguanas (Romero & Wikelski 2006). Blood samples were then collected 30 min after injection (60 min after capture) to assess ACTH's ability to elevate corticosterone titres above titres from stress alone. Injection of exogenous ACTH is intended to stimulate the inter-renal to ascertain whether the gland is functioning maximally in response to handling and restraint (Cyr & Romero 2009).
The third group (n = 39) was injected intramuscularly with 1 mg kg−1 DEX immediately following the second blood sample. DEX is a synthetic glucocorticoid that artificially stimulates negative feedback. This results in a decrease in circulating endogenous corticosterone titres if feedback is functioning normally (McDonald et al. 1986; Sapolsky & Altmann 1991). This decrease can be monitored because DEX does not bind to the commonly used antibodies in the radioimmunoassays (RIAs). The dose we used is effective in marine iguanas (Romero & Wikelski 2006). After injection, blood samples were collected at 60, 120 and 240 min post-capture. Since we were interested in the absolute strength of negative feedback, we selected the lowest DEX-induced corticosterone titre during the 240 min.
Prior to release, we weighed each animal, measured their snout-to-vent length (Wikelski & Trillmich 1997), computed a body condition index ((body mass/snout–vent length3)×106). Although this condition index is crude, it adequately describes the physical condition of an iguana (Laurie 1989; Wikelski & Trillmich 1997). All animals were given a small identifying brand on their flanks for later targeted recapturing and a unique brand on their bellies for individual identification.
A mild El Niño struck the Galápagos in late December/early January of 2002/2003, resulting in moderate marine iguana mortality. We returned in July 2003 and captured every previously marked animal that could be found over the course of a week (77%). Those iguanas we were unable to find were classified as having died (23%). Given the strong site fidelity of this species (Wikelski & Trillmich 1994) and that El Niños are virtually the only source of mortality of adults on this island (Berger et al. 2007), it is unlikely that many iguanas were misclassified. We also returned again in January 2006 and January 2008 and recaptured or resighted all marked iguanas. We did not find any animal that was previously characterized as ‘dead’, indicating that our categorization was presumably correct. We then analysed the data from the previous December to determine whether variation in an individual iguana's ability to mount, sustain and turn off corticosterone release could predict survival.
In a subset of 30 iguanas still alive in July 2003, we collected baseline and stress-induced (30 min) samples. We then injected 14 with DEX and the rest with ACTH, as described above. We compared these values and body mass to the values obtained from the same animals seven months earlier. Unfortunately, we were unable to appropriately remeasure body length.
Samples collected in the field were stored cool until returned to the field camp (less than 8 h). All samples were centrifuged and plasma removed and stored in an electric-powered freezer. A few blood samples were lost during centrifugation. Although samples remained cool, the freezer was unable to keep them frozen. Samples were then transported on ice to Tufts University. Corticosterone was assayed by RIA following Wingfield et al.'s (1992) methods that have been used in numerous studies on marine iguanas (Romero & Wikelski 2001, 2002; Wikelski et al. 2001; Romero & Wikelski 2006). Interassay and intra-assay coefficients of variation were less than 12 and 6 per cent, respectively.
A change to each aspect of corticosterone physiology was considered an independent hypothesis based upon previous work which indicates that each aspect is independently regulated (Romero 2001). Consequently, we analysed each aspect independently. We initially tested all comparisons for homogeneous variances using Levene's test. For samples collected prior to the El Niño, we compared animals later found dead with those later found alive using a t-test for unequal variances when Levene's test was significant and t-tests with equal variances when Levene's test was not significant. We further compared changes in parameters from pre- and post-El Niño in individual iguanas using repeated-measures ANOVA on log10-transformed data when Levene's test was significant and repeated-measures ANOVA on untransformed data when Levene's test was not significant. All tests were performed with JMP 501 (SAS Institute) with alpha set at 0.05.
