Abstract
The behavioural demands of group living and foraging have been implicated in both evolutionary and plastic changes in brain size. Desert locusts show extreme phenotypic plasticity, allowing brain morphology to be related to very different lifestyles in one species. At low population densities, locusts occur in a solitarious phase that avoids other locusts and is cryptic in appearance and behaviour. Crowding triggers the transformation into the highly active gregarious phase, which aggregates into dense migratory swarms. We found that the brains of gregarious locusts have very different proportions and are also 30 per cent larger overall than in solitarious locusts. To address whether brain proportions change with size through nonlinear scaling (allometry), we conducted the first comprehensive major axis regression analysis of scaling relations in an insect brain. This revealed that phase differences in brain proportions arise from a combination of allometric effects and deviations from the allometric expectation (grade shifts). In consequence, gregarious locusts had a larger midbrain∶optic lobe ratio, a larger central complex and a 50 per cent larger ratio of the olfactory primary calyx to the first olfactory neuropile. Solitarious locusts invest more in low-level sensory processing, having disproportionally larger primary visual and olfactory neuropiles, possibly to gain sensitivity. The larger brains of gregarious locusts prioritize higher integration, which may support the behavioural demands of generalist foraging and living in dense and highly mobile swarms dominated by intense intraspecific competition.
Keywords: phenotypic plasticity, phase change, allometry, brain scaling, mushroom body, insect brain
1. Introduction
Evolutionary changes in the size of the brain and in the proportions of its component regions are driven by behavioural demands and limited by metabolic costs and evolutionary history (Striedter 2005; Niven & Laughlin 2008). Brain size and proportions also show phenotypic plasticity—changes in response to individual experience—in both vertebrates and invertebrates (Bennett et al. 1964; Heisenberg et al. 1995). Evolutionary change and phenotypic change during an individual's lifetime are closely interrelated through common proximate mechanisms (West-Eberhard 2003).
The behavioural demands of group living and foraging have been implicated as major, although not necessarily independent, factors in vertebrate brain evolution (Harvey et al. 1980; Ratcliffe et al. 2006; Byrne & Bates 2007a; Dunbar & Shultz 2007). Sociality and foraging are also correlated to intraspecific differences in brain region size in insects, most notably in hymenopterans that display division of labour (e.g. Withers et al. 1993; Durst et al. 1994; Gronenberg et al. 1996; Molina & O'Donnell 2007, 2008). The desert locust is an insect that shows extreme phenotypic plasticity in morphology (electronic supplementary material, figure S1), physiology and behaviour (Uvarov 1966; Pener & Simpson 2009). The latter entails profound changes in foraging strategy and a shift from solitary to communal living. Locusts therefore present an opportunity to investigate the relationship between brain morphology and two very different lifestyles in the same species. In the wild, the plasticity arises in response to changing population density driven by large fluctuations in the quantity and spatial distribution of vegetation (Uvarov 1966; Collett et al. 1998; Despland et al. 2004). When population densities are low (typically 1–100 animals ha−1; Woldewahid et al. 2004), locusts occur in the solitarious phase. Solitarious locusts are cryptic in coloration and behaviour, active during dusk and dawn, show a narrow dietary range, and, most importantly, actively avoid other locusts. Forced crowding triggers a rapid transformation to gregarious behaviour that is mediated by serotonin and characterized by increased activity and mutual attraction (Anstey et al. 2009). Prolonged crowding eventually results in the full gregarious phase that is aposematically coloured (Sword 1999), smaller in body size, diurnal and highly mobile. Local aggregations may further coalesce into migrating swarms that cover several hundred km2 with densities exceeding 100 locusts m−2. Travelling up to 100 km d−1 to escape local host plant depletion, they encounter a wider range of plants than the comparatively sedentary solitarious phase and regularly arrive in regions that do not support their preferred host plants. They also use a wider range of plant species in a given habitat (Despland 2005). Both phases balance their nutritional intake across a range of foods in order to achieve an optimum intake of macronutrients, but gregarious locusts need to do this across a wider range of potential food plants and may therefore face increased foraging complexity (Simpson et al. 2002; van der Zee et al. 2002). Conversely, while certain social structures correlate with the evolution of large brains, group size per se is not necessarily a useful indicator of the cognitive demands of group living (Byrne & Bates 2007b; Dunbar & Shultz 2007). For locusts, living in a swarm might in fact ease the cognitive demands on individuals through reduced predation risk (Sword et al. 2005) and collective decision-making. Nevertheless, the environment of gregarious locusts is dominated by the presence of other locusts and they experience fierce competition for food and mates, even extending to a substantial risk of cannibalism (Bazazi et al. 2008). Studies on identified neurons in the two phases suggest that in gregarious locusts they maintain full functionality on repeated stimulation and maximize signal-to-noise in the face of an inherently highly complex visual environment (Matheson et al. 2004; Rogers et al. 2007, 2010).
We therefore investigated how the brains of solitarious and gregarious locusts differ in size and whether there is evidence for differential investment in different brain functions as indicated by the relative proportions of different brain regions. Where brains differ in overall size, differences in proportions can arise from grade shifts and/or as consequences of nonlinear (allometric) scaling between the component regions (Striedter 2005). In insects, the contribution of allometry has received little attention and proportional differences were thus usually implicitly interpreted as grade shifts (but see Mares et al. 2005).
2. Material and methods
(a). Animals
Male desert locusts (Schistocerca gregaria Forskål) of both phases were bred at the Department of Zoology, University of Oxford and the Department of Zoology, University of Cambridge, UK. Gregarious-phase locusts were taken from colonies that had been maintained under crowded conditions for many generations. Solitarious-phase locusts were produced from these gregarious stocks by isolation for three generations as described in Roessingh et al. (1993). Both phases were maintained on an identical diet of fresh seedling wheat and dry wheat germ under a 12 : 12 h photoperiod. Locusts develop through either five or six instars, which in the adult is manifest in six or seven dark vertical stripes across the eye. The additional moult occurs more frequently in the solitarious phase, but is not a phase character (electronic supplementary material, Supplemental Methods). All locusts used in this study had reached adulthood in five moults.
