The integral membrane protein Atg9 is delivered to the autophagosome in yeast and mammalian cells. We find that Atg9 does not originate from mitochondria and cannot reach the autophagosome directly from the ER. Instead, pairwise combinations of mutations in Golgi-endosomal traffic components cause defects in Atg9 delivery during starvation.
Abstract
While many of the proteins required for autophagy have been identified, the source of the membrane of the autophagosome is still unresolved with the endoplasmic reticulum (ER), endosomes, and mitochondria all having been evoked. The integral membrane protein Atg9 is delivered to the autophagosome during starvation and in the related cytoplasm-to-vacuole (Cvt) pathway that occurs constitutively in yeast. We have examined the requirements for delivery of Atg9-containing membrane to the yeast autophagosome. Atg9 does not appear to originate from mitochondria, and Atg9 cannot reach the forming autophagosome directly from the ER or early Golgi. Components of traffic between Golgi and endosomes are known to be required for the Cvt pathway but do not appear required for autophagy in starved cells. However, we find that pairwise combinations of mutations in Golgi-endosomal traffic components apparently only required for the Cvt pathway can cause profound defects in Atg9 delivery and autophagy in starved cells. Thus it appears that membrane that contains Atg9 is delivered to the autophagosome from the Golgi-endosomal system rather than from the ER or mitochondria. This is underestimated by examination of single mutants, providing a possible explanation for discrepancies between yeast and mammalian studies on Atg9 localization and autophagosome formation.
INTRODUCTION
Autophagy allows eukaryotic cells to recycle proteins and organelles by enveloping then in a double membrane to form autophagosomes which then fuse with lytic organelles for digestion. Initially identified as a response to starvation, autophagy has been found to be involved in many important biological processes including the clearance of pathogens and cytoplasmic aggregates, mobilizing lipid stores and remodeling of tissues (Rubinsztein et al., 2007; Xie and Klionsky, 2007; Farré et al., 2009). Proteins required for autophagosome formation have been identified by screening for yeast mutations that affect starvation-induced autophagy or the related Cvt pathway that delivers oligomerized enzymes to the vacuole during normal growth (Tsukada and Ohsumi, 1993; Thumm et al., 1994; Harding et al., 1995; Nakatogawa et al., 2009). However, the origin of the double-membrane of the autophagosome is less well understood (Juhasz and Neufeld, 2006; Longatti and Tooze, 2009). It has been suggested that membrane could extend out from the endoplasmic reticulum (ER) to wrap the target for digestion, or alternatively that autophagosomes could grow by the delivery of vesicular carriers that bud from other organelles such as mitochondria or endosomes (Reggiori et al., 2004b; Axe et al., 2008; Hayashi-Nishino et al., 2009; Ylä-Anttila et al., 2009; Hailey et al., 2010).
One integral membrane protein, Atg9, has been found on the forming autophagosome in both yeast and mammals (Lang et al., 2000; Noda et al., 2000; Young et al., 2006), and because it has six transmembrane domains its delivery must be accompanied by the delivery of membrane. In yeast Atg9 is found at the forming autophagosome and in peripheral sites that have been suggested to be mitochondria (Reggiori et al., 2004a; He et al., 2006; Reggiori and Klionsky, 2006). Yeast Atg9 appears to cycle between the forming autophagosome and the peripheral sites, however the precise nature of these sites and the membrane trafficking routes taken by Atg9 remain unclear. The genetic screens for proteins required for autophagy have found only a few known components of membrane traffic, and these are typically proteins such the SNARE Vam3 which act on the vacuole to mediate fusion with the completed autophagosome (Darsow et al., 1997). The related Cvt pathway has been found to require several proteins involved in traffic between the Golgi apparatus and endosomes, including the SNARE Tlg2, the GARP/VFT tethering complex, the sorting nexins Atg20 and Atg24, and the TRAPP subunit Gsg1/Trs85 (Abeliovich et al., 1999; Nice et al., 2002; Reggiori et al., 2003; Meiling-Wesse et al., 2005). However, these proteins are not required for bulk autophagy and so do not appear relevant to the delivery of membrane to the autophagosome during starvation (Juhasz and Neufeld, 2006). In mammalian cells Atg9 is found in the TGN and endosomal system and has been proposed to be delivered to the forming autophagosome from one or more of the compartments that constitute this system (Young et al., 2006; Razi et al., 2009). However, such a source for autophagosomal membranes would appear to lack support from yeast studies where the components that mediate traffic in the Golgi-endosomal system are only required for the Cvt pathway.
We have examined the trafficking of Atg9 as a means of understanding more about the routes by which membrane gets to the forming autophagosome during starvation. Restraining Atg9 to the ER and early Golgi reveals that it must move to later parts of the endomembrane system to be able to reach the autophagosome and exert its function. Examination of a range of membrane traffic components reveals the SNARE Gos1 to be a further component of Golgi-endosome traffic that is required for the Cvt pathway but, as with those found previously, it does not appear to be required for starvation-induced autophagy. However, we find that combining mutations that appear only to affect the Cvt pathway results in profound synergistic defects in autophagy in starved cells. This suggests that in yeast the Golgi-endosome system, rather that the ER or mitochondria, provides Atg9-containing membrane to the forming autophagosome.
MATERIALS AND METHODS
Yeast Strains and Plasmids
Yeast strains were based on BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), and all single deletion strains were in the BY4741 background and obtained from Open Biosystems (Huntsville, AL). Double mutants were created using the PCR method with KanMX or S. pombe HIS5 markers, and fluorescent markers RFP-APE1, ATG9-GFP, RFP-SCS2, Cherry-TLG1, and TOM20-RFP were introduced by homologous recombination [RFP was TagRFP (Evrogen, Moscow, Russia) or tdTomato (Campbell et al., 2002) as indicated, Supplemental Table S1]. An integration plasmid to express Atg9-EGFP under the TPI1 promoter was constructed follows: ATG9 promoter-TPI1 promoter-ATG9-EGFP-NatMX in pBluescriptII(KS-) (Supplemental Table S2). Homologous recombination was performed using a unique XbaI site in the ATG9 promoter region. Strains carrying this construct showed a similar distribution of Atg9-EGFP to that described previously (Sekito et al., 2009). For the GFP-Atg8 and ALP assays integration plasmids carrying EGFP-ATG9-MYC, KKXX, and RxR, were created by inserting the coding region between the TPI1 promoter and NatMX of pYOintF8, and introduced into Δatg9 genome by homologous recombination in the ATG9 promoter.
Yeast Growth and Protein Extraction
YEPD (1.1% yeast extract, 2.2% bactopeptone, 2% glucose, and 0.0055% adenine sulfate) or -URA medium was used for vegetative phase growth. For nitrogen starvation, overnight cultures in YEPD or -URA were diluted 1/50 and grown at 30°C to early log phase in YEPD or -URA medium, then resuspended in nitrogen starvation medium (SD-N, 0.17% yeast nitrogen base lacking ammonium sulfate and amino acids with 2% glucose) and incubated for 4 h. For protein extraction, 5 OD600 of cells were collected and resuspended in 100 ml of sample buffer, disrupted with an equal volume of glass beads using a homogenizer (FastPrep-24, MP Biomedicals, Aurora, OH, 45 s at intensity 6.5). Immunoblots were with anti-Ape1 (Dan Klionsky, University of Michigan), anti-GFP raised in rabbits, or anti-FLAG (Sigma-Aldrich, St. Louis, MO, A-8592). CPY pulse-chase was performed as described previously (Sapperstein et al., 1996), using anti-CPY (Rockland, Gilbertsville, PA 100-401-135).
