This report demonstrated selenite is transported through a monocarboxylate transporter Jen1p in Saccharomyces cerevisiae. Jen1p determined selenite sensitivity and uptake. Selenite had a similar affinity for Jen1p and a similar transport mechanism to the monocarboxylate lactate, which are both proton driven and exhibit reciprocal inhibition.
Abstract
Selenium is a micronutrient in most eukaryotes, including humans, which is well known for having an extremely thin border between beneficial and toxic concentrations. Soluble tetravalent selenite is the predominant environmental form and also the form that is applied in the treatment of human diseases. To acquire this nutrient from low environmental concentrations as well as to avoid toxicity, a well-controlled transport system is required. Here we report that Jen1p, a proton-coupled monocarboxylate transporter in S. cerevisiae, catalyzes high-affinity uptake of selenite. Disruption of JEN1 resulted in selenite resistance, and overexpression resulted in selenite hypersensitivity. Transport assay showed that overexpression of Jen1p enables selenite accumulation in yeast compared with a JEN1 knock out strain, indicating the Jen1p transporter facilitates selenite accumulation inside cells. Selenite uptake by Jen1p had a Km of 0.91 mM, which is comparable to the Km for lactate. Jen1p transported selenite in a proton-dependent manner which resembles the transport mechanism for lactate. In addition, selenite and lactate can inhibit the transport of each other competitively. Therefore, we postulate selenite is a molecular mimic of monocarboxylates which allows selenite to be transported by Jen1p.
INTRODUCTION
Selenium was once believed to be purely a toxin until the requirement was observed in mammals (Stadtman, 2002). The boundary between required and toxic concentrations of selenium is very narrow, particularly in the form of inorganic selenite (HSeO3−1 at physiological pH) (Gromer et al., 2005). Selenite toxicity is related to oxidative stress (Lewinska and Bartosz, 2008), which occurs spontaneously upon uptake. The requirement of selenium as a micronutrient is mostly attributable to the formation of essential antioxidant selenoproteins, in which selenocysteine is incorporated into their active sites (Stadtman, 1990; Veres et al., 1990; Burk et al., 2003). Recently, some low-molecular-weight selenium compounds have been shown to be responsible for clinical benefits of selenium (Davis and Uthus, 2003). These nonprotein selenium compounds, such as methylselenocysteine (Vadhanavikit et al., 1993) and inorganic selenite, have diverse roles, including scavenging radicals (Yamashita and Yamashita, 2010), attenuating heavy metal toxicity (Heath et al., 2010), serving as an anaerobic electron acceptor (Kabiri et al., 2009), preventing mutation of DNA (Seo et al., 2002), affecting signal transduction (Park et al., 2000a; Park et al., 2000b), maintaining metal homeostasis (Blessing et al., 2004), and other not fully characterized functions. Some eukaryotes, such as S. cerevisiae, have lost their selenoprotein biosynthetic machinery while retaining utilization of selenium compounds (Novoselov et al., 2002). Thus yeast is a convenient model organism to study nonselenoprotein roles of selenium. For example, yeast is useful to study selenite-induced antioxidant stress, because selenoproteins in mammals and microbes mask the antioxidant stress induced by early metabolism of selenite (Tarze et al., 2007). In addition, yeast is the primary source of commercial selenium supplementation in humans because it accumulates inorganic selenite and converts it to less toxic selenomethione (Schrauzer, 2003; McSheehy et al., 2005). Yeast-based selenium supplementation is approved as a feed additive for livestock and poultry (FDA, 2000, 2007). Both selenoyeast and selenite are used in clinical trials as a preventative agent for prostate cancer, as well as chemotherapeutic agents for other cancers (Etminan et al., 2005).
For selenite to exert either its physiological roles or its toxic effects it must first be transported inside cells, yet no selenite transporter has been identified in any organism. The more oxidized selenate is taken up by sulfate pathways in bacteria, but selenite is unlikely to be facilitated through the same pathway (Brown and Shrift, 1980). Previous studies of selenite uptake in yeast suggest that toxicity is increased under anaerobic conditions and with nonfermentative carbon sources (Lewinska and Bartosz, 2008). Biphasic selenite transport was observed with Km values of 54 μM and 435 μM in a glucose-sensitive manner (Gharieb and Gadd, 2004). Extracellular thiols have been observed to stimulate selenite uptake by human cancer cell lines (Ganyc and Self, 2008; Olm et al., 2009), and selenite uptake was proposed to be due to reduction by extracellular thiols to the highly unstable hydrogen selenide (HSe−) (Tarze et al., 2007).
Here we report the identification of a high-affinity S. cerevisiae selenite transporter, Jen1p, that mediates uptake of tetravalent selenite, not a selenium thiol conjugate or hydrogen selenide. Jen1p bidirectionally transports monocarboxylates including lactate, pyruvate, and other short chain carboxylic acids, and its expression is tightly regulated (Casal et al., 1999; Akita et al., 2000). Carbon sources such as glucose repress JEN1 transcription and result in degradation of JEN1 mRNA (Chambers et al., 2004). Glucose also produces endocytosis and degradation of Jen1p via lysine-linked ubiquitinylation (Paiva et al., 2009). JEN1 can be also derepressed by Cat8p (Bojunga and Entian, 1999), a transcriptional activator of gluconeogenic genes in the presence of lactate or pyruvate. Recent microarray analysis suggests that JEN1 may be up-regulated by methanol in the presence of glucose, indicating multiple levels of regulation (Yasokawa et al., 2010). This precise regulation of JEN1 may reflect its importance in uptake and utilization of monocarboxylates as energy sources and of lactate efflux to prevent build-up and inhibition of glycolysis. Selenite transport by Jen1p would be expected to be regulated by the same mechanisms. This regulation can explain why contrasting patterns of selenite uptake are seen between different studies (Gharieb and Gadd, 2004; Tarze et al., 2007; Olm et al., 2009).
