Cell–cell adhesion and communication maintains epithelial tissue homeostasis. This study demonstrates that ephrin ligands target the EphA2 receptor to dampen Erk1/2 signaling without affecting cell proliferation and identifies a novel link with desmoglein 1, a desmosomal cadherin that enhances epidermal adhesion and differentiation.
Abstract
EphA2 is a receptor tyrosine kinase that is engaged and activated by membrane-linked ephrin-A ligands residing on adjacent cell surfaces. Ligand targeting of EphA2 has been implicated in epithelial growth regulation by inhibiting the extracellular signal-regulated kinase 1/2 (Erk1/2)-mitogen activated protein kinase (MAPK) pathway. Although contact-dependent EphA2 activation was required for dampening Erk1/2-MAPK signaling after a calcium switch in primary human epidermal keratinocytes, the loss of this receptor did not prevent exit from the cell cycle. Incubating keratinocytes with a soluble ephrin-A1-Fc peptide mimetic to target EphA2 further increased receptor activation leading to its down-regulation. Moreover, soluble ligand targeting of EphA2 restricted the lateral expansion of epidermal cell colonies without limiting proliferation in these primary cultures. Rather, ephrin-A1-Fc peptide treatment promoted epidermal cell colony compaction and stratification in a manner that was associated with increased keratinocyte differentiation. The ligand-dependent increase in keratinocyte adhesion and differentiation relied largely upon the up-regulation of desmoglein 1, a desmosomal cadherin that maintains the integrity and differentiated state of suprabasal keratinocytes in the epidermis. These data suggest that keratinocytes expressing EphA2 in the basal layer may respond to ephrin-A1–based cues from their neighbors to facilitate entry into a terminal differentiation pathway.
INTRODUCTION
Eph receptors comprise the largest family of mammalian receptor tyrosine kinases that mediate cell–cell communication by interacting with ephrin ligands on adjacent cells (Miao and Wang, 2009; Pasquale, 2010). The Eph receptors are subdivided into A and B subfamily members depending on their binding preference for glycosylphosphatidylinositol (GPI)-linked ephrin-A or transmembrane ephrin-B ligands. Interestingly, Eph/ephrin complexes signal in a bidirectional manner with ‘forward’ pathways emanating through the receptor and ‘reverse’ signaling activated via the ephrins. A variety of Eph receptors and ephrin ligands are expressed during embryogenesis, where they play critical and often overlapping roles in brain and blood vessel development. Eph/ephrin expression is altered in many tumors and leads to changes in cancer cell adhesion, cytoskeleton, migration, proliferation, and survival. Eph receptors and ephrins are also present in adult epithelial tissues, where their roles are beginning to be elucidated.
EphA2 was originally cloned from a human epithelial (HeLa) cDNA library (Lindberg and Hunter, 1990). The expression and activation of EphA2 in epithelial cells is tightly regulated by the calcium-dependent cell adhesion molecule, E-cadherin (Zantek et al., 1999; Orsulic and Kemler, 2000; Miura et al., 2009). In addition to its role as a homophilic adhesion molecule in adherens junctions and in initiating the assembly of other junctional complexes (e.g., desmosomes, tight junctions and gap junctions), E-cadherin is thought to facilitate the engagement of EphA2 with ephrin-A ligands, leading to receptor autophosphorylation (Miao and Wang, 2009; Green et al., 2010; Pasquale, 2010). In support of this possibility, EphA2 phosphorylation is stimulated by conditions that enhance the extent of cell–cell contacts, such as higher seeding densities or increasing extracellular calcium (Fang et al., 2005; Tanaka et al., 2005; Taddei et al., 2009). In contrast, interfering with E-cadherin–mediated adhesion using functional blocking antibodies disrupts EphA2 signaling (Zantek et al., 1999; Miura et al., 2009).
Paradoxically, EphA2 has been shown to either enhance or disrupt epithelial intercellular junctions. For example, EphA2 overexpression or ligand engagement with ephrin-A ectodomain peptides interferes with adherens junctions in human breast epithelial cell lines (Fang et al., 2008). EphA2 also increases paracellular permeability by phosphorylating the tight junction protein, claudin-4, suggestive of a role in junction destabilization (Tanaka et al., 2005). In contrast, pharmacological activation of EphA2 in Madine-Darby canine kidney (MDCK) epithelial cells enhances E-cadherin stability at cell–cell contacts (Miura et al., 2009). The extent to which these opposing effects on adhesion reflect differences in cell type or EphA2 expression levels remains unclear.
Given that EphA2 transmits chemical signals after cell–cell contact, it was reasonable to speculate that this receptor tyrosine kinase contributed to contact inhibition. Indeed, many studies have examined the ability of EphA2 to regulate mitogenic signaling pathways, such as Erk1/2-MAPK, and the resultant effects on proliferation (Pasquale, 2010). Importantly, recombinant ephrin-A1-Fc peptides target EphA2 to stimulate or inhibit Erk1/2, depending on whether it interacts with the Grb2:Sos1 complex, a GTP exchange factor that activates Ras, or p120RasGAP, a negative regulator of Ras (Pratt and Kinch, 2002; Tong et al., 2003; Parri et al., 2005). EphA2 is itself a transcriptional target of epidermal growth factor receptor (EGFR)-Erk1/2 signaling, serving in a negative feedback loop that limits Ras activity and prevents fibroblast cell transformation (Macrae et al., 2005; Larsen et al., 2007; Menges and McCance, 2008). However, EphA2 also promotes mammary adenocarcinoma development by enhancing Erk1/2-MAPK in ErbB2 expressing breast carcinomas (Brantley-Sieders et al., 2008). Loss of EphA2 alone can impair Erk1/2-MAPK signaling and limit proliferation of cancer cell lines as well as normal mammary epithelial cells (Nasreen et al., 2006; Brantley-Sieders et al., 2008; Margaryan et al., 2009; Vaught et al., 2009). This dual potential of EphA2 to regulate mitogenic signaling might help explain its roles as both tumor suppressor and promoter depending on the context and presence of ephrins within the surrounding tissue.
Although EphA2 expression is increased in skin cancer, it limits epidermal tumor growth in mice (Guo et al., 2006). Moreover, the activation of EphA2 by recombinant ephrin-A1 peptide suppresses Erk1/2-MAPK and the clonal growth capacity of wild-type but not EphA2 null mutant mouse keratinocytes (Guo et al., 2006), consistent with the inhibition in proliferation found in several human cell lines (Miao et al., 2001; Parri et al., 2005). These observations suggest that EphA2 limits epithelial growth by inhibiting Erk1/2-MAPK activity and reducing proliferation. Further support for this possibility was recently obtained when soluble ephrin or Eph receptor ectodomain peptide mimetics were systemically delivered to mice, leading to increased keratinocyte proliferation in hair follicles and the interfollicular epidermis (Genander et al., 2010).
In the present study, we took advantage of the fact that primary human epidermal keratinocytes express both EphA2 and ephrin-A1 to promote contact-dependent activation of this pathway and complemented this approach with peptidomimetic activation and gene silencing of EphA2. Interestingly, ligand activation of EphA2 was required for the transient suppression of Erk1/2-MAPK but did not inhibit keratinocyte proliferation. Alternatively, soluble ephrin ligands were capable of increasing desmosomal adhesion and terminal differentiation, revealing a new understanding for how EphA2 contributes to epithelial morphogenesis.
MATERIALS AND METHODS
Cell Culture
Primary human epidermal keratinocytes were isolated from neonatal foreskins as previously described (Simpson et al., 2010). Cells were propagated in medium 154 (M154; Cascade Biologics, Portland, OR) supplemented with human keratinocyte growth supplement (HKGS; Cascade Biologics), 0.25 μg/ml amphotericin B (Mediatech, Manassas, VA), 10 μg/ml gentamicin (Sigma-Aldrich, St. Louis, MO), and 0.07 mM CaCl2. Keratinocytes were transduced with retroviral supernatants produced in Phoenix amphotropic packaging cells (provided by G. Nolan, Stanford University) as previously described (Getsios et al., 2004a). For calcium switch assays, keratinocytes were maintained in medium containing 0.03 mM CaCl2 (low calcium) for 24 h and subsequently switched into 1.2 mM CaCl2 (high calcium) for the specified time points. In some cases, cells were incubated in the presence of a functional blocking antibody targeted against the ectodomain of E-cadherin (SHE78–7; Zymed, Carlsbad, CA) or control mIgG (Jackson Labs, West Grove, PA) at a final concentration of 2.0 μg/ml. To cleave extracellular glycosylphosphatidylinositol (GPI)-linked ligands, keratinocytes were treated with 0.5 U/ml phosphatidylinositol-specific phospholipase C (PI-PLC; Invitrogen, Carlsbad, CA) for 20 min at 4°C as previously described (Wykosky et al., 2008). To inhibit ephrin ligand-mediated activation of EphA2, keratinocytes were treated with 400 μM of a 2,5-dimethylpyrrolyl benzoic acid derivative (DMBA; Matrix Scientific, Columbia, SC) as described by Noberini et al. (2008). Keratinocytes were incubated in the presence of recombinant mouse ephrin-A1-Fc, ephrin-B1-Fc (R&D Systems, Minneapolis, MN) or human Fc (Jackson Labs) as a control. The dimeric peptides were added directly to the culture medium or after preclustering with an anti-human Fc IgG (1:5 ratio of antibody to peptide) for 30 min as described previously (Shi et al., 2007). All experiments were performed using three separate isolates of keratinocyte cultures initiated from different donors to account for possible clonal variations.