Alkaline Phosphatase Assay
Phosphatase assays for autophagy using a cytosolic form of Pho8 (Pho8Δ60) were as described previously (Noda and Ohsumi, 1998). Briefly, 1–2 × 107 cells were collected by centrifugation, washed with water, and resuspended in 0.2 ml assay buffer (250 mM Tris-HCl (pH 9.0), 10 mM MgSO4, 10 μM ZnSO4) and vortexed with glass beads. After centrifugation, 50 μl of supernatant was transferred to 0.5 ml of assay buffer. Fifty microliters of 55 mM α-naphthyl phosphate (Sigma) was added to start the reaction and, after incubation at 30°C, was stopped by addition of 0.5 ml of 2 M glycine-NaOH (pH 11.0). Fluorescent emission at 472 nm after excitation at 345 nm was measured with a fluorometer (Perkin-Elmer, Foster City, CA). One unit is the release of 1 μmol α-naphthol/min/mg protein. Protein concentration was measured using by the Bradford method (Bio-Rad, Hercules, CA).
Microscopy of Yeast Strains
Cells expressing fluorescent fusion proteins were photographed with an 100 × 1.49 NA objective on a Nikon Eclipse TE2000 epifluorescent microscope using a CCD camera (CoolSNAP-HQ2, Roper Scientific, Tucson, AZ) and RFP and GFP filters (Chroma Technology, Rockingham, UT). Images were acquired and analyzed using MetaMorph and ImageJ, and levels adjusted with Adobe Photoshop. For FM4-64 labeling cells were incubated in 8 μM FM4-64 (Molecular Probes, Eugene, OR) at 30°C, washed twice and incubated in medium lacking FM4-64 at 30°C, timing being indicated in the figure legends.
RESULTS
Atg9 Can Be Retained in the ER and Early Golgi
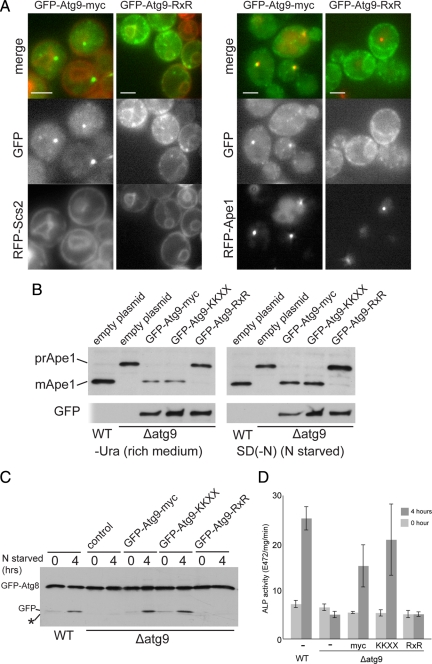
To be able to investigate whether Atg9 can travel to the autophagosome by direct delivery of membrane from the ER we attempted to create a version of the protein whose localization is restricted to this organelle. Both RXR and KKXX motifs cause membrane proteins to accumulate in the ER by directing retrieval from the cis-Golgi in COPI-coated vesicles. The C-terminal cytoplasmic tail of Atg9 is relatively long (∼250 residues), and although recognition of the KKXX signal by the COPI coat is reduced by increased separation from the membrane, recognition of RXR is apparently independent of distance (Shikano and Li, 2003). Atg9 is normally found in the forming autophagosome and in a peripheral pool of small structures (He et al., 2006; Reggiori and Klionsky, 2006). When Atg9 was expressed with an RXR motif at its C terminus it instead accumulated in the ER as well as in some additional puncta, which presumably reflect cycling through the cis Golgi (Figure 1A). To examine delivery to the autophagosome we compared GFP-Atg9 to aminopeptidase1 (Ape1). Ape1 forms oligomers in the cytoplasm and is delivered directly to the vacuole by the Cvt pathway in normal growth conditions and by bulk autophagy during starvation (Harding et al., 1995). Under both conditions the Ape1 aggregates are associated with the forming autophagosome (Shintani et al., 2002). Comparison with RFP-Ape1 revealed that the addition of RXR inhibited the delivery of Atg9-GFP to Ape1 aggregates (Figure 1B; 95% of RFP-Ape1 structures were labeled with GFP-Atg9 vs. 8% with GFP-Atg9-RXR, n = 100). As expected, the Atg9 cytoplasmic tail is too long to allow recognition of a C-terminal KKXX motif. However, Atg9 retains some functionality when the tail is truncated to just 38 residues (He et al., 2008), and this truncated form was found to be relocated to the ER when a KKXX motif was attached to the C terminus (Supplemental Figure S1A).
Figure 1.
Atg9 cannot get directly from the ER to the forming autophagosome. (A) Fluorescence micrographs of yeast expressing GFP-Atg9 with a C-terminal myc-tag followed by nothing or an RXR motif [KLRRRRI (Michelsen et al., 2007)]. The fusions were expressed with a TPI1 promoter from a centromeric vector and the cells also express either RFP-Ape1, or the ER protein RFP-Scs2. The RXR motif increases the amount of Atg9 in the ER and reduces that present at aggregates of mRFP-Ape1. Scale bars = 2 μm. (B) Anti-Ape1 immunoblots of lysates from wild-type cells, or from an ATG9 deletion strain transformed with either an empty plasmid, or the same GFP-Atg9 plasmids shown in A, and also one with the C-terminal myc-tag followed by a KKXX motif (SKKSL). Cells were harvested after growth in rich medium or after four hours of nitrogen starvation to induce autophagy. Attachment of the RXR motif to Atg9 blocks its activity under both conditions. (C) Anti-GFP immunoblots of lysates from cells expressing GFP-Atg8 from the ATG8 promoter on a CEN plasmid. The yeast strains are as in A and B but with the ATG9 fusions integrated into the genome. The cells were grown to midlog phase and either harvested (0) or starved for nitrogen for 4 h. Deletion of Atg9 prevents delivery of GFP-Atg8 to the vacuole and release of free GFP. (D) Alkaline phosphatase activity in strains expressing a cytosolic form of Pho8 (Pho8Δ60) that lacks a transmembrane domain and so only becomes active upon delivery to the vacuole by bulk autophagy (Noda and Ohsumi, 1998). Strains are as in C but with PHO13 deleted and PHO8 truncated to PHO8Δ60 by integration of TDH3 promoter. Cells were grown and starved for 4 h as in C (error bars indicate SD of three independent experiments).
The Cvt Pathway and Autophagy Depend on Atg9 Being Able to Leave the ER/Early Golgi
We next examined whether the relocation of Atg9 to the ER results in a defect in autophagy. We initially assayed the conversion of Ape1 from the precursor (proApe1) to the mature form which occurs when autophagosomes fuse to the vacuole. The rescue of Ape1 maturation in an Atg9 null strain by GFP-Atg9 was blocked by attachment of the RXR signal, and hence ER retention, in both rich medium and during nitrogen starvation (Figure 1C). Although the activity of full-length Atg9 was, as expected, unaffected by the KKXX motif, the version with a 38 residue tail which could be relocated to the ER by KKXX also showed a loss of activity when this motif was attached (Figure 1B and Supplemental Figure S1A). It is possible that the lack of processing of proApe1 during autophagy could reflect a cargo-specific defect. We thus examined two other widely used reporters for starvation-induced autophagy. The first is the release of free GFP from a fusion to the protein Atg8 that is covalently attached to lipids in the autophagosome and is digested after fusion to the vacuole (Shintani and Klionsky, 2004). The second is the vacuolar delivery and hence activation of a cytosolic form of the proenzyme alkaline phosphatase (Noda and Ohsumi, 1998). With both assays we again found that the RXR motif blocked the ability of GFP-Atg9 to allow autophagy during starvation conditions (Figure 1, C and D). Taken together these results indicate that Atg9 normally has access to the ER and early Golgi but that it has to move beyond the early Golgi to be able reach the autophagosome.
