How centrosomes amplify after DNA damage is unclear. Cell fusions demonstrated that only irradiated centrosomes duplicate when fused with untreated partners, suggesting a licensing signal that does not move from one centrosome to another. Our data indicate that centriole disengagement occurs after irradiation, suggesting this as the signal.
Abstract
DNA damage can induce centrosome overduplication in a manner that requires G2-to-M checkpoint function, suggesting that genotoxic stress can decouple the centrosome and chromosome cycles. How this happens is unclear. Using live-cell imaging of cells that express fluorescently tagged NEDD1/GCP-WD and proliferating cell nuclear antigen, we found that ionizing radiation (IR)-induced centrosome amplification can occur outside S phase. Analysis of synchronized populations showed that significantly more centrosome amplification occurred after irradiation of G2-enriched populations compared with G1-enriched or asynchronous cells, consistent with G2 phase centrosome amplification. Irradiated and control populations of G2 cells were then fused to test whether centrosome overduplication is allowed through a diffusible stimulatory signal, or the loss of a duplication-inhibiting signal. Irradiated G2/irradiated G2 cell fusions showed significantly higher centrosome amplification levels than irradiated G2/unirradiated G2 fusions. Chicken–human cell fusions demonstrated that centrosome amplification was limited to the irradiated partner. Our finding that only the irradiated centrosome can duplicate supports a model where a centrosome-autonomous inhibitory signal is lost upon irradiation of G2 cells. We observed centriole disengagement after irradiation. Although overexpression of dominant-negative securin did not affect IR-induced centrosome amplification, Plk1 inhibition reduced radiation-induced amplification. Together, our data support centriole disengagement as a licensing signal for DNA damage-induced centrosome amplification.
INTRODUCTION
The centrosome is the major microtubule-organizing center in animal somatic cells and is composed of two orthogonally arranged centrioles surrounded by an amorphous proteinaceous structure, the pericentriolar material (PCM). γ-Tubulin ring complexes in the PCM nucleate microtubules to form the mitotic spindle. To ensure that cells form only the bipolar spindle required for accurate mitotic chromosome segregation during mitosis, the number of centrosomes is strictly controlled. Centrosome duplication normally occurs only once per cell cycle (reviewed by Hinchcliffe and Sluder, 2001; Doxsey et al., 2005; Bettencourt-Dias and Glover, 2007; Nigg, 2007). Centrosome duplication is coordinated with DNA replication by the activation of cyclin-dependent kinase (CDK)-2–cyclin E in late G1 phase (Matsumoto et al., 1999; Bettencourt-Dias and Glover, 2007; Nigg, 2007). However, centrosome duplication is not solely dependent on CDK2–cyclin E activity, because cell fusion experiments have shown that G2 phase centrosomes do not duplicate in otherwise permissive environments (Wong and Stearns, 2003). Resolution of this centrosome-intrinsic block to reduplication has been termed “licensing” (Tsou and Stearns, 2006). It has been proposed that a potential inhibitory signal is the physical association of the centrioles (Tsou and Stearns, 2006; Tsou et al., 2009). This association arises during the centriole duplication process and is resolved in late mitosis when centrioles disengage for the next round of duplication (Piel et al., 2000). The disengagement that licenses centriole duplication is mediated by the action of Polo-like kinase 1 (Plk1) and by separase, the anaphase promoting complex/cyclosome (APC/C)-controlled protease that cleaves Scc1 cohesin at the metaphase–anaphase transition, thus linking licensing to the completion of mitosis (Tsou and Stearns, 2006; Tsou et al., 2009).
Aberrant centrosome number is a common feature of tumor cells (Nigg, 2006; Fukasawa, 2007). Centrosome abnormalities may contribute to aneuploidy and tumorigenesis by increasing the frequency of abnormal mitoses (Brinkley, 2001; Nigg, 2002; Fukasawa, 2007; Ganem et al., 2009). Centrosome aberrations are a potential consequence of a broad range of genotoxic insults. Amplification of the centrosome occurs in cells that carry mutations in DNA repair or checkpoint genes (Fukasawa et al., 1996; Mantel et al., 1999; Yamaguchi-Iwai et al., 1999; Griffin et al., 2000; Kraakman-van der Zwet et al., 2002; Tutt et al., 2002; Bertrand et al., 2003; Dodson et al., 2004), express mutant forms of telomerase (Guiducci et al., 2001), or express viral oncogenes (Duensing et al., 2000; Watanabe et al., 2000; Duensing and Munger, 2003; Duensing et al., 2006). Abnormal amplification of centrosomes also has been observed after DNA damage induced by irradiation (Sato et al., 2000a,b; Sato et al., 2000b; Dodson et al., 2007) or DNA replication stress (Balczon et al., 1995; Meraldi et al., 2002). We have shown that ionizing radiation (IR)-induced centrosome amplification is a Chk1-dependent process that can result in the formation of multipolar mitotic cells and in some cases, cell death (Bourke et al., 2007; Dodson et al., 2007), although the precise mechanism of how centrosome amplification arises after DNA damage is not clear.
Two hypotheses exist for the mechanism of amplification: 1) that the centrioles undergo multiple disengagement/procentriole formation steps in S phase, or 2) that normally duplicated centrosomes undergo a second disengagement and procentriole formation in G2 phase. Determination of which of these hypotheses is correct would be valuable in establishing the initial steps in overduplication and indicating candidate effector pathways. The overduplication model proposed for S phase-arrested cells (Guarguaglini et al., 2005; Duensing et al., 2007), in which multiple, immature daughter centrioles arise from a single mother, does not explain the outcome after irradiation, in which the majority of centrosomes carry the maturation marker Cep170 (Bourke et al., 2007; Saladino et al., 2009). It is possible that irradiation induces overduplication in S phase during a temporary checkpoint-mediated delay and that the duplicated centrosomes acquire mature markers as they proceed through the cell cycle. Alternatively, centrosome duplication may occur during the G2 phase delay induced by DNA damage, as we have proposed previously (Dodson et al., 2004). Despite the requirement for an intact G2-to-M checkpoint in allowing DNA damage-induced centrosome amplification (Dodson et al., 2004; Bourke et al., 2007), templated centrosome reduplication outside S phase has not been visualized, formally speaking. This is important because G2 phase duplication after IR would indicate that the DNA damage response overcomes the centrosome-intrinsic block to reduplication, rather than allowing reduplication during an extended, already licensed period.
A second issue is the nature of the signal allowing centrosome overduplication that is transmitted after genotoxic stress. Of two possible models for how centrosome amplification can occur as a result of DNA damage, the first envisages an activating signal that stimulates centriole duplication (activation) and the second invokes the loss of an inhibitory signal that normally limits the duplication of the centrosome to once per cell cycle (licensing) (Tsou and Stearns, 2006; Nigg, 2007). Wong and Stearns, (2003) used cell fusion experiments to demonstrate that the limitation on reduplication of the centrosome in G2 phase is centrosome associated, consistent with the license for duplication being the separase-/Plk1-dependent physical disengagement of the centrioles.
In this study we further dissect the timing of IR-induced centrosome amplification in human U2OS cells by using live-cell imaging. Furthermore, we investigate the nature of the signal that leads to centrosome amplification by using cell fusion assays. Our findings suggest that, unlike normal centrosome duplication, IR-induced centrosome amplification can occur outside S phase. Consistent with IR-induced centrosome amplification being distinct from S phase duplication, we also show that DNA damage incurred in G2 leads to more centrosome amplification than G1 phase damage. Also, our fusion assays suggest that only the irradiated centrosomes can duplicate, supporting a model in which centrosome amplification is permitted by the loss of a centrosome-intrinsic inhibitory signal that prevents duplication. Together, our results define the timing of DNA damage-induced centrosome amplification and the nature of the signal that drives centrosome amplification in response to genotoxic stress.
MATERIALS AND METHODS
Cell Culture and Transfections
U2OS cells were obtained from the American Type Culture Collection (Middlesex, United Kingdom) and grown in DMEM (Lonza, Cologne, Germany) with 10% FCS at 37°C in 5% CO2. Chicken DU249 hepatoma cells were obtained from W. C. Earnshaw (Wellcome Trust Centre for Cell Biology, Edinburgh, United Kingdom) and were cultured in RPMI 1640 medium plus 10% fetal bovine serum (FBS) with 5% chicken serum. Cells were irradiated using a 137Cs source (Mainance Engineering, Waterlooville, United Kingdom). Plk1 inhibitor BI 2536 (JS Research Chemicals Trading, Wedel, Germany) was dissolved in dimethylsulfoxide and stored at –20°C. Stable U2OS cell lines were generated by transfection of linearized plasmid into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and selection with G418 (0.4 mg/ml). Cells were placed under selection 24 h after transfection, until colonies could be picked using cloning rings.
