Abstract
Polyandry, where females mate with multiple males, means that a male's reproductive success will depend both on his ability to acquire mates and the ability of his sperm to compete effectively for fertilizations. But, how do males partition their reproductive investment between these two episodes of selection? Theory predicts that increases in ejaculate investment will come at a cost to investment in other reproductive traits. Although evidence revealing such trade-offs is accumulating, we know little about their genetic basis. Here, I report patterns of genetic (co)variation for a range of traits subject to pre- and post-copulatory sexual selection in the guppy Poecilia reticulata, a promiscuous livebearing fish in which males alternate between courtship and sneak matings to obtain copulations. The analyses of genetic variation and covariation for these behaviours revealed a strong genetic predisposition for one tactic over the other. Both mating tactics were also strongly genetically integrated with the level of sexual ornamentation and ejaculate quality. Males that predominantly performed sneak matings were less ornamented but had faster swimming sperm than those that predominantly used courtship. These patterns of genetic variation and covariation reveal potential evolutionary constraints on the direction of selection of pre- and post-copulatory traits, and support sperm competition theory by revealing a trade-off between sexual attractiveness and investment in ejaculates.
Keywords: heritability, trade-off, genetic constraints, postcopulatory sexual selection, cryptic female choice
1. Introduction
Sexual selection was originally conceived as a process resulting exclusively from differences in reproductive success caused by competition over mates, usually through male–male competition or female choice (Darwin 1871). We now know, however, that this view can be restrictive, because it ignores the fact that in the majority of species females mate multiply during a single reproductive episode (polyandry). Polyandry, in turn, provides the opportunity for sexual selection beyond mating in the form of sperm competition, where ejaculates from rival males compete for fertilization (Parker 1970), and cryptic female choice (Eberhard 1996), where females influence this competition. Both of these mechanisms of postcopulatory sexual selection are now recognized as common and powerful evolutionary forces generating selection on ejaculate traits (Birkhead & Pizzari 2002).
Understanding how pre- and postcopulatory episodes of selection interact to influence male reproductive success has been a key goal of many recent studies, although many of these reveal that such interactions can be complex. On the one hand, concordant patterns of pre- and post-copulatory sexual selection have been described in species where competitive fertilization success favours highly attractive males (Evans et al. 2003; Hosken et al. 2008). Such patterns may arise when pre- and post-copulatory traits reflect a male's underlying condition (Simmons & Kotiaho 2002), or where females mediate the outcome of sperm competition to favour attractive males (Edvardsson & Arnqvist 2000). On the other hand, pre- and post-copulatory sexual selection may work in opposing directions, possibly reflecting trade-offs between the males' investment in pre- and postcopulatory traits (Simmons & Emlen 2006), or evolutionary conflicts between the reproductive interests of males and females (Arnqvist & Rowe 2005). Indeed, trade-offs between pre- and postcopulatory sexual selection are expected under sperm competition theory, where investment in obtaining matings is predicted to come at a cost to investment in ejaculates (Parker 1998). Such trade-offs may account for negative associations between fertilization success and mating success (Danielsson 2001; Preston et al. 2001), and/or promote the evolution of alternative mating strategies in which males compensate for the inability to monopolize mates by investing relatively heavily in more competitive ejaculates (see reviews in Oliveira et al. 2008).
Despite the accumulating evidence that pre- and postcopulatory sexual selection can be phenotypically integrated, there is limited evidence for an underlying genetic basis for these relationships. Yet, establishing patterns of genetic covariance for correlated traits is important, as the sign of these relationships reveals their potential to either facilitate or impede coevolutionary responses to selection (Lynch & Walsh 1998). Among studies that have explored genetic covariances for pre- and postcopulatory sexually selected traits, evidence from the fruitfly, Drosophila simulans, based on a correlation between family means for copulation latency and paternity success, supports the existence of a positive genetic correlation between male attractiveness and sperm competitiveness (Hosken et al. 2008). Similarly, in the dung beetle, Onthophagus taurus, soma weight, an estimate of male condition that is positively genetically correlated with attractiveness (Kotiaho et al. 2001) is also positively genetically correlated with testes weight (Simmons & Kotiaho 2002). Both studies, therefore, suggest that heritable variation in traits linked to sperm competitiveness can promote positive, reinforcing selection on traits involved in the competition for mates.