Intracellular Distribution of Atg9
Direct delivery from the ER is not the only route that has been proposed for delivery of membrane to the forming autophagosome. In yeast Atg9 is located in peripheral puncta as well as the forming autophagosome, and it has been proposed that these puncta correspond to mitochondria (Reggiori et al., 2004a; He et al., 2006; Reggiori and Klionsky, 2006; Yen et al., 2007). When we examined the distribution of Atg9-GFP we were able to detect some labeled puncta close to mitochondria, particularly under starvation conditions (Figure 2A). However, when mitochondrial morphology was altered genetically, there was not a concomitant large-scale relocalization of Atg9-GFP (Figure 2A). In addition, time-lapse imaging revealed that the Atg9-GFP puncta were much more mobile than the mitochondria and appeared to be moving past them and also through other parts of the cell were there are no mitochondria (Supplemental Video 1). Similar observations have been recently reported by Sekito and coworkers (Sekito et al., 2009), and the apparent transient colocalization of Atg9-GFP puncta and mitochondria may be linked to mitophagy or could simply reflect the fact that both structures can move along actin cables (Frederick and Shaw, 2007; Monastyrska et al., 2008). When we compared Atg9-GFP to other organelle markers the only substantial colocalization we observed was with endocytic compartments as labeled by the late endosomal sodium/proton exchanger Nhx1 (Bowers et al., 2000), the endocytosed fluorescent dye FM4-64 (Vida and Emr, 1995), or the SNARE Tlg1 whose steady state distribution is primarily in endosomes as it cycles between endosomes and the Golgi (Holthuis et al., 1998) (Figure 2, B and C). This colocalization was maintained in the absence of Atg11 which is required for the formation of autophagosomal, but not the peripheral, pool of Atg9 (Figure 2C), and was also seen in the enlarged endosomes that are induced by deletion of the multivesicular body forming protein Vps4 (Figure 2C). These results indicate that Atg9 traffics through the Golgi and then resides in both the forming autophagosome and, at least in part, in endosomal compartments rather than being in mitochondria.
Figure 2.
Intracellular distribution of Atg9-GFP. (A) Fluorescent micrographs comparing Atg9-GFP with the mitochondrial outer membrane marker Tom20-RFP in wild-type cells or cells lacking the mitochondrial fusion protein Ugo1 and grown in YEPD or starved for nitrogen for four hours [SD(-N)] as indicated. The loss of Ugo1 causes the mitochondria to form clumps of fragments (Sesaki and Jensen, 2001), but Atg9-GFP remains scattered, with colocalization only observed occasionally, usually in forming buds. (B) Fluorescence micrographs comparing Atg9-GFP to the late Golgi protein Sec7-RFP or the late endosomal protein Nhx1-RFP in wild-type cells in rich medium. (C) Fluorescence micrographs comparing Atg9-GFP to RFP-Ape1, the endocytic tracer FM4-64 (10 min pulse, 10 min chase), or mCherry-Tlg1 (Chry-Tlg1) in wild-type cells in rich medium. (D) As for C except that the cells are either lacking the autophagosome scaffold protein Atg11 which is required for membrane to accumulate at the autophagosome (Shintani and Klionsky, 2004), or lacking the endosomal protein Vps4, in which the endocytic compartment expands (Babst et al., 1997).
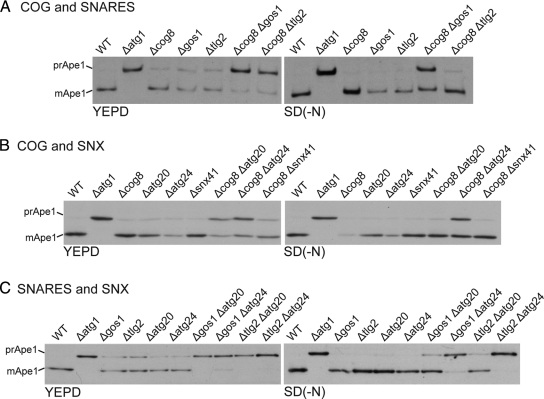
Analysis of SNARE Requirements for Cvt Pathway and Autophagy
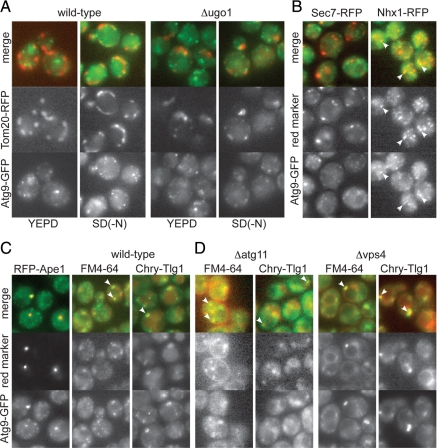
To obtain further insight into the route by which Atg9-containing membrane reaches the autophagosome we looked for trafficking components that are required for autophagy. We initially examined the SNARE proteins as these act in all known trafficking steps within the endomembrane system. To obtain a comprehensive picture of the involvement of SNARE proteins in the Cvt pathway and autophagy, Ape1 maturation was examined in individual mutants for all eleven of the SNAREs that are not essential for viability (Figure 3A). As reported previously the vacuolar SNAREs Vam3 and Vam7 that mediate fusion of the autophagosome with the vacuole are required for both autophagy and the Cvt pathway (Figure 3A; Darsow et al., 1997). In addition, we found that deletion of the Golgi SNARE Gos1 caused an accumulation of proApe1 in rich medium comparable to that seen with loss of the SNARE Tlg2 that has been previously reported to act in the Cvt pathway (Abeliovich et al., 1999). The stability of mature Ape1 means that the mature protein generated during starvation in stationary phase persists in the vacuole long after initiation of log-phase growth, but imaging of GFP-Apel in strains lacking either Gos1 or Tlg2 confirmed the accumulation of unprocessed Ape1 aggregates, and hence a defect in the Cvt pathway, during log-phase growth in rich medium (Figure 3B). However, neither Gos1 nor Tlg2 were required for Ape1 processing when autophagy was induced by starvation (Figure 2A).
Figure 3.
Golgi SNAREs and COG subunits are involved in the Cvt pathway. (A) Ape1 immunoblot analysis in SNARE mutants grown in rich medium (YEPD) for three hours or under conditions of nitrogen starvation for four hours [SD(-N)]. Vam3 and Vam7 are involved in the fusion of vacuolar membranes and so are required for maturation of Ape1 in vegetative and starvation conditions. Deletion of Pep12 inhibits Ape1 maturation, which at least in part appears to be because of its known role in the delivery of vacuolar hydrolases (Becherer et al., 1996). In starved cells some GFP-Ape1 could be seen in undigested autophagosomes inside the vacuole (Supplemental Figure 1B), but in rich medium we also observed an accumulation of GFP-Ape1 aggregates adjacent to the vacuole and so there may also be defects in autophagosome formation in the Cvt pathway. (B) Localization of GFP-Ape1 by fluorescence microscopy in the indicated strains. Cells were grown in YEPD or in SD(-N) and labeled with FM4-64. In YEPD a puncta of GFP-Ape1 was observed in 4% of wild-type cells, but this rose in Δatg1 (31%), Δcog8 (29%), Δgos1 (12%), and Δtlg2 (24%). In SD(-N), GFP signals were mainly observed inside the vacuole in all the strains except Δatg1. (C) Ape1 immunoblot analysis in wild-type (WT) or the indicated deletion mutants. Mutants lacking COG lobe B subunits, or YPT6 or its exchange factor RIC1 show reduced Ape1 maturation only in vegetative phase (YEPD).