MATERIALS AND METHODS
Plasmids and Yeast Strains
Plasmids and S. cerevisiae strains used in this study are described in Table 1. E. coli strain Top10 (NEB, USA) was used for molecular cloning.
Table 1.
Plasmids and yeast strains
| Genotype and/or description | Source | |
|---|---|---|
| Plasmids | ||
| pDR196 | Shuttle vector with PMA1 promoter | Reinders et al., 2002 |
| pJen1 | JEN1 was cloned into pDR196 | This study |
| Yeast strains | ||
| WT | BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ) | Open Biosystems |
| QZ1 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ jen1Δ | Open Biosystems |
| QZ1pDR196 | pDR196 is transformed into QZ1 | This study |
| QZ1pJen1 | pJen1 is transformed into QZ1 | This study |
Media
S. cerevisiae strains were grown at 30°C in rich Yeast-Peptone-Dextrose (YPD) medium (Sherman et al., 1974) or a minimal medium (6.7 g/l Bacto-yeast nitrogen base) (Sherman et al., 1974) supplemented with either 2% galactose or 2% glucose and with auxotrophic amino acid drop-out (Clontech, Palo Alto, CA). E. coli cells were grown in Luria-Bertani medium supplemented when necessary with 125 μg/ml ampicillin (Sigma, St. Louis, MO).
Cloning JEN1 into S. cerevisiae Expression Vector pDR196
JEN1 was amplified by PCR (PCR) of yeast genomic DNA using forward primer 5′-GGAATTCATGTCGTCGTCAATTACAG-3′ and reverse primer 5′-CTCGAGTTAAACGGTTTCAATATGCT-3′. PCR products were first cloned into pGEMT-easy vector (Promega, Madison, WI) and then digested with EcoRI and XhoI. The resulting restriction fragment was cloned into EcoRI and XhoI-digested vector pDR196 (Reinders et al., 2002), producing plasmid pJEN1. The pDR196 contains a URA3 selectable marker and a multiple cloning site flanked by a PMA1 promoter sequence upstream and an ADH terminator sequence downstream, allowing for constitutive overexpression of the inserted JEN1 genes. The plasmid was transformed into yeast strain QZ1 using a commercial kit (Qbiogene, Montreal, Quebec, Canada). Transformants were selected on minimal SD medium without uracil.
Metal Ion Resistance Assays
Strains were grown overnight at 30°C in liquid minimal medium (SD) medium (Sherman et al., 1974) with either 2% glucose or 2% galactose and appropriate supplements. Serial dilutions were applied to solid SD medium (1.5% agar) supplemented with the indicated concentrations of sodium selenite and incubated for 3 d at 30°C. Images were acquired using an Epson scanner.
Transport Assays
In vivo uptake assays were performed as described previously (Ghosh et al., 1999). In brief, cells were grown to exponential phase at 30°C in SD medium with either 2% glucose or galactose. The cells were harvested, washed twice with a transport buffer consisting of 75 mM HEPES, 150 mM KCl, and 1 mM MgCl2 (pH 5.5), and suspended to a density of 2 × 109 cells/ml in the same buffer, all at room temperature. Transport buffer with pH 6.5 and 7.5 were used to test pH affection. To initiate the assay, sodium selenite was added at the indicated concentrations to 0.2 ml of cells. After incubation at 30°C for 30 min or indicated intervals, portions (0.1 ml) were withdrawn and filtered through nitrocellulose filters (0.45-μm pore size; Milipore). The filters were washed with 5 ml of room temperature transport buffer three times and then digested with 0.3 ml of 70% nitric acid. When used as an inhibitor, mercuric chloride (Sigma), carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (Sigma), nigericin (Sigma), sodium arsenite (Sigma), or various monocarboxylates were added to the indicated final concentrations before addition of sodium selenite. Selenium was quantified by inductively coupled plasma mass spectrometry (ICP-MS, ELAN 9000, Perkin Elmer, Norwalk, CT).
Lactate transport assays were performed similarly. 14C-labeled lactic acid (Perkin Elmer) along with unlabeled lactate was added to cells at indicated concentrations. After 30 min, cells were passed through membrane filter and collected. The radioactivity was measured using a scintillation counter (Beckman, USA). All mean values and SEs are the average of three assays. Data were processed using SigmaPlot 10.0.
To determine the inhibition properties between lactate and selenite, inhibitor was added at indicated concentration before addition of substrate. Lactate transport was measured after 2 min and selenite uptake was measured after five minutes. Dixon plots were derived as previously reported (Martin-Venegas et al., 2007). Inhibition constants (Ki values) were obtained from Dixon plots.