The present study explores patterns of genetic variation and covariation for a range of pre- and postcopulatory sexually selected traits in the guppy, Poecilia reticulata, a promiscuous livebearing freshwater fish that is emerging as an important model for linking pre- and postcopulatory sexual selection. During courtship, male guppies reveal their highly polymorphic colour patterns to females using sigmoid displays, a ritualized courtship posture in which males attempt to persuade sexually receptive females to mate (Houde 1997). Males can also switch from courtship to a coercive mating tactic, termed gonopodial thrusting (hereafter ‘sneaking’). In the wild, females can be subjected to up to one sneaky mating attempt per minute (Magurran & Seghers 1994), which may account for the high levels of multiple paternity reported in natural guppy populations (Neff et al. 2008). Both the intensity of the male's courtship displays and the area of orange in the male's colour patterns have been shown to be positively phenotypically correlated with ejaculate quality (Matthews et al. 1997; Locatello et al. 2006; Pitcher et al. 2007; but see Skinner & Watt 2006), and these relationships are thought to explain why sperm competition favours males with relatively high levels of orange (Evans et al. 2003; Pitcher et al. 2003) and correspondingly high levels of courtship (Evans & Magurran 2001). However, females also differentially retain (or reject) ejaculates according to their perception of male attractiveness (Pilastro et al. 2004), and therefore the positive association between male sexual attractiveness and paternity success (Evans & Magurran 2001; Evans et al. 2003) may be influenced by female-mediated processes that favour relatively attractive males in sperm competition (e.g. Andersson & Simmons 2006). Thus, the extent to which these concordant patterns of pre- and postcopulatory sexual selection are due to an underlying causative relationship between attractiveness and sperm competitiveness has yet to be established.
I used a quantitative genetic breeding design to study genetic variation in pre- and postcopulatory traits in guppies, and to reveal their patterns of genetic covariation. I focused on traits that are known to confer a mating advantage to male guppies, including courtship intensity, colour ornamentation and caudal fin size (Houde 1997; Brooks & Endler 2001b). I also focused on variation in male body size, as previous work has shown that body size can be positively associated with male sexual attractiveness (Reynolds & Gross 1992) but negatively associated with sperm competitiveness (Evans et al. 2003; Becher & Magurran 2004), suggesting a possible trade-off between ejaculate quality and somatic growth. For the analysis of postcopulatory traits, I considered two components of sperm quality: sperm swimming velocity (estimated as average path velocity (VAP); see §2) and sperm viability (the proportion of live sperm in the ejaculate). Both of these sperm traits are important determinants of competitive fertilization success in a range of taxa (Snook 2005).
2. Material and methods
(a). Breeding design and rearing conditions
Fish from the parental generation were descendents of guppies captured in 2006 from the Alligator Creek River in Queensland, Australia. From this source population, a nested half-sib breeding design was established by mating 40 males (sires) to five females each (dams, 200 in total). Matings were conducted using artificial insemination (AI; see Evans et al. 2003 for details) to minimize possible differential maternal effects based on the female's perception of male attractiveness, which can inflate estimates of genetic variances attributable to sires (Kotiaho et al. 2003).
When offspring were produced by each dam, they were isolated from the female and reared in family groups until they could be sexed by eye (always less than 13 weeks). At this point, each male offspring was placed individually into a 2 l (19 × 11 × 11 cm) plastic tank with a gravel substrate. Raising males individually ensured that common environmental effects were minimized during the rearing period. Such effects were further reduced by rearing offspring in random positions in a room with constant temperature (26 ± 0.5°C and a light/dark cycle of 12/12 h) and by feeding individuals on standardized portions (approx. 6% body weight) of dried fish feed each morning (Specialty Feeds, Glen Forrest, WA, Australia). An anti-algae treatment (3 g l−1 2-chloro-4, 6-bis-[ethylamino]-s-triazine; Aquamaster) was applied to each male's tank every 7 days to prevent algal growth (and therefore the build-up of non-administered carotenoids) in the tanks, and to maintain water quality (Grether 2000). Offspring traits (see below) were measured when males were aged seven months (mean age in days ± s.e. = 204.10 ± 1.48). These strictly regulated breeding and rearing conditions were imposed to ensure that differential maternal and environmental effects, and variation in male age, had a minimum impact on estimates of genetic variance in the measured traits. Nevertheless, as offspring within full-sibling families shared the same gestational and early rearing environment (up to 13 weeks), common environmental effects are likely to have contributed towards the dam variances (which are not interpreted in this study; see below).
(b). Mating behaviour
Mating behaviour trials took place in 8 l tanks (28.5 × 14.5 × 19 cm, filled to 14 cm) containing aquarium gravel and artificial pond weed. In each trial, a female was taken from mixed-stock aquaria, placed in the observation tank and allowed to settle for approximately 30 mins. Females were size-matched (by eye) for each trial and were not re-used among trials. (The UWA institutional animal ethics permit did not allow non-terminal anaesthesia; thus for humane reasons, I did not take formal length measures under anaesthesia.) Each male was placed with the female and allowed to settle for a further 20 mins. The number of sigmoid displays (courtship) and gonopodial thrusts (sneaking) that males directed towards the female was then recorded for 5 min. Courtship was recorded when the male moved in front of the female, adopted an s-shaped posture and quivered (Houde 1997). Sneaking was recorded when a male approached a female from behind, circumducted (swung forward) his intromittent organ (the gonopodium) beyond 90° and attempted to copulate with the female without prior courtship (Houde 1997).
(c). Sperm assays
Following the behaviour trials, males were anaesthetized, carefully dried and placed on a glass slide under a dissecting microscope. A micropipette was then used to add 40 µl of an extender medium to the base of the male's gonopodium to ensure that sperm bundles remained intact and quiescent until they were used for the sperm assays (see Gardiner 1978 for formula). Light pressure was then applied to each male's abdomen to expel strippable sperm into the extender medium (Matthews et al. 1997). From this total sperm pool, eight spermatozeugmata (unencapsulated sperm bundles) were extracted to assess sperm viability using a live/dead sperm viability assay (Invitrogen, Molecular Probes). The proportion of live sperm in each sample was then estimated from 200 sperm cells per sample (for full details of this assay see Evans 2009).
