Abstract
Nature is rich with many different examples of the cohesive motion of animals. Previous attempts to model collective motion have primarily focused on group behaviours of identical individuals. In contrast, we put our emphasis on modelling the contributions of different individual-level characteristics within such groups by using stochastic asynchronous updating of individual positions and orientations. Our model predicts that higher updating frequency, which we relate to perceived threat, leads to more synchronized group movement, with speed and nearest-neighbour distributions becoming more uniform. Experiments with three-spined sticklebacks (Gasterosteus aculeatus) that were exposed to different threat levels provide strong empirical support for our predictions. Our results suggest that the behaviour of fish (at different states of agitation) can be explained by a single parameter in our model: the updating frequency. We postulate a mechanism for collective behavioural changes in different environment-induced contexts, and explain our findings with reference to confusion and oddity effects.
Keywords: collective behaviour, oddity effect, confusion effect, collective motion, synchrony, threat
1. Introduction
The ubiquitous features observed in animal collectives have inspired researchers from a range of disciplines to describe, model and reproduce these extraordinary displays of coordinated behaviour (Sumpter 2006). Most group-living animals are able to move coherently and collectively, preserving common features such as coordinated turns, maintenance of internal structures and apparently leaderless movement. Examples include the tightly bound tori exhibited by large shoals of sardines under predation pressure (Parrish et al. 2002) and the striking pre-roosting displays of starlings (Ballerini et al. 2008). Despite considerable research interest in group coordination there is still a significant gap between theory and experimental data. Attempts to bridge this gap are hindered by the emergent nature of collective motion (Viscido et al. 2004), and matching modelling studies to empirical data—as, for example, in Buhl et al. (2006) and Yates et al. (2009)—remains a challenging goal in this field.
Models have been central to understanding the mechanisms behind collective animal motion (Krause & Ruxton 2002). Individual-based models in particular have allowed researchers to examine the emergence of different collective behaviours resulting from simple mechanisms at the level of the individual (Krause & Ruxton 2002). These models typically assume that identical individuals react to the position and movement of their nearest conspecifics by a combination of alignment, attraction and repulsion (Krause & Ruxton 2002). Originally based on the extensive work by Aoki (1982), these simple ideas were also adopted in physics (Vicsek et al. 1995), computer science (Reynolds 1987) and control engineering (Liu et al. 2003). In biology, the connection between the metric inter-individual distance and the subsequent behaviour has given rise to a family of models investigated computationally (Couzin et al. 2002) and tested empirically (Tien et al. 2004). Models have been successful in shaping explanations and understanding mechanisms for different collective behaviours (Couzin et al. 2002, 2005; Hoare et al. 2004; Viscido et al. 2005; Hemelrijk & Hildenbrandt 2008), but only at a qualitative level.
One of the earliest empirical studies to quantify individual trajectories in collective motion was performed three decades ago (Aoki 1980). In his experiments, Aoki filmed shoals of tamoroko (Gnathopogon elongatus) and Japanese horse mackerel (Trachurus japonicus) under controlled conditions and extracted time series of the positions of individual fish from his films. Aoki assembled the distribution of speeds and nearest-neighbour distance distributions of individuals within fish shoals. It is surprising that so few models incorporate these findings and no model explains them. Most modelling studies, for example, use a constant and homogeneous speed (e.g. Couzin et al. 2002). Some studies have used Aoki's data by drawing an instantaneous speed at each time step from an appropriate distribution (Aoki 1982; Huth & Wissel 1992), but they do not explain the emergence of this distribution from first principles. In this article, we construct a model in which the speed distributions are emergent purely from the local interactions between the group members, and discuss its consequences in the context of experimental work on fish shoals.
2. Material and methods
(a). Computational model
We have developed an individual-based model of group interactions, based on local rules, that replicates the speed distributions found in Aoki's and our experiments. The basis of our approach is to adopt stochastic asynchronous updating of individual fish positions and orientations; rather than using deterministic and sequential updating at each time step, fish can react to external stimuli with a stochastic rate. The average behaviour of individuals over short time intervals then varies probabilistically. Models with asynchronous updating have been previously introduced in simple one- and two-dimensional models (O'Loan & Evans 1999; Liu et al. 2003; Raymond & Evans 2006; Şamiloğlu et al. 2006), but we believe their potential to explain empirical observations in real animals, such as effectively modelling fish speed distributions, has been overlooked.