(b). Immunofluorescence staining and stereology
Synaptic neuropiles were revealed in whole-brain preparations by immunofluorescence staining against the presynaptic protein synapsin (monoclonal antibody 3C11; obtained from DSHB, University of Iowa, IA, USA; Klagges et al. 1996). Brains were fixed in situ in zinc-formaldehyde (Ott 2008). The staining, clearing and mounting techniques used are described in detail in Ott (2008). The volumes of synapsin-immunofluorescent neuropile regions were measured by point-counting stereology on confocal planes as described in the electronic supplementary material, Supplemental Methods. The results are based on 10 solitarious and nine gregarious brains. In one solitarious preparation, the pigmented basal layer of the retina had not been completely removed and cast a shadow that precluded accurate measurement of lamina size. The sample size (listed in the electronic supplementary material, tables S1–S3) was therefore 9 per phase for the lamina, optic lobe and total brain volumes, and for proportional volumes of brain regions relative to total brain.
(c). Statistical analysis
Statistical analysis was carried out in the R v. 2.6.1 framework. Whether brain size is predicted by body weight and/or phase was tested by analysis of covariance (ANCOVA), with total brain size as a dependent variable, bodyweight as an independent variable and phase as a fixed factor (full model; F3,13 = 5.301, r2 = 0.5502, p = 0.0132). In this model, we included a phase–body weight interaction term to determine whether the scaling relationship between body weight and brain size might differ between phases, but the interaction term was non-significant (F1,13 = 0.6868, p = 0.422). Therefore, there was no evidence that the scaling between brain and body size differed between phases. In consequence, the interaction was dropped from the model (F2,14 = 7.65, r2 = 0.454, p = 0.00568).
The scaling relationship between two brain regions x, y was modelled as y = κxβ. This is the standard allometric equation widely used in scaling studies (Striedter 2005). It is often written as log y = α+β log x (where α = log κ), because logarithmic transformation permits estimating α and β from the intercept and slope of a regression line; the terms allometric intercept and slope are therefore commonly used for α and β. We estimated αsol, αgreg and βsol, βgreg in solitarious and gregarious brains by fitting bivariate standardized major axis lines to the log-transformed variates (smatr v. 2.1 for R). For statistical inference, the following tests implemented in the smatr package were used as recommended by Warton et al. (2006): a log-likelihood test for common slope (H0: βsol = βgreg), and Wald tests for common elevation (H0: αsol = αgreg) and for no shift along the major axis (H0: equal axis means across groups). βsol and βgreg did not differ significantly at p = 0.1 for any two neuropiles and lines were therefore fitted with a common slope β.
3. Results
(a). Phase differences in brain size and body size
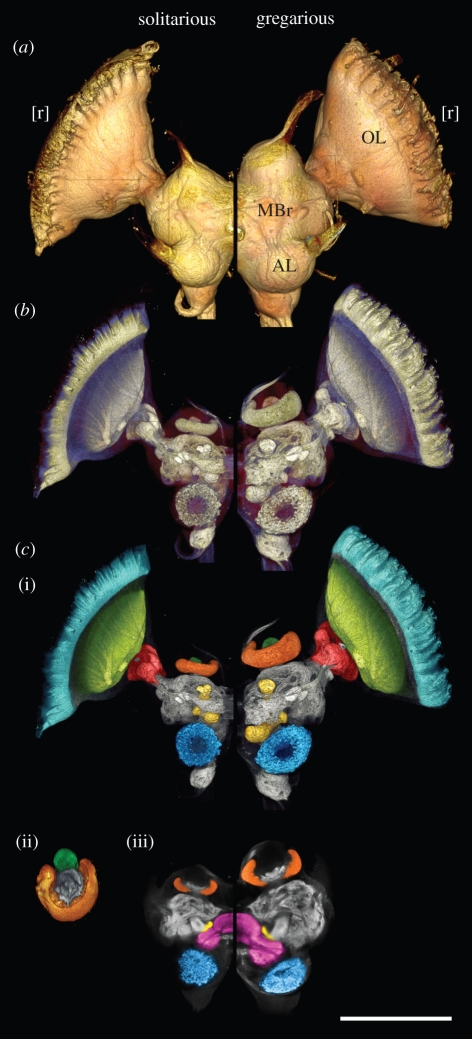
The locust brain comprises a midbrain flanked by paired optic lobes (figure 1a). The midbrain is itself formed from two distinct, bilaterally symmetrical halves. A narrow isthmus separates each optic lobe from the midbrain. Distally, the optic lobe attaches directly to the retina of the compound eye (removed in figure 1a). Figure 1a shows half the brain of a solitarious male (left) and of a gregarious male (right) at the same scale; the two animals were very closely matched in body size (body weight: solitarious, 1.28 g; gregarious, 1.26 g; head width, solitarious, 5.99 mm; gregarious, 5.93 mm). It is immediately apparent that the brain of the gregarious animal was considerably larger.
Figure 1.
Half-brains of a solitarious locust (left) and gregarious locust (right) in frontal view to the same scale (scale bar, 1 mm). The animals were of near-identical body size. (a) Surface view. The gregarious brain is substantially larger, but its optic lobe (OL) and antennal lobe (AL) are proportionally smaller relative to the midbrain (MBr). The retinae, here removed, would occupy the space [r] adjacent to the outer surface of the optic lobe. (b) The internal neuropiles as revealed by synapsin immunofluorescence. (c) The neuropile regions considered in this study. (i) The visual neuropiles of the optic lobe comprise the lamina (cyan), the medulla (lime) and the lobula (red). The midbrain houses the olfactory neuropiles: the antennal lobe (blue), and the primary calyx (orange) and lobes (yellow) of the mushroom body. (ii) Calyx of the mushroom body tilted forward by approximately 45° to show the accessory calyx (dark green). (iii) Frontal half of midbrain removed to expose the central complex (pink).