COG Complex Subunits Contribute to Efficient Functioning of the Cvt Pathway
The SNAREs Tlg2 and Gos1 act in distinct pathways for recycling of membrane back from the endosomes to the Golgi, or within the Golgi itself, and are believed to participate in distinct SNARE complexes (Holthuis et al., 1998; McNew et al., 1998; Parlati et al., 2002). Gos1 has been proposed to interact with the octameric COG complex which comprises two lobes, A and B, with the latter lobe containing four nonessential subunits (Cog5-8) that appear to be involved in recycling back to the Golgi from endosomes (Whyte and Munro, 2001; Ungar et al., 2006). Thus we asked whether mutants in lobe B subunits show defects in the Cvt pathway and autophagy. Figure 3C shows that in mutants lacking any one of Cog5-8 there was a small but reproducible accumulation of unprocessed Ape1 in rich (i.e., nonstarvation) medium. In these COG subunit mutants there was also an increase in the number of cells that where aggregates of GFP-Ape1 had accumulated in the cytoplasm (Figure 3B). As with the Δgos1 mutant, accumulation of proApe1 in the COG mutants was lost when bulk autophagy was induced by starvation (Figure 3, B and C). Thus Cog5-8 appear to be only required for autophagosome formation under nonstarvation conditions, and while this article was in preparation similar effects of cog5-8 mutants on Ape1 processing in the Cvt pathway but not autophagy were reported by Klionsky and coworkers (Yen et al., 2010).
Synthetic Genetic Interactions Reveal Role of Cvt Pathway Components in Autophagy
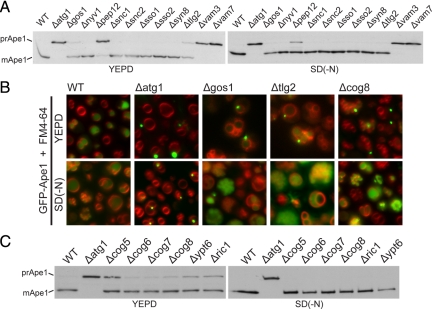
The above results reveal that Atg9 needs to travel past the early Golgi to reach the autophagosome in starved cells and that it does not appear to accumulate in mitochondria but rather it is, at least in part, localized to endosomes. However, the only endosome/Golgi SNAREs that are required for the Cvt pathway, Gos1 and Tlg2, do not appear to be required for autophagy during starvation. Nonetheless, previous studies on recycling between the Golgi and endosomes have revealed a remarkable degree of redundancy with many double mutants showing synergistic interactions (Tong et al., 2004; Sciorra et al., 2005; Burston et al., 2009). This appears to be due to there being multiple routes between endosomes and Golgi, and also to the ability of some cargo proteins to use a different route when their normal route is missing (Hettema et al., 2003; Quenneville et al., 2006). This led us to wonder whether the Golgi and endosomal proteins previously thought to contribute only to the Cvt pathway are in fact also contributing to autophagy but in a manner that is redundant and hence only visible when more than one protein is missing. We first analyzed strains lacking one or other of the SNAREs Gos1 and Tlg2, and the COG subunit Cog8. Not only were the Cvt defects observed in the single mutants enhanced in the double mutations, but now defects could be clearly seen under starvation conditions in the case of the Δcog8Δgos1 double mutant (Figure 4A).
Figure 4.
Combinations of Golgi-endosomal mutations cause defects in autophagy. Ape1 maturation was analyzed both in YEPD and SD(-N) by immunoblotting in the indicated mutants. (A) Analysis of Δcog8 and two SNARE mutants, Δgos1 and Δtlg2. (B) Analysis of Δcog8 and single mutants for three related sorting nexins, Atg20, Atg24, and Snx41. (C) Analysis of two SNARE mutants (Δgos1 and Δtlg2) and two sorting nexin mutants.
We next examined the sorting nexins Atg20 and Atg24 (Snx4). These proteins form a heterodimer and act in traffic in one of the routes from endosomes to the Golgi and are required for the Cvt pathway but not bulk autophagy (Nice et al., 2002; Hettema et al., 2003). Atg24 also forms heterodimers with a relative of Atg20, called Snx41, but the Atg24/Snx41 complex appears to be exclusively involved in endosome to Golgi traffic and not the Cvt pathway (Hettema et al., 2003). Figure 4B shows that the Δcog8Δatg24 double mutant has a clear defect in autophagy despite there being no detectable defect with either single mutation. Likewise, combining mutants in Atg20 or Atg24 with those in the SNAREs Gos1 or Tlg2 results in defects in Ape1 maturation in starved cells, with a near complete block in Δgos1Δatg24 and Δtlg2Δatg24, despite the individual mutations showing little defect (Figure 4C). These strong phenotypes are due to the loss of the deleted genes as they could be rescued by the relevant genes on plasmids (Supplemental Figure S2).
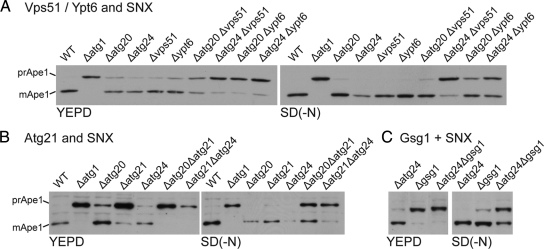
To test whether these findings were generally relevant to Cvt-specific proteins we examined double mutants in a range of other genes encoding components of endosome-Golgi traffic that appear to contribute to the Cvt pathway but not to autophagy. These were the GTPase Ypt6, its effector the GARP/VFT subunit Vps51, and the TRAPP subunit Gsg1 (Trs85) (Siniossoglou and Pelham, 2001; Reggiori et al., 2003; Meiling-Wesse et al., 2005; Lynch-Day et al., 2010). We also examined the PtdIns(3)P binding protein Atg21 that is required for the Cvt pathway but has no reported role in traffic (Krick et al., 2008). In every case combining mutations in these proteins with mutants in sorting nexin subunits again resulted in synergistic defects in autophagy (Figure 5).
Figure 5.
Combinations of Cvt pathway mutations cause defects in autophagy. Ape1 maturation was analyzed both in YEPD and SD(-N) by immunoblotting in the indicated mutants. (A) Analysis of mutants lacking GARP/VFT subunit Vps51, or Ypt6 that recruits GARP/VFT, and the sorting nexins (Δatg20 and Δatg24). (B) Analysis of mutants in Cvt component Atg21 and sorting nexins (Δatg20 and Δatg24). (C) Analysis of mutants in TRAPP subunit Gsg1 and sorting nexin Atg24.
The Effects of Double Mutants of Cvt Pathway Components on Other Assays for Autophagy
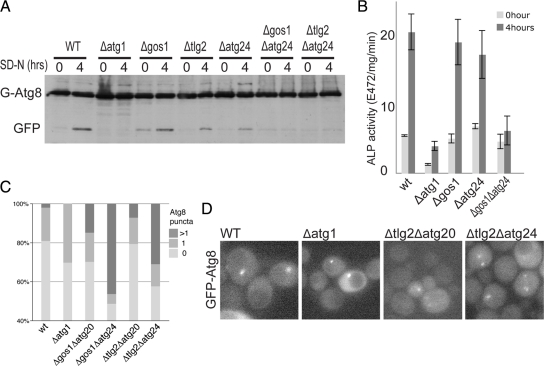
To confirm that the above synergistic effects on starvation-induced autophagy seen with combinations of Cvt pathway components were not confined to Ape1 processing we tested some mutant combinations with other assays for autophagy. The strongly synergistic combinations Δgos1Δatg24 and Δtlg2Δatg24 showed blocks in both the GFP-Atg8 processing assay and the Pho8Δ60 vacuolar activation assay that were not seen with the single mutants (Figure 6, A and B). In addition, GFP-Atg8 accumulated in more than one puncta in the cytosol of the double mutants (Figure 6, C and D), a phenotype associated with defects in autophagosome formation (Reggiori et al., 2004b; Meiling-Wesse et al., 2005; Yen et al., 2010).