Individuals are represented by points on the plane moving continuously in a toroidal space (a square box of side length L with periodic boundary conditions). The notation and nomenclature below follows that of Couzin et al. (2002). Let N be the number of individuals indexed i, with positions xi = (xj, yj) and direction of motion θi. We assume that each individual reacts with an identical stochastic rate, enabling us to exploit a particle-picking approach to exactly simulate the implicit underlying master equation of the system. The algorithmic implementation of our model contains aspects of earlier work (Tsitsiklis et al. 1986; O'Loan & Evans 1999; Couzin et al. 2002) and proceeds as follows:
Choose individual j at random, where j ∈ {1, …, N} (equal probabilities).
Decide which one of the two behavioural rules j will follow in this step (probabilities p and (1 − p), respectively).
Update xj and θj according to the behavioural rule chosen in step (2).
N realizations of steps (1)–(3) constitute one update step of length Δt seconds. The duration of this update step corresponds to the reciprocal value of the rate at which individuals update. The output of the model is obtained by recording the positions of all individuals every T = λΔt seconds, where λ ≥ 1. This is analogous to how data of animal motion are obtained empirically where individual positions and orientations are sampled according to the frame rate of video recordings (Aoki 1980; Buhl et al. 2006). In our simulations, we keep T fixed and only vary Δt and therefore also λ.
We use purely metric behaviour rules in this implementation, based on those of Couzin et al. (2002). We will defer commenting on the appropriateness of this approach given recent findings (Ballerini et al. 2008) until the discussion. Each individual obtains information from interaction zones—zone of repulsion (zor), zone of orientation (zoo) and zone of attraction (zoa)—which are described by concentric circles, centred on the individual, of radius rR, rO and rA, respectively. Both the zoo and the zoa are punctured by a ‘blind angle’, α, in which individuals cannot perceive other individuals. Suppose individual j has been chosen in the algorithm described above; our first behavioural rule, which is selected with probability p, implements either alignment or repulsion. The individual tries to move away from conspecifics within its zor or aligns to conspecifics in its zoo, where, in common with other models of collective motion, repulsive motion takes precedence over alignment. The distance of j to its nearest neighbour determines the behaviour. Let R ∈ {1, …, N} be the set of individuals within the zor of j, excluding j. If |R| ≥ 1, the desired direction of motion of j is given by
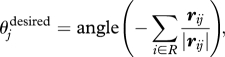 |
where angle (y) denotes the angle the vector y makes with the horizontal axis and rij = (xi − xj) is the vector in the direction from j to i. However, if the distance from j to its nearest neighbour is larger than rR, then j aligns with its conspecifics. Let O ∈ {1, …, N} be the set of individuals within the zoo of j, excluding j. If |O| ≥ 1, the desired direction of motion of j is given by
If both R and O are empty, then 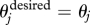 , and the individual does not deviate its direction. It executes this move with an instantaneous speed v = vO.
, and the individual does not deviate its direction. It executes this move with an instantaneous speed v = vO.
In the alternative case, we select our second behavioural rule with probability (1 − p). In this case, individual j gets attracted to conspecifics in its zoa and the distance rij once more determines its behaviour. Let A ∈ {1, …, N} be the set of individuals within the zoa of j, excluding j. If |A| ≥ 1, the desired direction of motion of j is given by
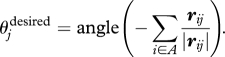 |
Once more, if A is empty, then 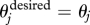 , and no deviation occurs. Subsequent movement happens at instantaneous speed v = vA. Throughout this study, we choose p = 0.5, in agreement with previous research in which an equal weight is assigned to orientation and attraction in individuals (Couzin et al. 2002). For both behavioural rules, once the desired direction of motion for j is calculated, the updated direction of motion, new(θj), is found by rotating the individual j by at most βΔt from θj towards
, and no deviation occurs. Subsequent movement happens at instantaneous speed v = vA. Throughout this study, we choose p = 0.5, in agreement with previous research in which an equal weight is assigned to orientation and attraction in individuals (Couzin et al. 2002). For both behavioural rules, once the desired direction of motion for j is calculated, the updated direction of motion, new(θj), is found by rotating the individual j by at most βΔt from θj towards 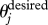 . Here, β denotes the maximum turning rate for individuals. Every time an individual j is updated, it is moved by v units in the updated direction
. Here, β denotes the maximum turning rate for individuals. Every time an individual j is updated, it is moved by v units in the updated direction
where v is selected to be either vO (alignment or repulsion) or vA (attraction), as described above. The average speed of an individual, over many update steps, is consequently given by vav = pvO + (1 − p)vA. Parameters used in the model simulations are as follows: N = 8, L = 168.73 cm, T = 0.04 s, p = 0.5, α = 270°, β = 40°, vO = 8.44 cm s−1, vA = 2vO, rR = 5.06 cm, rO = 20.25 cm, rA = 33.75 cm; values of Δt are given in the figure legends and justified in the electronic supplementary material.