Spermatozeugmata from the remainder of each male's sperm sample were activated with 40 µl of a 150 mM KCl solution (Billard & Cosson 1990) with 2 mg l−1 bovine serum albumin (BSA) added to prevent sperm from sticking to the glass slide and coverslip (Pitcher et al. 2007). From these activated sperm samples, two aliquots—each containing three spermatozeugmata (approx. 80 × 103 sperm cells)—were immediately used for the computer-assisted sperm analysis (CASA) trials. Both sub-samples were tested independently to ensure consistency in the CASA measures. Each sample was placed in a separate well of a 12-cell multi-test slide (MP Biomedicals, Aurora, OH, USA) coated with 1 per cent polyvinyl alcohol (Wilson-Leedy & Ingermann 2007) and analysed using the Ceros Sperm Tracking software package (Hamilton Thorne Research, Beverly, MA, USA). This assay generated a measure of average path velocity (VAP; the average velocity of sperm cells over a smoothed cell path) for each sperm sample. Within-sample repeatability (Lessells & Boag 1987) for VAP measures was significant (intra-class correlation coefficient: R = 0.68 ± 0.025 s.e.; 1-way ANOVA: p < 0.0001) and therefore the mean of the two VAP values for each male was used in the subsequent analysis. Sperm velocity measures were based on a mean of 69.9 ± 1.8 s.e. sperm tracks per sample.
(d). Male ornamentation
After the sperm analyses, each male was digitally photographed. The surface area of the various colour spots was then analysed using UTHSCSA Image Tool (University of Texas Health Science Center, San Antonio, TX, USA). These colour spots were measured on the left side of each male's body and included the area of carotenoid and pteridine pigments (orange and yellow spots, hereafter summed as ‘orange’) and structural colours (blue, green, purple, silver and white, summed as ‘iridescence’; Brooks & Endler 2001b). The total number of colour spots was also recorded as an index of colour complexity (Nicoletto 1993; Brooks & Endler 2001a). Standard length (the distance in millimetres from the snout to the tip of caudal peduncle) was estimated to test for a possible trade-off between somatic growth and sperm quality. Caudal fin area (tail), which is linked to male sexual attractiveness in the focal population (Brooks & Endler 2001b) was also measured. Thus, the analysis of male sexual ornamentation included five separate variables: orange, iridescence, total spot number, body length and tail area.
(e). Quantitative genetic analyses
Although AIs were conducted for 40 sires, 11 replicates were excluded because inseminations failed to generate at least two male offspring from each replicate. The final dataset therefore encompassed offspring from 29 sires, coming from 87 dams, with varying numbers of dams per sire and variation in the number of offspring assayed among dam families. Given this unbalanced design, analyses of genetic variation and (co)variance-component estimation were conducted using restricted maximum-likelihood (REML) methods (see below), which unlike least-squares approaches do not place any special demands on the balance data (Lynch & Walsh 1998). In a few cases, measurements for some assays were unavailable (e.g. where photographs were unusable, or where CASA analyses failed). Sample sizes, therefore, varied slightly among the different assays (N offspring tested = 449–508; see table 1 for all sample sizes).
Table 1.
Number of male offspring (N), trait means and their standard errors (s.e.), and REML estimates of sire, dam (within sire) and residual variance components (Varsire, Vardam & Varresidual) taken from nested mixed-models. Tests for significance for sire and dam estimates (psire & pdam; significant p-values highlighted in italics) are from likelihood-ratio tests, where the G statistics for each component are equivalent to twice the difference in log-likelihoods between hierarchically structured models (see main text for details). Coefficients of additive genetic variation (CVA) are provided for each trait and are calculated from the formula: CVA = 100*√VA/X, where VA = 4 x Varsire and X = trait mean (Houle 1992).