Immunological Detection of JEN1 Expression
A rabbit polyclonal antibody for Jen1p was raised commercially using the synthetic peptide (QDQGVEYEEDEEDKPNLSA) derived from a putative extracellular loop of Jen1p conjugated with carrier Keyhole Limpet hemocyanin (Genemed Synthesis, San Antonio, TX). Indirect immunofluorescence microscopy was used to detect Jen1p in the yeast membrane. Yeast was grown with minimal medium supplied with 2% glucose or galactose to A600 of ∼1.0. The cells were fixed using formaldehyde and converted to spheroplasts using zymolase (ICN Biomedical, USA) and developed for immunofluorescent microscopy as described previously (Roth et al., 2002). Jen1p was detected using a 1:7000 dilution of anti-Jen1p as primary antibody, followed by a 1:3000 dilution of donkey Cy3-conjugated AffiniPure anti-rabbit IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Digital images were collected using a ×125 oil objective lens on a Nikon LABOPHOT-2 microscope (Nikon Co., Japan), and digital photos were taken using SPOT PURSUIT software (Diagnostic Instruments). Images were contrast-enhanced with Photoshop (Adobe Systems, Mountain View, CA).
RESULTS
Jen1p Expression Correlates with Selenite Sensitivity
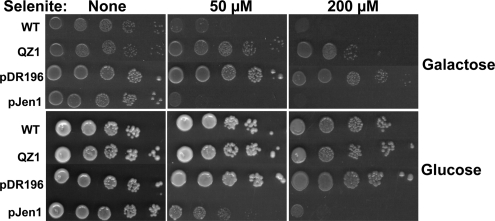
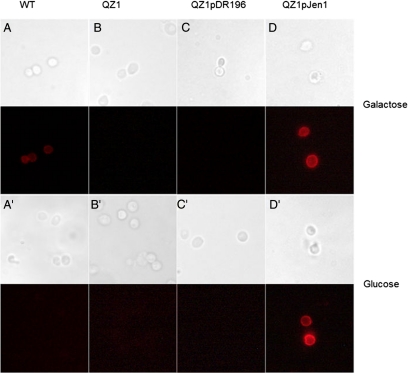
Selenite sensitivity differs when yeast cells are grown on different carbon sources, which could be due to a number of factors (Lewinska and Bartosz, 2008). To examine whether Jen1p expression is responsible for the variations in sensitivity, four yeast strains were used for controlled expression of Jen1p. Selenite tolerance of a wild-type (WT) strain, BY4741 (OpenBiosystem), QZ1 (JEN1Δ), QZ1 bearing vector plasmid pDR196 (QZ1pDR196) and QZ1 bearing plasmid pJEN1 (QZ1pJEN1), were assayed for selenite tolerance growing in minimal SD medium with either 2% galactose or glucose medium as carbon source. In galactose medium, Jen1p is expressed in WT cells, which exhibited selenite sensitivity compared with cells of QZ1 (Figure 1A). When Jen1p was overexpressed from a plasmid, the yeast cells were even more sensitive to extracellular selenite than the WT. WT cells grown with glucose as a carbon source exhibited resistance to selenite that was comparable to the tolerance of QZ1 (Figure 1B). Cells of QZ1pJEN1, in which JEN1 is constitutively expressed from a plasmid, exhibited selenite sensitivity even when grown in glucose containing medium (Figure 1B). Expression of JEN1 under different culture conditions was determined by immunofluorescence (Figure 2). When the WT was grown in glucose, immunofluorescence results showed that Jen1p was repressed (Figure 2B) compared with growth in galactose (Figure 2A), consistent with previous reports (Gharieb and Gadd, 2004). When expressed from a plasmid with a constitutive promoter, Jen1p was expressed and localized in the plasma membrane whether grown in galactose (Figure 2A) or glucose (Figure 2B). As expected, no Jen1p was observed in cells of QZ1 under any growth conditions. These results demonstrate that Jen1p expression and localization in the plasma membrane is responsible for selenite sensitivity.
Figure 1.
JEN1 expression leads to sensitive phenotype to selenite. Four yeast strains [BY4741 (WT), QZ1 (JEN1Δ), QZ1pDR196, and QZ1pJEN1] were grown in minimal SD medium containing either 2% glucose or galactose and supplied with the appropriate amino acids, and 10-fold serial dilutions were spotted onto 1.5% agar SD plates. Selenite was added to medium at final concentration of 50 or 200 μM, respectively. The plates were scanned and aligned for comparison.
Figure 2.
Detection of JEN1 expression in yeast membrane by immunofluorescence. Immunofluorescence staining of the four yeast strains from Figure 1 was performed using cells grown in SD medium containing either 2% glucose or galactose, as indicated. Images of bright field and immunofluorescence fields are shown side by side.
Jen1p Is the Major Selenite Transporter in S. cerevisiae
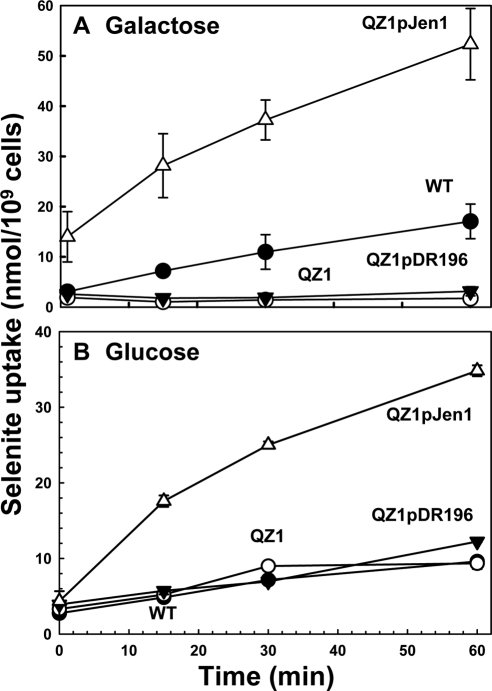
Selenite uptake was assayed in cells of yeast with or without Jen1p. Cells were cultured in minimal SD media supplied with either 2% galactose (Figure 3A) or glucose (Figure 3B). In galactose containing medium, WT cells exhibited increased selenite accumulation compared with the JEN1 knock out strain QZ1. Cells of QZ1 constitutively expressing JEN1 from plasmid pJEN1 accumulated more selenite than the WT (Figure 3A) and was insensitive to glucose repression (Figure 3B). When WT cells were cultured in glucose to repress JEN1, selenite uptake was comparable to the JEN1 knock out strain (Figure 3B). These results clearly demonstrate that Jen1p is the major transporter for selenite.