In a simple stochastic implementation, all individuals would have an identical instantaneous speed, independent of the rule they follow and the behaviour of their fellow individuals. This would produce an unskewed Poisson distribution for the individual speeds (when averaged over time) that is not supported by empirical data. The novelty of our implementation is that individuals adopt differing speeds according to the behavioural rule they follow. In such a way, we can obtain skewed distributions for the individual speeds as observed in empirical data (see below).
An inherent parameter in our model is the length of the update step Δt (in seconds). This parameter reciprocally rescales the reaction rates in the system: small values of Δt imply rapid updates, while large values of Δt imply slow updates. It is important to stress at this stage that we are not explicitly relating the size of Δt to biological or neurological reaction times of animals (but discuss the possibility of a connection later in this article). In addition, no direct physical meaning should be attached to the instantaneous positions on time scales close to Δt. The fact that some individual might not move for one or more update steps does not imply they have stopped; rather that they are reacting more slowly to their surrounding than their fellows. At first glance it may appear that the same effect we obtain by varying Δt could be obtained by varying the speed at which individuals move. This is not the case. In our model, different values of Δt do not alter the average speed at which individuals move but they do alter the average rate at which individuals act upon information from within their sensory zones. Imposing different average speeds (i.e. changing vA and vO) changes the relationship between the average speed of individuals and the extent of their sensory zones.
(b). Experimental methods
We extended Aoki's experiments (Aoki 1980), using small shoals of eight three-spined sticklebacks, Gasterosteus aculeatus, within an indoor circular tank of 1 m radius. From individual movement trajectories, we constructed the distributions of the individual speeds of fish within a shoal and the distribution of the individuals' distances to their nearest shoal mates (nearest-neighbour distances). To test predictions from our model, we designed a number of new experimental treatments (table 1) to produce varying levels of agitation in the fish, comprising most agitation (treatment 1), least agitation (treatment 4) and intermediate agitation levels. This allowed us to compare different model outcomes under different conditions to experimental data under parallel conditions. Evidence suggests that sticklebacks are in a greater state of agitation or excitement in higher light levels since, in experiments, fish of this species have preferred shaded regions in tanks (Ward et al. 2008). Fish that perceive a higher predation threat tend to use more shaded areas than fish that do not perceive the same level of threat (McCartt et al. 1997). This suggests that they perceive a lower predation threat in shaded areas than in well-lit areas. An explanation for this could be that fish can see approaching predators that are not in shaded areas better from the shade, and that fish are less likely to be seen in shade (Helfman 1981). We also varied the water depth in our experimental tank. Given the white background of the tank, the fish bodies are clearly visible and thus make fish potentially conspicuous to overhead predators, such as kingfishers and herons. In this situation sticklebacks show a strong tendency to move into deeper water (J. Krause 2008, unpublished data).
Table 1.