3. Results
(a). Comparison of animals destined to survive and destined to die
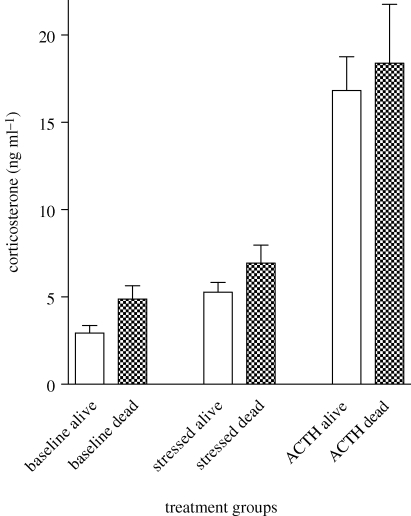
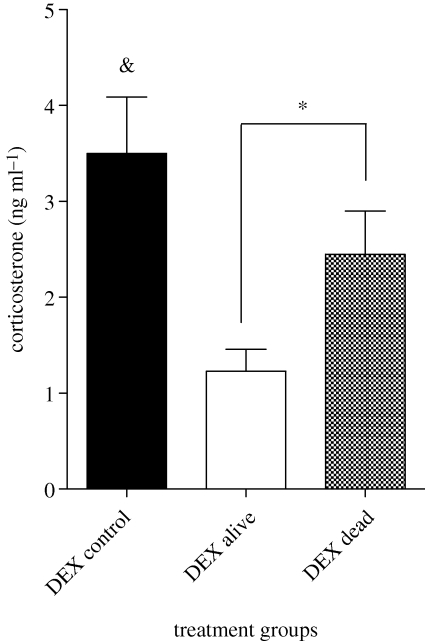
All animals were in excellent body condition prior to the El Niño and there was no difference between iguanas that ended up surviving or dying (t = 0.21, p = 0.83). Prior to the El Niño, there was no difference in baseline corticosterone (t = 1.50, d.f. = 24.49, p = 0.15), corticosterone after 30 min of restraint (t = 0.97, d.f. = 23.56, p = 0.34) or in the capacity to respond to a stressor as shown by the response to ACTH (t = 0.41, d.f. = 47, p = 0.69), between those iguanas destined to survive and those destined to die (figure 1). There was, however, a difference in the animals' sensitivity to negative feedback (figure 2). Although all animals reduced their corticosterone concentrations in response to DEX injection compared with uninjected controls (t = 3.16, d.f. = 44, p < 0.003), those iguanas destined to survive had a significantly stronger response (t = 2.40, d.f. = 37, p = 0.022).
Figure 1.
Comparison of corticosterone titres in marine iguanas captured prior to an El Niño. Assignment of individual iguanas to alive or dead categories was made subsequent to the El Niño. Baseline, initial corticosterone titres collected at capture (n = 73 and 23 for alive and dead, respectively). Stressed, stress-induced titres collected 30 min after capture and restraint (n = 74 and 22 for alive and dead, respectively). ACTH, HPA capacity assessed from titres collected 30 min after exogenous ACTH injection (n = 37 and 12 for alive and dead, respectively). Each bar represents the mean ± s.e.m.
Figure 2.
Comparison of corticosterone titres following dexamethasone (DEX) administration in marine iguanas captured prior to an El Niño. Assignment of individual iguanas to alive or dead categories was made subsequent to the El Niño. DEX injection tested the efficacy of negative feedback assessed from the lowest titres at 240 min post-capture following injection of exogenous DEX (n = 8, 31 and 7 for control (uninjected), alive and dead, respectively). Each bar represents the mean ± s.e.m. & p < 0.003 compared with all DEX-injected animals. *p = 0.022.