Figure 3.
Jen1p facilitates selenite uptake. Yeast strains BY4741 (●), QZ1 (○), QZ1pDR196 (▾), and QZ1pJEN1 (▵) were grown in minimal SD medium supplied with either 2% galactose or glucose to exponential phase (OD600 ∼1.0). The cells were harvested and suspended in transport buffer. Sodium selenite was added to the yeast suspensions at a final concentration of 1 mM, and at the indicated times portions (0.1 ml) were passed though the membrane filters. The filters were digested, and total selenium was quantified by ICP-MS. Three repeats of each were used to derive the means and SEs.
Properties of Selenite Transport via Jen1p
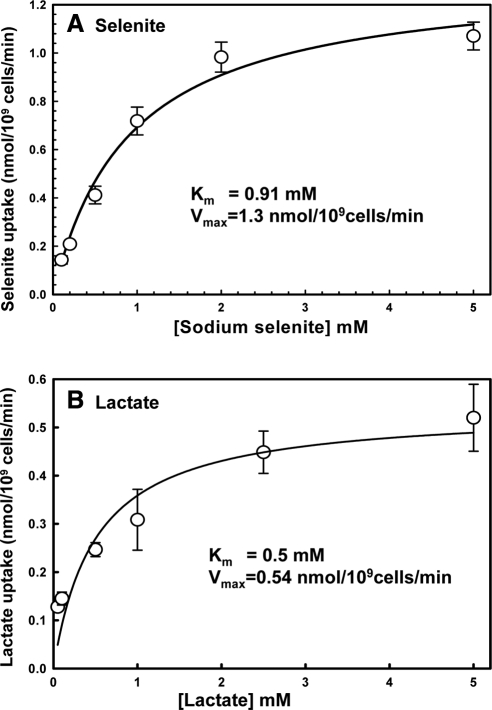
The kinetic parameters of Jen1p catalyzed selenite transport were determined using QZ1pJEN1. The Km for selenite was determined to be 0.91 ± 0.13 mM (Figure 4A), which is comparable to that of lactate [0.5 ± 0.21 mM in Figure 4B and 0.69 mM in a previous study (Casal et al., 1999)], indicating the affinities for both substrates are similar. The Vmax of selenite was determined to be 1.3 nmol/109 cells per min and for lactate was 0.53 nmol/109 cells per min. Thus Jen1p catalyzes selenite transport as efficiently as it does lactate.
Figure 4.
Jen1p catalyzes high-affinity uptake of both selenite and lactate. Uptake of selenite (A) or lactate (B) by strain QZ1pJEN1 was determined after 30 min, as described under Materials and Methods. The values from the deletion strain QZ1 were subtracted at each time point to correct for nonspecific binding. The Km and Vmax values were determined using a hypobolic fit of the data with SigmaPlot.
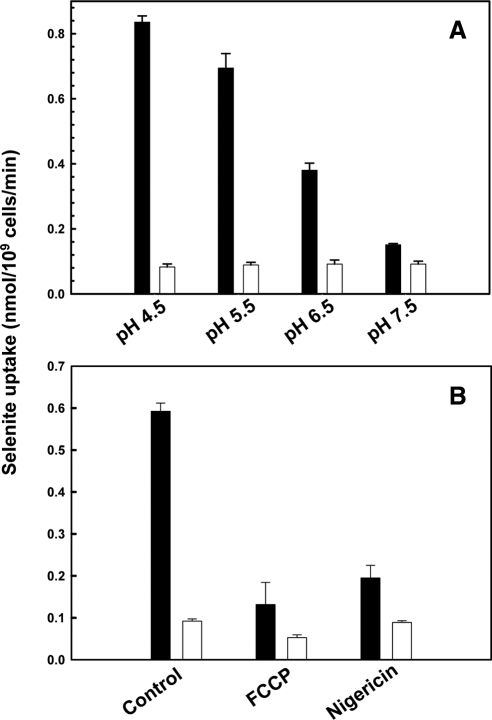
Jen1p is a monocarboxylate/proton symporter (Casal et al., 1999), which couples transport to the transmembrane pH gradient (ΔpH) (Soares-Silva et al., 2003; Soares-Silva et al., 2007). Selenite uptake was pH-dependent, with maximum transport occurring at lower pH (Figure 5A), consistent with proton dependence. The ionophore FCCP and protonophore nigericin, both of which dissipate the proton gradient, strongly inhibited selenite transport via Jen1p (Figure 5B). These results are consistent with selenite being taken up in a proton-coupled manner, dependent on ΔpH.
Figure 5.
(A) Selenite uptake as a function of pH. Selenite transport in QZ1pJEN1was assayed at the indicated pHs at a final concentration of 1 mM. (B) FCCP and nigericin inhibit selenite uptake. Cells of QZ1pJEN1 (black bar) were incubated with FCCP (100 μM) or nigericin (300 μM) for 5 min before addition of selenite at a final concentration of 1 mM, compared with QZ1 (white bar). Selenium accumulation was measured after 30-min incubation.