Figure 6.
Combined mutants in Golgi-endosomal components show synergistic defects in starvation-induced autophagy. (A) Anti-GFP immunoblot of the indicated strains expressing GFP-Atg8. Cells were grown without uracil (rich medium) to midlog phase and either harvested or shifted to starvation conditions for four hours (SD-N). The positions of GFP-Atg8 and the free GFP that is released after autophagic delivery to the vacuole are indicated. (B) Alkaline phosphatase activity in the indicated strains expressing Pho8Δ60 that lacks a transmembrane domain and so is only activated after delivery to the vacuole by autophagy. Starvation and strains (with PHO13 deleted) are as in A, and error bars indicate the SD of three independent experiments. (C) Quantitation of the distribution of GFP-Atg8 in cells grown as in A. For each strain >150 cells were counted. The values show the proportion of the population with one, or more than one puncta of GFP-Atg8, and are based on a projections of six focal planes. The increases in frequency of cells with multiple GFP-Atg8 puncta in all the double mutants are statistically significant (χ2 test, two-tailed values p < 0.0001). (D) Representative single focal plane images of the cells quantified in C. Combination of SNARE and Atg24 mutations results in the appearance of multiple puncta of GFP-Atg8 consistent with defects in autophagosome formation.
The Ape1 processing assay, the GFP-Atg8 cleavage assay, and the Pho8 activation assay all depend on the SNARE Vam3 and active hydrolases being present in the vacuole (Darsow et al., 1997). However, pulse-chase analysis showed that the maturation of the vacuolar hydrolase carboxypeptidase Y is not affected in the double mutants that show strong Ape1 processing defects (e.g., Δgos1Δatg24 and Δtlg2Δatg24), whereas it is, as expected, blocked in the Δvam3 control (Supplemental Figure S3A). Likewise the double mutations did not show severe vacuole fragmentation defects, or indeed perturbation of growth rate (Supplemental Figure S3, B and C). Defects in endosomal components can also result in the increased turnover of membrane proteins by vacuolar degradation, but there was no detectable reduction in levels of a FLAG-tagged form of Atg9 in the double mutants (Supplemental Figure S3D). Taken together these data indicate that the vacuole in these double mutants is fusion competent and contains active hydrolases, and therefore the defects in autophagy seen with double mutants of Cvt pathway components must be upstream of vacuolar processing and hence reflect defects in autophagosome formation.
Accumulation of Atg9-GFP in the Autophagosome Is Impaired in Double Mutants of Cvt Pathway Components
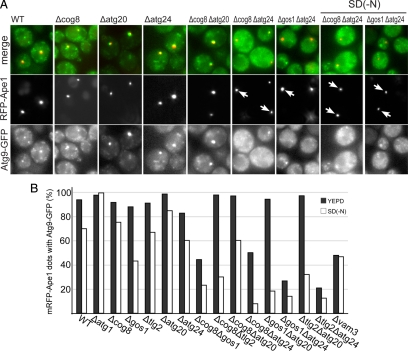
Because Atg9-GFP appears to be at least in part located in endosomal compartments, it seemed possible that the above combinations of mutations in components of Golgi-endosomal traffic that affect autophagy were affecting delivery of Atg9 to the autophagosome. To investigate the effects of the various double mutants on the delivery of Atg9 to the forming autophagosome, we compared the distribution of Atg9-GFP to that of RFP-Ape1. In the single mutants there was no substantial change in the number of cells that showed labeling of the Ape1 aggregate with Atg9-GFP (Figure 7, A and B). However, in double mutants with a severe defect in Ape1 processing there was in general a large reduction in the number of cells where Atg9-GFP colocalized with RFP-Ape1 in both rich medium and starvation conditions. This suggests that combining mutations in Cvt pathway can result in the delivery of Atg9 to the forming autophagosome being impaired, even in starvation conditions, which would provide an explanation for the observed defects in autophagy. The only exceptions to this general trend were those mutants containing Atg20, where Atg9 could still reach the forming autophagosome in rich medium even though Ape1 processing was defective. This could reflect a role for Atg20 in retrieval of Atg9 to peripheral sites, with elevated Atg9 being detrimental to autophagosome maturation. This has been suggested previously for Atg18 (Reggiori et al., 2004a), and Atg20 mutants consistently showed elevated levels of Atg9 associated with Ape1 aggregates (Figure 7A).
Figure 7.
Atg9 does not reach Ape1-containing autophagosomes in double mutants for Golgi-endosomal trafficking components. (A) Fluorescence micrographs comparing the distribution of Atg9-GFP and RFP-Ape1 in the indicated representative strains in YEPD or SD(-N). Arrows indicate RFP-Ape1 dots which are not colocalized with Atg9-GFP. (B) Quantification of Atg9-GFP and RFP-Ape1 colocalization in both YEPD (rich) and SD(-N). For each strain 50–100 RFP-Ape1 containing structures were examined.
The SNAREs Gos1 and Tlg2 Are Required for Different Steps in Atg9 Recycling
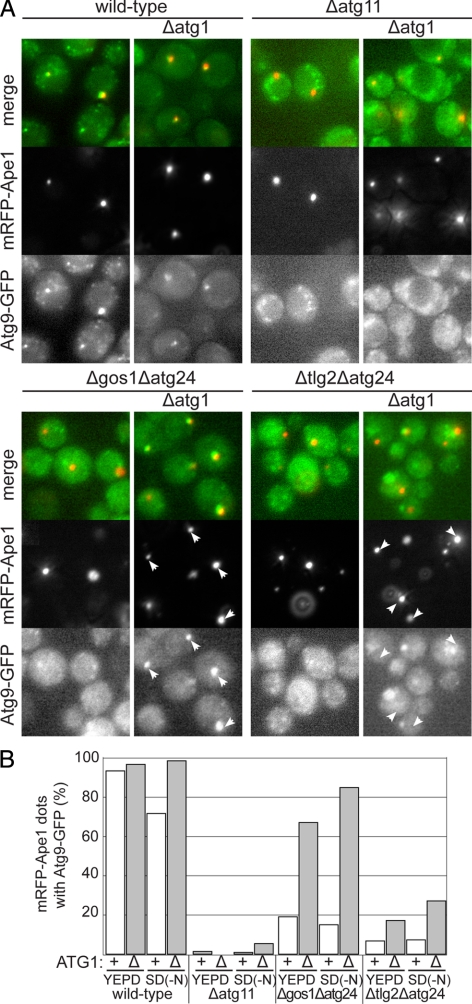
After Atg9 is delivered to the autophagosome it appears to be retrieved back to the peripheral pool, presumably allowing it to participate in multiple rounds of autophagosome formation (Reggiori et al., 2004a). Thus there must be membrane trafficking routes to deliver Atg9 to the forming autophagosome and also to remove it and deliver it back to the peripheral sites, at least some of which we suggest are endosomes. Thus if a mutant combination reduces the accumulation of Atg9 in the forming autophagosome it could reflect a direct defect in delivery, or it could be that delivery is normal but there is a defect in the retrieval route that occurs after Atg9 has exited the autophagosome. These possibilities can be distinguished using an epistasis analysis based on the Atg1 kinase, the so called “Take Atg1 kinase away (TAKA)” assay (Shintani and Klionsky, 2004). Atg1 is required for exit of Atg9 from the autophagosome at the start of the retrieval route (Reggiori et al., 2004a). Thus if a mutant, or mutant combination, affects delivery, when Atg1 is removed from the mutant background the accumulation of Atg9 at the autophagosome should remain inhibited. However, if the defect is in the retrieval route then removal of Atg1 should result in accumulation of Atg9 in the autophagosome as it cannot enter the defective retrieval route.