Antibodies and Reagents
The following mouse monoclonal antibodies were used in this study: D7 (anti–EphA2 extracellular domain; Millipore, Jaffrey, New Hampshire); 4G10 (anti–phospho-Tyrosine; Millipore); BU-33 (anti-BrdU; Sigma-Aldrich); 27B2 (anti-desmoglein 1 cytoplasmic domain; Zymed, Carlsbad, CA); U100 (anti-desmocollin 1 a/b cytoplasmic domain; Progen, Queensland, Australia); LL002 (anti-keratin 14 C terminus; Chemicon International, Billerica, MA); AK15 (anti-desmoglein 3 ectodomain; gift antibody from M. Amagai, Keio University); p120-catenin (anti-p120-catenin aa 326-632; BD Transduction Laboratories, San Jose, CA). The following rabbit monoclonal antibodies were purchased from Cell Signaling Tech., Danvers, MA: 56A6 (anti-p-Raf phospho-Ser338); 41G9 (anti-p-Mek1/2 phospho-Ser217/221); D13.14.4E (anti-p-Erk1/2, Thr202/Tyr204). Polyclonal antibodies used included: V114A (rabbit anti-ERK1/2; Promega, Madison, WI); AB9485 (rabbit, anti-glyceraldehyde-3-phosphate dehydrogenase; Abcam); C-20 (rabbit, anti-EphA2 C terminus; Santa Cruz Biotechnology, Santa Cruz, CA); V-18 (rabbit, anti-EphrinA1 C-terminus; Santa Cruz Biotechnology); 795 (rabbit, anti-E-cadherin; gift from R. Brackenbury, University of Cincinnati); C-2081 (rabbit, anti-α-catenin aa 890-901; Sigma-Aldrich); C-2206 (rabbit, anti-β-catenin aa 768-781); Raf (rabbit, anti-Raf Pro302; Cell Signaling Tech.); Rb-K10 (rabbit, anti-Keratin 10; gift from J. Segre, National Institutes of Health); Lor (rabbit, anti-loricrin C terminus; Covance); p-EphA (rabbit, anti-tyrosine phosphorylated EphA; gift from B.C. Wang, Case Western Reserve); AF638 (goat, anti-EphA1 extracellular domain; R&D Systems, Minneapolis, MN).
Secondary antibodies used for Western blotting included goat anti–mouse, mouse anti–rabbit, and bovine anti-goat IgG linked to peroxidase (Jackson ImmunoResearch Laboratories). Secondary antibodies used for immunofluorescence included goat anti–mouse and –rabbit or donkey anti-goat IgG linked to fluorophores of 488 nm and 555 nm (Alexa Fluor; Invitrogen). DAPI (Sigma-Aldrich) was used for nuclear staining. Fluorescein-conjugated Phalloidin (Invitrogen) was used to label F-actin. The Mek1/2 inhibitor, U0126, was purchased from Cell Signaling Tech. Recombinant Staphylococcus aureus exfoliative toxin A (ETA) was provided by John Stanley (University of Pennsylvania) and generated as previously described (Getsios et al., 2009). A retroviral vector harboring a constitutively active mutant of Raf-1 (pBabe-Raf22W) was provided by Natalia Mitin and Channing Der (University of North Carolina).
Gene Silencing
Gene silencing of EphA2 and EphA1 was performed using Stealth siRNA oligonucleotide duplexes or a GC-matched negative control (Invitrogen), which were introduced into 2 × 105 keratinocytes at a final concentration of 100 nM using Dharmafectin1 transfection reagent (Thermo Fisher Scientific) as previously described (Simpson et al., 2010). An LZRS-based miRNA mimetic retroviral vector containing hairpin sequences for lamin A/C or desmoglein 1 was used to silence the expression of these respective proteins as previously described (Getsios et al., 2009). All experiments were performed at least 72 h post-transduction.
Immunoprecipitation and Western Blot Analysis
Protein was extracted from cells in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris, pH 7.5, 140 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 5 mM EDTA, and 2 mM EGTA) containing 1× protease inhibitor cocktail (Roche, Indianapolis, IN) and 1× phosphatase inhibitor cocktail (Roche), and lysates were centrifuged at 15,000 rpm for 15 min at 4°C to remove insoluble proteins. Human EphA2 was immunoprecipitated from 200 to 300 μg of protein by incubating pre-cleared lysates with 2 μg of anti-EphA2 (C20; Santa Cruz Biotechnology,) for 1 h followed by 25 μl of gamma-bind Sepharose beads (GE Healthcare Biosciences, Piscataway, NJ) for 2 h at 4°C. Immune complexes were released using reducing Laemmli buffer. RIPA soluble fractions as well as immunoprecipitated complexes were denatured in SDS buffer and subjected to SDS-PAGE and Western blot analysis as previously described (Getsios et al., 2004a). For Western blot analysis of phosphorylated epitopes, cells were lysed in RIPA buffer. For the analysis of whole cell lysates, including the more insoluble structural proteins present in keratinocytes, cells were harvested in urea sample buffer (8 M urea, 1% SDS, 10 mM Tris, pH 7.5). Relative band intensities for phosphorylated proteins were normalized to total protein levels and calculated using ImageJ software (National Institutes of Health, Bethesda, MD).
Immunocytochemistry
Cells were grown on glass coverslips, rinsed in phosphate-buffered saline (PBS), fixed in anhydrous methanol for 2 min at −20°C or 4% paraformaldehyde for 5 min and permeabilized using 0.2% TritonX-100 for 5 min at 4°C, and then processed for immunofluorescence as previously described (Getsios et al., 2004a). Images were acquired using a 10 × 0.3 EC Plan-Neofluar, 40 × 0.5 EC Plan-Neofluar, or a 63 × 1.4 Plan-Apochromat objective, respectively, on an epifluorescence microscope system (AxioVision Z1; Carl Zeiss, Thornwood, NY) fitted with an Apotome slide module and a digital camera (AxioCam MRm; Carl Zeiss). Apotome-acquired Maximal Image Projections (MIPs) were taken at 0.25-μm steps, and cross sections were taken across the Z-X plane.
Cell Proliferation, Size and Colony Growth Analysis
To assess proliferation, keratinocytes were pulsed with 10 μM BrdU (Sigma-Aldrich) for 1 h, washed in PBS, and methanol fixed for 10 min at −20°C. DNA was denatured in 2 N hydrochloric acid (Fisher Scientific) for 30 min at 37°C. Residual acid was neutralized by washing coverslips in 0.1 M Borax (Sigma-Aldrich), followed by rinsing coverslips in PBS. Cells were digested in 0.1% trypsin (Sigma-Aldrich) for 10 min and blocked in 5% normal calf serum, 1% BSA and 0.05% Tween (Sigma-Aldrich). Coverslips were incubated overnight with an antibody against BrdU (BU-33, Sigma-Aldrich) at 4°C. BrdU-positive cells were divided by the total number of DAPI-stained nuclei from 10 random fields; >500 cells were counted for each condition, and the percentage was graphed ± SD. Cell density analysis was performed by counting the number of DAPI-stained nuclei in a 100-μm radius from five random fields. To measure cell compaction, ImageJ software was used to determine cell area from actin-immunostained images; >300 cells were counted from 10 random fields for each condition.
For cell cycle analysis, keratinocytes were typsinized and resuspended in PBS followed by fixation in 80% ethanol at 4°C for 2 h. Cells were pelleted at 500 × g for 5 min at 4°C to remove PBS/ethanol and resuspended in propidium iodide staining solution [0.1% Triton-X100, 50 μg/ml propidium iodide (Sigma-Aldrich), and 0.2 mg/ml RNaseA in PBS], vortexed, and incubated for 20 min at 37°C. For some experiments, cultures were treated with 10 μM U0126 (Cell Signaling Technology) for the duration of the calcium switch.
To assess colony growth capacity, a crystal violet assay was performed on keratinocytes plated at 1.5 × 103 cells/cm2 in a six-well plate, switched into 0.03 or 1.2 mM calcium, and treated with Fc or ephrin-A1-Fc peptides every 48 h. Cultures were harvested 7 d after ligand treatment. Plates were washed gently at room temperature with PBS and then incubated with 0.5% crystal violet (Sigma-Aldrich) in a 20% methanol solution for 15 min at room temperature. Plates were then washed twice in water, and colony images were acquired on a Leica MZ6 dissecting stereomicroscope with a Hamamatsu Orca digital camera (model C4742-95) using MetaVue software (Universal Imaging) and analyzed using ImageJ software. Particle analysis was performed on duplicate wells for each condition to obtain the surface area (in pixels2) of resultant keratinocyte colonies. The watershed function was used on each image to separate colonies that overlapped.
Dispase-Based Mechanical Dissociation Assay
Keratinocytes were seeded to confluency (3 × 105 cells/cm2) for 24 h in M154 containing 0.07 mM calcium and switched into 0.4 mM calcium containing Fc or ephrin-A1-Fc peptides for an additional 48 h or 1.2 mM calcium for 2 h. In some experiments, 2.0 μg/ml ETA was added 2 h before the addition of dispase to cleave the extracellular domain of desmoglein 1 as previously described (Getsios et al., 2009). The cultures were then washed twice with PBS and incubated in the presence of 2.4 U/ml Dispase II (Roche) at 37°C for 20 min. Released cell monolayers were transferred to a 15-ml conical tube containing 5 ml of PBS, inverted ten times, and transferred into a six-well plate. Images were acquired on a Leica MZ6 dissecting stereomicroscope with a Hamamatsu Orca digital camera using MetaVue software. Fragment size and total number were measured using ImageJ software as described in Klessner et al. (2009). Data are represented as the average number of fragments ± SD from triplicate wells.