Selenite and Lactate Transport via Jen1p Are Inhibited by Monocarboxylic Acids, Arsenite, and Mercury
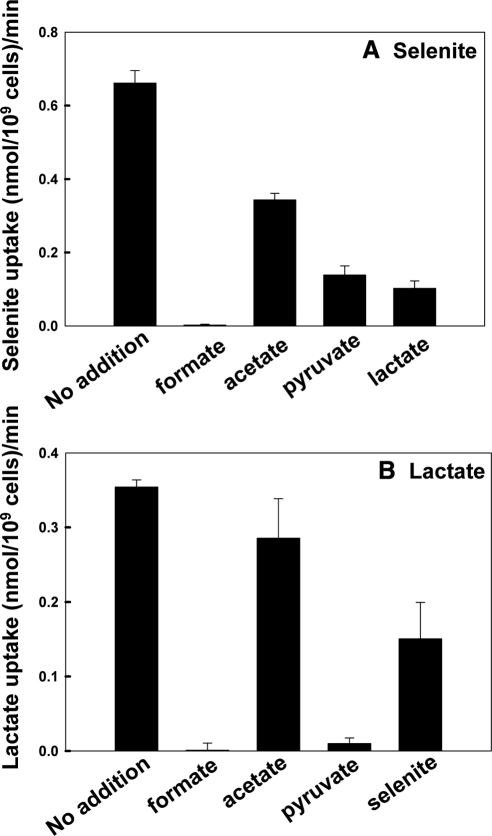
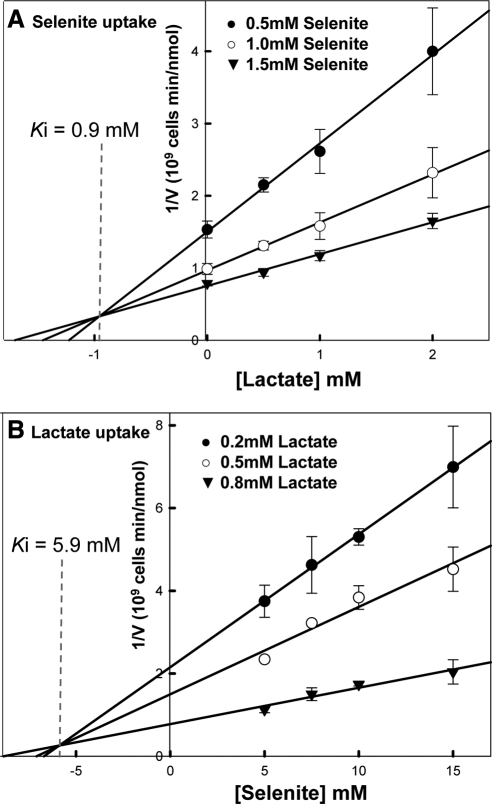
Selenite and lactate inhibit uptake of each other via Jen1p (Figure 6). In addition, transport of both selenite and lactate transport were inhibited by the carboxylic acids formate and pyruvate (Figure 6). Reciprocal inhibition by lactate and selenite demonstrated a competitive inhibition pattern (Figure 7), which supports lactate and selenite translocation sharing a similar mechanism. The Ki of lactate on selenite transport was 0.9 mM (Figure 7A) and the Ki of selenite on lactate transport is 5.8 mM (Figure 7B).
Figure 6.
Inhibition of selenite and lactate uptake by monocarboxylic acids. Uptake of 0.5 mM of either selenite (A) or lactate (B) by strain QZ1pJEN1 was determined after 30 min in the presence 5 mM of the indicated monocarboxylates, as described under Materials and Methods. The values from the deletion strain QZ1 were subtracted at each time point to correct for nonspecific binding.
Figure 7.
Inhibition kinetics between selenite and lactate. Uptake of indicated concentration of either selenite (A) or lactate (B) by strain QZ1pJEN1 was determined after 5 and 2 min in the presence of indicated concentration of lactate (A) or selenite (B), which is used as inhibitor. Each point represents three to four replications. Mean values and SEs were calculated from Sigma Plot 10.0. Each plot was linearized using Sigma Plot 10.0.
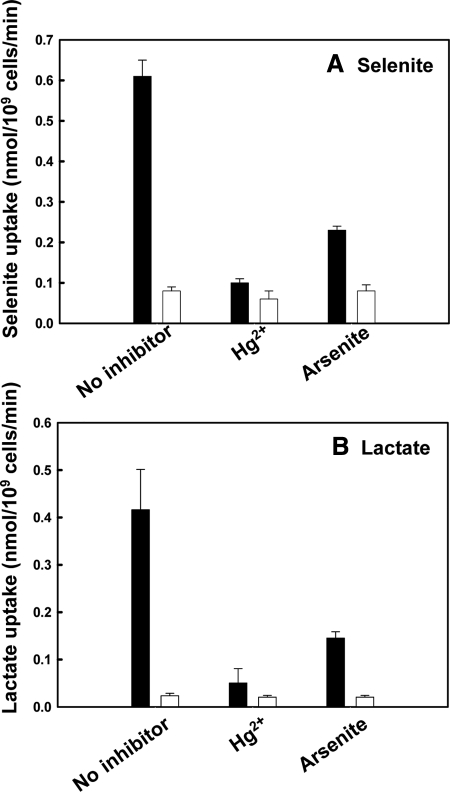
Mercury is a general thiol inhibitor which inhibits many membrane transporters (Agre et al., 2002). It usually acts by binding to cysteine or histidine residues located in or near the solute permeation pathway. Addition of mercury was found to block Jen1p-mediated selenite and lactate uptake in yeast (Figure 8, A and B). Jen1p has 10 cysteine residues in predicted transmembrane segments, as well as a histidine residue in a conserved sequence critical for monocarboxylate translocation in JEN1 family members (Soares-Silva et al., 2007). These residues are possible mercury binding sites. Similarly arsenite, which binds strongly to dithiols, inhibited uptake of both selenite and lactate (Figure 8, A and B). It is not clear whether Jen1p also transports arsenite because there are many arsenic uptake systems in yeast (Wysocki et al., 2001; Liu et al., 2004). However, inhibition of selenite uptake by arsenite may explain in part the reciprocal inhibitory interactions between these two metalloids.