Plasmids and Cloning
We used pEGFPC2-NEDD1 (Haren et al., 2006) to generate green fluorescent protein (GFP)-NEDD1–expressing cells. To generate red fluorescent protein (RFP)-proliferating cell nuclear antigen (PCNA)–expressing cells, PCNA coding sequence was subcloned from GFP-PCNA (Essers et al., 2005) into pRFP (Dodson et al., 2007), yielding pRFP-PCNA. To generate Centrin2-GFP–expressing cells, full-length Centrin-2 was obtained by reverse transcription-polymerase chain reaction (RT-PCR) from U2OS cells using the following set of primers: forward, 5′-CCGCTCGAGATGGCCTCCAACTTTAAG-3′ and reverse, 5′-CGGGATCCCAATAGAGGCTGGTCTTTTTC-3′. Centrin2 was then cloned into pEGFP-N1 (Clontech, Takara, Saint-Germain-en-Laye, France) to make the expression construct pCentrin2-GFP. Expression vectors for myc-tagged wild-type and nondegradable KAA-DM securin were provided by M. Brandeis (Zur and Brandeis, 2001).
Flow Cytometry
Cells were fixed in 70% ice-cold ethanol in phosphate-buffered saline (PBS), stored overnight at 4°C, and then washed in PBS and resuspended in 0.5 ml of 40 μg/ml propidium iodide and 0.1 mg/ml RNase-A solution in PBS and incubated at room temperature for 1 h before flow cytometry analysis. Cells were analyzed using an FACSCalibur flow cytometer (BD Biosciences, Erembodegem, Belgium). To quantitate cells in S phase, 5-bromo-2′-deoxyuridine (BrdU) was added to the cells to a final concentration of 20 μΜ and incubated for 15 min at 37°C. After fixation in 70% ice-cold ethanol, the cells were treated with 2 M HCl/0.5% Triton X-100 for 30 min at 37°C and then incubated with anti-BrdU (BD Biosciences) for 1 h with shaking at 37°C. After washing, the cells were incubated with fluorescein isothiocyanate-conjugated anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA) and incubated with propidium iodide solution as described above.
Cell Synchronization
U2OS cells were synchronized (enriched) in G1 phase by a thymidine/mimosine double block procedure, and an enriched G2 population was obtained by releasing G1 cells into fresh growth medium for a defined time. U2OS cells were grown to 60% confluence and treated with 2 mM thymidine (Sigma, Dublin, Ireland) for 12 h. Cells were then washed three times with warmed PBS and released into fresh growth medium for 10 h. A second block was induced with 0.4 mM mimosine (Sigma) treatment for 14 h to arrest cells at the G1/S boundary. Cells were then washed three times with warmed PBS and released into fresh medium for 11 h to obtain a cell population enriched in G2 phase cells.
Cell Fusions
Wild-type U2OS cells were stained with the fluorochrome probe CellTracker Blue 7-amino-4-chloromethylcoumarin (CMAC; Invitrogen) before fusion. CellTracker Blue CMAC was prepared as a 10 mM stock solution in dimethyl sulfoxide. Cells were incubated at 37°C for 15 min in serum-free DMEM containing 3 μM of this dye. After washing with PBS, cells were resuspended in growth media and incubated for 30 min at 37°C. Cells at G2 phase received 5 Gy of IR before fusion. For cell fusions, 1 × 106 GFP-NEDD1–expressing U2OS cells and wild-type U2OS cells stained with CellTracker Blue CMAC were trypsinized and washed with 10 ml of warmed growth media. Next, cells were resuspended and mixed in 600 μl of 50 μM SDS in PBS for 3 min at 37°C, as described previously (Wong and Stearns, 2003). Cells were then centrifuged and 0.5 ml of PEG-1450 solution Hybri-Max (Sigma), and 50 μl of serum-free DMEM mixture was added dropwise to the cells with gentle tapping for 2 min, after which 5 ml of serum-free DMEM medium was added to the mixture for 5 min at 37°C with continuous stirring. Next, 10 ml of serum-free DMEM medium was added, and the cell mixture was incubated at 37°C for 5 min before centrifugation. The cells were then washed twice with 10 ml serum-free media before being resuspended in growth media and seeded onto coverslips. U2OS and DU249 cells were fused as described above using PEG-1500 (Roche, West Sussex, United Kingdom). U2OS-DU249 fusions were incubated in DMEM plus 10% FBS at 37°C in 5% CO2.
Microscopy
For live cell imaging cells were grown in phenol-red free DMEM with 10% fetal calf serum (FCS) and 25 mM HEPES (Invitrogen). Cells were grown on glass-bottomed 35-mm dishes (MatTek, Ashland, MA), and the lid was sealed with parafilm during the imaging experiment. All live microscopy was carried out on a DeltaVision integrated microscope system controlled by softWoRx software (Applied Precision, Issaquah, WA) mounted on an IX71 microscope (Olympus, Melville, NY) with a PlanApo N 60× objective, 1.42 numerical aperture (NA), and a 37°C environmental chamber (WeatherStation; Precision Control).
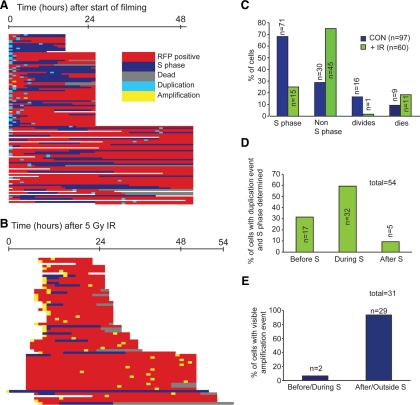
Centrosome duplication events were recorded when a change between one GFP-NEDD1 spot in a cell to two spots could be seen between time frames (as defined in Figure 2B). Centrosome amplification events were recorded as more than two GFP-NEDD1 spots per cell.
Figure 2.
Determination of cell cycle timing of centrosome amplification using GFP-NEDD1 and RFP-PCNA. (A) Line diagram showing history of each cell studied relative to S phase status (RFP-PCNA) and centrosome duplication (1 centrosome becoming 2) or amplification (>2 centrosomes forming) (GFP-NEDD1) as determined by live-cell imaging. (B) Line diagram showing history of each cell studied relative to S phase status (RFP-PCNA) and centrosome duplication or amplification (GFP-NEDD1) as determined by live-cell imaging. (C) Histogram showing the fate of control and irradiated cells with respect to S phase, division, and death. Note that the same cell may be represented more than once depending on what it was observed to do. (D) Histogram showing the timing of centrosome duplication in control cells relative to S phase during live cell imaging. (E) Histogram showing the timing of IR-induced centrosome amplification relative to S phase in U2OS cells during live imaging.
Cells were fixed in methanol as described previously (Dodson et al., 2004) and stained with the following primary antibodies: γ-tubulin (T3559; Sigma), γ-tubulin (T6557; Sigma), γ-tubulin (7396; Santa Cruz, Heidelberg, Germany), γ-H2AX (JBW301; Millipore, Carrigtwohill, Ireland), BrdU (347580; BD Biosciences), Cenp-F (Hussein and Taylor, 2002), Centrin-2 (27793; Santa Cruz), Centrobin (70448; Abcam, Cambridge, United Kingdom), Cep170 (Guarguaglini et al., 2005), C-NAP1 (611374; BD Biosciences), Kizuna (Oshimori et al., 2006), Nek2 (610593; BD Biosciences), and Ninein (Ou et al., 2002). Cell counting and imaging were performed using a BX51 microscope (Olympus), 60× (NA 1.4) and 100× (NA 1.35) objectives, by using Openlab software (Improvision/PerkinElmer Life and Analytical Sciences, Boston, MA). Deconvolved (nearest neighbor differential interference contrast) images were saved as Photoshop CS version 8.0 files (Adobe Systems, Mountain View, CA).
Immunoblotting
Mouse monoclonal anti-myc 9E10 and rabbit anti-actin A2066 (Sigma-Aldrich) were used as primary antibodies at 1:1000 and 1:5000, respectively.