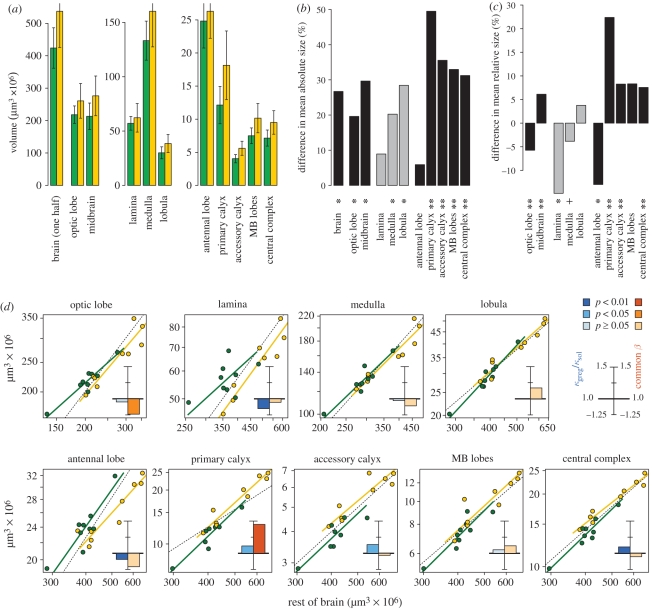
The average neuropile volume of the brain (figure 1b) was 27 per cent larger in gregarious animals (figure 2a,b; electronic supplementary material, table S1). Differences in body size did not explain this difference in brain size. Indeed, gregarious locusts were on average 21 per cent smaller (body mass: 1.19 ± 0.186 g, n = 19) than solitarious (1.51 ± 0.160 g, n = 19; t = −5.61, p = 2.33 × 10−6). Within each phase, heavier animals tended to have larger brains (ANCOVA, F1,14 = 4.865, p = 0.0446), but the brains of gregarious locusts were significantly larger than expected from their body weight (F1,14 = 14.61, p = 0.00187).
Figure 2.
Phase differences in absolute brain size and in the proportion of the brain occupied by different neuropile regions. (a) Mean absolute volumes for one side of the brain in solitarious locusts (green) and gregarious locusts (yellow); error bars indicate standard deviations. (b) Differences in mean absolute neuropile volumes in gregarious over solitarious locusts (**p < 0.01; *p < 0.05; electronic supplementary material, table S1). (c) Differences in the mean proportion of the total brain neuropile occupied by different neuropile regions in gregarious over solitarious brains (**p < 0.01; *p < 0.05; +p < 0.1; electronic supplementary material, table S2). (d) Phase differences in brain proportions arise from a combination of grade shifts and allometries in the scaling of different neuropile regions (y-axes) against the rest of the brain (x-axes). Data for solitarious (green) and gregarious (yellow) locusts are shown with separate standardized major axis fit lines; phase differences in slope were non-significant in all cases (electronic supplementary material, table S3). Dashed lines of slope 1.0 (isometric scaling) are shown for reference. The insets summarize the scaling relationships: blue bars indicate grade shifts by showing the deviation of the grade shift index (κgreg/κsol) from 1, and orange bars indicate allometry by showing the deviation of the estimated common allometric slope β from 1.
(b). Phase differences in the proportional size of brain regions
Next, we examined whether the two phases allocate different proportions of their total brain neuropile to different brain regions. In the optic lobe, we measured the three visual neuropiles arranged in series from distal to proximal: the lamina (coloured cyan in figure 1c), which receives direct input from photoreceptor cells of the retina; the medulla (lime in figure 1c); and the lobula complex (red in figure 1c), which attaches to the midbrain. In the midbrain, we measured the neuropile of the antennal lobe (AL; blue in figure 1c), which receives the axons of the olfactory receptor neurons (ORNs) on the antenna, and three major neuropiles of the mushroom body (MB): the olfactory primary calyx (orange in figure 1c); the gustatory accessory calyx (dark green in figure 1c); and the sum total of the lobes (yellow in figure 1c), which contain the axons of the intrinsic Kenyon cells (KCs) of the MB. Lastly, we also included the central complex (CC; magenta in figure 1c), a higher motor control region spanning the midline.
Only two of these eight neuropiles showed no significant phase difference in absolute size, and both were primary sensory neuropiles: the lamina and the AL. Neuropiles that were larger in gregarious locusts differed in the extent to which they followed the overall expansion of the brain (figure 2a,b; electronic supplementary material, table S1). Most extreme among them was the primary calyx of the MB, with an average volume 49 per cent larger than in solitarious animals. Consequently, average brain proportions were also different between solitarious and gregarious locusts (figure 2c; electronic supplementary material, table S2). Relative to the total brain neuropile, gregarious locusts had a 15 per cent smaller lamina and 13 per cent smaller AL, but a 22 per cent larger MB primary calyx, a 13 per cent larger accessory calyx and an 8 per cent larger CC. The optic lobe : midbrain ratio was on average 11 per cent smaller in gregarious brains, but trends for a smaller medulla, a larger lobula and larger MB lobes relative to total brain size in gregarious locusts were not significant.
(c). The contribution of allometry to phase differences
Because gregarious brains were on average larger, we considered whether allometry might contribute to the phase differences in brain proportions. We modelled the scaling between two brain regions x and y by the allometric equation y = κ xβ, and estimated κ and β by standardized major axis regression (electronic supplementary material, table S3). Because β was not significantly different between phases for any of the scaling relations, differences in brain proportions arose in two (not mutually exclusive) ways. First, κ may be different in solitarious and gregarious locusts. In this case, animals with matching sizes of brain region x but of different phase will have different sizes of brain region y, and in plots of log y against log x, the two phases fall on separate lines. When comparing between species, such a deviation from allometric expectation is known as a grade shift (Striedter 2005), and we adopt this term here for discrete differences between phases. We estimate the extent of the shift by the grade shift index (gsi), κgreg/κsol, an estimate of how much larger region y is in gregarious brains for a given size of region x. Second, if β ≠ 1, the relationship between brain regions x and y is allometric, meaning that the proportion y : x changes with size. If brain region x is larger on average in one phase, a phase difference in the average ratio y : x will result, even though both solitarious and gregarious locusts exist along the same allometric continuum. In double-log plots, this shows as a shift between phases along a common allometric axis of slope β ≠ 1. Throughout the text below, 95% confidence intervals for β are indicated by [βlower, βupper], and significant differences between phases in their position along the common allometric axis by pd.
We first analysed the scaling of each neuropile region against the rest of the brain (figure 2d) and found that the differences in average brain proportions seen in figure 2c do indeed arise from a combination of grade shifts and allometries.
(d). Visual pathway
In both phases, animals with larger midbrains had disproportionally small optic lobes (β = 0.76 [0.60, 0.97]). The allometry arises because the medulla, which occupies about 60 per cent of the optic lobe, is disproportionally small in locusts with larger midbrains (β = 0.77 [0.60, 0.98]). Since gregarious locusts had, on average, larger midbrains and optic lobes (pd = 0.017), this allometry contributed to their 11 per cent smaller average optic lobe∶midbrain ratio. There was no grade shift between the total optic lobe and midbrain volumes between phases: solitarious and gregarious locusts of matching midbrain size showed no significant difference in the size of their optic lobes (gsi = 0.95, p = 0.19). Gregarious locusts did, however, have smaller laminae than solitarious locusts of matching brain size (gsi = 0.85, p = 0.0071).