Figure 8.
Mercury and arsenite inhibit selenite and lactate transport via Jen1p. Uptake of 1 mM of either selenite (A) or lactate (B) by strain QZ1pJEN1 (black bar) was determined after 30 min in the presence of either 0.2 mM mercuric chloride (Hg2+) or 5 mM sodium arsenite final concentrations. The cells were preincubated with mercury or arsenite for 5 min before initiation of the assay. Strain QZ1 was used as a control (white bar).
DISCUSSION
Uptake is the first step for selenium to exert its cellular function as a toxin or micronutrient. It is a key regulator of selenite toxicity, as the potent prooxidant action of selenite is spontaneous once inside the cell. Because selenium is required by most eukaryotic organisms, efficient transporter systems are needed to facilitate selenite. With such a narrow range between toxicity and essentiality, selenite transport proteins would be expected to play a critical role in its homeostasis. Despite this importance, no transporter for selenite has been identified in any organism.
Yeast is a well-accepted model organism for the study of selenite detoxification and the effect of selenite on transcription and cellular redox (Wysocki and Tamas, 2010; Lewinska and Bartosz, 2008; Salin et al., 2008). Yeast efficiently accumulates selenite and subsequently metabolizes inorganic selenite, primarily to selenomethionine, depending on the culture conditions (Ponce de Leon et al., 2002). In this study, we have shown that a monocarboxylate transporter, Jen1p, which catalyzes transport of cellular metabolites such as pyruvate and lactate, facilitates selenite with high affinity and is responsible for the majority of selenite accumulation in yeast. JEN1 expression is regulated by culture conditions and carbon sources such as glucose (Chambers et al., 2004). This is reasonable because monocarboxylates are used as fuel when more preferred fermentative carbon sources are absent (Casal et al., 2008). As expected, selenite transport is also regulated by culture conditions and glucose, in agreement with previous observations showing selenite toxicity (reflecting selenite uptake) is increased under anaerobic conditions with nonfermentative carbon sources, conditions under which JEN1 expression is up-regulated (Lewinska and Bartosz, 2008).
The identification of Jen1p as the major yeast selenite transporter clarifies previous hypotheses about selenite uptake. One hypothesis is that the real substrate for cellular uptake is not selenite, but is a GSH reduced product, hydrogen selenide (HSe−) (Tarze et al., 2007). Here we conclusively demonstrate Jen1p transports selenite and not selenide. Yeast cells accumulate selenite via Jen1p without addition of any exogenous reducing agent, and addition of equimolar GSH to the transport buffer before the transport assay did not increase selenite uptake (data not shown).
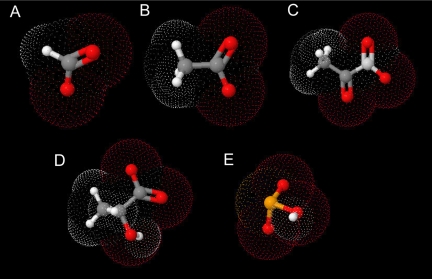
Why is selenite facilitated by a nutrient transporter? It is likely that selenite is adventitiously transported by molecular mimicry of carboxylic acids. Selenite shares significant structural similarity with the other Jen1p substrates such as formate, acetate, pyruvate and lactate (Figure 9). The first pKa of 2.62 for selenite is similar to those of pyruvate and lactate (2.39 and 3.08, respectively). At physiological pH these molecules are all monovalent oxyanions. Selenite and lactate reciprocally inhibited uptake of each other in a competitive manner. Jen1p transports multiple carboxylic acids such as pyruvate and lactate which similarly exhibit competitive inhibition of one another (Cassio et al., 1987; Casal et al., 1996). Therefore it is likely that selenite and monocarboxylate substrates are translocated via Jen1p using a similar mechanism. Interestingly, arsenite, which is known to be a selenite antagonistic agent, also inhibited the transport of both selenite and lactate. Further studies are required to elucidate whether arsenite is a substrate of Jen1p, a regulator of JEN1 expression or exerts its effect as a thiol modifying agent.
Figure 9.
Selenite is an inorganic molecular mimic of monocarboxylates. Shown are the structures of (A) formate (PubChem CID: 284), (B) acetate (PubChem CID: 176), (C) pyruvate (PubChem CID: 450648), (D) lactate (PubChem CID: 612), and (E) selenite (Larsen and Søtofte Inger, 1971). The bond angles used to create the pdb files were derived from published data (PubChem data base) and the structures rendered with JMOL.