Experimental conditions and corresponding treatment identities (IDs) from most agitation (1) to least agitation (4).
| treatment ID | experimental conditions |
|---|---|
| 1 | shallow water (2 cm), bright light (690 lux) |
| 2 | deep water (8 cm), bright light (690 lux) |
| 3 | shallow water (2 cm), dimmed light (20 lux) |
| 4 | deep water (8 cm), dimmed light (20 lux) |
3. Results
(a). Model output
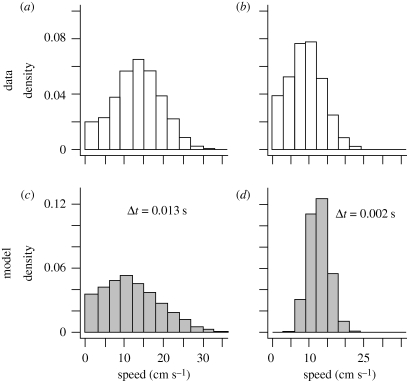
Our model produces skewed speed distributions (in two dimensions) similar to empirical data as an emergent property of our novel update scheme (figure 1c,d). Individual speeds are approximated by calculating the distance covered by fish over a fixed time step, with each time step T comprising many multiples of Δt. This is analogous to how speed distributions are determined empirically, where fish speeds are averaged over a range of video frames (see electronic supplementary material; see also Aoki 1980). The effect of varying Δt is striking: large values of Δt promote a strongly positively skewed distribution, and small values reduce the skewness and give rise to speed distributions that resemble normal, or Gaussian, distributions (figure 1c,d). We do not claim to reproduce speed distributions of real fish quantitatively as the influence of important factors on the speed distributions is unknown (e.g. interaction with environment). Rather, we show that our model is capable of producing similar speed distributions to the data without a priori assumptions or explicit addition of stochastic noise. Furthermore, our model suggests that the shape of the speed distributions can be varied by changing one parameter in our model.
Figure 1.
In (a) and (b) we show empirical speed distributions for two different shoals of eight fish over 10 min in identical experimental conditions (treatment 1 in table 1). Note how the shape of the speed distributions varies between groups. In (c) and (d) we show simulated speed distributions for different values of Δt, which illustrate the model's capability to produce qualitatively similar speed distributions to those observed in the empirical data. To facilitate comparison, we have ensured that all histograms in this figure have area 1. Summary statistics are given for comparison: (a) mean = 13.3 ± 6.2 (s.d.) cm s−1, skewness = 0.1; (b) mean = 9.1 ± 4.8 (s.d.) cm s−1, skewness = 0.3; (c) mean = 12.7 ± 7.3 (s.d.) cm s−1, skewness = 0.6; (d) mean = 12.7 ± 2.8 (s.d.) cm s−1, skewness = 0.2. See text for details of the data analysis and the model simulations.
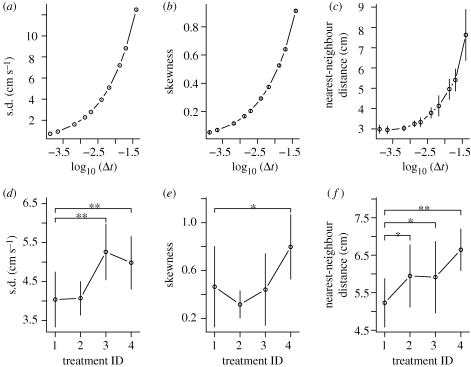
From the speed and nearest-neighbour distance distributions of the simulated shoals, we extracted three summary statistics: the standard deviation of the speed distributions, skewness of the speed distributions and the median of the nearest-neighbour distance distributions. Substantial changes in the summary statistics for different values of Δt are observed (figure 2a–c). Most prominent is the reduction in nearest-neighbour distance (for a given fish, this is the distance between this fish and the fish closest to it). Such a decrease in nearest-neighbour distances, or more compact group structures have previously been observed in empirical experiments for increasing threat or agitation levels (Krause 1993; Tien et al. 2004; Carere et al. 2009). The effect of Δt on the summary statistics highlights that this parameter is important for biological interpretation and is not just an invisible model implementation parameter.
Figure 2.
Summary statistics for a shoal of eight fish for model simulations (a–c; five replicates) and empirical data (d–f; eight replicates) for varying Δt and different treatments, respectively. The model simulations are not fitted to the data. Error bars show 1 standard deviation from the mean; in (a) and (b), the error bars are smaller than the symbols. In (a) and (b) we show the mean of the standard deviations and skewness of normalized speed distributions (to account for varying group speeds). Both these statistics, as well as (c) the mean of the median nearest-neighbour distances, increase with increasing values of Δt (note the log scale on the x-axis, Δt, is measured in seconds). This trend is qualitatively replicated in the empirical data for decreasing perceived agitation levels (d–f). The effect of the treatments is analysed using a GLMM with predicting factors (categorical) treatment ID + sequential treatment order and random factor (categorical) replicate ID (see also electronic supplementary material). Significant differences between treatment 1 and other treatments are indicated by asterisks above the brackets (*p < 0.05, **p < 0.001).