We therefore examined the effect of deleting Atg1 in combinations of Cvt mutants that show strong autophagy defects. Figure 8 shows that when Atg1 activity is removed from the Δtlg2Δatg24 strain then delivery of Atg9 remains substantially inhibited. In contrast, when the SNARE Gos1 is absent in combination with Δatg24, then removal of Atg1 results in Atg9 accumulating at the forming autophagosome. This indicates that when cells are sensitized by removal of Atg24, then the route that Atg9 must travel to the forming autophagosome is dependent upon Tlg2, but not Gos1. In contrast the retrieval route from the autophagosome has a requirement for Gos1, but not Tlg2, that appears to occur after the Atg1-dependent exit of Atg9 from the autophagosome. Distinct roles for Gos1 and Tlg2 would also be consistent with the two SNAREs being known to participate in distinct SNARE complexes and hence different trafficking processes (McNew et al., 1998; Parlati et al., 2002; Burri and Lithgow, 2004). These results suggest that during starvation-induced autophagy, Cvt components are making a redundant contribution to both the delivery of Atg9-containing membrane to the forming autophagosome and also to its retrieval.
Figure 8.
Combining loss of Atg24 with loss of different SNAREs affects different parts of the Atg9 recycling itinerary. (A) Fluorescence micrographs comparing the distribution of Atg9-GFP and RFP-Ape1 in the indicated strains with or without Atg1 deleted. Cells were grown in YEPD to midlog phase and shifted to starvation conditions for four hours (SD-N). Deletion of Atg1 blocks exit of Atg9 from the autophagosome that forms around Ape1 aggregates (Reggiori et al., 2004a). The autophagosome does not form in the absence of Atg11, and so this serves as a negative control. In Δgos1Δatg24 the majority of forming autophagosomes show an accumulation of Atg9 in the absence of Atg1 (arrows), whereas in Δtlg2Δatg24, the forming autophagosomes tend to lack Atg9 (arrows), indicating that delivery rather than retrieval is defective. (B) Quantitation of the experiments in A in which the presence of Atg9 in forming autophagosomes was quantified by counting how many Ape1 aggregates had associated Atg9-GFP. 100–200 mRFP-Ape1 puncta were analyzed for each condition.
DISCUSSION
The results reported here demonstrate that in yeast Atg9 has to move beyond the ER and early Golgi to be able to function in autophagy. This finding suggests that substantial amounts of bilayer membrane are not delivered directly from the ER to the forming autophagosome, although we cannot exclude the possibility that there is a process that transports some bilayer directly from the ER to the autophagosome in carriers that exclude Atg9. The conclusion that at least Atg9 must traffic out of the ER via the Golgi is consistent with previous reports that conditional mutations that block ER to Golgi traffic affect autophagy (Ishihara et al., 2001; Reggiori et al., 2004b), although it could have been argued in this case that the general, and ultimately lethal, block in secretion used in these experiments could have affected other processes apart from Atg9 delivery, or could reflect indirect effects mediated via the stress pathways known to be induced when secretion is blocked (Mizuta and Warner, 1994; Nanduri and Tartakoff, 2001; Geng et al., 2010). In addition, we do not find evidence to support previous suggestions that Atg9 is localized to mitochondria and hence bilayer membrane can be delivered from mitochondria to the forming autophagosome (Reggiori et al., 2004a; He et al., 2006; Reggiori and Klionsky, 2006; Yen et al., 2007), a route that would have required unprecedented membrane trafficking processes and machinery.
Instead, our results demonstrate that during starvation the delivery of Atg9, and hence membrane, to the autophagosome is perturbed by removal of combinations of components of the membrane traffic routes that link the Golgi and endosomal systems. These components had previously been shown to be required for efficient functioning of the Cvt pathway, but did not appear to be required for starvation-induced autophagy (Abeliovich et al., 1999; Nice et al., 2002; Reggiori et al., 2003; Meiling-Wesse et al., 2005). The ability of the yeast Golgi-endosomal system to compensate for many single gene deletions under laboratory conditions would explain why some of these genes need to be deleted in combination before defects in autophagy become apparent and why these genes were not found in the seminal genetic screens that identified the proteins that regulate and initiate autophagy (Tsukada and Ohsumi, 1993; Thumm et al., 1994; Harding et al., 1995; Nakatogawa et al., 2009). The fact that single mutants show defects only in the Cvt pathway and not in starvation conditions might reflect that fact that during starvation it could become important to deliver membrane from more sources in the Golgi-endosome system so that no one aspect of endocytic traffic becomes depleted when there is a larger flux through the autophagic pathway. This would also explain why a block in the flow of membrane from the ER into the secretory pathway rapidly affects autophagy but has a slower effect on the Cvt pathway (Ishihara et al., 2001; Reggiori et al., 2004b).
Our results imply that the Cvt-pathway is more similar to starvation-induced autophagy than previously appreciated, and hence the two processes differ more in rate than in mechanism. The majority of cellular components thought to be specific to the Cvt-pathway are components of Golgi-endosomal traffic which we show here are also important for autophagy. Of the remainder, Atg19 is a receptor for a specific cargo (Ape1) that needs to efficiently directed into forming autophagosomes in nonstarved cells, and the Cvt-specific scaffold protein Atg11 appears to be also required for autophagy if Atg17 is absent (Suzuki et al., 2007). Moreover, Atg11 is distantly related to FIP200 (S.M., unpublished observation), a mammalian protein which is required for starvation-induced autophagy (Hara et al., 2008).
The link between Golgi-endosome components and starvation-induced autophagy is also consistent with the fact that the forming autophagosome has many of the properties of an endocytic compartment including enrichment in PtdIns(3)P and its effector proteins (Hettema et al., 2003; Krick et al., 2008; Obara et al., 2008), the apparent exchange of membrane with compartments in the Golgi-endosome system, and the ability to mature to fuse with the vacuole. Indeed we note that Atg2, a protein found on the autophagosome and required for autophagy, is distantly related to the endosomal trafficking component Vps13 (S.M., unpublished observation). Delivery of membrane and Atg9 from compartments of the endocytic system would also be consistent with recent findings that conditional inactivation of either of the essential Golgi GTPase activities Arf1/2 or Ypt31/32 inhibits autophagy, as these activities are likely to be required directly and indirectly for both traffic through the Golgi and also recycling from endocytic compartments (Hicke et al., 1997; Geng et al., 2010; van der Vaart et al., 2010). This requirement does not appear to reflect a need to traffic Atg9 to the cell surface, and indeed we do not see Atg9 accumulating at the surface in endocytic mutants such as Δend3 (YO and SM, unpublished observations). Our finding that a RXR motif is recognized when attached to Atg9 indicates that the protein must move through the cis Golgi where COPI acts to recycle RXR-containing proteins to the ER. Further work will be needed to determine where Atg9 leaves the Golgi, as recent studies indicate that inactivation of the late Golgi Arf exchange factor Sec7 does not result in accumulation of Atg9 in the late Golgi (van der Vaart et al., 2010). Given the complexities of the yeast Golgi-endosome system, further work will also be required to map which precise routes Atg9 can use and when, and indeed it is possible that some components have a role on the autophagosome itself as well as being required for the traffic of Atg9 into a post-Golgi compartment and on to the autophagosome. This has recently been suggested for the essential (lobe A) subunits of the COG complex (Yen et al., 2010), but because these subunits are also required for traffic of membrane proteins through the Golgi then further work will be required to determine the point at which these particular mutations are blocking Atg9 delivery (VanRheenen et al., 1999; Ram et al., 2002).