It is uncertain whether the transport of selenite is advantageous or adventitious for yeast. The increased proton-coupled transport will affect selenium utilization, perhaps beneficially, as it couples selenite transport to a more reducing environment which attenuates its toxicity. It is important to note that the growth conditions of yeast, including pH and carbon source (which regulate selenite uptake), alter the pathway of selenite metabolism, which produces metabolites of different functions. Additionally, the tight regulation of selenite by different culture conditions limits selenium toxicity in certain environments. However, it is possible that this restricted uptake is merely adventitious. The accidental transport of toxic metalloids through nutrient transporters is not rare. For example, it has been previously shown that toxic arsenic is able to be facilitated by aquaglyceroporins and glucose transporters in yeast, indicating this transport mechanism may be popular in nature (Wysocki et al., 2001; Liu et al., 2004).
As selenium function is dependent on chemical form and concentration, the effects of selenite are therefore dependent on the transport mechanisms. The importance of extracellular concentration has been acknowledged in selenite studies, but the role of transport have been neglected in the absence of knowledge of transporter regulation. It is important to know the transporter identity and regulation mechanisms so that studies using selenite in yeast may be properly compared. This finding will also shed light on the study of selenite uptake mechanisms in other organisms. As selenite is used in trials for the treatment of various diseases (Rayman, 2005), as well as a promising antagonistic reagent for arsenic and other toxic metals (Zeng et al., 2005), it is critical to identify the selenite transporters in humans. It is possible that mammalian monocarboxylate transporters have similar function in the uptake of inorganic selenite.
ACKNOWLEDGMENTS
We are most grateful to Nicholas G. Davis (Wayne State University) and Shravan K. Chintala (Oakland University) for help with immunofluorescence assays. We also thank John Ward (University of Minnesota) for his generous gift of plasmid pDR196 and Andrew Halestrap (University of Bristol, UK), Eckhard Boles (Goths University, Germany), and John Reddan (Oakland University) for their constructive advice and critical comments on the manuscript. This work is supported by National Institutes of Health Grants ES016856 (to Z.L.) and GM55425 (to B.P.R.).
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0513) on September 22, 2010.
REFERENCES
- Agre P., King L. S., Yasui M., Guggino W. B., Ottersen O. P., Fujiyoshi Y., Engel A., Nielsen S. Aquaporin water channels–from atomic structure to clinical medicine. J. Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita O., Nishimori C., Shimamoto T., Fujii T., Iefuji H. Transport of pyruvate in Saccharomyces cerevisiae and cloning of the gene encoded pyruvate permease. Biosci Biotechnol Biochem. 2000;64:980–984. doi: 10.1271/bbb.64.980. [DOI] [PubMed] [Google Scholar]
- Blessing H., Kraus S., Heindl P., Bal W., Hartwig A. Interaction of selenium compounds with zinc finger proteins involved in DNA repair. Eur. J. Biochem. 2004;271:3190–3199. doi: 10.1111/j.1432-1033.2004.04251.x. [DOI] [PubMed] [Google Scholar]
- Bojunga N., Entian K. D. Cat8p, the activator of gluconeogenic genes in Saccharomyces cerevisiae, regulates carbon source-dependent expression of NADP-dependent cytosolic isocitrate dehydrogenase (Idp2p) and lactate permease (Jen1p) Mol. Gen. Genet. 1999;262:869–875. doi: 10.1007/s004380051152. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Shrift A. Assimilation of selenate and selenite by Salmonella typhimurium. Can. J. Microbiol. 1980;26:671–675. doi: 10.1139/m80-117. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Hill K. E., Motley A. K. Selenoprotein metabolism and function: evidence for more than one function for selenoprotein. P. J. Nutr. 2003;133:1517S–1520S. doi: 10.1093/jn/133.5.1517S. [DOI] [PubMed] [Google Scholar]
- Casal M., Cardoso H., Leao C. Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology. 1996;142:1385–1390. doi: 10.1099/13500872-142-6-1385. [DOI] [PubMed] [Google Scholar]
- Casal M., Paiva S., Andrade R. P., Gancedo C., Leao C. The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. J. Bacteriol. 1999;181:2620–2623. doi: 10.1128/jb.181.8.2620-2623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal M., Paiva S., Queiros O., Soares-Silva I. Transport of carboxylic acids in yeasts. FEMS Microbiol. Rev. 2008;32:974–994. doi: 10.1111/j.1574-6976.2008.00128.x. [DOI] [PubMed] [Google Scholar]
- Cassio F., Leao C., van Uden N. Transport of lactate and other short-chain monocarboxylates in the yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1987;53:509–513. doi: 10.1128/aem.53.3.509-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers P., Issaka A., Palecek S. P. Saccharomyces cerevisiae JEN1 promoter activity is inversely related to concentration of repressing sugar. Appl. Environ. Microbiol. 2004;70:8–17. doi: 10.1128/AEM.70.1.8-17.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. D., Uthus E. O. Dietary folate and selenium affect dimethylhydrazine-induced aberrant crypt formation, global DNA methylation and one-carbon metabolism in rats. J. Nutr. 2003;133:2907–2914. doi: 10.1093/jn/133.9.2907. [DOI] [PubMed] [Google Scholar]
- Etminan M., FitzGerald J. M., Gleave M., Chambers K. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control. 2005;16:1125–1131. doi: 10.1007/s10552-005-0334-2. [DOI] [PubMed] [Google Scholar]