RESULTS
Although an intact G2-to-M checkpoint signal is required for centrosome amplification in response to DNA damage (Dodson et al., 2004; Bourke et al., 2007), the issue of whether amplification actually occurs during G2 has not been formally resolved.
We used the human U2OS osteosarcoma cell line to ask whether centrosome amplification occurs during G2. First, we used microscopy to monitor the time course of IR-induced cell cycle arrest and centrosome amplification in U2OS cells, with BrdU incorporation indicating S phase cells and Cenp-F signal revealing G2 cells (Liao et al., 1995; Hussein and Taylor, 2002; Fletcher et al., 2003; Supplemental Figure 1A). As shown in Supplemental Figure 1B, by 24 h after 10 Gy of IR, we saw a dramatic decrease in the number of S phase cells and an increase in the percentage of cells in G2. Microscopy of γ-tubulin revealed that 20–30% of the cells with amplified centrosomes were in G2 phase, with fewer than 10% in S phase (Supplemental Figure 1C). These observations suggest that little IR-induced centrosome amplification occurred during S phase in U2OS cells, consistent with a model where DNA damage induces centrosome amplification during G2. However, this analysis of fixed cells does not indicate when in its cell cycle an individual cell was irradiated.
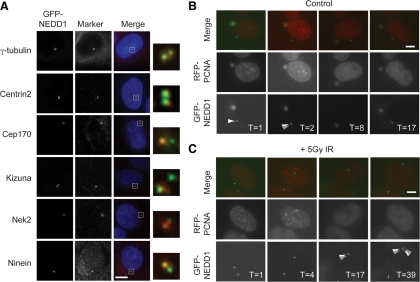
Therefore, we developed a model for live-cell imaging of centrosome amplification. We generated U2OS cell lines that stably expressed fluorescently tagged fusions of different centrosomal proteins, including Centrin-1, Centrin-2, CEP170 (data not shown), and NEDD1/GCP-WD (hereafter NEDD1). Of these, we chose cells with GFP-NEDD1 as a centrosome marker, because it showed a strong and stable fluorescent signal, with minimal noncentrosomal background in cells. As shown in Figure 1A, GFP-NEDD1 colocalized with a range of centrosome markers, confirming its centrosome localization, as appropriate for a component of the γ-tubulin ring complex (Haren et al., 2006; Luders et al., 2006). To confirm whether GFP-NEDD1 was a marker of centrioles or centrosomes we carried out careful analysis in G2 and mitotic cells by using an antibody to Centrin-2 that identifies the centrioles (Supplemental Figure 2A). We observed only one GFP-NEDD1 spot at each centrosome in G2 and mitotic cells when two Centrin-2 spots (centrioles) are visible. Normally, only one or two GFP-NEDD1 spots are seen in any cell. We then fluorescently tagged the replication marker PCNA and confirmed that RFP-tagged PCNA localized to replicative foci in U2OS cells, as shown by colocalization with BrdU (Supplemental Figure 2B) and that RFP-PCNA was a robust marker in live-cell imaging of the cell cycle in a stable cell line (Supplemental Movies 1 and 2). We then generated cells with both GFP-NEDD1 and RFP-PCNA (Supplemental Figure 2C). Importantly, cells that stably expressed GFP-NEDD1 and RFP-PCNA showed centrosome amplification after IR, with similar penetrance and kinetics to those observed in wild-type cells, indicating that this cellular response is unaffected by expression of the transgenes (Supplemental Figure 2D and Supplemental Movies 3–6). The cell line that expressed GFP-NEDD1 and RFP-PCNA was then used to study centrosome behavior in combination with S phase dynamics by live-cell imaging over extended (12- to 48-h) periods (Figure 1, B and C).
Figure 1.
Live-cell imaging of centrosomes and S phase timing. (A) Human U2OS cells expressing GFP-NEDD1 (green) were fixed in methanol and stained with the following antibodies to centrosomal or centrosome-associated proteins—γ-tubulin, Centrin-2, Cep170, Kizuna, Nek2, and Ninein (red)—and counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). Bar, 10 μm. (B) Representative example of stills from control filming experiment, showing centrosome duplication during S phase. Centrosome duplication was scored as observing a transition between one GFP-NEDD1 spot and two GFP-NEDD1 spots in a cell. GFP-NEDD1 is shown in green and RFP-PCNA in red. Numbers indicate times in hours after the start of filming. Bar, 10 μm. (C) Representative example of stills from experimental filming experiment showing IR-induced amplification after S phase. Centrosome amplification was scored as observing more than two GFP-NEDD1 spots per cell. Imaging was initiated 5 h after 5 Gy of IR, and other details are as described in B.
In all experiments, 20 fields were selected with cells expressing both GFP-NEDD1 and RFP-PCNA. In each field, every cell expressing both GFP-NEDD1 and RFP-PCNA was studied. We recorded the number of centrosomes at each time point, the PCNA status of the cell, and whether the cell divided or died. If punctate RFP-PCNA was observed, the cell was recorded as S phase. Control data were collected from three independent control experiments in which cells were imaged for between 15 and 48 h. In total, 97 cells were studied, and events within each cell over the course of the experiment are depicted as a line in Figure 2A. To examine radiation responses, cells were treated with 5 Gy of IR and then filmed immediately or after a recovery period of 5–9 h after IR. Data were collected for 15–48 h from 15 independent filming experiments after irradiation. As in the control experiments, every cell expressing GFP-NEDD1 and RFP-PCNA was analyzed. In total, 919 irradiated cells were examined to identify centrosome amplification. We saw 60 cells with more than two centrosomes (6.5% of the irradiated cells analyzed; Figure 2B). This population was studied to address the specific question of the timing of IR-induced centrosome amplification relative to S phase.
More than 65% of the control cells entered S phase, 15% divided and >10% died during the filming experiment (Figure 2C). The timing of centrosome duplication was examined relative to S phase, and we observed that the majority (>90%) of centrosome duplication occurred before or during S phase in untreated U2OS cells (Figure 2D). The average length of S phase was determined in those cells that entered and exited S phase, and this was found to be 14 ± 6.1 h long under our experimental conditions. This period is longer than the ≤10 h observed in other experiments using U2OS cells (Bugler et al., 2010), but we attribute this to the additional stresses imposed by the imaging. Some level of checkpoint activation also may explain the low proportion of dividing cells and why we did not see centrosome duplication in all of the cells over the imaging period.
During our filming, we observed S phase in only 15 irradiated cells (25%) with centrosome amplification (Figure 2C). This is due to treatment with 5 Gy of IR in U2OS cells significantly reducing the number of cells in S phase (Supplemental Figure 1, B and C). Only one cell divided after 5 Gy of IR, and 11 died during the course of filming (Figure 2C). We were able to see centrosome amplification happening in 31 (52%) of cells that possessed more than two centrosomes. Of these cells, >90% of them amplified their centrosomes either after or outside S phase (Figure 2E). This shows that there is a significant difference between the timing of centrosome duplication and IR induced centrosome amplification relative to S phase. Centrosome duplication is tightly coupled to S phase in U2OS cells, whereas centrosome amplification is decoupled from S phase after irradiation.