RESULTS
EphA2 Is Required for Contact-Dependent Erk1/2-MAPK Signaling but not Keratinocyte Cell Cycle Exit
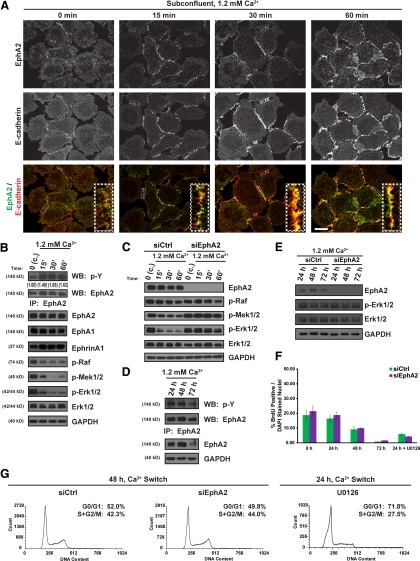
EphA2 activation by ephrin-A ligands has been implicated in contact inhibition of epithelial cells by suppressing Erk1/2-MAPK signaling (Pasquale, 2010). To formally test whether EphA2 is required for negative regulation of normal epithelial growth, we used conditions where this receptor becomes activated by native ephrin-A ligands in a contact-dependent manner. A precedent for this approach comes from work using MDCK cell lines, where EphA2 tyrosine phosphorylation is enhanced by switching cultures from low to high extracellular calcium (Miura et al., 2009). Instead of testing the role of EphA2 in epithelial growth regulation using an immortalized cell line, we used primary cultures of human epidermal keratinocytes that normally exit the cell cycle in response to a calcium switch. Importantly, primary keratinocytes abundantly express EphA2 along with its high-affinity ligand, ephrin-A1, and the related receptor subfamily member, EphA1 (Figure 1B). This overlapping EphA/ephrin-A expression pattern is consistent with the presence of these receptor-ligand pairs in the epidermis (de Saint-Vis et al., 2003; Guo et al., 2006; Hafner et al., 2006).
Figure 1.
Contact-dependent activation of EphA2 dampens Erk1/2-MAPK signaling without affecting keratinocyte proliferation. (A) EphA2 (in green) and E-cadherin (in red) immunostaining of subconfluent keratinocytes grown in low (0.03 mM) calcium and switched into high (1.2 mM) calcium for 0, 15, 30, or 60 min. Insets depict magnified images of regions outlined in white dotted rectangles (scale bar, 20 μm). (B) Keratinocytes cultured under confluent (c.) conditions and switched into 1.2 mM calcium for 0, 15, 30, or 60 min; (C) keratinocytes transfected with siControl (siCtrl) or siEphA2 oligonucleotide duplexes and switched into 1.2 mM calcium for 0, 15, 30, or 60 min; (D) keratinocytes switched into high calcium for 24, 48, or 72 h; (E) siCtrl or siEphA2 keratinocytes switched into 1.2 mM calcium for 24, 48 or 72 h. Protein lysates were harvested for EphA2 immunoprecipitation (IP) and probed for total phospho-tyrosine (p-Y) or EphA2 (ratio of p-Y:EphA2 is shown in B). Western blots were probed for EphA2, EphA1, ephrin-A1, p-Raf, p-Mek1/2, p-Erk1/2, total Erk1/2 or GAPDH. (F) siCtrl or siEphA2 keratinocytes were switched into 1.2 mM calcium for 0, 24, 48, or 72 h and incubated with BrdU for 60 min before fixation. Keratinocytes were also treated with 10 μM U0126 for Mek-Erk1/2 inhibition. BrdU incorporation was calculated as a percentage of the total number of BrdU-positive nuclei divided by the total number of DAPI-stained nuclei from >500 cells in each experiment (n = 3). The bar graphs are from a representative experiment. (G) Histograms of PI fluorescence in siCtrl or siEphA2 keratinocytes switched into 1.2 mM calcium for 48 h or untransfected keratinocytes switched into 1.2 mM calcium and treated with 10 μM U0126 for 24 h. The percentage of cells in Go/G1 or S + G2/M phases of the cell cycle are shown in the top right corner.
To confirm that calcium-induced stabilization of E-cadherin-based cell–cell contacts was capable of activating EphA2 in primary keratinocytes, we examined the subcellular distribution and tyrosine phosphorylation of this receptor after a switch from low (0.03 mM) to high (1.2 mM) calcium. EphA2 was broadly distributed throughout the cell in cultures maintained in low calcium and did not extensively colocalize with the more diffuse E-cadherin except in areas of direct cell–cell apposition; these likely reflect transient contact points that form even under low calcium conditions (Figure 1A). As expected, EphA2 and E-cadherin were both concentrated at nascent contacts concomitant with a marked increase in EphA2 tyrosine phosphorylation after a calcium switch (Figure 1, A and B). EphA2 border localization was disrupted by a function-blocking antibody that interferes with E-cadherin–mediated adhesion (Supplemental Figure S1A). Moreover, contact-dependent activation of EphA2 was perturbed by a 2,5 dimethylpyrrolyl benzoic acid (DMBA) derivative (Supplemental Figure S1B) that inhibits ephrin-A1 binding to EphA2 (Noberini et al., 2008), providing more direct evidence that ligands and not other kinases trigger EphA2 phosphorylation in response to cell–cell contact. However, long-term treatment with DMBA also altered EphA1 expression levels, limiting its utility for further studies. Collectively, these data indicated that contact-dependent native EphA2/ephrin-A signaling complexes form in keratinocytes.
Epidermal Erk1/2-MAPK activity promotes keratinocyte proliferation and is dampened to allow for cell cycle exit (Scholl et al., 2004; Dumesic et al., 2009). In addition, the contact-dependent increase in EphA2 phosphorylation was associated with a reduction in the activation state of the Raf-Mek-Erk1/2 pathway in keratinocytes (Figure 1B); this correlation has also been noted in MDCK cells (Miura et al., 2009). To determine whether EphA2 was required for Erk1/2 suppression following a calcium switch, we silenced its expression. Whereas Erk1/2 phosphorylation was markedly reduced in confluent control keratinocytes after a calcium switch, it remained elevated in keratinocytes lacking EphA2 (Figure 1C). The EphA2-dependent dampening of Erk1/2-MAPK signaling further suggested a role for this receptor in the negative regulation of keratinocyte proliferation.
The phosphorylation of EphA2 remained relatively stable for up to 48 h after a calcium switch (Figure 1D); this time period corresponded with a reduction in cell proliferation in these confluent cultures (Figure 1F). To determine whether EphA2 was required for contact-dependent cell cycle exit, EphA2-deficient keratinocytes were seeded to confluency and subjected to a calcium switch before BrdU incorporation or propidium iodide-FACS analysis (Figure 1, F and G). As expected, keratinocyte proliferation was reduced progressively with time after a calcium switch, with <2% of the cells found in the S phase after 72 h (Figure 1F). Notably, the lack of EphA2 had no measurable effect on keratinocyte proliferation or cell cycle dynamics in low calcium or after a calcium switch (Figure 1F and G). As a positive control for cell cycle exit, we treated keratinocytes for 24 h with the Mek1/2 inhibitor, U0126, to block the Erk1/2-MAPK pathway. Finally, there was no difference in Erk1/2 activation from control or EphA2-deficient keratinocytes 24 h after a calcium switch (Figure 1E). We conclude from these observations that EphA2 plays a critical role in early phases of contact-dependent Erk1/2-MAPK signaling but is dispensable for cell cycle exit.
Ligand Targeting of EphA2 Restricts Epidermal Cell Colony Size without Inhibiting Proliferation
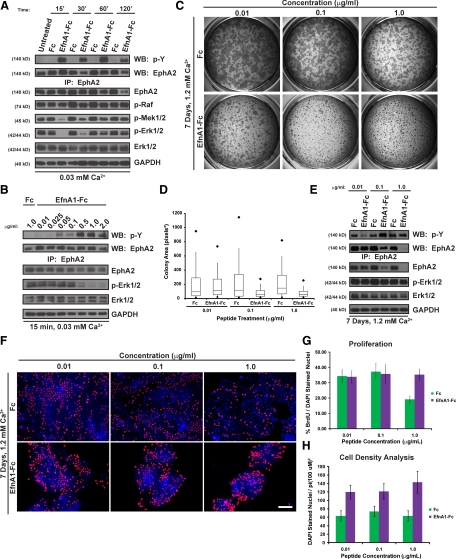
EphA2 can be targeted by a recombinant ephrin-A1-Fc peptide mimetic, leading to the suppression of Erk1/2-MAPK and limiting the clonal expansion of epithelial cell colonies, including mouse epidermal keratinocytes (Miao et al., 2001; Parri et al., 2005; Guo et al., 2006); this ‘growth restriction’ has been attributed to a reduction in proliferation. Yet, native EphA2/ephrin-A1 complexes were not required for keratinocyte cell cycle exit (Figure 1, F and G). Hence, we sought to directly test whether peptidomimetic targeting of EphA2 indeed restricted keratinocyte colony growth via the inhibition of Erk1/2-MAPK and proliferation.
To focus specifically on ‘forward’ signaling events downstream of EphA2, we determined the ability of ephrin-A1-Fc peptide to target EphA2 and modulate the Erk1/2-MAPK signaling pathway in sparse cultures maintained in low calcium, where the receptor is not expected to be extensively engaged by endogenous ephrin ligands. We first confirmed that EphA2 could be effectively activated by ephrin-A1-Fc under these experimental conditions by adding a soluble dimeric peptide to sparse (<5 × 104 cells/cm2) or dense (>3 × 105 cells/cm2) cultures that were maintained in low (0.03 mM) or high (1.2 mM) calcium. As expected, EphA2 tyrosine phosphorylation was increased by high calcium and, to a lesser extent, high-density conditions, consistent with the contact-dependent formation of native EphA2/ephrin-A1 complexes (Supplemental Figure S2). Furthermore, the addition of ephrin-A1-Fc markedly enhanced EphA2 phosphorylation levels under all experimental conditions, demonstrating that these recombinant peptides can be used to activate EphA2 independent of its localization at cell–cell borders.