| trait | N | mean (s.e.) | Varsire | Vardam | Varresidual | Gsire | Gdam | psire | pdam | CVA |
|---|---|---|---|---|---|---|---|---|---|---|
| VAP (µs−1) | 450 | 85.28 (0.73) | 47.34 | 15.19 | 182.0 | 35.6 | 9.9 | <0.0001 | 0.002 | 16.21 |
| sperm viability (%) | 449 | 59.78 (0.10) | 83.80 | ∼0 | 199.55 | 74.6 | 0.16 | <0.0001 | 0.69 | 0.31 |
| courtship | 508 | 5.59 (0.26) | 2.17 | 0.80 | 30.13 | 15.2 | 1.7 | <0.0001 | 0.19 | 59.76 |
| sneaking | 508 | 4.58 (0.26) | 2.25 | 1.25 | 29.55 | 15.7 | 2.7 | <0.0001 | 0.10 | 77.96 |
| body length (mm) | 499 | 14.82 (0.03) | 0.14 | 0.08 | 0.35 | 35.8 | 44.1 | <0.0001 | <0.0001 | 5.27 |
| tail area (mm2) | 482 | 12.75 (0.10) | 0.39 | 0.32 | 4.42 | 7.94 | 10.2 | 0.005 | 0.001 | 10.50 |
| orange (mm2) | 499 | 2.62 (0.13) | 1.03 | 0.21 | 1.38 | 80.8 | 21.1 | <0.0001 | <0.0001 | 139.43 |
| iridescence (mm2) | 499 | 4.95 (0.09) | 0.50 | 0.25 | 3.15 | 19.6 | 9.1 | <0.0001 | 0.003 | 28.57 |
| spot number (N) | 499 | 8.25 (0.09) | 0.82 | 0.51 | 2.53 | 30.5 | 40.1 | <0.0001 | <0.0001 | 21.82 |
All genetic analyses (and associated standard error estimates) were conducted using the program ASReml 3.0 (Gilmour et al. 2009). For the estimation of genetic variances, I conducted separate univariate linear mixed-effects models (with sire, and dam nested within sire, entered as random effects). The variance components from these univariate models were used to calculate additive genetic variances required for the estimation of narrow-sense heritabilities using standard formulae for half-sib designs (Falconer & Mackay 1996). Coefficients of additive genetic variation (CVA) were calculated following Houle (1992) and are reported in table 1 (see table heading for formula). The significance levels for the variance components were estimated using likelihood-ratio tests (Lynch & Walsh 1998). This involved removing the sire term from the full model and calculating twice the difference in log-likelihoods between hierarchically structured models. The resultant goodness-of-fit statistic (G) was then tested against a χ2 distribution with the degrees of freedom matching the number of parameters removed (Lynch & Walsh 1998). I estimated heritabilities owing to both sires and dams and compared these estimates using a one-sample t-test of the paired sire and dam pseudovalues estimated through the jackknife procedure (Roff 2008).
For genetic correlations, only covariances owing to sires are presented, since dam estimates are potentially contaminated by dominance variance, common environmental and maternal effects (Roff 1997). Calculations of genetic correlations and their standard errors were conducted in ASReml using a bivariate model with an unstructured variance matrix (Gilmour et al. 2009). To test the significance of each genetic correlation, I noted the log-likelihood of the full model with an unfixed covariance structure, then re-ran each model with the covariances fixed at zero, again noting the log-likelihood of the modified model. Likelihood-ratio tests were then conducted using twice the difference in log-likelihoods between fixed and unfixed models, as described above for univariate tests.
3. Results
(a). Descriptive genetic analyses
All traits exhibited highly significant additive genetic variation attributable to sires (see table 1 for descriptive genetic statistics). Dam effects, which include genetic, common environmental and maternal effects, and possibly dominance variance, were also significant for the majority of traits (table 1). In all cases, estimates of narrow-sense heritabilities owing to sires (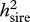 ) exceeded those attributable to dams, although these differences were significant only in the cases of VAP, sperm viability and orange area (table 2). The asymmetries between sire and dam heritabilities for these traits are consistent with patterns of Y-linked genetic variation, which in the case of orange area has been well documented in guppies (Winge 1927; Haskins et al. 1961; Houde 1992). In these cases, heritability estimates are likely to be inflated by up to twice their actual value, as they were calculated on the assumption of autosomal inheritance (Falconer & Mackay 1996; Roff 1997). This may explain why some
) exceeded those attributable to dams, although these differences were significant only in the cases of VAP, sperm viability and orange area (table 2). The asymmetries between sire and dam heritabilities for these traits are consistent with patterns of Y-linked genetic variation, which in the case of orange area has been well documented in guppies (Winge 1927; Haskins et al. 1961; Houde 1992). In these cases, heritability estimates are likely to be inflated by up to twice their actual value, as they were calculated on the assumption of autosomal inheritance (Falconer & Mackay 1996; Roff 1997). This may explain why some 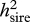 values, and notably orange area, exceeded the theoretical maximum of 1.0 (see also Brooks & Endler 2001a, where narrow sense heritability for orange area in the same population also exceeded 1.0).
values, and notably orange area, exceeded the theoretical maximum of 1.0 (see also Brooks & Endler 2001a, where narrow sense heritability for orange area in the same population also exceeded 1.0).
Table 2.
Heritabilities (with REML estimates of s.e.) owing to sires (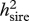 ) and dams (
) and dams (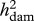 ). Sire and dam heritabilities were compared using the delete-one sire jackknife procedure (Roff 2008). p-values from paired t-tests (pdiff; significant p-values highlighted in italics) are given for these comparisons.