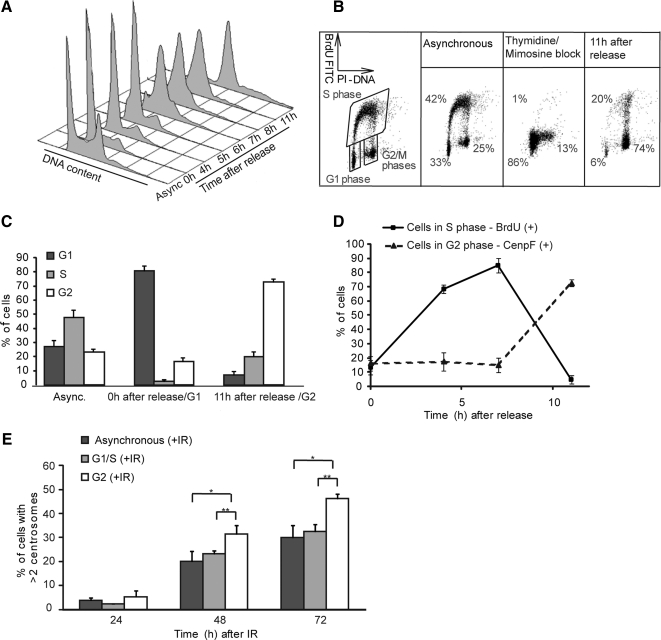
The model that a significant level of DNA damage-induced centrosome amplification occurs in G2 phase predicts that irradiation of a G2 population should permit high levels of centrosome amplification. Conversely, if the signal to allow centrosome overduplication is afforded only when cells incur DNA damage during the permissive (licensed) G1/S phase of the cell cycle when centrosomes normally duplicate (Tsou and Stearns, 2006), irradiation of a G2 population should not cause much amplification. We tested these two possibilities by treating defined, synchronized populations of U2OS cells with γ-irradiation. A double thymidine/mimosine block was used to obtain a highly enriched G1 phase population (Figure 3A). Collection of cells at defined times after release allowed us to achieve an enriched population of G2 cells, with >70% of cells in G2 at 11 h after release (Figure 3A). We confirmed these initial flow cytometry data by more detailed two-dimensional fluorescence-activated cell sorting analysis (Figure 3, B and C) and microscopy (Figure 3D), showing that high and reproducible synchrony was obtained using this approach. Next, we irradiated the defined, synchronized populations and followed centrosome numbers over time post-IR. Significantly higher levels of centrosome amplification were observed after irradiation of G2-enriched populations compared with populations of G1-enriched or asynchronous cells (Figure 3E). These data suggest that a DNA damage response signal in G2 phase can allow centrosome overduplication. Irradiation of a G1-enriched population also caused centrosome amplification. Extended S phase delay allows centrosome overduplication in rodent cells (Balczon et al., 1995; Kuriyama et al., 2007); so, for irradiated G1 cells, it is not clear whether the amplification resulted from DNA damage incurred while licensed, or from postreplicative repair of the radiation damage leading to a later, G2 phase DNA damage response that amplifies the centrosomes.
Figure 3.
IR-induced centrosome amplification in synchronized populations of U2OS cells. (A) Cell cycle profile of cells stained with propidium iodide after synchronization and release from a thymidine/mimosine double block method. Async., asynchronous population. (B) Indicative cell cycle profile determination using flow cytometry to quantitate BrdU incorporation. Data shows a representative example of BrdU analysis after block and release. The percentage of cells in G1, S, and G2/M at each time point was calculated as shown. (C) Histogram showing population breakdown by cell cycle stage in unsynchronized, G1-enriched and G2-enriched populations used for fusions. Data are expressed as the means of three independent experiments ± SD. (D) Microscopy analysis of cell cycle status of U2OS cells after thymidine/mimosine block and release. Cells in S phase were assayed by BrdU labeling, and cells in G2 phase were assayed by anti-CenpF antibody staining. Data show the mean ± SD of three separate experiments in which at least 100 cells were scored. (E) Quantitation by γ-tubulin microscopy of centrosome amplification at the indicated times after 5-Gy IR treatment of differently synchronized populations. Data show the mean ± SD of three separate experiments in which at least 100 cells were scored. Statistical significances were calculated by Student's unpaired t test and are indicated on the histogram as *p ≤ 0.1, **p ≤ 0.05, and ***p ≤ 0.01.
Having demonstrated that a G2 damage signal is permissive for centrosome overduplication, we wanted to determine the nature of this signal. We considered two possible models for how IR-induced centrosome amplification can occur. The first of these envisaged an activating signal that stimulates centriole duplication and the second invokes the loss of an inhibitory signal that normally limits the duplication of the centrosome to once per chromosome cycle (Tsou and Stearns, 2006; Nigg, 2007; Tsou et al., 2009). An activating signal should be transmissible from an irradiated centrosome to an untreated centrosome, whereas loss of an inhibitory signal should be specific to the treated centrosome.
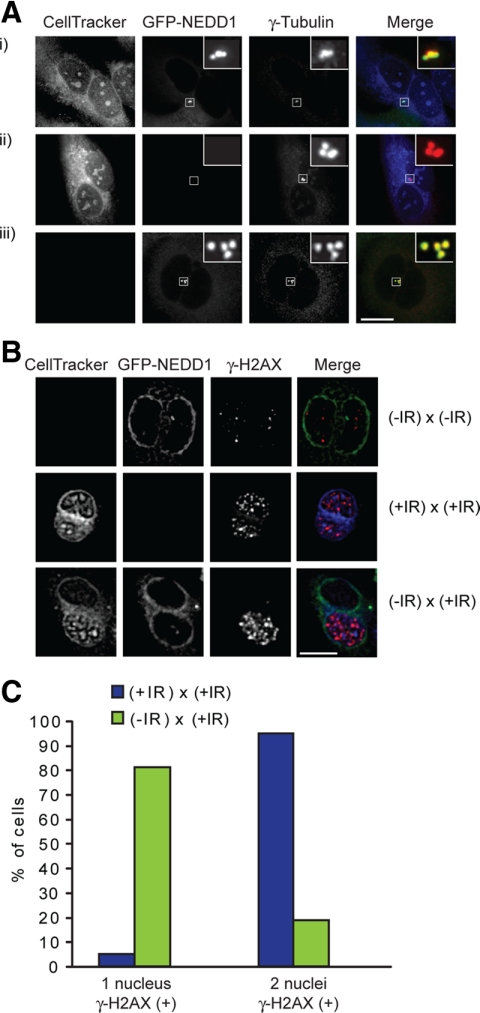
To perform an experiment capable of distinguishing between these models, we used PEG-mediated cell fusions, following previous work that demonstrated the centrosome-intrinsic limit of reduplication during the normal cell cycle (Wong and Stearns, 2003). Different populations were labeled using a cytoplasmic dye (CellTracker Blue) and expression of GFP-NEDD1. Fused cells were therefore identifiable as having both blue and green fluorescence in the same cell (Figure 4A). We confirmed that the GFP-NEDD1–expressing cells showed the same synchronization and release kinetics as wild type before initiating the fusion experiments (Supplemental Figure 3A). Populations of U2OS cells enriched at defined cell cycle stages were irradiated or untreated; fused; incubated for 24, 36, or 48 h; and then fixed and stained for γ-tubulin to quantify the number of centrosomes in the fused cells (Figure 4A). As shown in Figure 4, B and C, the DNA damage signal, as monitored by the appearance of γ-H2AX foci, was limited to the irradiated fusion partner.
Figure 4.
Fluorescence microscopy analysis of cell fusions. (A) Centrosome analysis in cell fusions. Wild-type U2OS cells were stained with CellTracker Blue and fused with GFP-NEDD1–expressing U2OS cells. Fused cells were fixed in methanol and stained for γ-tubulin (red). (i) Heterotypic fused cells could be identified by blue and green cytoplasmic fluorescence, with homotypic fusions showing (ii) only blue or (iii) only green fluorescence. The CellTracker Blue dye mixed in (the cytoplasm) fused cells but was not transferable between adjacent unfused cells. Staining with γ-tubulin antibodies revealed that GFP-NEDD1 incorporates into the centrosomes of the wild-type (nonexpressing) fusion partner. (B) Limitation of DNA damage signal to irradiated fusion partners. Cells were untreated or irradiated with 5 Gy and then fused with the indicated partners. Fixation and staining were performed as described in A, with antibodies to γ-H2AX (red) in place of those for γ-tubulin. (C) Quantitation of γ-H2AX signal in nuclei of untreated and 5-Gy-treated cells fused to irradiated partners. Representative example of experiment in which at least 100 cells were scored.