We propose that values of Δt in our model correspond to states of agitation in animals. For example, low values of Δt (that is, rapid updates) would correspond to high states of agitation, which might occur when animals feel threatened or at risk (Krause & Ruxton 2002). Our model predicts that increasing agitation makes the speed distribution of the shoal become more uniform and causes the nearest-neighbour distribution to contract. This allows us to form the following empirically testable hypothesis: the speed distributions and nearest-neighbour distributions of fish at different levels of agitation should qualitatively correspond to distributions in our model, where Δt is varied appropriately. Specifically, our model predicts that:
— High states of agitation (low values of Δt) should result in strongly peaked, unskewed speed distributions and a contraction of nearest-neighbour distances.
— Low states of agitation (high values of Δt) should result in well-spread distributions with positive skew and an increase in nearest-neighbour distances.
(b). Empirical findings
Using our empirical system, we confirmed previous results (Aoki 1980) in finding long-tailed and positively skewed speed distributions (figure 1a,b). We then extracted the same three summary statistics from the empirical data as we did for the simulated data. To investigate the statistical significance of differences in the measurements of the summary statistics across treatments, we used a generalized linear mixed model (GLMM), taking into account the differences between shoals and the order in which the treatments were applied. In our analysis of the empirical data, we found that all three summary statistics were affected by one or more of the treatments in a statistically significant way (figure 2). Statistically significant differences between treatment 1 and treatments 2 and 3 (e.g. figure 2d,f) illustrate that water depth and light intensity can separately affect the animal's movement patterns. The lack of monotony in some of the trends is due to behavioural factors we cannot control, which are discussed in the electronic supplementary material. The fact that not all of the summary statistics show statistically significant differences between treatment 1 and treatments 2 and 3 is likely to be due to the fact that the contrast between these treatments is not large enough.
Overall, our experimental findings confirmed the predictions from our model that increasing agitation in fish makes the speed distribution of the shoal become more uniform (i.e. it decreases the distribution's standard deviation and skewness, and makes the nearest-neighbour distribution contract; figure 2).
4. Discussion
This study is a combined modelling and empirical effort that has successfully predicted and reproduced emergent empirical properties of coordinated group behaviour from a model based entirely on local interactions. Our model is relatively simple and therefore provides an ideal starting point for the inclusion of individual characteristics and excitement levels into models of collective motion based on stochasticity. Our model produces novel predictions as to how group properties will alter in different behavioural contexts, and our experiments provide supporting evidence for these predictions. This reveals the importance of threat or risk levels perceived by fish for the composition of their movement trajectories and coordination. It has been suggested that fish react more quickly to shoal mates in situations of higher perceived risk or threat levels (Ward et al. 2008). However, to our knowledge this is the first time that the updating frequency of individuals has been modelled and tested against empirical data explicitly.
Our parameter Δt is the mean inter-update time that captures the relative frequency of updates within a given sampling time frame. It is important to emphasize that we are not making an explicit claim that this parameter is derivable from neurological information; we regard this parameter as controlling the dynamic averaging of the positional information from nearby conspecifics. The precise mechanism and quantities in our model provide an interesting avenue to be tested in further empirical research, focused on understanding the physiological interpretation of the stochastic rates we find emerging from our qualitative model comparisons. The simulations presented in figures 1 and 2 use values of Δt that are significantly lower than the 0.1 s that has been previously recorded as the reaction time for fish (Partridge & Pitcher 1980), indicating that many multiples of Δt make up a responsive reaction from the organism. The effect of reducing the step size in algorithms such as ours has previously been considered, but not in a biological context (Tsitsiklis et al. 1986).