). Sire and dam heritabilities were compared using the delete-one sire jackknife procedure (Roff 2008). p-values from paired t-tests (pdiff; significant p-values highlighted in italics) are given for these comparisons.
| trait |
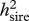 (s.e.) (s.e.) |
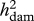 (s.e.) (s.e.) |
pdiff |
|---|---|---|---|
| VAP | 0.78 (0.26) | 0.25 (0.16) | 0.035 |
| sperm viability | 1.20 (0.27) | ∼0 | <0.0001 |
| courtship | 0.26 (0.13) | 0.10 (0.13) | 0.083 |
| sneaking | 0.27 (0.14) | 0.15 (0.14) | 0.517 |
| body length | 1.00 (0.31) | 0.56 (0.20) | 0.263 |
| tail area | 0.30 (0.18) | 0.25 (0.17) | 0.934 |
| orange | 1.56 (0.31) | 0.32 (0.15) | 0.021 |
| iridescence | 0.51 (0.21) | 0.25 (0.16) | 0.369 |
| spot number | 0.85 (0.28) | 0.52 (0.19) | 0.317 |
(b). Patterns of genetic covariance
The analysis of genetic covariance revealed a significant negative genetic correlation between the intensity of courtship and sneaking, indicating a genetic predisposition for one mating tactic over the other. Underlying this negative genetic association between the two mating tactics were opposing genetic correlations between both tactics and sperm velocity. The intensity of sneaking was positively genetically correlated with sperm velocity (table 3), while the intensity of courtship was negatively genetically correlated with sperm velocity (table 3). Sperm viability was also negatively genetically correlated with sneaking, but uncorrelated with courtship (table 3).
Table 3.
Genetic and phenotypic correlations among pre- and post-copulatory traits in male guppies. REML estimates of genetic covariance and their standard errors (in parentheses) are listed above the diagonal and phenotypic correlations are listed below the diagonal. Significance levels for genetic covariance estimates come from likelihood-ratio tests from models with fixed and unfixed covariance structures. *p < 0.05; **p < 0.01; ***p < 0.001; see §2 for full details.
| trait | VAP | sperm viability | courtship | sneaking | body length | tail area | orange | iridescence | spot number |
|---|---|---|---|---|---|---|---|---|---|
| VAP | — | 0.062 (0.25) | −0.931*** (0.18) | 0.600* (0.26) | −0.333 (0.25) | −0.38 (0.33) | −0.08 (0.25) | −0.993*** (0.16) | 0.478 (0.25) |
| sperm viability | 0.112 | — | −0.054 (0.29) | −0.839* (0.32) | −0.274 (0.23) | 0.544 (0.25) | 0.191 (0.22) | −0.593* (0.21) | 0.437 (0.23) |
| courtship | −0.101 | 0.019 | — | −0.839* (0.32) | 0.345 (0.29) | 0.848* (0.36) | 0.07 (0.29) | 0.427 (0.30) | −0.05 (0.33) |
| sneaking | 0.064 | 0.034 | −0.049 | — | 0.151 (0.32) | −1.56 (0.73) | −0.813* (0.27) | −0.495 (0.31) | −0.767* (0.31) |
| body length | 0.049 | −0.075 | 0.128 | 0.128 | — | 0.045 (0.35) | −0.171 (0.24) | 0.413 (0.24) | −0.200 (0.27) |
| tail area | 0.020 | 0.120 | 0.091 | −0.043 | 0.233 | — | 0.648* (0.23) | −0.235 (0.38) | 0.726* (0.28) |
| orange | −0.047 | −0.070 | 0.097 | −0.036 | 0.033 | 0.204 | — | 0.051 (0.26) | 0.577* (0.17) |
| iridescence | −0.041 | −0.095 | 0.253 | 0.028 | 0.380 | 0.193 | 0.136 | — | −0.532* (0.26) |
| spot number | 0.095 | 0.124 | 0.124 | −0.026 | 0.067 | 0.171 | 0.247 | −0.021 | — |
Both mating tactics were also genetically associated with body ornamentation. Sneaking was negatively genetically correlated with the area of orange spots and the total number of colour spots (the measure of ‘colour complexity’; table 3). By contrast, courtship was genetically uncorrelated with the orange area and the number of colour spots, but significantly positively genetically correlated with tail area (table 3). Thus, both mating tactics largely exhibited opposing genetic correlations with phenotypic measures of male sexual attractiveness; males that increasingly used sneaking were less ornamented than their highly courting counterparts.
The analyses of genetic covariance also revealed evidence for a trade-off between colour ornamentation and sperm quality. The negative genetic correlation between iridescent area and VAP was especially pronounced (table 3), further highlighting a trade-off between investment in precopulatory sexually selected traits and ejaculate quality. Likewise, iridescent area and sperm viability were negatively genetically correlated. Interestingly, there were no significant phenotypic or genetic correlations between the area of orange pigmentation and either measure of the sperm quality (table 3).
4. Discussion
The patterns of genetic covariation revealed by this study provide support for a key component of sperm competition theory by revealing a trade-off between investment in ejaculate quality and investment in traits conferring a mating advantage (Parker 1998). The results complement an increasing body of evidence for such patterns across several taxa, but they offer the first evidence that these relationships have a strong genetic basis. The accumulated evidence from the current study suggests that males that predominantly use sneak matings are likely to be less attractive but better equipped to engage in sperm competition than phenotypically attractive males that predominantly perform courtship.