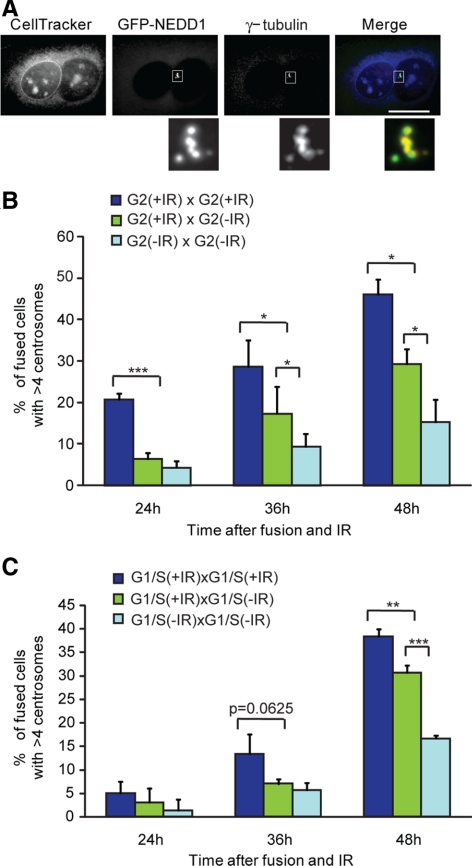
In control experiments, <15% of fused, unirradiated cells had more than four centrosomes (Figure 5, A and B), indicating that the fusion procedure itself did not cause high levels of DNA damage or centrosome overduplication. As shown in Figure 5B, irradiated G2-irradiated G2 cell fusions had significantly higher levels of centrosome amplification than did irradiated G2-unirradiated G2 fusions at 36 h and 48 h post-IR (Figure 5B). Similarly, irradiated G1/S-irradiated G1/S fusions showed significantly higher levels of centrosome amplification than did irradiated G1/S-unirradiated G1/S cell fusions at 48 h post-IR (Figure 5C). Based on our prediction that an activating signal should drive centrosome amplification in the unirradiated fusion partner as well, these results indicate that no such signal is generated. These data therefore support a model in which a centrosome-autonomous inhibitory signal is lost upon irradiation, allowing only the irradiated centrosomes to duplicate.
Figure 5.
IR-induced centrosome amplification in fused cells. (A) Micrograph of centrosome amplification in fused cells. Fused binucleate cells 36 h after treatment with 5-Gy irradiation, containing multiple centrosomes. GFP-NEDD1 (green) and anti-γ-tubulin antibody staining (red) were used as markers of IR-induced centrosome amplification. (B) Histogram showing the percentage of fused, G2-enriched populations with more than four centrosomes at the indicated times after treatment, where indicated, with 5 Gy of IR. Data show the mean ± SD of three separate experiments in which at least 35 cells were scored. Statistical significances were calculated by Student's unpaired t test and are indicated on the histogram as *p ≤ 0.1, **p ≤ 0.05, and ***p ≤ 0.01. (C) Histogram showing the percentage of fused, G1/S-enriched populations with more than four centrosomes at the indicated times after treatment, where indicated, with 5 Gy of IR. Quantitation and statistical analysis were as described in B.
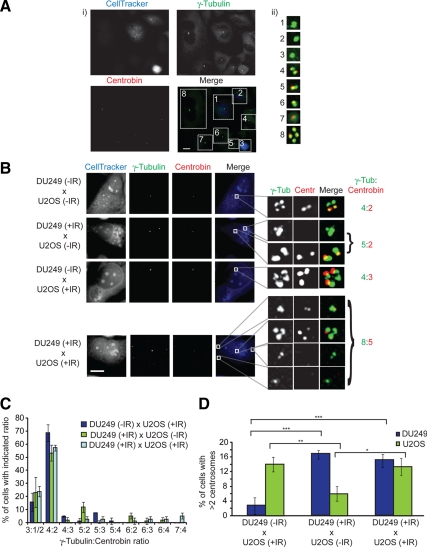
An important question in drawing conclusions from these experiments is the source of the centrosomes that amplify in fusions between irradiated and unirradiated cells. We tried to address this issue by fusing cells that express GFP-tagged centrosome components with wild-type cells. However, the high mobility of GFP-NEDD1 and GFP-Centrin1 within fused cytoplasms meant that all centrosomes were labeled and thus indistinguishable by the time any analysis was performed. We also failed to find any DNA damage-induced alteration that persisted as a marker of irradiated centrosomes until analysis. Therefore, we took advantage of the fact that certain of our antibodies to centrosome components are specific for the human protein and do not recognize their chicken orthologue. Centrobin is a core component of the centrosomes that preferentially localizes to daughter centrioles, and centrobin staining consistently colocalizes with that of γ-tubulin (Zou et al., 2005). As shown in Figure 6A, when chicken DU249 hepatoma cells are labeled with CellTracker Blue and then included in a fusion experiment with U2OS cells, nonfused DU249 cells or homokaryons were not stained with anti-centrobin antibodies (Zou et al., 2005). In the same field, nonfused or homokaryotic U2OS cells can be seen with all their centrosomes labeled with the anti-centrobin antibodies (Figure 6A). In DU249/U2OS heterokaryons, we saw one set of centrosomes that remained positive for centrobin staining, and one set that did not, indicating that the centrobin from one species does not replace that of the other in fused cells over our experimental period (Figure 6B). Once we had established that DU249 cells amplify their centrosomes when irradiated (Supplemental Figure 3B), this allowed us to test which of the centrosomes amplifies after IR. In fusions between DU249 cells and U2OS cells where one population was irradiated and the other not, the irradiated population was the only one to show centrosome amplification (Figure 6, C and D). Importantly, when both populations were irradiated and fused, we saw amplification in both partners, indicating no inhibitory effect of the interspecies fusion. A notable caveat to interpreting these observations is the possibility that a putative diffusible signal from chicken cells may not function in human cells, and vice versa. However, these results show clearly that irradiated centrosomes amplify in cell fusions, rather than those in the untreated partner. Even though the formal possibility remains that the unirradiated centrosomes may also amplify in fusions of the same species, the level of amplification seen in the irradiated U2OS fusions with the unirradiated DU249 cells is entirely sufficient to account for the entire amplification seen in the irradiated G2-unirradiated G2 U2OS fusion experiment (Figure 5B). We conclude that the amplification we see is limited to the irradiated partner, supporting a centrosome-autonomous activity in allowing reduplication.
Figure 6.
Centrosome analysis in U2OS and DU249 cell fusions. (A) DU249 cells were stained with CellTracker Blue and fused with wild-type U2OS cells. Fused cells were fixed in methanol and stained for γ-tubulin (green) and centrobin (red). U2OS cells were recognized by both centrobin and γ-tubulin antibodies, whereas DU249 cells were only stained with γ-tubulin antibody. (i) DU249 cells could be distinguished from U2OS cells by blue cytoplasmic fluorescence. U2OS cells could be distinguished from DU249 cells by coimmunostaining for centrosomes with antibodies to γ-tubulin and centrobin. (ii) Blow-ups of the boxed insets in i showing (1–3) DU249 and (4–8) U2OS centrosomes. Note that images 1 and 8 are from homokaryotic DU249 and U2OS fusions, respectively. Bar, 10 μm. (B) Micrographs of fusions between U2OS and DU249 cells. Cells were untreated or irradiated with 5 Gy and then fused with the indicated partners. Fixation and staining were performed as described in A. Boxed insets are shown at right along with the ratio of γ-tubulin stained centrosomes (green, U2OS and DU249) to centrobin-stained centrosomes (red, U2OS only). Bar, 10 μm. (C) Quantitation of centrosome numbers by γ-tubulin:centrobin ratio in U2OS-DU249 fusions following treatment, where indicated, with 5 Gy of IR. For example, a ratio of 3:1 means that there were three γ-tubulin spots and one centrobin spot, indicating a G2 DU249 fused with a G1 U2OS cell; 6:2 indicates four DU249 centrosomes and two U2OS centrosomes. Data show the mean ± SD of three separate experiments in which 25 cells were scored. (D) Histogram showing the percentage of DU249 and U2OS cells with more than two centrosomes in U2OS-DU249 heterokaryons after treatment, where indicated, with 5 Gy of IR. Fixation, staining, and scoring were performed as described in A. Data show the mean ± SD of three separate experiments in which 25 cells were scored. Statistical significances were calculated by Student's unpaired t test (two-tailed) and are indicated on the histogram as *p < 0.05, **p < 0.01, and ***p < 0.001.