Overall, our results suggest that Atg9-containing bilayer is recycling between the forming autophagosome and multiple compartments in the Golgi-endosome system rather than the ER or mitochondria. This provides a possible resolution of the discrepancy between previous work in yeast and recent studies in mammalian cells where the two orthologues of Atg9 are found to be localized to TGN and endosomes (Young et al., 2006; Razi et al., 2009). It should be added that while our results suggest that bilayer may not be delivered directly from the ER to the autophagosome, this need not preclude some phospholipids being transferred from the ER or mitochondria by cytosolic carrier proteins (Voelker, 2009). Our demonstration that the Golgi–endosome system rather than the ER or mitochondria is apparently involved in Atg9 delivery to the forming yeast autophagosome will hopefully allow the yeast system to guide study of these and other issues in both yeast and mammalian cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alison Gillingham and Maki Ohashi for comments on the manuscript and Daniel Klionsky for reagents. This work was supported by a grant from the Medical Research Council (to S.M.), and by postdoctoral fellowships (to Y.O.) from the European Molecular Biology Organization, the Human Frontiers Scientific Program, and the Japanese Society for the Promotion of Science.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-05-0457) on September 22, 2010.
REFERENCES
- Abeliovich H., Darsow T., Emr S. D. Cytoplasm to vacuole trafficking of aminopeptidase I requires a t-SNARE-Sec1p complex composed of Tlg2p and Vps45p. EMBO J. 1999;18:6005–6016. doi: 10.1093/emboj/18.21.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G., Ktistakis N. T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell. Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M., Sato T. K., Banta L. M., Emr S. D. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer K. A., Rieder S. E., Emr S. D., Jones E. W. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol. Biol. Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K., Levi B. P., Patel F. I., Stevens T. H. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 2000;11:4277–4294. doi: 10.1091/mbc.11.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri L., Lithgow T. A complete set of SNAREs in yeast. Traffic. 2004;5:45–52. doi: 10.1046/j.1600-0854.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- Burston H. E., Maldonado-Baez L., Davey M., Montpetit B., Schluter C., Wendland B., Conibear E. Regulators of yeast endocytosis identified by systematic quantitative analysis. J. Cell. Biol. 2009;185:1097–1110. doi: 10.1083/jcb.200811116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. E., Tour O., Palmer A. E., Steinbach P. A., Baird G. S., Zacharias D. A., Tsien R. Y. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T., Rieder S. E., Emr S. D. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J. Cell. Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré J.-C., Krick R., Subramani S., Thumm M. Turnover of organelles by autophagy in yeast. Curr. Opin. Cell. Biol. 2009;21:522–530. doi: 10.1016/j.ceb.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick R. L., Shaw J. M. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J., Nair U., Yasumura-Yorimitsu K., Klionsky D. J. Post-Golgi Sec proteins Are required for autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell. 2010;21:2257–2269. doi: 10.1091/mbc.E09-11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey D. W., Rambold A. S., Satpute-Krishnan P., Mitra K., Sougrat R., Kim P. K., Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Takamura A., Kishi C., Iemura S.-I., Natsume T., Guan J.-L., Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell. Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding T. M., Morano K. A., Scott S. V., Klionsky D. J. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J. Cell. Biol. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Nishino M., Fujita N., Noda T., Yamaguchi A., Yoshimori T., Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell. Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- He C., Baba M., Cao Y., Klionsky D. J. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Mol. Biol. Cell. 2008;19:5506–5516. doi: 10.1091/mbc.E08-05-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Song H., Yorimitsu T., Monastyrska I., Yen W.-L., Legakis J. E., Klionsky D. J. Recruitment of Atg9 to the preautophagosomal structure by Atg11 is essential for selective autophagy in budding yeast. J. Cell. Biol. 2006;175:925–935. doi: 10.1083/jcb.200606084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema E. H., Lewis M. J., Black M. W., Pelham H.R.B. Retromer and the sorting nexins Snx4/41/42 mediate distinct retrieval pathways from yeast endosomes. EMBO J. 2003;22:548–557. doi: 10.1093/emboj/cdg062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L., Zanolari B., Pypaert M., Rohrer J., Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol. Biol. Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis J. C., Nichols B. J., Dhruvakumar S., Pelham H. R. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N., Hamasaki M., Yokota S., Suzuki K., Kamada Y., Kihara A., Yoshimori T., Noda T., Ohsumi Y. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol. Biol. Cell. 2001;12:3690–3702. doi: 10.1091/mbc.12.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G., Neufeld T. P. Autophagy: a forty-year search for a missing membrane source. PLoS Biol. 2006;4:e36. doi: 10.1371/journal.pbio.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick R., Henke S., Tolstrup J., Thumm M. Dissecting the localization and function of Atg18, Atg21 and Ygr223c. Autophagy. 2008;4:896–910. doi: 10.4161/auto.6801. [DOI] [PubMed] [Google Scholar]
- Lang T., Reiche S., Straub M., Bredschneider M., Thumm M. Autophagy and the cvt pathway both depend on AUT9. J. Bacteriol. 2000;182:2125–2133. doi: 10.1128/jb.182.8.2125-2133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longatti A., Tooze S. Vesicular trafficking and autophagosome formation. Cell. Death Differ. 2009 doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- Lynch-Day M. A., Bhandari D., Menon S., Huang J., Cai H., Bartholomew C. R., Brumell J. H., Ferro-Novick S., Klionsky D. J. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc. Natl. Acad. Sci. USA. 2010;107:7811–7816. doi: 10.1073/pnas.1000063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew J. A., Coe J. G., Søgaard M., Zemelman B. V., Wimmer C., Hong W., Söllner T. H. Gos1p, a Saccharomyces cerevisiae SNARE protein involved in Golgi transport. FEBS Lett. 1998;435:89–95. doi: 10.1016/s0014-5793(98)01044-8. [DOI] [PubMed] [Google Scholar]
- Meiling-Wesse K., Epple U. D., Krick R., Barth H., Appelles A., Voss C., Eskelinen E.-L., Thumm M. Trs85 (Gsg1), a component of the TRAPP complexes, is required for the organization of the preautophagosomal structure during selective autophagy via the Cvt pathway. J. Biol. Chem. 2005;280:33669–33678. doi: 10.1074/jbc.M501701200. [DOI] [PubMed] [Google Scholar]
- Michelsen K., Schmid V., Metz J., Heusser K., Liebel U., Schwede T., Spang A., Schwappach B. Novel cargo-binding site in the beta and delta subunits of coatomer. J. Cell. Biol. 2007;179:209–217. doi: 10.1083/jcb.200704142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta K., Warner J. R. Continued functioning of the secretory pathway is essential for ribosome synthesis. Mol. Cell. Biol. 1994;14:2493–2502. doi: 10.1128/mcb.14.4.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastyrska I., He C., Geng J., Hoppe A. D., Li Z., Klionsky D. J. Arp2 links autophagic machinery with the actin cytoskeleton. Mol. Biol. Cell. 2008;19:1962–1975. doi: 10.1091/mbc.E07-09-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H., Suzuki K., Kamada Y., Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell. Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- Nanduri J., Tartakoff A. M. The arrest of secretion response in yeast: signaling from the secretory path to the nucleus via Wsc proteins and Pkc1p. Mol. Cell. 2001;8:281–289. doi: 10.1016/s1097-2765(01)00312-4. [DOI] [PubMed] [Google Scholar]