(b). Comparison of pre- and post-El Niño responses in surviving iguanas
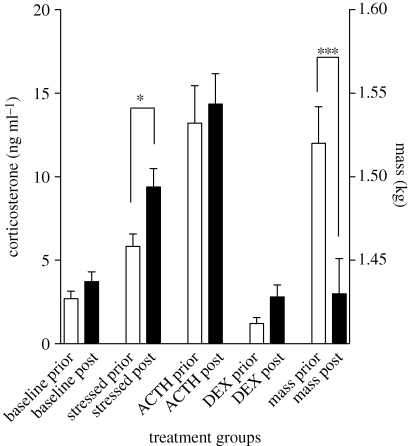
Only one aspect of HPI function changed in surviving iguanas after the El Niño (figure 3). Surviving iguanas had a significantly more robust response to the acute stressor of capture, handling and restraint (F1,29 = 5.09, p = 0.032), but this was not accompanied by changes in baseline corticosterone (F1,27 = 3.35, p = 0.078), the capacity to respond as indicated by response to ACTH (F1,26 = 0.23, p = 0.63) or the sensitivity to negative feedback (F1,12 = 1.47, p = 0.25). However, surviving iguanas lost a significant amount of weight (figure 3) and were approximately 6 per cent lighter after the El Niño (F1,72 = 30.32, p < 0.0001).
Figure 3.
Comparison of changes from prior- to post-El Niño in corticosterone titres and mass in surviving marine iguanas. n = 28, 30, 27, 13 and 73 for baseline, stressed, ACTH, DEX and mass, respectively. See figure 1 for description of treatments. Each bar represents the mean ± s.e.m. *p = 0.032. ***p < 0.0001.
4. Discussion
The iguanas in this study were clearly affected by the food shortage conditions during the El Niño. Not only did 23 per cent of them die, but those that survived weighed less. The El Niño was a major stressor that required an appropriate physiological response to survive. Despite this impact on these largest iguanas, however, this El Niño had little overall impact on the population with low mortality of smaller animals (L. M. Romero & M. Wikelski 2003, personal observations).
The only statistically significant correlation with survival of an El Niño was an individual iguana's ability to terminate the corticosterone stress response. This is the first study to show that a robust negative feedback response is important in survival. Because corticosterone induces negative feedback by binding to receptors in the brain (Dallman et al. 1992), we can speculate that the underlying cause of an attenuated feedback was relatively fewer corticosterone receptors in the brains of those who died.
These data fit an overall framework of stress responses arising from the biomedical literature and a few field studies. Negative feedback is an important aspect of HPI axis function (Dallman et al. 1992). Failure of negative feedback can lead to persistent elevated corticosterone levels that effectively lengthen and strengthen the overall stress response (Romero 2004). Disrupted negative feedback can result in many deleterious effects in free-living baboons (Sapolsky & Altmann 1991; Sapolsky 1992a) and can lead to stress-related disease in humans (Sapolsky 1992b). Ageing also can attenuate negative feedback in reptiles (Moore et al. 2000), but even though we preferentially selected the largest males, it is not clear that we selected the oldest individuals. Size alone cannot be a reliable indicator of age in this species because individuals are known to shrink during El Niño conditions (Wikelski & Thom 2000).
Traditionally, long-term elevations in corticosterone were thought to provide aid in survival, but four lines of converging evidence now suggest the opposite. First, in many species, physiological responses to starvation can be partitioned into the three phases, with phase 1 typified by carbohydrate breakdown, phase 2 by lipid metabolism and phase 3 by protein metabolism (Vleck & Vleck 2002; McCue 2010). Phase 2 of starvation does not elicit increased glucocorticoid release in rats (Dallman et al. 1999), but the transition to phase 3 appears to be triggered by glucocorticoids in penguins (Cherel et al. 1988). Second, previous evidence from marine iguanas indicated that corticosterone only increased when body condition crossed a threshold presumed to be the transition to phase 3 (Romero & Wikelski 2001). This created a strong inverse relationship between both baseline and stress-induced corticosterone titres and survival—the higher the average corticosterone in a population, the higher the mortality. Third, glucocorticoids appear to be the proximate mechanism for death in semelparous species such as salmon (Wingfield & Romero 2001). Elevated glucocorticoids is the proximate mechanism for death, and preventing that elevation increases survival in these species.