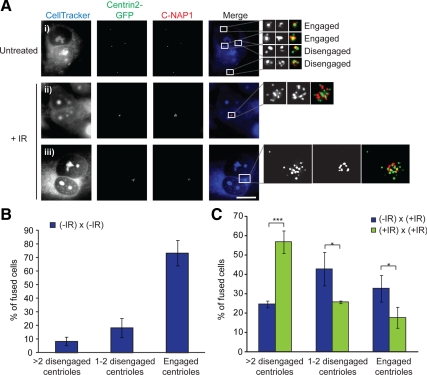
With centriole disengagement being a control mechanism for centrosome duplication (Tsou and Stearns, 2006), it was an obvious candidate for the control of IR-induced centrosome amplification. Therefore, we tested the impact of DNA damage on centriole engagement. We used the same criteria for centrosome engagement as published previously, by using microscopy of the centriolar marker Centrin2 and the linker protein C-NAP1 (Tsou and Stearns, 2006). A 2:1 ratio of Centrin2:C-NAP1 foci indicated engagement and a ratio of 1:1 or 1:0 ratio, disengagement (Figure 7A). This approach allowed us to determine centriole engagement even in fusions with large numbers of clustered centrosomes (Figure 7A). In fusions of unirradiated cells, the majority of centrioles were engaged, with a small number of disengaged centrioles that are likely to reflect the cell cycle stage at which fusions were performed (Figure 7B). However, when one or both of the fusion partners was irradiated, we saw a notable increase in centriole disengagement, with approximately twice the number of cells being seen with disengaged centrioles where both partners were irradiated (Figure 7C). From this result, we conclude that irradiation induces centriole disengagement.
Figure 7.
IR induces centriole disengagement. (A) Centriole engagement/disengagement in cell fusions. Wild-type U2OS cells were stained with CellTracker Blue and fused with Centrin2-GFP–expressing U2OS cells. Cells were fixed in methanol and stained for C-NAP1 (red). Centriole engagement was determined with Centrin2-GFP and anti-C-NAP1 antibody. (i) Micrograph of untreated, fused cell with engaged and disengaged centrioles, as indicated. Micrographs of fused binucleate cells with amplified (ii) engaged and (iii) disengaged centrioles at 48 h after treatment with 5 Gy of irradiation. Boxed insets are shown at right. Bar, 10 μm. (B) Histogram showing the percentages of 5-Gy-treated fused cells with more than eight Centrin2 spots. Data show the mean ± SD of three separate experiments in which 40 cells were scored. Statistical significances were calculated by Student's unpaired t test (two-tailed) and are indicated on the histogram as *p < 0.05, **p < 0.01, and ***p < 0.001. (C) Histogram showing centriole engagement in untreated fused cells with four to eight Centrin2 spots. Quantitation and analysis were as described in B.
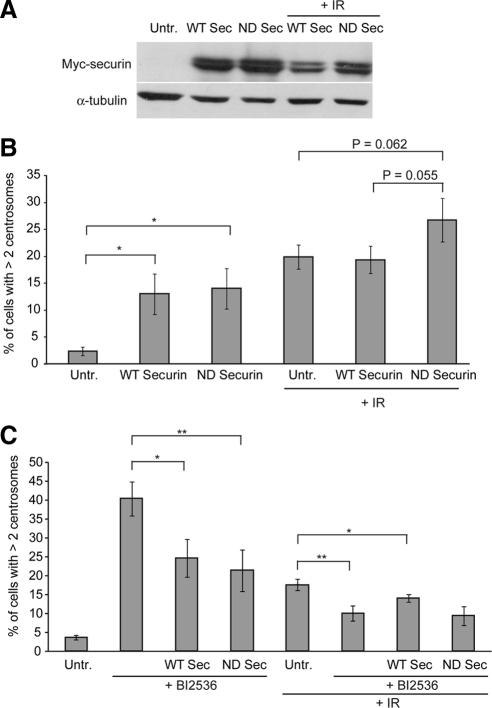
We next set out to test whether centriole disengagement is the permissive signal for centrosome amplification after DNA damage. Although separase is a major mechanism of disengagement, recent data have also implicated Plk1 in this regulation (Tsou et al., 2009). To disrupt separase function, we overexpressed a nondegradable form of its regulator, securin, which is normally degraded by the anaphase-promoting complex/cyclosome. Expression of this mutant securin has been shown previously to impede sister chromatid separation (Zur and Brandeis, 2001). As shown in Figure 8, A and B, transfection of either wild-type or nondegradable securin led to centrosome amplification, which may reflect a DNA damage response induced by transfection or an impact of securin overexpression. After irradiation of the transfected cells, we observed no impact of the overexpression of wild-type securin, but unexpectedly, a small increase in centrosome amplification in cells that expressed nondegradable securin. Next, we used a specific pharmacological inhibitor of Plk1, BI 2536, to inhibit the other pathway of centriole disengagement (Johnson et al., 2007; Steegmaier et al., 2007). Treatment with BI 2536 alone led to a high level of cells with more than two centrosomes (Figure 8C), which we attribute to failure in cytokinesis, as has been reported previously (Brennan et al., 2007; Burkard et al., 2007; Lenart et al., 2007; Petronczki et al., 2007). Treatment of wild-type or nondegradable securin-expressing cells with BI 2536 reduced the levels of cells with more than two centrosomes, but the mechanism of this reduction is not clear. Interestingly, IR-induced centrosome amplification was significantly reduced by BI 2536 treatment, although no further reduction in IR-induced centrosome amplification was seen when cells were both transfected with securin and treated with BI 2536 (Figure 8C). Together, these data implicate the known pathways of centriole disengagement in IR-induced centrosome amplification, consistent with a link between DNA damage and disengagement in licensing centrosome reduplication.
Figure 8.
Impact of securin overexpression and PLK1 inhibition on centrosome amplification. (A) Immunoblot of cell lysates from cells transfected with expression vectors for myc-Securin or myc-DM-KAA-Securin at 72 h after transfection. Where indicated, cells were irradiated at 24 h after transfection with 5 Gy of IR. (B) Effects of securin overexpression on IR-induced centrosome amplification. Cells were treated as described in A, and centrosome numbers were assessed by scoring γ-tubulin in myc-positive cells. Data show the mean + SD of three separate experiments in which at least 100 cells were scored. Statistical significances were calculated by Student's unpaired t test (two-tailed) and are indicated on the histogram as the p value or as *p < 0.05, **p < 0.01, and ***p < 0.001. (C) Impact of Plk1 inhibition and of combined Plk1 inhibition and securin overexpression on centrosome amplification. Where indicated, cells were transfected with the securin expression construct and then irradiated 8 h after transfection. BI 2536 was added to the cells 1 h before irradiation with 10 Gy. All cells were analyzed 38 h after irradiation as described in B.
DISCUSSION
The frequency with which centrosome abnormalities are seen in tumor cells and the correlation of elevated centrosome numbers with aneuploidy and chromosomal instability have long prompted the idea that control of centrosome number is important in maintaining genome stability (Lingle et al., 1998; Duensing et al., 2000; Pihan et al., 2001; D'Assoro et al., 2002; Nigg, 2002). Multiple or fragmented centrosomes can lead to mitotic spindle multipolarity (Keryer et al., 1984; Hut et al., 2003; Dodson et al., 2004; Oshimori et al., 2006; Thein et al., 2007; Ganem et al., 2009). However, several studies have shown that although multiple centrosomes lead to multipolar mitotic spindles, cells eventually cluster their centrosomes to assemble bipolar spindles before initiating anaphase (Ring et al., 1982; Quintyne et al., 2005; Basto et al., 2008; Ganem et al., 2009). Recent data have shown that the transient multipolarity itself may cause problems in kinetochore–spindle pole attachments (Ganem et al., 2009), so numerical control of the centrosome is clearly important, although different models exist for the mechanism by which genome instability arises in cells with multiple centrosomes.
Here, we explore in detail the mechanism by which DNA damage drives centrosome duplication, a phenomenon that has been observed after irradiation or radiomimetic drug treatment (Sato et al., 2000a,b; Dodson et al., 2007; Saladino et al., 2009) or DNA replication stress (Balczon et al., 1995; Meraldi et al., 2002). In previous work, we had used pulse-chase analysis of fixed cells to demonstrate that amplified centrosomes that arose in conditionally Rad51-deficient cells were detectable only in G2 phase cells (Dodson et al., 2004). Genetic analyses then demonstrated that the DNA damage response, which ensures a robust G2-to-M checkpoint through ataxia telangiectasia, mutated/ATM and Rad3-related-Chk1 signaling, was required for IR and other DNA double-strand break-inducing treatments, to cause centrosome amplification (Dodson et al., 2004; Bourke et al., 2007; Saladino et al., 2009). However, these analyses did not formally show that the centrosome duplication process occurred outside S phase, the normal stage at which centrosome duplication occurs under the control of CDK2 (Hinchcliffe et al., 1999; Lacey et al., 1999; Matsumoto et al., 1999). Therefore, we undertook a series of live-cell imaging experiments to explore the cell cycle timing of centrosome overduplication.