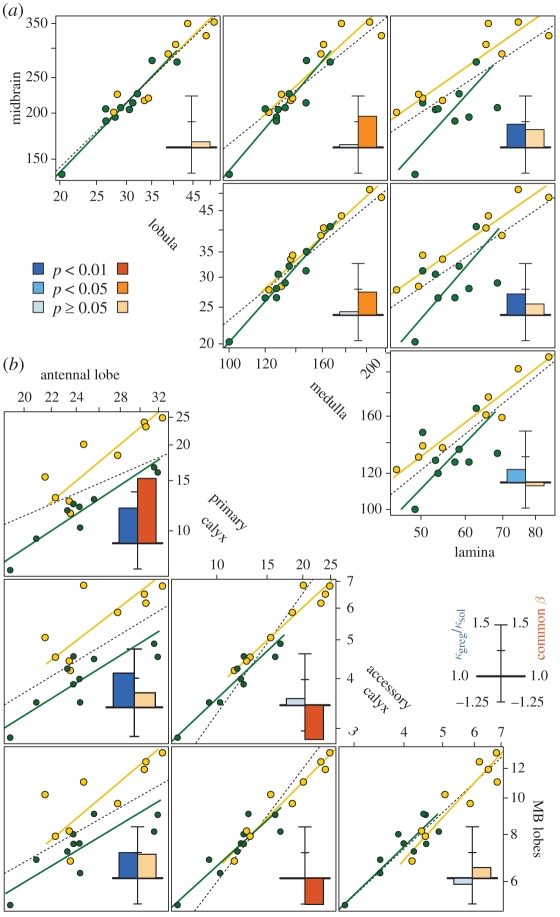
Phase also affected the relative proportions of the neuropiles within the optic lobe (figure 3a). Gregarious locusts had 12 per cent more medulla per unit volume of lamina (i.e. the primary sensory neuropile, the lamina, was proportionally smaller; t16 = 2.33, p = 0.033), a difference fully explained by a pronounced grade shift (gsi = 1.13, p = 0.019). Gregarious locusts also had an average of 7 per cent more lobula per unit volume of medulla (t17 = 2.53, p = 0.021), but there was no evidence for a grade shift (gsi = 1.03, p = 0.31). The difference was instead explained by an allometric relationship between the medulla and the lobula (β = 1.22 [1.04, 1.46]; pd = 0.023). There was no significant phase difference in midbrain size for a given lobula size (gsi = 0.98, p = 0.94), and the scaling between the two was approximately isometric (β = 1.06 [0.88, 1.30]).
Figure 3.
Scaling (a) between visual neuropiles and the midbrain and (b) between olfactory and gustatory (accessory calyx) neuropiles within the midbrain in solitarious (green) and gregarious (yellow) locusts. All units are µm3 × 10−6. See figure 2d for further explanation and electronic supplementary material, table S3, for statistical analysis.
(e). Olfactory pathway and other midbrain neuropiles
Gregarious locusts had smaller ALs than solitarious locusts of matching brain size (gsi = 0.905, p = 0.0089). This grade shift accounts for most of the 13 per cent smaller average AL : brain ratio. In addition, the negative allometry of the AL against the rest of the brain was weakly supported (β = 0.79 [0.61, 1.04]; pd = 0.063). The phase difference in the AL : midbrain ratio was even more prominent: gregarious locusts had an average of 18 per cent less AL per unit volume midbrain than solitarious locusts. This difference arose from a pronounced grade shift (gsi = 0.87; p = 0.00053) that was compounded by allometry; the AL was disproportionally small in large midbrains, irrespective of phase (β = 0.71 [0.56, 0.91]), but gregarious locusts had larger midbrains on average (even though a shift along the common allometric axis was only weakly supported; pd = 0.092).
From the AL, axons of projection neurons (PNs) transmit olfactory information to the primary calyx of the MB. One might therefore expect that the proportionally smaller ALs in gregarious brains would feed forward into smaller primary calyces. Against this expectation, in gregarious locusts, the primary calyx was on average 22 per cent larger relative to the brain. A grade shift accounted only in part for this difference (gsi = 1.11, p = 0.023). In both phases, however, the primary calyx was also disproportionally large in larger brains (β = 1.45 [1.22, 1.73]). This pronounced allometry, together with the larger brains and primary calyces in gregarious locusts (pd = 0.0044), accounted for the remainder of the difference. When the primary calyx is considered in relation to just the AL, the grade shift and the allometry become even more pronounced (figure 3b). The primary calyx was substantially larger in gregarious brains of matching AL size (gsi = 1.34, p = 2.16 × 10−6), and extreme positive allometry over the AL (β = 1.63 [1.29, 2.09]) is likely to further widen the phase difference (pd = 0.053). The result was a 41 per cent greater primary calyx : AL ratio in gregarious compared with solitarious brains (t17 = 4.868, p = 0.00014).
Positive allometry of the primary calyx was also evident against other midbrain neuropiles. This is particularly noteworthy with respect to the MB lobes (β = 1.34 [1.11, 1.63]; figure 3b), because they contain the axons of the KCs, whereas the calyx contains their dendrites (§4). We also compared the olfactory primary calyx in relation to the medulla, a visual neuropile. A grade shift towards larger primary calyces was only weakly supported (gsi = 1.11, p = 0.11), but extreme allometry (β = 1.62 [1.27, 2.09]; pd = 0.011) resulted in 24 per cent more primary calyx per unit volume medulla in gregarious locusts (t17 = 3.40, p = 0.0034). Remarkably, these strong positive allometries were specific to the primary calyx. The accessory calyx, which receives gustatory input from the mouthparts (Farris 2008a), showed a grade shift towards larger size in gregarious brains (gsi = 1.14, p = 0.012), but scaled approximately isometrically with the brain (β = 0.97 [0.74, 1.28]). The lobes of the MB showed no significant grade shift between phases (gsi = 1.05, p = 0.35) and scaled approximately isometrically with the brain (β = 1.12 [0.86, 1.45]). The 8 per cent larger average CC : brain ratio of gregarious locusts was explained entirely as a grade shift (gsi = 1.10; p = 0.0018).