The picture emerging from these three lines of evidence is that it is extremely important to delay the onset of phase 3 and the subsequent metabolizing of key proteins (Sapolsky et al. 2000). Higher corticosterone titres might help individuals survive by providing a last-ditch mobilization of protein and thereby extend survival a bit longer in the hope that El Niño conditions end. However, because corticosterone does not appear to play an important role in mobilizing fat during phase 2 of starvation, it appears instead that higher corticosterone titres reflect phase 3 and the failure of homeostatic mechanisms. Consequently, studies that find elevated corticosterone titres with starvation are probably sampling animals in phase 3 that are unlikely to survive. This probably explains the fourth line of evidence: lower, not higher, glucocorticoid concentrations are associated with fitness in many studies (Breuner et al. 2008; Bonier et al. 2009).
Survival of the iguanas that have stronger negative feedback fits into this emerging picture. Robust negative feedback would help conserve energy by damping any increase in corticosterone and thus delaying the transition from phase 2 to phase 3. Given corticosterone's important role in mobilizing proteins for energy (Sapolsky et al. 2000), any elevation in corticosterone could break down more protein, thereby decreasing reserves, and make the animal succumb to starvation earlier than an animal with more robust negative feedback. Consequently, even though short-term increases in glucocorticoids are likely important for survival, turning off that response appears to be a key feature for allowing long-term successful coping with prolonged stressors.
A few other studies have examined the connection between glucocorticoid concentrations and survival. Lower baseline corticosterone levels have been associated with increased survival in some species (e.g. Brown et al. 2005), but there do not appear to be consistent patterns across studies (Bonier et al. 2009). In addition, higher stress-induced glucocorticoid titres are associated with increased survival in some (Cabezas et al. 2007; Angelier et al. 2009), but not all (Blas et al. 2007), species. However, lengthy periods (often years) between correlating corticosterone responses with survival and confounding variables such as recent captivity limit the value of those studies. Currently, data from marine iguanas (this study and Romero & Wikelski 2001) provide the only association of corticosterone responses with survival in response to a single well-defined source of mortality.
Importantly, the El Niño did not appear to significantly alter the functioning of the HPA axis in the surviving iguanas. Although baseline titres, inter-renal capacity and efficacy of negative feedback were all slightly elevated compared with prior to the El Niño, these differences were not significant. Only the response to capture and handling was elevated, and it was higher even than in those iguanas that ultimately died. However, the elevated stress response is probably due to the repeated capture of these animals. Although seasonal changes cannot be excluded, previous work showed that marine iguanas learn that humans can be potential predators and have a more robust response upon subsequent captures (Rödl et al. 2007).
In conclusion, individual differences in HPI axis regulation, particularly in the ability to terminate corticosterone release, could be a substrate for natural selection. Severe El Niño events can exert profound selective pressure on morphological traits (Grant 1986; Wikelski & Trillmich 1997), and these data suggest that El Niño events may also exert selective pressure on physiological traits such as the efficacy of negative feedback.
Acknowledgements
All procedures were approved by the Tufts University Institutional Animal Care and Use Committee.
We thank T. Rödl and S. Berger for help in catching animals and J. M. Reed for statistical advice. We also thank the Charles Darwin Foundation, the Parque Nacional de Galapagos and TAME airlines for logistical assistance. Funding was provided by U.S. National Science Foundation DEB-0545592 to both L.M.R. and M.W. This is contribution no. 1267 of the Charles Darwin Foundation.