The pattern of genetic covariance between the two mating tactics was striking. Although both tactics are expressed conditionally in response to various environmental and ecological cues (Houde 1997), this study shows that there is a genetic basis for these conditionally expressed behaviours (Tomkins & Hazel 2007). Underlying the trade-off between these behaviours were opposing genetic correlations between both mating tactics and sperm swimming velocity (estimated as average path velocity; VAP), suggesting a compensatory pattern by which males that predominantly employ the sneaking tactic invest relatively heavily in this component of ejaculate quality. This positive genetic association between sneaking and VAP may be adaptive because males that predominantly perform sneak copulations are likely to routinely face sperm competition, since these matings are typically directed at sexually unreceptive (i.e. previously mated) females (Houde 1997). However, the genetic correlation between sneaking and sperm viability was negative, suggesting a trade-off between these traits. Although we currently lack studies that link sperm viability to sperm competitiveness in guppies, sperm viability can be an important determinant of competitive fertilization success (e.g. García-González & Simmons 2005). Thus, the negative genetic correlation between sperm viability and sneaking may partially offset any advantage enjoyed by sneaking males in sperm competition, although we clearly need to understand the relative importance of sperm viability and sperm swimming velocity in influencing the competitive performance of guppy ejaculates to test this possibility.
The relationships between body size and both components of ejaculate quality (VAP and sperm viability) were not statistically significant. However, the sign (negative) of both relationships is consistent with prior evidence for a resource allocation trade-off between somatic growth and ejaculate investment. For example, previous work has shown that relatively small males have a competitive advantage during both controlled sperm competition trials (Evans et al. 2003) and in long term and replicated mating experiments (Becher & Magurran 2004). Negative correlations between somatic growth and ejaculates are common in species in which males exhibit alternative reproductive strategies (e.g. Vladic & Järvi 2001). Clearly, we need more work documenting the genetic basis for such trade-offs, both in species exhibiting fixed alternative reproductive strategies and those with more plastic reproductive tactics (such as guppies).
Previous studies on Trinidadian guppy populations have revealed positive phenotypic associations between the area of orange pigment spots and both sperm swimming velocity and viability (Locatello et al. 2006; Pitcher et al. 2007). By contrast, the current study revealed no evidence for either genetic or phenotypic integration among these traits. This may explain why orange area (along with other aspects of male coloration) and paternity were previously shown to be uncorrelated in the focal (Australian) population used in the current study (Evans & Rutstein 2008), which contrasts with other (Trinidadian) populations, where positive relationships between orange area and ejaculate traits have been reported (Locatello et al. 2006; Pitcher et al. 2007; but see Skinner & Watt (2006) for lack of evidence in a different feral population of guppies). Instead, the present analysis revealed that the area of iridescence was strongly negatively genetically correlated with both measures of sperm quality, suggesting that in this population, males trade iridescent structural coloration for sperm quality. This finding may have important implications in the light of recent studies on birds and insects revealing that variation in iridescent colour markings can reflect male condition (Johnsen et al. 2003; Hill et al. 2005; Kemp & Rutowski 2007). Given the importance of iridescence in determining male attractiveness in the focal guppy population (Brooks & Endler 2001b), the trade-off between iridescence and ejaculate quality indicates that pre- and postcopulatory sexual selection may act antagonistically on this component of male sexual ornamentation.
In conclusion, this study reveals high levels of additive genetic variation for several pre- and postcopulatory sexually selected traits in guppies. The study also reveals high levels of genetic integration among these traits, highlighting potential constraints on their evolvability. These patterns, and in particular the negative genetic associations between ejaculate and display traits, may explain the high levels of genetic polymorphisms in the guppy's sexual traits, despite persistent directional sexual selection which is expected to erode such variability (Brooks 2002). Nevertheless, several studies suggest that pre- and postcopulatory sexual selection can act concordantly in guppies (Evans & Magurran 2001; Evans et al. 2003; Pitcher et al. 2003), which may in part be due to the influence of cryptic female choice, which clearly has the potential to reinforce patterns of precopulatory sexual selection in guppies (Pilastro et al. 2004). Thus, postcopulatory mechanisms of female choice may yet provide a mechanism by which females override selection against attractive males during sperm competition.
Acknowledgements
All animal work was conducted according to the University of Western Australia's Animal Ethics Committee (Research Integrity Office, permit number 05/100/513).
I am especially grateful to Cameron Duggin for his help with animal breeding, maintenance and with the behavioural, and sperm viability assays. I am also grateful to Andrea Pilastro and Joe Tomkins for their patient advice with ASReml and Rob Brooks, John Fitzpatrick, Paco García-González, Leigh Simmons, Joe Tomkins and three anonymous reviewers for providing critical feedback on previous drafts of this manuscript. This work was funded by a research fellowship and Discovery Grant from the Australian Research Council.