4. Discussion
Desert locusts show pronounced phase-related phenotypic plasticity in both absolute and relative investment in brain tissue. Long-term gregarious locusts were smaller than long-term solitarious locusts, yet had brains nearly 30 per cent larger. Whereas locust swarms are notorious for their catholic appetite, solitarious locusts show markedly narrower dietary preferences and associate with specific host plants (Woldewahid et al. 2004; Despland 2005). Solitarious locusts also reject food that contains toxic secondary plant compounds, whereas such food is readily eaten by gregarious locusts (Despland & Simpson 2005). Yet gregarious locusts are not indiscriminate eaters; they tightly regulate their nutrient intake when given the opportunity (Simpson et al. 2002). The catholic diet adopted by gregarious locusts allows them to have longer-term trade-offs in dietary choice in the absence of their preferred host plants; their mobile lifestyle leads to an increased probability of encountering foods of complementary composition in the future. The dietary intake strategy of solitarious locusts strongly suggests they eat to minimize the immediate imbalance in macronutrient uptake. The larger brain of gregarious locusts, and particularly their larger MBs, may support their more generalist foraging strategy, a behavioural context invoked in the evolution of large brains in vertebrates and, more recently, in insects: dietary generalism in scarab beetles correlates with anatomical elaboration of the MB (Farris & Roberts 2005; Farris 2008b). At the phenotypic level, MB volume increases with foraging experience in the workers of eusocial bees and ants (Withers et al. 1993; Durst et al. 1994; Gronenberg et al. 1996; Ismail et al. 2006), as it does in the solitary bee Osmia (Withers et al. 2008). Foraging is not, however, the only behavioural demand that may lead to increased brain size. In primitively eusocial wasps, for example, larger MBs are associated not with foraging experience but with social dominance (Molina & O'Donnell 2007, 2008). More generally, phenotypic changes in brain morphology in response to population density have been reported in vertebrates (e.g. teleost fish: Gonda et al. 2009) and insects (e.g. Drosophila melanogaster: Heisenberg et al. 1995). The behavioural demands of group living and foraging may therefore appear as prominent drivers of both phenotypic and evolutionary change in brain size. In locusts, foraging and group living are further entwined because the interactions with conspecifics that they experience in a swarm include intense food competition, even leading to widespread cannibalism, and this intraspecific predation is a major factor in driving the swarm forwards (Bazazi et al. 2008). An indirect consequence of group living is that the close proximity of so many other locusts and the olfactory, visual and tactile stimuli they produce may well decrease the salience of important non-locust stimuli, making the task of the central nervous system to extract these from the ambient noise intrinsically much more difficult.
(a). Neurogenesis as a source of volume differences
Differences in neuropile volume may reflect a greater number of neurons and/or an increase in neuron size (through increased branching, longer or wider neurites, or a greater number of synaptic spines). Many types of sensory neuron increase in number during post-embryonic development. In hemimetabolous insects such as locusts, their numbers can thus be subject to phenotypic plasticity depending on sensory experience (Rogers & Simpson 1997) and phase (Heifetz & Applebaum 1995; Rogers et al. 2003). Within the central nervous system, post-embryonic neurogenesis in the optic lobe (locusts: Anderson 1978) and in the MB (crickets: Malaterre et al. 2002) yields variation in cell number within some classes of interneurons. Many interneurons show absolute constancy in number between individuals, however, at least within the gregarious phase (Burrows 1996). For such neurons, numeric differences between phases might still arise through parental epigenetic programming, because phase state is passed on to the developing embryo (Simpson & Miller 2007).
(b). Allometry and grade shifts as causes for changes in brain proportions
The extent to which allometry contributes to differences in insect brain proportions has received little attention to date. Mares et al. (2005) studied brain scaling in honeybees and bumble-bees, identifying allometry for the CC in bumble-bees and for the MB lobes in both species. Our analysis reveals that the extensive differences in brain proportions between phases arise from a combination of allometric effects and grade shifts. Allometry contributes markedly to phase differences because of the considerably greater average brain size in gregarious locusts. Brain size affects brain proportions in the same way in both phases, however; with increasing brain size, locusts acquire a disproportionally larger midbrain, a larger lobula over the medulla and, most notably, a larger primary calyx. It is unknown to what extent this increasing over-representation of higher centres is adaptive or dictated by developmental or connectivity constraints that arise from growing a larger brain. Grade shifts, by contrast, contribute towards smaller primary sensory neuropiles (lamina and AL) in gregarious locusts, and also towards their larger accessory calyx and CC.
(c). Visual and olfactory neuropiles
In the visual system, gregarious locusts showed increased proportional investment in the second (medulla) and the third visual neuropile (lobula) over the first (lamina). This emphasis on higher visual centres in gregarious locusts is in striking contrast to the fact that they have smaller eyes (Dirsh 1953; Rogers et al. 2010). The increased lobula : lamina and medulla ratio in the gregarious phase is possibly linked to the more complex optic flowfields, and the increased risk of collision encountered in a swarm, particularly during flight (Matheson et al. 2004). In the olfactory system, gregarious locusts have fewer olfactory sensilla on the antenna (Locusta migratoria: Greenwood & Chapman 1984; S. gregaria: Ochieng' et al. 1998). Since the axons of the ORNs project into the AL, one would predict the AL to be smaller in gregarious locusts. We found that gregarious locusts do indeed have ALs more than 10 per cent smaller than solitarious locusts of matching brain size. This effect of phase on AL size is, however, masked by the effect of phase on the overall size of the brain, which explains why Anton et al. (2002) found no phase difference in AL diameter.
(d). Mushroom body
The primary calyx, which is composed mainly of PN axon terminals and KC dendrites, showed the most dramatic phase-related difference (50% absolute) of all brain regions investigated. Its greater size in gregarious locusts contrasts strongly with their lesser number of olfactory sensilla and their smaller ALs. For a given AL size, the primary calyx was 34 per cent larger in gregarious brains. The larger calyx may confer increased olfactory discrimination or better associative learning performance. Both would be adaptive when faced with a wide range of host plants, and hence with the need to balance nutrient intake as opportunities arise (Simpson et al. 2002). Conceivable structural causes of the enlargement are an increased number of KCs and increased branching of PN terminals and KC dendrites. Increased KC branching is implicated in the experience-driven expansion of the calyx in forager honeybees (Farris et al. 2001; Ismail et al. 2006). In crickets, social enrichment, olfactory stimuli and visual stimuli all promote adult KC neurogenesis (Scotto-Lomassese et al. 2002). The effect of group-rearing on KC neurogenesis has not yet been investigated in locusts, where KC neurogenesis shuts down in adults. Given the phylogenetic proximity of locusts and crickets, however, it is likely that crowd-reared locusts may acquire more KCs than solitarious locusts as nymphs.