The addition of ephrin-A1-Fc but not control Fc (Figure 2, A and B) or ephrin-B1-Fc (Supplemental Figure S3A) inhibited the Raf-Mek-Erk1/2 pathway in a time- and dose-dependent manner. Erk1/2-MAPK suppression corresponded with maximal phosphorylation of EphA2 at concentrations ≥0.5 μg/ml ephrin-A1-Fc (Figure 2B). Whereas EphA2 phosphorylation remained high after 120 min of peptide treatment in human keratinocytes, Erk1/2 began to recover as early as 30 min and returned to baseline levels at later time points (Figure 2A). Moreover, total EphA2 expression levels also began to decrease after 60 min, consistent with the ligand-dependent receptor down-regulation found in other cell types (Walker-Daniels et al., 2002). Importantly, ephrin-A1-Fc–mediated suppression of Erk1/2-MAPK was impaired in EphA2-deficient keratinocytes that continued to express EphA1 (Supplemental Figure S3B), demonstrating that EphA2 was the predominant receptor capable of responding to ephrin-A1-Fc peptide to transiently inhibit Erk1/2-MAPK signaling.
Figure 2.
Soluble ligand targeting of EphA2 dampens Erk1/2-MAPK signaling and restricts colony size in keratinocyte cultures that continue to proliferate. (A) The activation state of EphA2 and the Raf-Mek-Erk1/2 pathway was examined in keratinocytes treated with 1.0 μg/ml Fc or ephrin-A1-Fc for 0, 15, 30, 60, or 120 min in 0.03 mM calcium. (B) Keratinocytes treated with 0.01, 0.025, 0.05, 0.1, 0.5, 1.0, or 2.0 μg/ml ephrin-A1-Fc or 1.0 μg/ml Fc peptide for 15 min in 0.03 mM calcium. (C) Sparse keratinocytes were switched into 1.2 mM calcium and treated with 0.01, 0.1, or 1.0 μg/ml Fc or ephrin-A1-Fc for 7 days before being fixed and stained with crystal violet to measure colony expansion. Shown are representative low-power images of the resultant colonies from a single experiment that was performed in triplicate and repeated using three separate keratinocyte isolates. (D) The surface area of resultant keratinocyte colonies was measured using ImageJ software and analyzed by box-and-whisker plot. The extent of the box represents the 25th and 75th percentiles of the data, the line inside the box indicates the median, and the capped lines extending from the bottom and top of the box represent the 10th and 90th percentiles, respectively. The dark diamonds indicate the 5th and 95th percentiles. (E) The activation state of EphA2 and members of the Raf-Mek-Erk1/2 pathway. (F) BrdU incorporation and DAPI nuclear staining (scale bar, 100 μm) was examined in sparse keratinocytes, switched into 1.2 mM calcium and treated with 0.01, 0.1 or 1.0 μg/ml Fc or ephrin-A1-Fc for 7 days. (G) Bar graph of the percentage of BrdU-positive cells, and (H) the average number of DAPI-stained nuclei in a 100-μm radius.
To determine whether peptidomimetic activation of EphA2 reduced keratinocyte proliferation, we performed BrdU incorporation analysis in sparse keratinocytes (1.5 × 103 cells/cm2) treated with Fc or ephrin-A1-Fc peptide for 7 days in low calcium. Long-term treatment with ephrin-A1-Fc peptide led to a marked down-regulation of total EphA2 levels, although the residual receptor remained abundantly phosphorylated (Supplemental Figure S4A). In addition, Erk1/2-MAPK activation was no longer reduced after 7 days (Supplemental Figure S4A). Consequently, it was not too surprising that keratinocyte proliferation remained unchanged following chronic ephrin-A1-Fc peptide treatment in low calcium (Supplemental Figure S4B). These findings led us to conclude that ephrin-A1-Fc peptide targeting of EphA2 was, by itself, not sufficient to restrict the growth of primary human epidermal keratinocytes.
Keratinocytes maintained in low calcium grow as individual cells that are poorly associated with their neighbors, whereas cells maintained in high calcium are tightly connected to one another and expand as relatively discrete epithelial colonies. It was possible that the ephrin-A1-Fc peptide-mediated growth restriction found in several cell types (Miao et al., 2001; Parri et al., 2005; Guo et al., 2006) relied upon calcium-induced stabilization of cell–cell contacts. Therefore, we tested whether the expansion of human keratinocyte colonies was inhibited by targeting EphA2 under high-calcium conditions. EphA2 was basally phosphorylated in high calcium, but this activation state could be further enhanced by peptide treatment after 15 min (Supplemental Figure S2). Ephrin-A1-Fc peptide treatment for 7 days in high calcium markedly restricted the lateral expansion of keratinocyte colonies in a dose-dependent manner, as assessed by crystal violet staining (Figure 2, C and D). Keratinocyte colony size was limited in a manner that corresponded with EphA2 down-regulation in response to increasing concentrations of ephrin-A1-Fc peptide (≥0.1 μg/ml; Figure 2C–E). In spite of receptor down-regulation, tyrosine phosphorylation of EphA2 remained readily detectable after prolonged peptide treatment. The tyrosine phosphorylation detected by immunoprecipitation analysis likely reflected residual EphA2 activation after long-term peptide treatment because it was impaired by gene silencing (Figure 4C). These findings showed that long-term targeting of EphA2 by ephrin-A1-Fc treatment leads to its down-regulation and the calcium-dependent restriction in keratinocyte colony size.
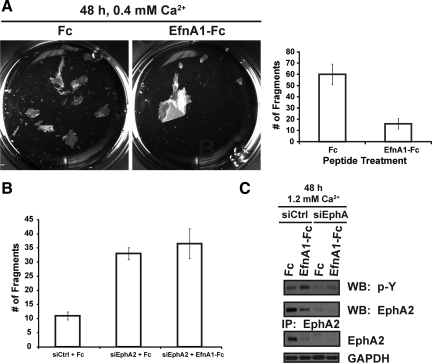
Figure 4.
EphA2 enhances the mechanical integrity of keratinocyte sheets. (A) A dispase-based dissociation assay was performed to measure the adhesive strength of confluent keratinocyte sheets treated with 1.0 μg/ml Fc or ephrin-A1-Fc peptides for 48 h in 0.4 mM calcium. Dispase was used to release intact keratinocyte sheets from the substrate, which were subsequently exposed to mechanical shear stress by inversion in conical tubes. Photomicrographs of the resultant fragments are shown. A bar graph of the average number of fragments (right panel), which is inversely related to adhesive strength, from three individual wells of a representative experiment; the assay was repeated on three separate occasions. (B) Dispase assay results from peptide-treated siCtrl or siEphA2 keratinocytes in 0.4 mM calcium are shown. (C) The activation state of EphA2 in siCtrl or siEphA2 transfected keratinocytes treated with 1.0 μg/ml Fc or ephrin-A1-Fc peptide for an additional 48 h in 1.2 mM calcium. Western blot analysis from these lysates were probed for EphA2 or GAPDH.
Because the reduction in keratinocyte colony size occurred at concentrations of ephrin-A1-Fc peptide (<0.5 μg/ml) that did not profoundly inhibit Erk1/2-MAPK after 15 min in low calcium (Figure 2B) or 7 d in high calcium (Figure 2E), it seemed unlikely that keratinocyte colony expansion required suppression of this mitogenic signaling pathway. To rule out the importance of Erk1/2-MAPK inhibition to peptide-mediated colony size restriction, we transduced keratinocytes with a retroviral vector harboring a constitutively active mutant of human Raf-1 (pBabe-Raf22W), thereby sustaining high levels of Mek-Erk1/2 signaling (Supplemental Figure S5A). Ephrin-A1-Fc treatment limited colony size to a similar extent in control (pBabe) or Raf22W mutant keratinocytes (Supplemental Figure S5B), providing direct evidence that Erk1/2-MAPK inhibition was not required for EphA2-mediated colony morphology changes.
It remained possible that the ephrin-A1-Fc–mediated decrease in colony size was due to a reduction in keratinocyte proliferation involving alternative mitogenic pathways. BrdU incorporation assays were performed to determine whether there was a reduction in proliferative capacity after chronic ligand stimulation in high calcium (Figure 2, F and G). BrdU-positive cells were readily detected under all concentrations of ephrin-A1-Fc examined, particularly at the periphery of colonies that were exposed to cell-free surfaces. Although the keratinocytes at the center of these colonies stained less frequently for BrdU, proliferation within the entire culture was even higher in 1.0 μg/ml ephrin-A1-Fc–treated cultures compared with the Fc control, likely as a result of ‘off-target’ effects from the human Fc peptide. The most striking morphological difference noted was instead increased cell density, which was measured by the greater number of DAPI-stained nuclei within the smaller ephrin-A1-Fc–treated colonies (Figure 2H). These collective observations demonstrated that ligand targeting of EphA2 restricted the lateral expansion of epidermal colonies via cellular mechanisms other than what has been most commonly proposed for ephrin-A1-Fc–mediated epithelial growth restriction; namely, the inhibition of Erk1/2-MAPK signaling and proliferation.