References
- Angelier F., Holberton R. L., Marra P. P.2009Does stress response predict return rate in a migratory bird species? A study of American redstarts and their non-breeding habitat. Proc. R. Soc. B 276, 3545–3551 (doi:10.1098/rspb.2009.0868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S., Wikelski M., Romero L. M., Kalko E. K., Roedl T.2007Behavioral and physiological adjustments to new predators in an endemic island species, the Galapagos marine iguana. Horm. Behav. 52, 653–663 (doi:10.1016/j.yhbeh.2007.08.004) [DOI] [PubMed] [Google Scholar]
- Blas J., Bortolotti G. R., Tella J. L., Baos R., Marchant T. A.2007Stress response during development predicts fitness in a wild, long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884 (doi:10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F., Martin P. R., Moore I. T., Wingfield J. C.2009Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- Breuner C. W., Patterson S. H., Hahn T. P.2008In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- Brown C. R., Brown M. B., Raouf S. A., Smith L. C., Wingfield J. C.2005Effects of endogenous steroid hormone levels on annual survival in cliff swallows. Ecol. (Washington DC) 86, 1034–1046 [Google Scholar]
- Cabezas S., Blas J., Marchant T. A., Moreno S.2007Physiological stress levels predict survival probabilities in wild rabbits. Horm. Behav. 51, 313–320 (doi:10.1016/j.yhbeh.2006.11.004) [DOI] [PubMed] [Google Scholar]
- Cherel Y., Robin J. P., Walch O., Karmann H., Netchitailo P., Le Maho Y.1988Fasting in king penguin. I. Hormonal and metabolic changes during breeding. Am. J. Physiol. 254, R170–R177 [DOI] [PubMed] [Google Scholar]
- Cyr N. E., Romero L. M.2009Identifying hormonal habituation in field studies of stress. Gen. Comp. Endocrinol. 161, 295–303 (doi:10.1016/j.ygcen.2009.02.001) [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Akana S. F., Scribner K. A., Bradbury M. J., Walker C.-D., Strack A. M., Cascio C. S.1992Stress, feedback and facilitation in the hypothalamo–pituitary–adrenal axis. J. Neuroendocrinol. 4, 517–526 (doi:10.1111/j.1365-2826.1992.tb00200.x) [DOI] [PubMed] [Google Scholar]
- Dallman M. F., et al. 1999Starvation: early signals, sensors, and sequelae. Endocrinology 140, 4015–4023 (doi:10.1210/en.140.9.4015) [DOI] [PubMed] [Google Scholar]
- Grant P. R.1986Ecology and evolution of Darwin's finches. Princeton, NJ: Princeton University Press [Google Scholar]
- Greenberg N., Wingfield J.1987Stress and reproduction: reciprocal relationships. In Hormones and reproduction in fishes, amphibians, and reptiles (eds Norris D. O., Jones R. E.), pp. 461–503 New York, NY: Plenum Press [Google Scholar]
- Kruuk H., Snell H.1981Prey selection by feral dogs from a population of marine iguanas (Amblyrhynchus cristatus). J. Appl. Ecol. 18, 197–204 (doi:10.2307/2402489) [Google Scholar]
- Laurie W. A.1989Effects of the 1982–1983 El Nino-Southern Oscillation event on marine iguana (Amblyrhynchus cristatus, Bell, 1825) populations in the Galapagos islands. In Global ecological consequences of the 1982–1983 El Nino-Southern Oscillation. (ed. Glynn P.), pp. 121–141 New York, NY: Elsevier [Google Scholar]
- McCue M. D.2010Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 156, 1–18 (doi:10.1016/j.cbpa.2010.01.002) [DOI] [PubMed] [Google Scholar]
- McDonald I. R., Lee A. K., Than K. A., Martin R. W.1986Failure of glucocorticoid feedback in males of a population of small marsupials (Antechinus swainsonii) during the period of mating. J. Endocrinol. 108, 63–68 (doi:10.1677/joe.0.1080063) [DOI] [PubMed] [Google Scholar]
- Moore I. T., Lemaster M. P., Mason R. T.2000Behavioural and hormonal responses to capture stress in the male red-sided garter snake, Thamnophis sirtalis parietalis. Anim. Behav. 59, 529–534 (doi:10.1006/anbe.1999.1344) [DOI] [PubMed] [Google Scholar]
- Rödl T., Berger S., Romero L. M., Wikelski M.2007Tameness and stress physiology in a predator-naive island species confronted with novel predation threat. Proc. R. Soc. B 274, 577–582 (doi:10.1098/rspb.2006.3755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L. M.2001Mechanisms underlying seasonal differences in the avian stress response. In Avian endocrinology (eds Dawson A., Chaturvedi C. M.), pp. 373–384 New Delhi, India: Narosa Publishing House [Google Scholar]