A greater number of KCs in the gregarious phase cannot, however, by itself explain why the calyx increases disproportionally with MB size in both phases. A similar disproportional increase in the calyx over the lobes also occurs in bees (Mares et al. 2005) and may therefore reflect a general scaling rule in the insect MB. We propose that the allometry may arise from different wiring overheads in the calyx and lobes. If larger MBs contain more KCs, each additional KC will obviously increase the calyx by some volume, and the lobes by some (other) volume. Regardless of what these two volumes are (both may well be larger in larger MBs), as long as their ratio is fixed, adding KCs would expand the calyx and lobes isometrically (β = 1). The pronounced allometry (β = 1.34) suggests that the total branch length in the calyx increases disproportionally over that in the lobes. PN axon terminals and KC dendrites are highly branched, whereas KC axons form a tight parallel bundle and only have very short collaterals that contact arbours of extrinsic neurons. Because of the very different anatomy (which presumably reflects a different type of connectivity pattern), the wiring overhead for integrating additional KC dendrites in the calyx circuitry may increase at a greater rate compared with that for integrating additional KC axons in the circuitry in the lobes. This hypothesis also tallies with our observation that the accessory calyx showed no significant allometry over the lobes. The KCs of the accessory calyx are generated during embryogenesis, so that their number cannot respond to sensory experience, and in the absence of large variation in neuron number, wiring overheads are inconsequential for how the calyx scales over the lobes. The accessory calyx receives input from gustatory sensilla on the mouthparts via PNs in the lobus glomerulatus (Ernst et al. 1977; Farris 2008a). The number of such sensilla is clearly plastic (Rogers & Simpson 1997) and, on the legs at least, higher in gregarious locusts (Rogers et al. 2003).
(e). Central complex
The CC has long been implicated in motor coordination, but its functions are only now becoming more clearly understood. In cockroaches, which rely heavily on their antennae to negotiate obstacles when walking, the CC contains neurons that receive antennal mechanosensory input, and mechanical CC lesions impair turning and climbing over blocks (Ridgel et al. 2007; Ritzmann et al. 2008). Genetic lesions to the CC in Drosophila affect visually guided locomotion, spatial working memory and visual pattern memory (Strauss 2002; Liu et al. 2006; Neuser et al. 2008). Some CC neurons in the locust are sensitive to polarized light, suggesting a role in sky compass navigation (Heinze & Homberg 2007; Heinze et al. 2009). The larger CC in gregarious locusts could subserve an increased requirement for compass navigation during swarming migration, although there is as yet no evidence that locusts actively navigate in this situation.
(f). Biological significance of phase-related brain plasticity
Phase change affects virtually all aspects of locust biology, from body size, shape and coloration to endocrinology, metabolism and many different aspects of behaviour. To these, we may now add extreme phenotypic changes in brain size and proportions. For many of these characters, similar phenotypic responses are known to occur in non-swarming grasshoppers, in other insects and in vertebrates. They are thus manifestations of ubiquitous mechanisms of plasticity shared by many different animals. In phase change, these mechanisms have been recruited to yield plasticity that is extreme in extent, yet coordinated. The sheer extent of changes in brain structure between phases suggests that it has no simple causal explanation, but arises from multiple differences in diet, foraging strategy, mobility and environmental complexity engendered by the change to the swarming gregarious phase. In the brain, solitarious locusts privilege primary visual and olfactory neuropiles (coupled with larger eyes and antennae), suggesting a focus on sensitivity and distance sensation compared with gregarious locusts, whose sensory range is strongly curtailed by the presence of many other locusts in their immediate environment. The sensory complexity of this environment may require more complex processing of sensory signals; accordingly, gregarious locusts show a general trend towards emphasizing higher centres over primary sensory neuropiles. Unlike the expansion of sensory neuropiles, which will increase sensitivity or resolution, expansion of higher centres may allow qualitatively different behaviours to emerge (Chittka & Niven 2009). The present findings invite the identification of the behavioural consequences of these size differences.
Acknowledgements
This work was supported by a University Research Fellowship to S.R.O. from the Royal Society (London, UK) and grant BB/D018854/1 from the BBSRC (UK). We thank our Cambridge colleagues Malcolm Burrows and Jeremy Niven for valuable comments.