Although long-term imaging of irradiated cells for multiple fluorescently labeled components is technically challenging (Dodson et al., 2007), we obtained sufficient data to offer clear support for the model that IR-induced centrosome amplification occurs after S phase. We also considered the possibility that IR might induce a transient S phase delay, which could be permissive for centrosome duplication. Multiple, immature daughter centrioles have been shown to arise in S phase-arrested cells (Guarguaglini et al., 2005; Duensing et al., 2007). This does not seem to be the case after IR, where multiple centrosomes carry the maturation marks Cep170 and ninein (Bourke et al., 2007; Saladino et al., 2009), which lends further support to the notion of G2 phase centrosome duplication after IR. Our interspecies fusion experiments, where irradiation induces the amplification of centrioles that retain species identity, support the idea of directly-templated centriole duplication after IR, because multiple, immature daughters forming de novo would be expected to have been assembled stochastically from the proteins in the fused cytoplasm.
For IR to decouple centrosome duplication from the DNA replication cycle, the DNA damage response must remove the normally present brake on G2 phase reduplication. This brake is normally intrinsic to G2 phase centrosomes, because they cannot reduplicate even in an environment that is permissive of centrosome duplication (Wong and Stearns, 2003). Current models suggest that centriole engagement acts as a brake on reduplication, so that resolution of this engagement confers a license for duplication on the centrosome. We tested whether the reduplication of irradiated G2 phase centrosomes was permitted by a transmissible signal, such as would be provided by a mobile enzyme activity, or whether the license continued to reside at the irradiated centrosome alone. Our cell fusion data indicate that there is no mobile activity in irradiated cells that is sufficient to signal centrosome reduplication to untreated, nonpermissive centrioles. This observation suggests that licensing occurs only at irradiated centrosomes. Our finding that irradiation induces high levels of disengagement is consistent with this being a licensing signal.
Centriolar disengagement requires separase activity, potentially on centrosomal Scc1, and involves Plk1 (Tsou and Stearns, 2006; Nakamura et al., 2009; Tsou et al., 2009). Taking the model where separase is the principal signal that allows duplication, such a restricted licensing implies that the mobility of activated separase is limited or that the cleavage of its targets requires an additional, IR-induced signal for licensing. Our overexpression of nondegradable securin did not offer clear support for a role of separase in licensing centrosome overduplication after IR, but the APC/C-regulated cleavage of securin may not be the mechanism by which separase activation occurs after DNA damage. However, our use of BI 2536 provided clear support for the involvement of Plk1 in licensing centrosome amplification after IR. An issue with incorporating this observation into a mechanism is that DNA damage signaling inhibits Plk1 activity (Smits et al., 2000; van Vugt et al., 2001; Zhang et al., 2005), although recent evidence for IR-induced CDK2 activation (Bourke et al., 2010) indicates that up-regulation of cell cycle regulatory kinases can occur after DNA damage and may serve as a coregulatory signal to license aberrant centrosome duplication. The next step in establishing how the DNA damage response allows the centrosome cycle to continue while delaying the cell cycle will be the identification of the target(s) of separase/Plk1 activity in centriole disengagement.
Supplementary Material
ACKNOWLEDGMENTS
We thank Michael Brandeis, Laurence Haren, Roland Kanaar, Andreas Merdes, Miho Ohsugi, Tadashi Yamamoto, and Steve Taylor for plasmids and antibodies. We also thank Andreas Merdes for critical reading of the manuscript. This work was supported by Science Foundation Ireland Principal Investigator award 08/IN.1/B1029.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-02-0124) on September 22, 2010.
REFERENCES
- Balczon R., Bao L., Zimmer W. E., Brown K., Zinkowski R. P., Brinkley B. R. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R., Brunk K., Vinadogrova T., Peel N., Franz A., Khodjakov A., Raff J. W. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand P., Lambert S., Joubert C., Lopez B. S. Overexpression of mammalian Rad51 does not stimulate tumorigenesis while a dominant-negative Rad51 affects centrosome fragmentation, ploidy and stimulates tumorigenesis, in p53-defective CHO cells. Oncogene. 2003;22:7587–7592. doi: 10.1038/sj.onc.1206998. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M., Glover D. M. Centrosome biogenesis and function: centrosomics brings new understanding. Nat. Rev. Mol. Cell Biol. 2007;8:451–463. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- Bourke E., Brown J. A., Takeda S., Hochegger H., Morrison C. G. DNA damage induces Chk1-dependent threonine-160 phosphorylation and activation of Cdk2. Oncogene. 2010;29:616–624. doi: 10.1038/onc.2009.340. [DOI] [PubMed] [Google Scholar]
- Bourke E., Dodson H., Merdes A., Cuffe L., Zachos G., Walker M., Gillespie D., Morrison C. G. DNA damage induces Chk1-dependent centrosome amplification. EMBO Rep. 2007;8:603–609. doi: 10.1038/sj.embor.7400962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan I. M., Peters U., Kapoor T. M., Straight A. F. Polo-like kinase controls vertebrate spindle elongation and cytokinesis. PLoS One. 2007;2:e409. doi: 10.1371/journal.pone.0000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 2001;11:18–21. doi: 10.1016/s0962-8924(00)01872-9. [DOI] [PubMed] [Google Scholar]
- Bugler B., Schmitt E., Aressy B., Ducommun B. Unscheduled expression of CDC25B in S-phase leads to replicative stress and DNA damage. Mol. Cancer. 2010;9:29. doi: 10.1186/1476-4598-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard M. E., Randall C. L., Larochelle S., Zhang C., Shokat K. M., Fisher R. P., Jallepalli P. V. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc. Natl. Acad. Sci. USA. 2007;104:4383–4388. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Assoro A. B., Lingle W. L., Salisbury J. L. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–6153. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- Dodson H., Bourke E., Jeffers L. J., Vagnarelli P., Sonoda E., Takeda S., Earnshaw W. C., Merdes A., Morrison C. Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM. EMBO J. 2004;23:3864–3873. doi: 10.1038/sj.emboj.7600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson H., Wheatley S. P., Morrison C. G. Involvement of centrosome amplification in radiation-induced mitotic catastrophe. Cell Cycle. 2007;6:364–370. doi: 10.4161/cc.6.3.3834. [DOI] [PubMed] [Google Scholar]
- Doxsey S., McCollum D., Theurkauf W. Centrosomes in cellular regulation. Annu. Rev. Cell Dev. Biol. 2005;21:411–434. doi: 10.1146/annurev.cellbio.21.122303.120418. [DOI] [PubMed] [Google Scholar]
- Duensing A., Liu Y., Perdreau S. A., Kleylein-Sohn J., Nigg E. A., Duensing S. Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene. 2007;26:6280–6288. doi: 10.1038/sj.onc.1210456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing A., Liu Y., Tseng M., Malumbres M., Barbacid M., Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene. 2006;25:2943–2949. doi: 10.1038/sj.onc.1209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S., Lee L. Y., Duensing A., Basile J., Piboonniyom S., Gonzalez S., Crum C. P., Munger K. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc. Natl. Acad. Sci. USA. 2000;97:10002–10007. doi: 10.1073/pnas.170093297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing S., Munger K. Human papillomavirus type 16 E7 oncoprotein can induce abnormal centrosome duplication through a mechanism independent of inactivation of retinoblastoma protein family members. J. Virol. 2003;77:12331–12335. doi: 10.1128/JVI.77.22.12331-12335.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J., Theil A. F., Baldeyron C., van Cappellen W. A., Houtsmuller A. B., Kanaar R., Vermeulen W. Nuclear dynamics of PCNA in DNA replication and repair. Mol. Cell. Biol. 2005;25:9350–9359. doi: 10.1128/MCB.25.21.9350-9359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher L., Yen T. J., Muschel R. J. DNA damage in HeLa cells induced arrest at a discrete point in G2 phase as defined by CENP-F localization. Radiat. Res. 2003;159:604–611. doi: 10.1667/0033-7587(2003)159[0604:ddihci]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat. Rev. Cancer. 2007;7:911–924. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- Fukasawa K., Choi T., Kuriyama R., Rulong S., Vande Woude G. F. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- Ganem N. J., Godinho S. A., Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C. S., Simpson P. J., Wilson C. R., Thacker J. Mammalian recombination-repair genes XRCC2 and XRCC3 promote correct chromosome segregation. Nat. Cell Biol. 2000;2:757–761. doi: 10.1038/35036399. [DOI] [PubMed] [Google Scholar]