EphA2 Triggers Keratinocyte Compaction, Stratification and Differentiation
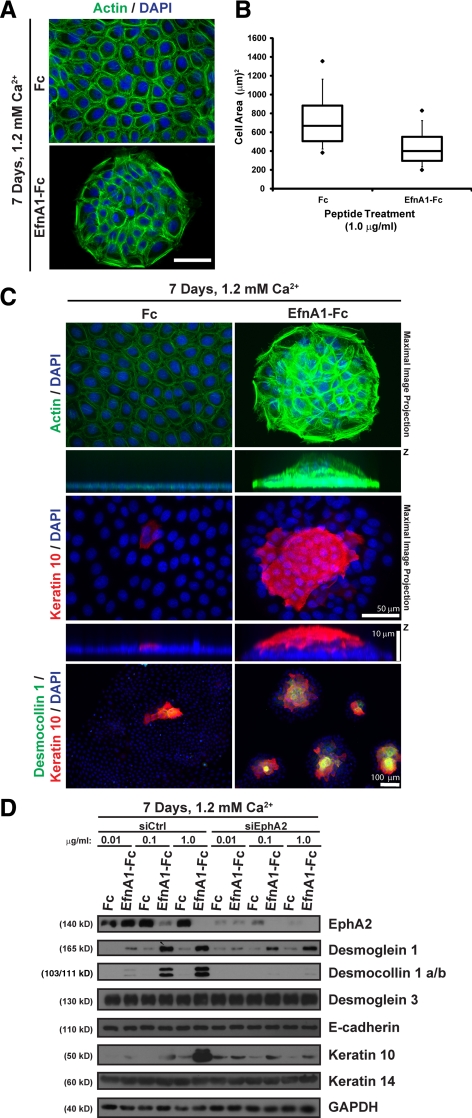
The addition of ephrin-A1-Fc peptides promotes compaction and enhances apical-basal polarity in MDCK cell cultures (Miura et al., 2009). Although proliferation or colony formation capacity was not specifically examined in the study by Miura et al., cell compaction served as a likely alternative explanation for why the lateral expansion of keratinocytes was restricted by ligand targeting of EphA2. To determine whether long-term treatment with ephrin-A1-Fc altered keratinocyte morphology and promoted cell compaction, we examined the organization of the actin cytoskeleton using phalloidin staining. Whereas control Fc–treated cultures exhibited a relatively uniform cortical F-actin array, ephrin-A1-Fc–treated keratinocytes demonstrated an increase in F-actin staining and a corresponding decrease in overall cell size (Figure 3, A and B). Interestingly, enhanced F-actin staining was also observed in peptide-treated keratinocytes maintained in low calcium without gross changes in cell morphology (Supplemental Figure S4C). These findings demonstrated that ephrin-A1-Fc peptide promoted keratinocyte compaction and further suggested that calcium-mediated stabilization of cell–cell contacts contributed to this morphological transformation.
Figure 3.
Ligand targeting of EphA2 triggers keratinocyte colony compaction, stratification and differentiation. (A) Phalloidin (F-actin; in green) and DAPI (nuclei; in blue) staining of sparse keratinocytes that were treated with 1.0 μg/ml Fc or ephrin-A1-Fc for 7 d in 1.2 mM calcium (scale bar, 50 μm). (B) Cell compaction was analyzed by using ImageJ software to measure the surface area of individual cells and presented in a box-and-whisker plot. (C) Maximal image projections (top panels) from peptide-treated colonies that were stained with phalloidin to image F-actin (in green), an antibody against keratin 10 (in red) or DAPI to visualize nuclei (in blue). Apotome-processed images were acquired from stratified keratinocyte colonies at 0.25 μm increments (scale bar, 50 μm). Images below depict cross-sections through the z–x axis that were magnified in the z plane to better resolve keratinocyte piles (scale bar, 10 μm). Colonies that had formed in the presence of Fc or ephrin-A1-Fc peptide in 1.2 mM calcium were also fixed and immunostained for desmocollin 1 a/b (in green) or keratin 10 (in red) to detect stratified keratinocytes (scale bar, 100 μm). (D) siCtrl or siEphA2 keratinocytes were seeded under sparse conditions and treated with 0.01, 0.1, or 1.0 μg/ml Fc or ephrin-A1-Fc peptide for 7 days in 1.2 mM calcium. Western blot analysis of lysates prepared from these cultures were probed for EphA2, desmoglein 1, desmocollin 1 a/b, desmoglein 3, E-cadherin, keratin 10, keratin 14, or GAPDH.
Proliferating keratinocytes in the basal layer of the epidermis give rise to more differentiated cells in the suprabasal layers (Fuchs, 2008). Keratinocytes in culture can also be induced to undergo stratification and form differentiated piles of cells in a manner that relies on calcium-dependent cadherin-mediated adhesion (Wheelock and Jensen, 1992; Hines et al., 1999). Because the lateral expansion of keratinocytes was restricted by ephrin-A1-Fc peptide treatment, we tested whether these primary cultures alternatively expanded in the vertical plane by undergoing stratification. For this purpose, we imaged the actin cytoskeleton in three dimensions. Control keratinocytes grew as relatively flat (<3 μm) monolayers with uniform F-actin staining throughout the colonies (Figure 3C; top panels). In contrast, keratinocyte colonies expanded vertically to form stratified piles of cells with increased F-actin staining after long-term ephrin-A1-Fc peptide treatment.
Keratinocyte stratification is associated with changes in structural proteins, with basal keratinocytes expressing the desmosomal cadherin, desmoglein 3, in association with an intermediate filament network containing keratin-5 and -14 and suprabasal keratinocytes expressing desmoglein 1 and desmocollin 1 a/b in association with keratin 1 and 10 (Fuchs, 2008). Immunostaining for keratin 10 confirmed that the ephrin-A1-Fc peptide treated cultures had undergone stratification (Figure 3C; middle panels). Moreover, keratin 10 and desmocollin 1 a/b were commonly detected in the compacted keratinocyte colonies (Figure 3C; bottom panels). In contrast, these two markers of suprabasal keratinocytes were found infrequently in control Fc conditions. These results showed that ligand targeting of EphA2 promoted keratinocyte stratification and enhanced their differentiated phenotype.
To measure the extent of differentiation more precisely and test whether EphA2 was required for these effects on keratinocyte colony expansion, we examined the expression of markers for basal or suprabasal keratinocytes following EphA2 knockdown and peptide treatment. A marked increase in desmoglein 1, desmocollin 1 a/b, and keratin 10 was found in siControl (siCtrl) transfected keratinocytes that corresponded to the dose of ephrin-A1-Fc peptide (Figure 3D). In contrast, structural proteins in basal keratinocytes (i.e., E-cadherin, desmoglein 3 and keratin 14) remained unchanged. Importantly, EphA2 gene silencing by itself had little effect on colony morphology but impaired the ability of the ephrin-A1-Fc peptide to up-regulate suprabasal differentiation markers and restrict colony size (Figure 3D and Supplemental Figure S6, A–C). These data suggested that EphA2 loss is not sufficient to promote keratinocyte stratification and supported the idea that peptide-mediated activation and receptor down-regulation were both required for this morphological transformation. Ephrin-A1-Fc peptide treatment had residual effects on colony morphology and differentiation in siEphA2 transfected keratinocytes beyond the levels of Fc controls. This might be explained by incomplete knockdown because gene silencing of another major epidermal EphA receptor, namely EphA1, had little impact on the lateral expansion of epidermal colonies as well as desmoglein-1, desmocollin 1 a/b, or keratin 10 expression (Supplemental Figure S6, A–C). Collectively, these findings defined a novel function for EphA2 in promoting a differentiated phenotype in keratinocytes responding to soluble ligand activation.
EphA2 Strengthens Keratinocyte Adhesion
Keratinocyte adhesion is strengthened by desmosomes that increase in number and change in molecular composition as these cells differentiate in the upper layers of the epidermis (Getsios et al., 2004b). The effects of ephrin-A1-Fc on keratinocyte colony compaction and the expression of differentiation-associated desmosomal cadherins suggested that ligand targeting of EphA2 might enhance epidermal adhesion. To functionally test the ability of ephrin-A1-Fc peptide treatment to increase epidermal adhesion strength, we used a mechanical dissociation assay employing the bacterial enzyme, dispase, to release intact keratinocyte sheets from the substrate (Calautti et al., 1998; Klessner et al., 2009). Shear stress applied to epithelial sheets results in fragmentation that is inversely related to the strength of adhesion and depends on the levels of extracellular calcium. Because we predicted an increase in adhesive strength after peptide treatment, the dispase assays were performed in 0.4 mM calcium to enhance fragmentation as previously shown (Klessner et al., 2009). At this intermediate calcium concentration, ephrin-A1-Fc peptide treatment increased the expression of several adhesion proteins, including a marginal increase in E-cadherin and desmoglein 3 and a more pronounced increase in desmoglein 1 and desmocollin 1 a/b (Supplemental Figure S7). Accordingly, confluent keratinocyte sheets treated with 1.0 μg/ml ephrin-A1-Fc for 48 h generated fewer fragments following shear stress when compared with Fc controls (Figure 4A), providing functional evidence for an increase in cell–cell adhesion after ligand targeting of EphA2.
To determine whether EphA2 was required for the ephrin-A1-Fc–mediated increase in adhesion and to gain further insight into its physiological role, dispase assays were performed using EphA2-deficient keratinocytes (Figure 4B). Not surprisingly, contact-dependent activation of EphA2 was enhanced by ephrin-A1-Fc peptide treatment in control but not EphA2-deficient keratinocytes (Figure 4C). Importantly, keratinocytes lacking EphA2 were generally less adhesive compared with controls. Moreover, the number of fragments was similar between Fc and ephrin-A1-Fc treatment in EphA2-deficient keratinocytes, indicating this receptor was primarily responsible for the peptide-mediated increase in keratinocyte adhesion (Figure 4B). We can conclude from these studies that ligand activation of EphA2 plays a key role in fortifying keratinocyte adhesion.