References
- Anderson H.1978Postembryonic development of the visual system of the locust, Schistocerca gregaria. I. Patterns of growth and developmental interactions in the retina and optic lobes. J. Embryol. Exp. Morph. 45, 55–83 [PubMed] [Google Scholar]
- Anstey M. L., Rogers S. M., Ott S. R., Burrows M., Simpson S. J.2009Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 323, 627–630 (doi:10.1126/science.1165939) [DOI] [PubMed] [Google Scholar]
- Anton S., Ignell R., Hansson B. S.2002Developmental changes in the structure and function of the central olfactory system in gregarious and solitary desert locusts. Microsc. Res. Tech. 56, 281–291 (doi:10.1002/jemt.10032) [DOI] [PubMed] [Google Scholar]
- Bazazi S., Buhl J., Hale J. J., Anstey M. L., Sword G. A., Simpson S. J., Couzin I. D.2008Collective motion and cannibalism in locust migratory bands. Curr. Biol. 18, 735–739 (doi:10.1016/j.cub.2008.04.035) [DOI] [PubMed] [Google Scholar]
- Bennett E. L., Diamond M. C., Krech D., Rosenzweig M. R.1964Chemical and anatomical plasticity of brain. Science 146, 610–619 (doi:10.1126/science.146.3644.610) [DOI] [PubMed] [Google Scholar]
- Burrows M.1996The neurobiology of an insect brain. Oxford, UK: Oxford University Press [Google Scholar]
- Byrne R. W., Bates L. A.2007aSociality, evolution and cognition. Curr. Biol. 17, R714–R723 (doi:10.1016/j.cub.2007.05.069) [DOI] [PubMed] [Google Scholar]
- Byrne R. W., Bates L. A.2007bBrain evolution: when is a group not a group? Curr. Biol. 17, R883–R884 (doi:10.1016/j.cub.2007.08.018) [DOI] [PubMed] [Google Scholar]
- Chittka L., Niven J. E.2009Are bigger brains better? Curr. Biol. 19, R995–R1008 (doi:10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- Collett M., Despland E., Simpson S. J., Krakauer D. C.1998Spatial scales of desert locust gregarization Proc. Natl Acad. Sci. USA 95, 13 052–13 055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despland E.2005Diet breadth and anti-predator strategies in desert locusts and other Orthoptera. J. Orthoptera Res. 14, 227–233 (doi:10.1665/1082-6467(2005)14[227:DBAASI]2.0.CO;2) [Google Scholar]
- Despland E., Simpson S. J.2005Food choices of solitarious and gregarious locusts reflect cryptic and aposematic antipredator strategies. Anim. Behav. 69, 471–479 (doi:10.1016/j.anbehav.2004.04.018) [Google Scholar]
- Despland E., Rosenberg J., Simpson S. J.2004Landscape structure and locust swarming: a satellite's eye view. Ecography 27, 381–391 (doi:10.1111/j.0906-7590.2004.03779.x) [Google Scholar]
- Dirsh V. M.1953Morphometrical studies on phases of the desert locust. Anti-Locust Bull. 16, 1–34 [Google Scholar]
- Dunbar R. I., Shultz S.2007Evolution in the social brain. Science 317, 1344–1347 (doi:10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- Durst C., Eichmüller S., Menzel R.1994Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behav. Neural Biol. 62, 259–263 (doi:10.1016/S0163-1047(05)80025-1) [DOI] [PubMed] [Google Scholar]
- Ernst K. D., Boeckh J., Boeckh V.1977A neuroanatomical study on the organization of the central antennal pathways in insects. Cell Tissue Res. 176, 285–306 [DOI] [PubMed] [Google Scholar]
- Farris S. M.2008aTritocerebral tract input to the insect mushroom bodies. Arthropod Struct. Dev. 37, 492–503 (doi:10.1016/j.asd.2008.05.005) [DOI] [PubMed] [Google Scholar]
- Farris S. M.2008bStructural, functional and developmental convergence of the insect mushroom bodies with higher brain centers of vertebrates. Brain Behav. Evol. 72, 1–15 (doi:10.1159/000139457) [DOI] [PubMed] [Google Scholar]
- Farris S. M., Roberts N. S.2005Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc. Natl Acad. Sci. USA 102, 17 394–17 399 (doi:10.1073/pnas.0508430102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris S. M., Robinson G. E., Fahrbach S. E.2001Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21, 6395–6404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda A., Herczeg G., Merilä J.2009Habitat-dependent and -independent plastic responses to social environment in the nine-spined stickleback (Pungitius pungitius) brain. Proc. R. Soc. B 276, 2085–2092 (doi:10.1098/rspb.2009.0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood M., Chapman R. F.1984Differences in numbers of sensilla on the antennae of solitarious and gregarious Locusta migratoria L. (Orthoptera: Acrididae). Int. J. Insect Morphol. Embryol. 13, 295–301 (doi:10.1016/0020-7322(84)90004-7) [Google Scholar]
- Gronenberg W., Heeren S., Hölldobler B.1996Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 199, 2011–2019 [DOI] [PubMed] [Google Scholar]
- Harvey P. H., Clutton-Brock T. H., Mace G. M.1980Brain size and ecology in small mammals and primates. Proc. Natl Acad. Sci. USA 77, 4387–4389 (doi:10.1073/pnas.77.7.4387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y., Applebaum S. W.1995Density-dependent physiological phase in a non-migratory grasshopper Aiolopus thalassinus. Entom. Exp. Appl. 77, 251–262 (doi:10.1007/BF02383059) [Google Scholar]
- Heinze S., Homberg U.2007Maplike representation of celestial e-vector orientations in the brain of an insect. Science 315, 995–997 (doi:10.1126/science.1135531) [DOI] [PubMed] [Google Scholar]
- Heinze S., Gotthardt S., Homberg U.2009Transformation of polarized light information in the central complex of the locust. J. Neurosci. 29, 11783–11793 (doi:10.1523/JNEUROSCI.1870-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M., Heusipp M., Wanke C.1995Structural plasticity in the Drosophila brain. J. Neurosci. 15, 1951–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N., Robinson G. E., Fahrbach S. E.2006Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proc. Natl Acad. Sci. USA 103, 207–211 (doi:10.1073/pnas.0508318102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagges B. R., et al. 1996Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J. Neurosci. 16, 3154–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Seiler H., Wen A., Zars T., Ito K., Wolf R., Heisenberg M., Liu L.2006Distinct memory traces for two visual features in the Drosophila brain. Nature 439, 551–556 (doi:10.1038/nature04381) [DOI] [PubMed] [Google Scholar]
- Malaterre J., Strambi C., Chiang A. S., Aouane A., Strambi A., Cayre M.2002Development of cricket mushroom bodies. J. Comp. Neurol. 452, 215–227 (doi:10.1002/cne.10319) [DOI] [PubMed] [Google Scholar]