References
- Andersson M., Simmons L. W.2006Sexual selection and mate choice. Trends Ecol. Evol. 21, 296–302 (doi:10.1016/j.tree.2006.03.015) [DOI] [PubMed] [Google Scholar]
- Arnqvist G., Rowe L.2005Sexual conflict. New Jersey, NJ: Princeton University Press [Google Scholar]
- Becher S. A., Magurran A. E.2004Multiple mating and reproductive skew in Trinidadian guppies. Proc. R. Soc. Lond. B 271, 1009–1014 (doi:10.1098/rspb.2004.2701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard R., Cosson M. P.1990The energetics of fish sperm motility. In Controls of sperm motility: biological and clinical aspects (ed. Gagnon C.), pp. 153–173 Boca Raton, FL: CRC Press, Inc [Google Scholar]
- Birkhead T. R., Pizzari T.2002Postcopulatory sexual selection. Nat. Rev. Gen. 3, 262–273 (doi:10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- Brooks R.2002Variation in female mate choice within guppy populations: population divergence, multiple ornaments and the maintenance of polymorphism. Genetica 116, 343–358 (doi:10.1023/A:1021228308636) [PubMed] [Google Scholar]
- Brooks R., Endler J. A.2001aDirect and indirect sexual selection and quantitative genetics of male traits in guppies (Poecilia reticulata). Evolution 55, 1002–1015 (doi:10.1554/0014-3820(2001)055[1002:DAISSA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Brooks R., Endler J. A.2001bFemale guppies agree to differ: phenotypic and genetic variation in mate-choice behavior and the consequences for sexual selection. Evolution 55, 1644–1655 [DOI] [PubMed] [Google Scholar]
- Danielsson I.2001Antagonistic pre- and post-copulatory sexual selection on male body size in a water strider (Gerris lacustris). Proc. R. Soc. Lond. B 268, 77–81 (doi:10.1098/rspb.2000.1332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C.1871The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- Eberhard W. G.1996Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press [Google Scholar]
- Edvardsson M., Arnqvist G.2000Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc. R. Soc. Lond. B 267, 559–563 (doi:10.1098/rspb.2000.1037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. P.2009No evidence for sperm priming responses under varying sperm competition risk or intensity in guppies. Naturwissenschaften 96, 771–779 (doi:10.1007/s00114-009-0529-6) [DOI] [PubMed] [Google Scholar]
- Evans J. P., Magurran A. E.2001Patterns of sperm precedence and predictors of paternity in the Trinidadian guppy. Proc. R. Soc. Lond. B 268, 719–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. P., Rutstein A. N.2008Postcopulatory sexual selection favours intrinsically good sperm competitors. Behav. Ecol. Sociobiol. 62, 1167–1173 (doi:10.1007/s00265-008-0545-0) [Google Scholar]
- Evans J. P., Zane L., Francescato S., Pilastro A.2003Directional postcopulatory sexual selection revealed by artificial insemination. Nature 421, 360–363 (doi:10.1038/nature01367) [DOI] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F. C.1996Introduction to quantitative genetics. London, UK: Longman Group Ltd [Google Scholar]
- García-González F., Simmons L. W.2005Sperm viability matters in insect sperm competition. Curr. Biol. 15, 271–275 (doi:10.1016/j.cub.2005.01.032) [DOI] [PubMed] [Google Scholar]
- Gardiner D. M.1978Utilization of extracellular glucose by spermatozoa of two viviparous fishes. Comp. Biochem. Physiol. 59A, 165–168 [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., Thompson R.2009ASReml user guide release 3.0. Hemel Hempstead, UK: VSN International Ltd [Google Scholar]
- Grether G.2000Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata). Evolution 54, 1712–1724 [DOI] [PubMed] [Google Scholar]
- Haskins C. P., Haskins E. F., McLaughlin J. J. A., Hewett R. E.1961Polymorphism and population structure in Lebistes reticulatus, an ecological study. In Vertebrate speciation (ed. Blair W. F.), pp. 320–395 Austin, TX: University of Texas Press [Google Scholar]
- Hill G. E., Doucet S. M., Buchholz R.2005The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim. Behav. 69, 387–394 (doi:10.1016/j.anbehav.2004.03.013) [Google Scholar]
- Hosken D. J., Taylor M. L., Hoyle K., Higgins S., Wedell N.2008Attractive males have greater success in sperm competition. Curr. Biol. 18, R553–R554 (doi:10.1016/j.cub.2008.04.028) [DOI] [PubMed] [Google Scholar]
- Houde A. E.1992Sex-linked heritability of a sexually selected character in a natural population of Poecilia reticulata (Pisces: Poeciliidae) (guppies). Heredity 69, 229–235 (doi:10.1038/hdy.1992.120) [Google Scholar]
- Houde A. E.1997Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press [Google Scholar]