It has recently been argued that the behavioural rules of collectively moving animals are based on the number of conspecifics each individual tracks (‘topological framework’) rather than on the distance between individuals as in our model (Ballerini et al. 2008). Some of the phenomena Ballerini and co-workers observed in their data have been reproduced in extensive simulation studies (Hildenbrandt et al. 2009) by assuming a priori that individuals only interact with a limited number of shoal mates. However, we suggest that this is not necessarily the only way in which the observations made in Ballerini et al. (2008) may arise in a model. Furthermore, a simple implementation of a topological framework in which individuals only interact with a fixed number of their nearest neighbours would not affect the emergence of speed distributions in our model. For these reasons we have continued to use a distance-based approach.
We suggest that by moving in a more coherent fashion with shoal members, an individual is able to reduce the risk of being targeted by predators as the ‘odd one out’, often termed the oddity effect (Krause & Ruxton 2002). The confusion effect—where predators find it more difficult to target an individual in a group than to target an isolated individual—is easily broken if one individual differs morphologically or behaviourally from others (Krause & Ruxton 2002). For example, in a threatened group where nearest-neighbour distances are generally low, an individual with a large nearest-neighbour distance will stand out from the crowd and probably be targeted by predators. This provides a mechanistic explanation for our findings: greater risk produces higher updating frequencies and higher updating frequencies produce lower oddity. Therefore, we suggest that the oddity effect could be the driving force for the behavioural changes in different contexts and the high degree of synchrony characterizing threat-induced collective behaviours.
Finally, our method of measuring the uniformity of speed distributions and nearest-neighbour distances could provide a simple way of empirically assessing stress levels of collectively grouping animals in a remotely collectable and non-obtrusive way.
Acknowledgements
N.W.F.B.'s research is supported by the Natural Environment Research Council. J.J.F. acknowledges support from the Biotechnology and Biological Sciences Research Council. D.W.F. and A.J.W. are supported by RCUK Fellowships. D.W.F. acknowledges support from NERC grant no. NE/E016111/1. J.K. acknowledges support from NERC (NE/D011035/1).
References
- Aoki I.1980An analysis of the schooling behavior of fish: internal organization and communication process. Bull. Ocean Res. Inst., Univ. Tokyo 12, 1–62 [Google Scholar]
- Aoki I.1982A simulation study on the schooling mechanism in fish. Bull. Jpn. Soc. Sci. Fish 48, 1081–1088 [Google Scholar]
- Ballerini M., et al. 2008Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237 (doi:10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl J., Sumpter D. J. T., Couzin I. D., Hale J. J., Despland E., Miller E. R., Simpson S. J.2006From disorder to order in marching locusts. Science 312, 1402–1406 (doi:10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- Carere C., Montanino S., Moreschini F., Zoratto F., Chiarotti F., Santucci D., Alleva E.2009Aerial flocking patterns of wintering starlings, Sturnus vulgaris, under different predation risk. Anim. Behav. 77, 101–107 (doi:10.1016/j.anbehav.2008.08.034) [Google Scholar]
- Couzin I., Krause J., James R., Ruxton G. D., Franks N. R.2002Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11 (doi:10.1006/jtbi.2002.3065) [DOI] [PubMed] [Google Scholar]
- Couzin I. D., Krause J., Franks N. R., Levin S. A.2005Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 (doi:10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- Helfman G. S.1981The advantage to fishes of hovering in shade. Copeia 1981, 392–400 (doi:10.2307/1444228) [Google Scholar]
- Hemelrijk C. K., Hildenbrandt H.2008Self-organized shape and frontal density of fish schools. Ethology 114, 245–254 (doi:10.1111/j.1439-0310.2007.01459.x) [Google Scholar]