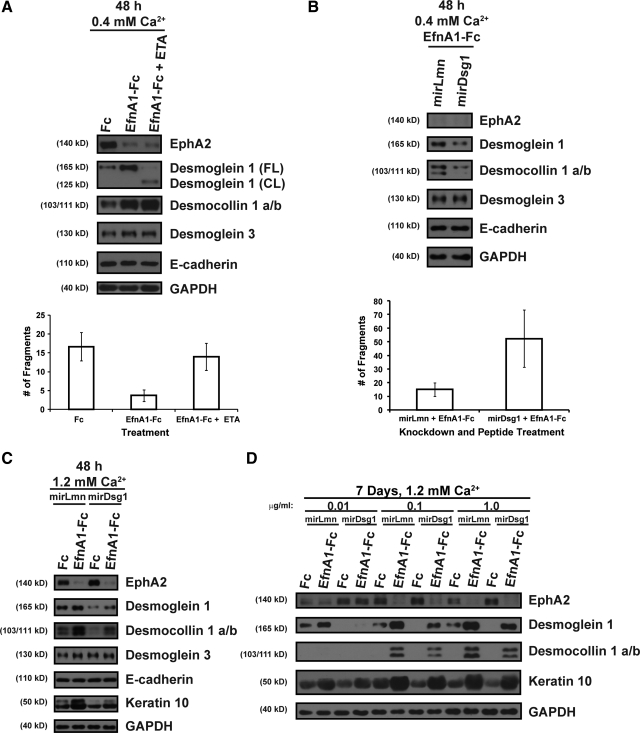
Desmoglein 1 Mediates the Ligand-Dependent Effects of EphA2 on Keratinocyte Adhesion and Differentiation
EphA2 decreases the adhesive strength of the MCF-10A breast epithelial cell line (Fang et al., 2008) but increases compaction in MDCK cells (Miura et al., 2009); interestingly, these distinct outcomes have each been attributed to differential effects on E-cadherin–mediated adhesion. In keratinocytes, ligand targeting of EphA2 enhanced compaction and adhesion without major changes in the expression of E-cadherin or desmoglein 3 (Figure 3D and Supplemental Figure S7). Instead, the expression of the suprabasal desmosomal cadherin partners, desmoglein 1 and desmocollin 1 a/b, was markedly up-regulated by ephrin-A1-Fc peptide treatment. Furthermore, we did not detect differences in keratinocyte adhesion after a short (2 h) calcium switch where E-cadherin and desmoglein 3 are abundantly expressed but desmoglein 1 and desmocollin 1 a/b levels remain undetectable (Supplemental Figure S8A). The ability of EphA2 to regulate desmosome function has not been previously examined, but our data suggested that ligand targeting of EphA2 may preferentially act upon differentiation-associated desmosomal cadherins to strengthen adhesion.
To determine whether EphA2 specifically enhanced desmoglein 1–mediated adhesion, keratinocyte sheets were incubated with recombinant Staphylococcus aureus exfoliative toxin A (ETA), a serine protease that causes blisters in the epidermis by removing the adhesive extracellular domain of desmoglein 1 but not other cadherins, including desmoglein 3 or E-cadherin (Amagai et al., 2000). ETA efficiently cleaved the desmoglein 1 ectodomain after ephrin-A1-Fc peptide stimulation without affecting the integrity or levels of E-cadherin, desmoglein 3, or desmocollin 1 a/b (Figure 5A). Ephrin-A1-Fc treatment no longer protected keratinocyte sheets from fragmentation after ETA treatment, indicating that the adhesive ectodomain of desmoglein 1 was required for ligand-mediated adhesion. Keratinocytes treated with Fc control peptide expressed low levels of desmoglein 1 (Figure 5A) and fragmented to a similar extent in the presence or absence of ETA (Supplemental Figure S8B). In addition, adhesion was similar between Fc and ephrin-A1-Fc–treated cultures exposed to ETA (Supplemental Figure S8C), indicating that the up-regulation of desmoglein 1 largely accounted for the ligand-mediated increase in adhesion. We silenced desmoglein 1 expression to confirm that it was required for the adhesive effects of EphA2. Desmoglein 1 knockdown has more global effects on keratinocyte adhesion because it also prohibits the induction of desmocollin 1 a/b, which is itself critical for epidermal integrity (Chidgey et al., 2001; Getsios et al., 2009). In contrast to controls, ephrin-A1-Fc treatment failed to strengthen adhesion of desmoglein 1–deficient keratinocytes (Figure 5B). Although we cannot rule out minor contributions of other adhesion molecules, these two distinct approaches provided strong support for the notion that desmoglein 1 is primarily responsible for ligand-mediated effects of EphA2 on adhesion.
Figure 5.
Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation in a desmoglein-1 dependent manner. (A) Confluent keratinocyte sheets were treated with 1.0 μg/ml Fc or ephrin-A1-Fc peptide for 48 h in 0.4 mM calcium and incubated in the presence of 2.0 μg/ml recombinant Staphylococcus aureus exfoliative toxin A (ETA) for an additional 2 h. Top panels show Western blot analysis of EphA2, full length (FL) and cleaved (CL) desmoglein 1, desmocollin 1 a/b, desmoglein 3, E-cadherin, or GAPDH. A mechanical dissociation assay was performed, and the bottom panel contains a bar graph depicting the average number of fragments. (B) Keratinocytes transduced with retroviruses harboring microRNA mimetic sequences targeting lamin A/C (mirLmn) as a control or desmoglein 1 (mirDsg1) were seeded to confluency and treated with 1.0 μg/ml ephrin-A1-Fc peptide for 48 h in 0.4 mM calcium. Top panels show Western blot analysis of EphA2, desmoglein 1, desmocollin 1 a/b, desmoglein 3, E-cadherin, or GAPDH. In parallel, a mechanical dissociation assay was performed (bottom panel). (C) Western blot analysis for EphA2, desmoglein 1, desmocollin 1 a/b, desmoglein 3, E-cadherin, keratin 10, or GAPDH from confluent keratinocytes transduced with mirLmn and mirDsg1 that were treated with 1.0 μg/ml Fc or ephrin-A1-Fc peptide for 48 h in 1.2 mM calcium. (D) Keratinocytes transduced with mirLmn and mirDsg1 were seeded under sparse conditions and treated with 0.01, 0.1, or 1.0 μg/ml Fc or ephrin-A1-Fc in 1.2 mM calcium for 7 days. Western blot analysis was performed for EphA2, desmoglein 1, desmocollin 1 a/b, keratin 10, or GAPDH.
Desmoglein 1 not only increases adhesion but further promotes keratinocyte differentiation (Getsios et al., 2009). To determine whether an increase in desmoglein 1 was required for soluble ligands to enhance keratinocyte differentiation, we examined the expression of desmocollin 1 a/b and keratin 10 in desmoglein 1–deficient confluent cultures subjected to a calcium switch and treated with ephrin-A1-Fc. Keratinocytes transduced with a miRNA (miR)-mimetic designed to target lamin A/C as a negative control underwent peptide-mediated differentiation (Figure 5C). In contrast, desmoglein 1 knockdown impaired the ability of EphA2 to up-regulate the expression of desmocollin 1 a/b and keratin 10. It was also possible to effectively silence desmoglein 1 expression during the formation of keratinocyte colonies for 7 d in high calcium but only at low concentrations of ephrin-A1-Fc (0.01 μg/ml; Figure 5D). Partial knockdown was observed at higher concentrations of peptide (≥0.1 μg/ml), suggesting that the pathway triggered by EphA2 to up-regulate desmoglein 1 expression overwhelmed the retroviral gene silencing machinery. Although this marginal reduction in desmoglein 1 expression was insufficient to reverse the effects on colony size (data not shown) or keratin 10 expression, the levels of desmocollin 1 a/b were notably reduced (Figure 5D). Collectively, these studies are consistent with the idea that ligand targeting of EphA2 up-regulated desmoglein 1 to enhance keratinocyte adhesion and differentiation.
DISCUSSION
Cell–cell adhesion and communication are coordinated events that help maintain epithelial tissue homeostasis. The present study defined the ability of EphA2 to dampen contact-dependent Erk1/2-MAPK signaling in primary human keratinocytes without affecting cell proliferation and identified the importance of this receptor tyrosine kinase in enhancing desmoglein 1–dependent adhesion and differentiation in cells derived from stratified epithelial tissues.
EphA2 is abundantly expressed in primary keratinocytes and becomes concentrated at cell–cell contacts where it is activated by ephrin ligands. Our studies were focused on the consequences of EphA2 ligand targeting and support a model whereby this receptor responds to ephrins present on adjacent cells to fortify differentiation-associated adhesion complexes that facilitate keratinocyte stratification. Under steady-state conditions, keratinocytes in the basal layer of the epidermis are tightly associated with one another and EphA2/ephrin-A1 signaling complexes are likely present at cell–cell contacts (Guo et al., 2006). Because EphA2 knockdown weakened adhesion in epidermal sheets, it seems likely that it plays a general role in maintaining tissue integrity even in the basal layer. The signaling mechanisms controlling the ability of basal keratinocytes to enter into suprabasal layers and adopt a more differentiated phenotype remain somewhat unclear. Our data suggest that one of the early signals that triggers keratinocyte differentiation may be increased forward signaling through EphA2 that elicits the expression of differentiation-associated desmosomal cadherins. Ephrin-A1-Fc peptidomimetics were used to enhance EphA2 forward signaling in our study, leading to receptor destabilization and down-regulation. It is possible that robust EphA2 activation and membrane destabilization may also normally occur in response to increased presentation of GPI-linked ephrin-A1 in the basal layer, possibly involving receptor internalization and subsequent down-regulation to promote differentiation in suprabasal keratinocytes.
Similar to peptide-mediated down-regulation of EphA2, the formation of native Eph receptor/ephrin signaling complexes often leads to their endocytosis and/or proteolysis in poorly adhesive cells, such as neurons (Miao and Wang, 2009; Pasquale, 2010). As previously shown in cell types with more robust intercellular junctions (Zantek et al., 1999; Orsulic and Kemler, 2000; Hess et al., 2006; Miura et al., 2009), cadherin-mediated adhesion stabilized EphA2 at keratinocyte borders. At present, it remains unclear whether EphA2 is preferentially stabilized on the cell surface or more efficiently recycled back to junctions after its activation by ephrin ligands. Interestingly, the lipid phosphatase SHIP2 interacts with EphA2 and regulates its endocytosis and degradation via inhibition of a phosphatidylinositol 3-kinase–dependent receptor internalization pathway that relies on Rac1 activity (Zhuang et al., 2007). It is therefore possible that the dampening of Rac1 induced by long-term stabilization of E-cadherin–based cell–cell contacts allows SHIP2 to more efficiently stabilize EphA2 at the cell surface (Braga et al., 2000; Perez et al., 2008). Alternatively, EphA2 may be preferentially recruited into an endosomal recycling pathway in keratinocytes instead of being targeted for proteosomal and/or lysosomal degradation by modulation of c-Cbl–dependent ubiquitinylation and subsequent interactions with the ankyrin domain containing protein, Odin (Wang et al., 2002; Kim et al., 2010). In the future, it will be interesting to determine whether the extent of ligand activation of EphA2 modulates its association with these trafficking effectors in epidermal cells, possibly leading to its destabilization and loss in more differentiated keratinocytes.