- Nice D. C., Sato T. K., Stromhaug P. E., Emr S. D., Klionsky D. J. Cooperative binding of the cytoplasm to vacuole targeting pathway proteins, Cvt13 and Cvt20, to phosphatidylinositol 3-phosphate at the pre-autophagosomal structure is required for selective autophagy. J. Biol. Chem. 2002;277:30198–30207. doi: 10.1074/jbc.M204736200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Kim J., Huang W. P., Baba M., Tokunaga C., Ohsumi Y., Klionsky D. J. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J. Cell. Biol. 2000;148:465–480. doi: 10.1083/jcb.148.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 1998;273:3963–3966. doi: 10.1074/jbc.273.7.3963. [DOI] [PubMed] [Google Scholar]
- Obara K., Sekito T., Niimi K., Ohsumi Y. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J. Biol. Chem. 2008;283:23972–23980. doi: 10.1074/jbc.M803180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F., Varlamov O., Paz K., Mcnew J. A., Hurtado D., Söllner T. H., Rothman J. E. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA. 2002;99:5424–5429. doi: 10.1073/pnas.082100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenneville N. R., Chao T.-Y., McCaffery J. M., Conibear E. Domains within the GARP subunit Vps54 confer separate functions in complex assembly and early endosome recognition. Mol. Biol. Cell. 2006;17:1859–1870. doi: 10.1091/mbc.E05-11-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram R. J., Li B., Kaiser C. A. Identification of Sec36p, Sec37p, and Sec38p: components of yeast complex that contains Sec34p and Sec35p. Mol. Biol. Cell. 2002;13:1484–1500. doi: 10.1091/mbc.01-10-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi M., Chan E.Y.W., Tooze S. A. Early endosomes and endosomal coatomer are required for autophagy. J. Cell. Biol. 2009;185:305–321. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Klionsky D. J. Atg9 sorting from mitochondria is impaired in early secretion and VFT-complex mutants in Saccharomyces cerevisiae. J. Cell. Sci. 2006;119:2903–2911. doi: 10.1242/jcs.03047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Tucker K. A., Stromhaug P. E., Klionsky D. J. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev. Cell. 2004a;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Reggiori F., Wang C.-W., Nair U., Shintani T., Abeliovich H., Klionsky D. J. Early stages of the secretory pathway, but not endosomes, are required for Cvt vesicle and autophagosome assembly in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004b;15:2189–2204. doi: 10.1091/mbc.E03-07-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F., Wang C.-W., Stromhaug P. E., Shintani T., Klionsky D. J. Vps51 is part of the yeast Vps fifty-three tethering complex essential for retrograde traffic from the early endosome and Cvt vesicle completion. J. Biol. Chem. 2003;278:5009–5020. doi: 10.1074/jbc.M210436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D. C., Gestwicki J. E., Murphy L. O., Klionsky D. J. Potential therapeutic applications of autophagy. Nature Rev. Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Sapperstein S. K., Lupashin V. V., Schmitt H. D., Waters M. G. Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell. Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra V. A., Audhya A., Parsons A. B., Segev N., Boone C., Emr S. D. Synthetic genetic array analysis of the PtdIns 4-kinase Pik1p identifies components in a Golgi-specific Ypt31/rab-GTPase signaling pathway. Mol. Biol. Cell. 2005;16:776–793. doi: 10.1091/mbc.E04-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito T., Kawamata T., Ichikawa R., Suzuki K., Ohsumi Y. Atg17 recruits Atg9 to organize the pre-autophagosomal structure. Genes Cells. 2009;14:525–538. doi: 10.1111/j.1365-2443.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- Sesaki H., Jensen R. E. UGO1 encodes an outer membrane protein required for mitochondrial fusion. J. Cell. Biol. 2001;152:1123–1134. doi: 10.1083/jcb.152.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikano S., Li M. Membrane receptor trafficking: evidence of proximal and distal zones conferred by two independent endoplasmic reticulum localization signals. Proc. Natl. Acad. Sci. USA. 2003;100:5783–5788. doi: 10.1073/pnas.1031748100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Huang W.-P., Stromhaug P. E., Klionsky D. J. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev. Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T., Klionsky D. J. Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J. Biol. Chem. 2004;279:29889–29894. doi: 10.1074/jbc.M404399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S., Pelham H. R. An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 2001;20:5991–5998. doi: 10.1093/emboj/20.21.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kubota Y., Sekito T., Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- Thumm M., Egner R., Koch B., Schlumpberger M., Straub M., Veenhuis M., Wolf D. H. Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett. 1994;349:275–280. doi: 10.1016/0014-5793(94)00672-5. [DOI] [PubMed] [Google Scholar]
- Tong A. H., Lesage G., Bader G. D., Ding H., Xu H., Xin X., Young J., Berriz G. F., Brost R. L., Chang M. Global mapping of the yeast genetic interaction network. Science. 2004;303:808–813. doi: 10.1126/science.1091317. [DOI] [PubMed] [Google Scholar]
- Tsukada M., Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Ungar D., Oka T., Krieger M., Hughson F. M. Retrograde transport on the COG railway. Trends Cell. Biol. 2006;16:113–120. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- van der Vaart A., Griffith J., Reggiori F. Exit from the Golgi is required for the expansion of the autophagosomal phagophore in yeast Saccharomyces cerevisiae. Mol. Biol. Cell. 2010;21:2270–2284. doi: 10.1091/mbc.E09-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRheenen S. M., Cao X., Sapperstein S. K., Chiang E. C., Lupashin V. V., Barlowe C., Waters M. G. Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p. J. Cell. Biol. 1999;147:729–742. doi: 10.1083/jcb.147.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T. A., Emr S. D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell. Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker D. R. Genetic and biochemical analysis of non-vesicular lipid traffic. Annu. Rev. Biochem. 2009;78:827–856. doi: 10.1146/annurev.biochem.78.081307.112144. [DOI] [PubMed] [Google Scholar]
- Whyte J. R., Munro S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell. 2001;1:527–537. doi: 10.1016/s1534-5807(01)00063-6. [DOI] [PubMed] [Google Scholar]
- Xie Z., Klionsky D. J. Autophagosome formation: core machinery and adaptations. Nat. Cell. Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Yen W.-L., Legakis J. E., Nair U., Klionsky D. J. Atg27 is required for autophagy-dependent cycling of Atg9. Mol. Biol. Cell. 2007;18:581–593. doi: 10.1091/mbc.E06-07-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen W.-L., Shintani T., Nair U., Cao Y., Richardson B. C., Li Z., Hughson F. M., Baba M., Klionsky D. J. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J. Cell. Biol. 2010;188:101–114. doi: 10.1083/jcb.200904075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Anttila P., Vihinen H., Jokitalo E., Eskelinen E. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5:1180–1185. doi: 10.4161/auto.5.8.10274. [DOI] [PubMed] [Google Scholar]
- Young A.R.J., Chan E.Y.W., Hu X. W., Köchl R., Crawshaw S. G., High S., Hailey D. W., Lippincott-Schwartz J., Tooze S. A. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J. Cell. Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.