- Guarguaglini G., Duncan P. I., Stierhof Y. D., Holmstrom T., Duensing S., Nigg E. A. The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol. Biol. Cell. 2005;16:1095–1107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C., Cerone M. A., Bacchetti S. Expression of mutant telomerase in immortal telomerase-negative human cells results in cell cycle deregulation, nuclear and chromosomal abnormalities and rapid loss of viability. Oncogene. 2001;20:714–725. doi: 10.1038/sj.onc.1204145. [DOI] [PubMed] [Google Scholar]
- Haren L., Remy M. H., Bazin I., Callebaut I., Wright M., Merdes A. NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 2006;172:505–515. doi: 10.1083/jcb.200510028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe E. H., Li C., Thompson E. A., Maller J. L., Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe E. H., Sluder G. “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 2001;15:1167–1181. doi: 10.1101/gad.894001. [DOI] [PubMed] [Google Scholar]
- Hussein D., Taylor S. S. Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J. Cell Sci. 2002;115:3403–3414. doi: 10.1242/jcs.115.17.3403. [DOI] [PubMed] [Google Scholar]
- Hut H. M., Lemstra W., Blaauw E. H., Van Cappellen G. W., Kampinga H. H., Sibon O. C. Centrosomes split in the presence of impaired DNA integrity during mitosis. Mol. Biol. Cell. 2003;14:1993–2004. doi: 10.1091/mbc.E02-08-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. F., Stewart K. D., Woods K. W., Giranda V. L., Luo Y. Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. Biochemistry. 2007;46:9551–9563. doi: 10.1021/bi7008745. [DOI] [PubMed] [Google Scholar]
- Keryer G., Ris H., Borisy G. G. Centriole distribution during tripolar mitosis in Chinese hamster ovary cells. J. Cell Biol. 1984;98:2222–2229. doi: 10.1083/jcb.98.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraakman-van der Zwet M., et al. Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions. Mol. Cell Biol. 2002;22:669–679. doi: 10.1128/MCB.22.2.669-679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Terada Y., Lee K. S., Wang C. L. Centrosome replication in hydroxyurea-arrested CHO cells expressing GFP-tagged centrin2. J. Cell Sci. 2007;120:2444–2453. doi: 10.1242/jcs.008938. [DOI] [PubMed] [Google Scholar]
- Lacey K. R., Jackson P. K., Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc. Natl. Acad. Sci. USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J. J., Hoffmann M., Rettig W. J., Kraut N., Peters J. M. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Liao H., Winkfein R. J., Mack G., Rattner J. B., Yen T. J. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle W. L., Lutz W. H., Ingle J. N., Maihle N. J., Salisbury J. L. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders J., Patel U. K., Stearns T. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 2006;8:137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- Mantel C., Braun S. E., Reid S., Henegariu O., Liu L., Hangoc G., Broxmeyer H. E. p21(cip-1/waf-1) deficiency causes deformed nuclear architecture, centriole overduplication, polyploidy, and relaxed microtubule damage checkpoints in human hematopoietic cells. Blood. 1999;93:1390–1398. [PubMed] [Google Scholar]
- Matsumoto Y., Hayashi K., Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr. Biol. 1999;9:429–432. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- Meraldi P., Honda R., Nigg E. A. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A., Arai H., Fujita N. Centrosomal Aki1 and cohesin function in separase-regulated centriole disengagement. J. Cell Biol. 2009;187:607–614. doi: 10.1083/jcb.200906019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Origins and consequences of centrosome aberrations in human cancers. Int. J. Cancer. 2006;119:2717–2723. doi: 10.1002/ijc.22245. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Centrosome duplication: of rules and licenses. Trends Cell Biol. 2007;17:215–221. doi: 10.1016/j.tcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Oshimori N., Ohsugi M., Yamamoto T. The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 2006;8:1095–1101. doi: 10.1038/ncb1474. [DOI] [PubMed] [Google Scholar]
- Ou Y. Y., Mack G. J., Zhang M., Rattner J. B. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J. Cell Sci. 2002;115:1825–1835. doi: 10.1242/jcs.115.9.1825. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Glotzer M., Kraut N., Peters J. M. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev. Cell. 2007;12:713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Piel M., Meyer P., Khodjakov A., Rieder C. L., Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 2000;149:317–330. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan G. A., Purohit A., Wallace J., Malhotra R., Liotta L., Doxsey S. J. Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 2001;61:2212–2219. [PubMed] [Google Scholar]
- Quintyne N. J., Reing J. E., Hoffelder D. R., Gollin S. M., Saunders W. S. Spindle multipolarity is prevented by centrosomal clustering. Science. 2005;307:127–129. doi: 10.1126/science.1104905. [DOI] [PubMed] [Google Scholar]
- Ring D., Hubble R., Kirschner M. Mitosis in a cell with multiple centrioles. J. Cell Biol. 1982;94:549–556. doi: 10.1083/jcb.94.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladino C., Bourke E., Conroy P. C., Morrison C. G. Centriole separation in DNA damage-induced centrosome amplification. Environ. Mol. Mutagen. 2009;50:725–732. doi: 10.1002/em.20477. [DOI] [PubMed] [Google Scholar]
- Sato N., Mizumoto K., Nakamura M., Tanaka M. Radiation-induced centrosome overduplication and multiple mitotic spindles in human tumor cells. Exp. Cell Res. 2000a;255:321–326. doi: 10.1006/excr.1999.4797. [DOI] [PubMed] [Google Scholar]
- Sato N., Mizumoto K., Nakamura M., Ueno H., Minamishima Y. A., Farber J. L., Tanaka M. A possible role for centrosome overduplication in radiation-induced cell death. Oncogene. 2000b;19:5281–5290. doi: 10.1038/sj.onc.1203902. [DOI] [PubMed] [Google Scholar]
- Smits V. A., Klompmaker R., Arnaud L., Rijksen G., Nigg E. A., Medema R. H. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat. Cell Biol. 2000;2:672–676. doi: 10.1038/35023629. [DOI] [PubMed] [Google Scholar]
- Steegmaier M., et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr. Biol. 2007;17:316–322. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Thein K. H., Kleylein-Sohn J., Nigg E. A., Gruneberg U. Astrin is required for the maintenance of sister chromatid cohesion and centrosome integrity. J. Cell Biol. 2007;178:345–354. doi: 10.1083/jcb.200701163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou M. F., Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- Tsou M. F., Wang W. J., George K. A., Uryu K., Stearns T., Jallepalli P. V. Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev. Cell. 2009;17:344–354. doi: 10.1016/j.devcel.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt A. N., van Oostrom C. T., Ross G. M., van Steeg H., Ashworth A. Disruption of Brca2 increases the spontaneous mutation rate in vivo: synergism with ionizing radiation. EMBO Rep. 2002;3:255–260. doi: 10.1093/embo-reports/kvf037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt M. A., Smits V. A., Klompmaker R., Medema R. H. Inhibition of Polo-like kinase-1 by DNA damage occurs in an ATM- or ATR-dependent fashion. J. Biol. Chem. 2001;276:41656–41660. doi: 10.1074/jbc.M101831200. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Yamaguchi T., Akimoto Y., Rattner J. B., Hirano H., Nakauchi H. Induction of M-phase arrest and apoptosis after HIV-1 Vpr expression through uncoupling of nuclear and centrosomal cycle in HeLa cells. Exp. Cell Res. 2000;258:261–269. doi: 10.1006/excr.2000.4908. [DOI] [PubMed] [Google Scholar]
- Wong C., Stearns T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat. Cell Biol. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y., et al. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 1999;18:6619–6629. doi: 10.1093/emboj/18.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Fletcher L., Muschel R. J. The role of Polo-like kinase 1 in the inhibition of centrosome separation after ionizing radiation. J. Biol. Chem. 2005;280:42994–42999. doi: 10.1074/jbc.M505450200. [DOI] [PubMed] [Google Scholar]
- Zou C., Li J., Bai Y., Gunning W. T., Wazer D. E., Band V., Gao Q. Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J. Cell Biol. 2005;171:437–445. doi: 10.1083/jcb.200506185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur A., Brandeis M. Securin degradation is mediated by fzy and fzr, and is required for complete chromatid separation but not for cytokinesis. EMBO J. 2001;20:792–801. doi: 10.1093/emboj/20.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.