Ephrins and Eph receptors have been shown to support the epithelial phenotype. For example, EphB forward signaling and ephrin-B reverse signaling enhance adherens junctions and tight junctions, respectively (Noren et al., 2006; Cortina et al., 2007; Lee et al., 2008; Chiu et al., 2009). Although ligand activation of EphA2 can disrupt adherens junctions by increasing RhoA activity when the receptor is overexpressed (Fang et al., 2008), these complexes also promote cadherin-dependent compaction through the recruitment of Nck and Git1, which leads to the suppression of the GTPase, Arf 6 (Miura et al., 2009). Moreover, EphA2 has been shown to regulate epithelial branching morphogenesis in the kidney and mammary gland, suggesting a dynamic interplay between this receptor tyrosine kinase and cell adhesion complexes (Miao et al., 2003; Vaught et al., 2009). Finally, EPHA2 mutations in humans or targeted deletion of this receptor or the ephrin-A5 ligand in mice leads to cataract development that has been associated with defects in N-cadherin–based junctions of lens fiber cells (Cooper et al., 2008; Jun et al., 2009); epithelial tissues, including the epidermis, appear to be relatively intact in this and independent lines of mice that lack EphA2 (Naruse-Nakajima et al., 2001; Guo et al., 2006), perhaps due to compensation by other EphA receptor subtypes. In the present study, we provide the first functional evidence for EphA2 to increase the strength of epithelial cell–cell adhesion. Notably, we did not find any evidence for marked changes in E-cadherin incorporation into junctions (data not shown) or changes in expression levels. Alternatively, a marked increase in the expression of desmosomal cadherins was observed. In particular, desmosomal cadherins restricted to the more differentiated layers of the epidermis, namely desmoglein 1 and desmocollin 1 a/b, were up-regulated by ligand targeting of EphA2, uncovering a novel link between EphA2 and desmosomes. EphA2 not only enhanced desmosomal adhesion but also increased terminal differentiation in a population of stratified keratinocytes while allowing cells in contact with the substrate to continue proliferating. Finally, we demonstrated that the increase in desmoglein 1 expression contributed to these differentiation-promoting effects of soluble ephrin ligands.
Although ligand targeting of EphA2 did not inhibit human keratinocyte proliferation, we believe that our results remain consistent with previous reports in mouse keratinocytes where colony growth was inhibited and proliferation was not directly measured (Guo et al., 2006). Instead of suppressing proliferation, the primary event in limiting the expansion of mouse and human keratinocyte colonies may be increased differentiation and stratification. The ability of ephrin ligands to enhance keratinocyte differentiation may also help explain how EphA2 serves as a tumor suppressor in the skin. Recent studies in mouse models of skin carcinogenesis have demonstrated a critical role for Raf-1 in suppressing differentiation; this differentiation phenotype was unrelated to the mitogenic effects of Raf-1 mediated through activation of Mek-Erk1/2 signaling cascade (Ehrenreiter et al., 2009). Importantly, tumors were resolved by Raf-1 ablation. Because EphA2 is abundantly expressed in skin cancer, it may serve as a good candidate to be targeted for the purpose of promoting tumor cell differentiation and regression. Systemic administration of ephrin peptidomimetics may not be the best approach to trigger EphA2 activation in the epidermis as this leads to an increase in keratinocyte proliferation (Genander et al., 2010). Because our studies show that EphA2 is not required for the negative regulation of keratinocyte proliferation, the growth-promoting effects observed in peptide-treated mice may reflect differences in cell culture or species, agonistic or antagonistic effects of these peptides on other EphA receptor subtypes, changes in epidermal differentiation and barrier function that indirectly lead to epidermal hyperplasia, or actions on other targeted cell types, such as immunoregulatory cells, that drive inflammatory-associated epidermal hyperproliferation (Segre, 2006; Hanifin, 2009). The differentiation-promoting effects of ephrins may be restricted to stratified epithelia and corresponds well with the spatiotemporal distribution of ephrin-A ligands in the basal layer and the more broad distribution of EphA receptors (EphA1 and EphA2) that extend into the upper layers of the epidermis (de Saint-Vis et al., 2003; Guo et al., 2006; Hafner et al., 2006).
Finally, it is important to consider that EphA2 expression in keratinocytes is increased in response to hypoxia and UV irradiation, where it plays an important role in promoting apoptosis (Vihanto et al., 2005; Zhang et al., 2008). Ligand targeting of EphA2 did not alter keratinocyte growth but effects on survival pathways may contribute to the differentiated phenotype because these distinct cellular processes share many of the same effector molecules and both ultimately lead to cell death (Candi et al., 2005). Taken together, these studies demonstrate that ligand targeting of EphA2 increases the adhesive strength and terminal differentiation of keratinocytes via up-regulation of desmoglein 1 and provide a novel mechanism beyond the inhibition in proliferation to explain ephrin-mediated restriction in the lateral expansion of epithelial colonies. Future studies will be aimed at delineating the pathways downstream of EphA receptors that help maintain epidermal homeostasis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Robert M Lavker, Sergey Troyanovsky, and Kathleen J. Green as well as members of their laboratories for their helpful suggestions on this work. We thank Drs. Masayuki Amagai (Keio University), Robert Brackenbury (University of Cincinnati), Channing Der and Natalia Mitin (University of North Carolina), Gary Nolan (Stanford University), Julia Segre (National Human Genome Research Institute, National Institutes of Health), John Stanley (University of Pennsylvania), Bing-Cheng Wang (Case Western Reserve), Kathleen J Green and Cory Simpson (Northwestern University) for kind gifts and reagents. The Robert H. Lurie Comprehensive Cancer Center (RHLCCC) Flow Cytometry Facility provided technical assistance with the PI-FACS analysis and Paul Hoover supplied primary keratinocyte cultures from the Northwestern University Skin Disease Research Center Keratinocyte Core Facility with support from National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (1P30AR057216-01). This work was supported in part from a Dermatology Foundation Research Grant and Career Development Award, Zell Family Foundation funds from the RHLCCC, and a Foglia Family Foundation grant from the Department of Dermatology (Northwestern University) to S.G. S.L. is the recipient of a Baseball Cancer Charities Fellowship from the RHLCCC.
Abbreviations used:
- EGFR
Epidermal growth factor receptor
- EphA2
erythropoietin producing hepatocyte-receptor 2
- Erk1/2
extracellular signal-regulated kinase 1/2
- ETA
exfoliative toxin A
- GPI
glycosyl phosphatidyl inositol
- MAPK
mitogen activated protein kinase
- MDCK
Madine-Darby canine kidney.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-03-0242) on September 22, 2010.
REFERENCES
- Amagai M., Matsuyoshi N., Wang Z. H., Andl C., Stanley J. R. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 2000;6:1275–1277. doi: 10.1038/81385. [DOI] [PubMed] [Google Scholar]
- Braga V. M., Betson M., Li X., Lamarche-Vane N. Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell–cell adhesion in normal human keratinocytes. Mol. Biol. Cell. 2000;11:3703–3721. doi: 10.1091/mbc.11.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley-Sieders D. M., Zhuang G., Hicks D., Fang W. B., Hwang Y., Cates J. M., Coffman K., Jackson D., Bruckheimer E., Muraoka-Cook R. S., Chen J. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E., Cabodi S., Stein P. L., Hatzfeld M., Kedersha N., Paolo Dotto G. Tyrosine phosphorylation and src family kinases control keratinocyte cell–cell adhesion. J. Cell Biol. 1998;141:1449–1465. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E., Schmidt R., Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Chidgey M., Brakebusch C., Gustafsson E., Cruchley A., Hail C., Kirk S., Merritt A., North A., Tselepis C., Hewitt J., Byrne C., Fassler R., Garrod D. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J. Cell Biol. 2001;155:821–832. doi: 10.1083/jcb.200105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. T., Chang K. J., Ting C. H., Shen H. C., Li H., Hsieh F. J. Over-expression of EphB3 enhances cell-cell contacts and suppresses tumor growth in HT-29 human colon cancer cells. Carcinogenesis. 2009;30:1475–1486. doi: 10.1093/carcin/bgp133. [DOI] [PubMed] [Google Scholar]
- Cooper M. A., Son A. I., Komlos D., Sun Y., Kleiman N. J., Zhou R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl. Acad. Sci. USA. 2008;105:16620–16625. doi: 10.1073/pnas.0808987105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina C., Palomo-Ponce S., Iglesias M., Fernandez-Masip J. L., Vivancos A., Whissell G., Huma M., Peiro N., Gallego L., Jonkheer S., Davy A., Lloreta J., Sancho E., Batlle E. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B., Bouchet C., Gautier G., Valladeau J., Caux C., Garrone P. Human dendritic cells express neuronal Eph receptor tyrosine kinases: role of EphA2 in regulating adhesion to fibronectin. Blood. 2003;102:4431–4440. doi: 10.1182/blood-2003-02-0500. [DOI] [PubMed] [Google Scholar]
- Dumesic P. A., Scholl F. A., Barragan D. I., Khavari P. A. Erk1/2 MAP kinases are required for epidermal G2/M progression. J. Cell Biol. 2009;185:409–422. doi: 10.1083/jcb.200804038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreiter K., Kern F., Velamoor V., Meissl K., Galabova-Kovacs G., Sibilia M., Baccarini M. Raf-1 addiction in Ras-induced skin carcinogenesis. Cancer Cell. 2009;16:149–160. doi: 10.1016/j.ccr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Fang W. B., Brantley-Sieders D. M., Parker M. A., Reith A. D., Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- Fang W. B., Ireton R. C., Zhuang G., Takahashi T., Reynolds A., Chen J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J. Cell Sci. 2008;121:358–368. doi: 10.1242/jcs.017145. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Skin stem cells: rising to the surface. J. Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M., Holmberg J., Frisen J. Ephrins negatively regulate cell proliferation in the epidermis and hair follicle. Stem Cells (Dayton, Ohio) 2010;28:1196–1205. doi: 10.1002/stem.442. [DOI] [PubMed] [Google Scholar]