- Mares S., Ash L., Gronenberg W.2005Brain allometry in bumblebee and honey bee workers. Brain Behav. Evol. 66, 50–61 (doi:10.1159/000085047) [DOI] [PubMed] [Google Scholar]
- Matheson T., Rogers S. M., Krapp H. G.2004Plasticity in the visual system is correlated with a change in lifestyle of solitarious and gregarious locusts. J. Neurophysiol. 91, 1–12 (doi:10.1152/jn.00795.2003) [DOI] [PubMed] [Google Scholar]
- Molina Y., O'Donnell S.2007Mushroom body volume is related to social aggression and ovary development in the paperwasp Polistes instabilis. Brain Behav. Evol. 70, 137–144 (doi:10.1159/000102975) [DOI] [PubMed] [Google Scholar]
- Molina Y., O'Donnell S.2008Age, sex, and dominance-related mushroom body plasticity in the paperwasp Mischocyttarus mastigophorus. Dev. Neurobiol. 68, 950–959 (doi:10.1002/dneu.20633) [DOI] [PubMed] [Google Scholar]
- Neuser K., Triphan T., Mronz M., Poeck B., Strauss R.2008Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244–1248 (doi:10.1038/nature07003) [DOI] [PubMed] [Google Scholar]
- Niven J. E., Laughlin S. B.2008Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804 (doi:10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- Ochieng' S. A., Hallberg E., Hansson B. S.1998Fine structure and distribution of antennal sensilla of the desert locust, Schistocerca gregaria (Orthoptera: Acrididae). Cell Tissue Res. 291, 525–536 (doi:10.1007/s004410051022) [DOI] [PubMed] [Google Scholar]
- Ott S. R.2008Confocal microscopy in large insect brains: zinc-formaldehyde fixation improves synapsin immunostaining and preservation of morphology in whole-mounts. J. Neurosci. Methods 172, 220–230 (doi:10.1016/j.jneumeth.2008.04.031) [DOI] [PubMed] [Google Scholar]
- Pener M. P., Simpson S. J.2009Locust phase polyphenism: an update. Adv. Insect Physiol. 36, 1–272 (doi:10.1016/S0065-2806(08)36001-9) [Google Scholar]
- Ratcliffe J. M., Fenton M. B., Shettleworth S. J.2006Behavioral flexibility positively correlated with relative brain volume in predatory bats. Brain Behav. Evol. 67, 165–176 (doi:10.1159/000090980) [DOI] [PubMed] [Google Scholar]
- Ridgel A. L., Alexander B. E., Ritzmann R. E.2007Descending control of turning behavior in the cockroach, Blaberus discoidalis. J. Comp. Physiol. A 193, 385–402 (doi:10.1007/s00359-006-0193-7) [DOI] [PubMed] [Google Scholar]
- Ritzmann R. E., Ridgel A. L., Pollack A. J.2008Multi-unit recording of antennal mechano-sensitive units in the central complex of the cockroach, Blaberus discoidalis. J. Comp. Physiol. A 194, 341–360 (doi:10.1007/s00359-007-0310-2) [DOI] [PubMed] [Google Scholar]
- Roessingh P., Simpson S. J., James S.1993Analysis of phase-related changes of desert locust nymphs. Proc. R. Soc. Lond. B 252, 43–49 (doi:10.1098/rspb.1993.0044) [Google Scholar]
- Rogers S. M., Simpson S. J.1997Experience-dependent changes in the number of chemosensory sensilla on the mouthparts and antennae of Locusta migratoria. J. Exp. Biol. 200, 2313–2321 [DOI] [PubMed] [Google Scholar]
- Rogers S. M., Matheson T., Despland E., Dodgson T., Burrows M., Simpson S. J.2003Mechanosensory-induced behavioural gregarization in the desert locust Schistocerca gregaria. J. Exp. Biol. 206, 3991–4002 (doi:10.1242/jeb.00648) [DOI] [PubMed] [Google Scholar]
- Rogers S. M., Krapp H. G., Burrows M., Matheson T.2007Compensatory plasticity at an identified synapse tunes a visuomotor pathway. J. Neurosci. 27, 4621–4633 (doi:10.1523/JNEUROSCI.4615-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. M., Harston G. W. J., Kilburn-Toppin F., Matheson T., Burrows M., Gabbiani F., Krapp H. G.2010Spatiotemporal receptive field properties of a looming-sensitive neuron in solitarious and gregarious phases of the desert locust. J. Neurophysiol. 103, 779–792 (doi:10.1152/jn.00855.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto-Lomassese S., Strambi C., Aouane A., Strambi A., Cayre M.2002Sensory inputs stimulate progenitor cell proliferation in an adult insect brain. Curr. Biol. 12, 1001–1005 (doi:10.1016/S0960-9822(02)00889-8) [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Miller G. A.2007Maternal effects on phase characteristics in the desert locust, Schistocerca gregaria: a review of current understanding. J. Insect. Physiol. 53, 869–876 (doi:10.1016/j.jinsphys.2007.05.011) [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Raubenheimer D., Behmer S. T., Whitworth A., Wright G. A.2002A comparison of nutritional regulation in solitarious- and gregarious-phase nymphs of the desert locust Schistocerca gregaria. J. Exp. Biol. 205, 121–129 [DOI] [PubMed] [Google Scholar]
- Strauss R.2002The central complex and the genetic dissection of locomotor behaviour. Curr. Opin. Neurobiol. 12, 633–638 (doi:10.1016/S0959-4388(02)00385-9) [DOI] [PubMed] [Google Scholar]
- Striedter G. F.2005Principles of brain evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- Sword G. A.1999Density-dependent warning coloration. Nature 397, 217 (doi:10.1038/16609) [Google Scholar]
- Sword G. A., Lorch P. D., Gwynne D. T.2005Insect behaviour: migratory bands give crickets protection. Nature 433, 703 (doi:10.1038/433703a) [DOI] [PubMed] [Google Scholar]
- Uvarov B. P.1966Grasshoppers and locusts, vol. I London, UK: Centre for Overseas Pest Research [Google Scholar]
- van der Zee B., Behmer S. T., Simpson S. J.2002Food mixing strategies in the desert locust: effects of phase, distance between foods, and food nutrient content. Entomol. Exp. Appl. 103, 227–237 (doi:10.1023/A:1021165102855) [Google Scholar]
- Warton D. I., Wright I. J., Falster D. S., Westoby M.2006Bivariate line-fitting methods for allometry. Biol. Rev. 81, 259–291 (doi:10.1017/S1464793106007007) [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J.2003Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- Withers G. S., Fahrbach S. E., Robinson G. E.1993Selective neuroanatomical plasticity and division of labour in the honeybee. Nature 364, 238–240 (doi:10.1038/364238a0) [DOI] [PubMed] [Google Scholar]
- Withers G. S., Day N. F., Talbot E. F., Dobson H. E. M., Wallace C. S.2008Experience-dependent plasticity in the mushroom bodies of the solitary bee Osmia lignaria (Megachilidae). Dev. Neurobiol. 68, 73–82 (doi:10.1002/dneu.20574) [DOI] [PubMed] [Google Scholar]
- Woldewahid G., van der Werf W., van Huis A., Stein A.2004Spatial distribution of populations of solitarious adult desert locust (Schistocerca gregaria Forsk.) on the coastal plain of Sudan. Agr. Forest Entomol. 6, 181–191 (doi:10.1111/j.1461-9555.2004.00221.x) [Google Scholar]