- Hildenbrandt H., Carere C., Hemelrijk C. K.2009Self-organised complex aerial displays of thousands of starlings: a model. See http://arXiv.org/abs/0908.2677 [Google Scholar]
- Hoare D., Couzin I., Godin J., Krause J.2004Context-dependent group size choice in fish. Anim. Behav. 67, 155–164 (doi:10.1016/j.anbehav.2003.04.004) [Google Scholar]
- Huth A., Wissel C.1992The simulation of the movement of fish schools. J. Theor. Biol. 156, 365–385 (doi:10.1016/S0022-5193(05)80681-2) [Google Scholar]
- Krause J.1993The effect of ‘Schreckstoff’ on the shoaling behaviour of the minnow: a test of Hamilton's selfish herd theory. Anim. Behav. 45, 1019–1024 (doi:10.1006/anbe.1993.1119) [Google Scholar]
- Krause J., Ruxton G. D.2002Living in groups. Oxford, UK: Oxford University Press [Google Scholar]
- Liu Y., Passino K. M., Polycarpou M.2003Stability analysis of m-dimensional asynchronous swarms with a fixed communication topology. IEEE Trans. Autom. Control 48, 76–95 [Google Scholar]
- McCartt A. L., Lynch W. E., Jr, Johnson D. L.1997How light, a predator, and experience influence bluegill use of shade and schooling. Environ. Biol. Fishes 49, 79–87 [Google Scholar]
- O'Loan O., Evans M.1999Alternating steady state in one-dimensional flocking. J. Phys. A-Math. Gen. 32, 99 [Google Scholar]
- Parrish J. K., Viscido S. V., Grűnbaum D.2002Self-organized fish schools: an examination of emergent properties. Biol. Bull. 202, 296–305 (doi:10.2307/1543482) [DOI] [PubMed] [Google Scholar]
- Partridge B. L., Pitcher T. J.1980The sensory basis of fish schools: relative roles of lateral line and vision. J. Comp. Physiol. A 135, 315–325 (doi:10.1007/BF00657647) [Google Scholar]
- Raymond J. R., Evans M. R.2006Flocking regimes in a simple lattice model. Phys. Rev. E 73, 036112 (doi:10.1103/PhysRevE.73.036112) [DOI] [PubMed] [Google Scholar]
- Reynolds C. W.1987Flocks, herds and schools: a distributed behavioral model. Comput. Graph. 21, 25–34 (doi:10.1145/37402.37406) [Google Scholar]
- Şamiloğlu A. T., Gazi V., Koku A. B.2006Effects of asynchronism and neighborhood size on clustering in self-propelled particle systems. Lect. Notes Comp. Sci. 4263, 665–676 (doi:10.1007/11902140_70) [Google Scholar]
- Sumpter D. J. T.2006The principles of collective animal behaviour. Phil. Trans. R. Soc. B 361, 5–22 (doi:10.1098/rstb.2005.1733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien J. H., Levin S. A., Rubenstein D. I.2004Dynamics of fish shoals: identifying key decision rules. Evol. Ecol. Res. 6, 555–565 [Google Scholar]
- Tsitsiklis J., Bertsekas D., Athans M.1986Distributed asynchronous deterministic and stochastic gradient optimization algorithms. IEEE Trans. Autom. Control 31, 803–812 (doi:10.1109/TAC.1986.1104412) [Google Scholar]
- Vicsek T., Czirók A., Ben-Jacob E., Cohen I., Shochet O.1995Novel type of phase transition in a system of self-driven particles. Phys. Rev. Lett. 75, 1226–1229 (doi:10.1103/PhysRevLett.75.1226) [DOI] [PubMed] [Google Scholar]
- Viscido S. V., Parrish J. K., Grűnbaum D.2004Individual behavior and emergent properties of fish schools: a comparison of observation and theory. Mar. Ecol. Prog. Ser. 273, 239–249 (doi:10.3354/meps273239) [Google Scholar]
- Viscido S. V., Parrish J. K., Grűnbaum D.2005The effect of population size and number of influential neighbors on the emergent properties of fish schools. Ecol. Model. 183, 347–363 (doi:10.1016/j.ecolmodel.2004.08.019) [Google Scholar]
- Ward A. J. W., Sumpter D. J. T., Couzin I. D., Hart P. J. B., Krause J.2008Quorum decision-making facilitates information transfer in fish shoals. Proc. Natl Acad. Sci. USA 105, 6948–6953 (doi:10.1073/pnas.0710344105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates C. A., Erban R., Escudero C., Couzin I. D., Buhl J., Kevrekidis I. G., Maini P. K., Sumpter D. J. T.2009Inherent noise can facilitate coherence in collective swarm motion. Proc. Natl Acad. Sci. USA 106, 5464–5469 (doi:10.1073/pnas.0811195106) [DOI] [PMC free article] [PubMed] [Google Scholar]