- Houle D.1992Comparing evolvability and variability of quantitative traits. Genetics 130, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen A., Delhey K., Andersson S., Kempenaers B.2003Plumage colour in nestling blue tits: sexual dichromatism, condition dependence and genetic effects. Proc. R. Soc. Lond. B 270, 1263–1270 (doi:10.1098/rspb.2003.2375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Rutowski R. L.2007Condition dependence, quantitative genetics, and the potential signal content of iridescent ultraviolet butterfly coloration. Evolution 61, 168–183 (doi:10.1111/j.1558-5646.2007.00014.x) [DOI] [PubMed] [Google Scholar]
- Kotiaho J. S., Simmons L. W., Tomkins J. L.2001Towards a resolution of the lek paradox. Nature 410, 684–686 (doi:10.1038/35070557) [DOI] [PubMed] [Google Scholar]
- Kotiaho J. S., Simmons L. W., Hunt J., Tomkins J. L.2003Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am. Nat. 161, 852–859 (doi:10.1086/375173) [DOI] [PubMed] [Google Scholar]
- Lessells C. M., Boag P. T.1987Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- Locatello L., Rasotto M. B., Evans J. P., Pilastro A.2006Colourful male guppies produce faster and more viable sperm. J. Evol. Biol. 19, 1595–1602 (doi:10.1111/j.1420-9101.2006.01117.x) [DOI] [PubMed] [Google Scholar]
- Lynch M., Walsh B.1998Genetics and analysis of quantitative traits. Sunderland, UK: Sinauer Associates, Inc [Google Scholar]
- Magurran A. E., Seghers B. H.1994Sexual conflict as a consequence of ecology: evidence from guppy, Poecilia reticulata, populations in Trinidad. Proc. R. Soc. Lond. B 255, 31–36 (doi:10.1098/rspb.1994.0005) [Google Scholar]
- Matthews I. M., Evans J. P., Magurran A. E.1997Male display rate reveals ejaculate characteristics in the Trinidadian guppy Poecilia reticulata. Proc. R. Soc. Lond. B 264, 695–700 (doi:10.1098/rspb.1997.0099) [Google Scholar]
- Neff B. D., Pitcher T. E., Ramnarine I. W.2008Inter-population variation in multiple paternity and reproductive skew in the guppy. Mol. Ecol. 17, 2975–2984 (doi:10.1111/j.1365-294X.2008.03816.x) [DOI] [PubMed] [Google Scholar]
- Nicoletto P. F.1993Female sexual response to condition-dependent ornaments in the guppy, Poecilia reticulata. Anim. Behav. 46, 441–450 (doi:10.1006/anbe.1993.1213) [Google Scholar]
- Oliveira R. F., Toborsky M., Brockmann H. J.(eds)2008Alternative reproductive tactics: an integrative approach Cambridge, UK: Cambridge University Press [Google Scholar]
- Parker G. A.1970Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567 (doi:10.1111/j.1469-185X.1970.tb01176.x) [Google Scholar]
- Parker G. A.1998Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead T. R., Møller A. P.), pp. 3–54 San Diego, CA: Academic Press [Google Scholar]
- Pilastro A., Simonato M., Bisazza A., Evans J. P.2004Cryptic female preferences for colorful males in guppies. Evolution 58, 665–669 [PubMed] [Google Scholar]
- Pitcher T. E., Neff B. D., Rodd F. H., Rowe L.2003Multiple mating and sequential mate choice in guppies: females trade up. Proc. R. Soc. Lond. B 270, 1623–1629 (doi:10.1098/rspb.2002.2280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher T. E., Rodd F. H., Rowe L.2007Sexual colouration and sperm traits in guppies. J. Fish. Biol. 70, 165–177 (doi:10.1111/j.1095-8649.2006.01292.x) [Google Scholar]
- Preston B. T., Stevenson I. R., Pemberton J. M., Wilson K.2001Dominant rams lose out by sperm depletion. Nature 409, 681–682 (doi:10.1038/35055617) [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Gross M. R.1992Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proc. R. Soc. Lond. B 250, 57–62 (doi:10.1098/rspb.1992.0130) [Google Scholar]
- Roff D. A.1997Evolutionary quantitative genetics. New York, NY: Chapman & Hall [Google Scholar]
- Roff D. A.2008Comparing sire and dam estimates of heritability: jackknife and likelihood approaches. Heredity 100, 32–38 (doi:10.1038/sj.hdy.6801048) [DOI] [PubMed] [Google Scholar]
- Simmons L. W., Emlen D. J.2006Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351 (doi:10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W., Kotiaho J. S.2002Evolution of ejaculates: patterns of phenotypic and genotypic variation and condition dependence in sperm competition traits. Evolution 56, 1622–1631 [DOI] [PubMed] [Google Scholar]
- Skinner A. M. J., Watt P. J.2006Phenotypic correlates of spermatozoon quality in the guppy, Poecilia reticulata. Behav. Ecol. 18, 47–52 (doi:10.1093/beheco/arl049) [Google Scholar]
- Snook R. R.2005Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53 (doi:10.1016/j.tree.2004.10.011) [DOI] [PubMed] [Google Scholar]
- Tomkins J. L., Hazel W.2007The status of the conditional evolutionarily stable strategy. Trends Ecol. Evol. 22, 522–528 (doi:10.1016/j.tree.2007.09.002) [DOI] [PubMed] [Google Scholar]
- Vladic T., Järvi T.2001Sperm quality in the alternative reproductive tactics of Atlantic salmon: the importance of the loaded raffle mechanism. Proc. R. Soc. Lond. B 268, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Leedy J. G., Ingermann R. L.2007Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 67, 661–672 [DOI] [PubMed] [Google Scholar]
- Winge Ø.1927The location of eighteen genes in Lebistes reticulatus. J. Gen. 18, 1–43 (doi:10.1007/BF03052599) [Google Scholar]