- Getsios S., Amargo E. V., Dusek R. L., Ishii K., Sheu L., Godsel L. M., Green K. J. Coordinated expression of desmoglein 1 and desmocollin 1 regulates intercellular adhesion. Differentiation. 2004a;72:419–433. doi: 10.1111/j.1432-0436.2004.07208008.x. [DOI] [PubMed] [Google Scholar]
- Getsios S., Huen A. C., Green K. J. Working out the strength and flexibility of desmosomes. Nat. Rev. 2004b;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Getsios S., Simpson C. L., Kojima S., Harmon R., Sheu L. J., Dusek R. L., Cornwell M., Green K. J. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. J., Getsios S., Troyanovsky S., Godsel L. M. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb. Perspect. Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Miao H., Gerber L., Singh J., Denning M. F., Gilliam A. C., Wang B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- Hafner C., Becker B., Landthaler M., Vogt T. Expression profile of Eph receptors and ephrin ligands in human skin and downregulation of EphA1 in nonmelanoma skin cancer. Mod. Pathol. 2006;19:1369–1377. doi: 10.1038/modpathol.3800660. [DOI] [PubMed] [Google Scholar]
- Hanifin J. M. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J. Invest. Dermatol. 2009;129:320–322. doi: 10.1038/jid.2008.252. [DOI] [PubMed] [Google Scholar]
- Hess A. R., Seftor E. A., Gruman L. M., Kinch M. S., Seftor R. E., Hendrix M. J. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol. Ther. 2006;5:228–233. doi: 10.4161/cbt.5.2.2510. [DOI] [PubMed] [Google Scholar]
- Hines M. D., Jin H. C., Wheelock M. J., Jensen P. J. Inhibition of cadherin function differentially affects markers of terminal differentiation in cultured human keratinocytes. J. Cell Sci. 1999;112:4569–4579. doi: 10.1242/jcs.112.24.4569. [DOI] [PubMed] [Google Scholar]
- Jun G., Guo H., Klein B. E., Klein R., Wang J. J., Mitchell P., Miao H., Lee K. E., Joshi T., Buck M., Chugha P., Bardenstein D., Klein A. P., Bailey-Wilson J. E., Gong X., Spector T. D., Andrew T., Hammond C. J., Elston R. C., Iyengar S. K., Wang B. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 2009;5:e1000584. doi: 10.1371/journal.pgen.1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Lee H., Kim Y., Yoo S., Park E., Park S. The SAM domains of Anks family proteins are critically involved in modulating the degradation of EphA receptors. Mol. Cell. Biol. 2010;30:1582–1592. doi: 10.1128/MCB.01605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessner J. L., Desai B. V., Amargo E. V., Getsios S., Green K. J. EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2. Mol. Biol. Cell. 2009;20:328–337. doi: 10.1091/mbc.E08-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen A. B., Pedersen M. W., Stockhausen M. T., Grandal M. V., van Deurs B., Poulsen H. S. Activation of the EGFR gene target EphA2 inhibits epidermal growth factor-induced cancer cell motility. Mol. Cancer Res. 2007;5:283–293. doi: 10.1158/1541-7786.MCR-06-0321. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Nishanian T. G., Mood K., Bong Y. S., Daar I. O. EphrinB1 controls cell-cell junctions through the Par polarity complex. Nat. Cell Biol. 2008;10:979–986. doi: 10.1038/ncb1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg R. A., Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol. Cell. Biol. 1990;10:6316–6324. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae M., Neve R. M., Rodriguez-Viciana P., Haqq C., Yeh J., Chen C., Gray J. W., McCormick F. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Margaryan N. V., Strizzi L., Abbott D. E., Seftor E. A., Rao M. S., Hendrix M. J., Hess A. R. EphA2 as a promoter of melanoma tumorigenicity. Cancer Biol. Ther. 2009;8:279–288. doi: 10.4161/cbt.8.3.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges C. W., McCance D. J. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27:2934–2940. doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- Miao H., Nickel C. H., Cantley L. G., Bruggeman L. A., Bennardo L. N., Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J. Cell. Biol. 2003;162:1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int. J. Biochem. Cell. Biol. 2009;41:762–770. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Wei B. R., Peehl D. M., Li Q., Alexandrou T., Schelling J. R., Rhim J. S., Sedor J. R., Burnett E., Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell. Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Miura K., Nam J. M., Kojima C., Mochizuki N., Sabe H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol. Biol. Cell. 2009;20:1949–1959. doi: 10.1091/mbc.E08-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse-Nakajima C., Asano M., Iwakura Y. Involvement of EphA2 in the formation of the tail notochord via interaction with ephrinA1. Mech. Dev. 2001;102:95–105. doi: 10.1016/s0925-4773(01)00290-8. [DOI] [PubMed] [Google Scholar]
- Nasreen N., Mohammed K. A., Antony V. B. Silencing the receptor EphA2 suppresses the growth and haptotaxis of malignant mesothelioma cells. Cancer. 2006;107:2425–2435. doi: 10.1002/cncr.22254. [DOI] [PubMed] [Google Scholar]
- Noberini R., Koolpe M., Peddibhotla S., Dahl R., Su Y., Cosford N. D., Roth G. P., Pasquale E. B. Small molecules can selectively inhibit ephrin binding to the EphA4 and EphA2 receptors. J. Biol. Chem. 2008;283:29461–29472. doi: 10.1074/jbc.M804103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren N. K., Foos G., Hauser C. A., Pasquale E. B. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat. Cell Biology. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- Orsulic S., Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J. Cell Sci. 2000;113:1793–1802. doi: 10.1242/jcs.113.10.1793. [DOI] [PubMed] [Google Scholar]
- Parri M., Buricchi F., Taddei M. L., Giannoni E., Raugei G., Ramponi G., Chiarugi P. EphrinA1 repulsive response is regulated by an EphA2 tyrosine phosphatase. J. Biol. Chem. 2005;280:34008–34018. doi: 10.1074/jbc.M502879200. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. Eph receptors and ephrins in cancer: bidirectional signaling and beyond. Nat. Rev. Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez T. D., Tamada M., Sheetz M. P., Nelson W. J. Immediate-early signaling induced by E-cadherin engagement and adhesion. J. Biol. Chem. 2008;283:5014–5022. doi: 10.1074/jbc.M705209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt R. L., Kinch M. S. Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade. Oncogene. 2002;21:7690–7699. doi: 10.1038/sj.onc.1205758. [DOI] [PubMed] [Google Scholar]
- Scholl F. A., Dumesic P. A., Khavari P. A. Mek1 alters epidermal growth and differentiation. Cancer Res. 2004;64:6035–6040. doi: 10.1158/0008-5472.CAN-04-0017. [DOI] [PubMed] [Google Scholar]
- Segre J. A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Fu W. Y., Hung K. W., Porchetta C., Hall C., Fu A. K., Ip N. Y. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc. Natl. Acad. Sci. USA. 2007;104:16347–16352. doi: 10.1073/pnas.0706626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C. L., Kojima S., Getsios S. RNA interference in keratinocytes and an organotypic model of human epidermis. Methods Mol. Biol. 2010;585:127–146. doi: 10.1007/978-1-60761-380-0_10. [DOI] [PubMed] [Google Scholar]
- Taddei M. L., Parri M., Angelucci A., Onnis B., Bianchini F., Giannoni E., Raugei G., Calorini L., Rucci N., Teti A., Bologna M., Chiarugi P. Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. Am. J. Pathol. 2009;174:1492–1503. doi: 10.2353/ajpath.2009.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Kamata R., Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J. Biol. Chem. 2005;280:42375–42382. doi: 10.1074/jbc.M503786200. [DOI] [PubMed] [Google Scholar]
- Tong J., Elowe S., Nash P., Pawson T. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J. Biol. Chem. 2003;278:6111–6119. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- Vaught D., Chen J., Brantley-Sieders D. M. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol. Biol. Cell. 2009;20:2572–2581. doi: 10.1091/mbc.E08-04-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihanto M. M., Plock J., Erni D., Frey B. M., Frey F. J., Huynh-Do U. Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J. 2005;19:1689–1691. doi: 10.1096/fj.04-3647fje. [DOI] [PubMed] [Google Scholar]
- Walker-Daniels J., Riese D. J., 2nd, Kinch M. S. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol. Cancer Res. 2002;1:79–87. [PubMed] [Google Scholar]
- Wang Y., Ota S., Kataoka H., Kanamori M., Li Z., Band H., Tanaka M., Sugimura H. Negative regulation of EphA2 receptor by Cbl. Biochem. Biophys. Res. Comm. 2002;296:214–220. doi: 10.1016/s0006-291x(02)00806-9. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J., Jensen P. J. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J. Cell Biol. 1992;117:415–425. doi: 10.1083/jcb.117.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykosky J., Palma E., Gibo D. M., Ringler S., Turner C. P., Debinski W. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27:7260–7273. doi: 10.1038/onc.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zantek N. D., Azimi M., Fedor-Chaiken M., Wang B., Brackenbury R., Kinch M. S. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell. Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- Zhang G., Njauw C. N., Park J. M., Naruse C., Asano M., Tsao H. EphA2 is an essential mediator of UV radiation-induced apoptosis. Cancer Res. 2008;68:1691–1696. doi: 10.1158/0008-5472.CAN-07-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang G., Hunter S., Hwang Y., Chen J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-kinase-dependent Rac1 activation. J. Biol. Chem. 2007;282:2683–2694. doi: 10.1074/jbc.M608509200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.