- Romero L. M.2004Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- Romero L. M., Reed J. M.2005Collecting baseline corticosterone samples in the field: is under three minutes good enough? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 140, 73–79 (doi:10.1016/j.cbpb.2004.11.004) [DOI] [PubMed] [Google Scholar]
- Romero L. M., Wikelski M.2001Corticosterone levels predict survival probabilities of Galápagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA 98, 7366–7370 (doi:10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L. M., Wikelski M.2002Exposure to tourism reduces stress-induced corticosterone levels in Galápagos marine iguanas. Biol. Conserv. 108, 371–374 (doi:10.1016/S0006-3207(02)00128-3) [Google Scholar]
- Romero L. M., Wikelski M.2006Diurnal and nocturnal differences in hypothalamic–pituitary–adrenal axis function in Galapagos marine iguanas. Gen. Comp. Endocrinol. 145, 177–181 (doi:10.1016/j.ygcen.2005.09.011) [DOI] [PubMed] [Google Scholar]
- Romero L. M., Reed J. M., Wingfield J. C.2000Effects of weather on corticosterone responses in wild free-living passerine birds. Gen. Comp. Endocrinol. 118, 113–122 (doi:10.1006/gcen.1999.7446) [DOI] [PubMed] [Google Scholar]
- Rubenstein D. R., Wikelski M.2005Steroid hormones and aggression in female Galapagos marine iguanas. Horm. Behav. 48, 329–341 (doi:10.1016/j.yhbeh.2005.04.006) [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M.1992aCortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology 17, 701–709 (doi:10.1016/0306-4530(92)90029-7) [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M.1992bStress, the aging brain, and the mechanisms of neuron death. Cambridge, MA: MIT Press [Google Scholar]
- Sapolsky R. M., Altmann J.1991Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol. Psych. 30, 1008–1016 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U.2000How do glucocorticoids influence stress-responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- Vleck C. M., Vleck D.2002Physiological condition and reproductive consequences in Adelie penguins. Integr. Comp. Biol. 42, 76–83 (doi:10.1093/icb/42.1.76) [DOI] [PubMed] [Google Scholar]
- Wikelski M.2005Evolution of body size in Galapagos marine iguanas. Proc. R. Soc. B 272, 1985–1993 (doi:10.1098/rspb.2005.3205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M., Thom C.2000Marine iguanas shrink to survive El Niño. Nature 403, 37–38 (doi:10.1038/47396) [DOI] [PubMed] [Google Scholar]
- Wikelski M., Trillmich F.1994Foraging strategies of the Galapagos marine iguana (Amblyrhynchus cristatus): adapting behavioral rules to ontogenetic size change. Behaviour 128, 255–279 (doi:10.1163/156853994X00280) [Google Scholar]
- Wikelski M., Trillmich F.1997Body size and sexual size dimorphism in marine iguanas fluctuate as a result of opposing natural and sexual selection: an island comparison. Evolution 51, 922–936 (doi:10.2307/2411166) [DOI] [PubMed] [Google Scholar]
- Wikelski M., Carrillo V., Trillmich F.1997Energy limits to body size in a grazing reptile, the Galapagos marine iguana. Ecology 78, 2204–2217 (doi:10.1890/0012-9658(1997)078[2204:ELTBSI]2.0.CO;2) [Google Scholar]
- Wikelski M., Romero L. M., Snell H. L.2001Marine iguanas oiled in the Galápagos. Science 292, 437–438 (doi:10.1126/science.292.5516.437c) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Romero L. M.2001Adrenocortical responses to stress and their modulation in free-living vertebrates. In Handbook of physiology; Section 7: the endocrine system; Volume IV: coping with the environment: neural and endocrine mechanisms (eds McEwen B. S., Goodman H. M.), pp. 211–234 New York, NY: Oxford University Press [Google Scholar]
- Wingfield J. C., Vleck C. M., Moore M. C.1992Seasonal changes of the adrenocortical response to stress in birds of the Sonoran Desert. J. Exp. Zool. 264, 419–428 (doi:10.1002/jez.1402640407) [DOI] [PubMed] [Google Scholar]