- Ganyc D., Self W. T. High affinity selenium uptake in a keratinocyte model. FEBS Lett. 2008;582:299–304. doi: 10.1016/j.febslet.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharieb M. M., Gadd G. M. The kinetics of 75[Se]-selenite uptake by Saccharomyces cerevisiae and the vacuolization response to high concentrations. Mycol. Res. 2004;108:1415–1422. doi: 10.1017/s0953756204001418. [DOI] [PubMed] [Google Scholar]
- Ghosh M., Shen J., Rosen B. P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1999;96:5001–5006. doi: 10.1073/pnas.96.9.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromer S., Eubel J. K., Lee B. L., Jacob J. Human selenoproteins at a glance. Cell. Mol. Life Sci. 2005;62:2414–2437. doi: 10.1007/s00018-005-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. C., Banna K. M., Reed M. N., Pesek E. F., Cole N., Li J., Newland M. C. Dietary selenium protects against selected signs of aging and methylmercury exposure. Neurotoxicology. 2010;31:169–179. doi: 10.1016/j.neuro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabiri M., Amoozegar M. A., Tabebordbar M., Gilany K., Salekdeh G. H. Effects of selenite and tellurite on growth, physiology, and proteome of a moderately halophilic bacterium. J. Proteome. Res. 2009;8:3098–3108. doi: 10.1021/pr900005h. [DOI] [PubMed] [Google Scholar]
- Larsen F.K.L., Søtofte Inger M. S. A neutron diffraction study of selenious acid, H2SeO3. Acta. Chemica Scandinavica. 1971;25:1233–1240. [Google Scholar]
- Lewinska A., Bartosz G. A role for yeast glutaredoxin genes in selenite-mediated oxidative stress. Fungal Genet. Biol. 2008;45:1182–1187. doi: 10.1016/j.fgb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Liu Z., Boles E., Rosen B. P. Arsenic trioxide uptake by hexose permeases in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:17312–17318. doi: 10.1074/jbc.M314006200. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Rodriguez-Lagunas M. J., Geraert P. A., Ferrer R. Monocarboxylate transporter 1 mediates DL-2-Hydroxy-(4-methylthio)butanoic acid transport across the apical membrane of Caco-2 cell monolayers. J. Nutr. 2007;137:49–54. doi: 10.1093/jn/137.1.49. [DOI] [PubMed] [Google Scholar]
- McSheehy S., Yang L., Sturgeon R., Mester Z. Determination of methionine and selenomethionine in selenium-enriched yeast by species-specific isotope dilution with liquid chromatography-mass spectrometry and inductively coupled plasma mass spectrometry detection. Anal. Chem. 2005;77:344–349. doi: 10.1021/ac048637e. [DOI] [PubMed] [Google Scholar]
- Novoselov S. V., Rao M., Onoshko N. V., Zhi H., Kryukov G. V., Xiang Y., Weeks D. P., Hatfield D. L., Gladyshev V. N. Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J. 2002;21:3681–3693. doi: 10.1093/emboj/cdf372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olm E., Fernandes A. P., Hebert C., Rundlof A. K., Larsen E. H., Danielsson O., Bjornstedt M. Extracellular thiol-assisted selenium uptake dependent on the x(c)-cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc. Natl. Acad. Sci. USA. 2009;106:11400–11405. doi: 10.1073/pnas.0902204106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva S., Vieira N., Nondier I., Haguenauer-Tsapis R., Casal M., Urban-Grimal D. Glucose-induced ubiquitylation and endocytosis of the yeast Jen1 transporter: role of lysine 63-linked ubiquitin chains. J. Biol. Chem. 2009;284:19228–19236. doi: 10.1074/jbc.M109.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. S., Huh S. H., Kim Y., Shim J., Lee S. H., Park I. S., Jung Y. K., Kim I. Y., Choi E. J. Selenite negatively regulates caspase-3 through a redox mechanism. J. Biol. Chem. 2000a;275:8487–8491. doi: 10.1074/jbc.275.12.8487. [DOI] [PubMed] [Google Scholar]
- Park H. S., Park E., Kim M. S., Ahn K., Kim I. Y., Choi E. J. Selenite inhibits the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) through a thiol redox mechanism. J. Biol. Chem. 2000b;275:2527–2531. doi: 10.1074/jbc.275.4.2527. [DOI] [PubMed] [Google Scholar]
- Ponce de Leon C. A., Bayon M. M., Paquin C., Caruso J. A. Selenium incorporation into Saccharomyces cerevisiae cells: a study of different incorporation methods. J. Appl. Microbiol. 2002;92:602–610. doi: 10.1046/j.1365-2672.2002.01562.x. [DOI] [PubMed] [Google Scholar]
- Rayman M. P. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005;64:527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- Reinders A., Schulze W., Thaminy S., Stagljar I., Frommer W. B., Ward J. M. Intra- and intermolecular interactions in sucrose transporters at the plasma membrane detected by the split-ubiquitin system and functional assays. Structure. 2002;10:763–772. doi: 10.1016/s0969-2126(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Roth A. F., Feng Y., Chen L., Davis N. G. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell. Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin H., Fardeau V., Piccini E., Lelandais G., Tanty V., Lemoine S., Jacq C., Devaux F. Structure and properties of transcriptional networks driving selenite stress response in yeasts. BMC Genomics. 2008;9:333. doi: 10.1186/1471-2164-9-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauzer G. N. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 2003;47:73–112. doi: 10.1016/s1043-4526(03)47002-2. [DOI] [PubMed] [Google Scholar]
- Seo Y. R., Sweeney C., Smith M. L. Selenomethionine induction of DNA repair response in human fibroblasts. Oncogene. 2002;21:3663–3669. doi: 10.1038/sj.onc.1205468. [DOI] [PubMed] [Google Scholar]
- Sherman F., Fink G. R., Lawrence C. W. New York: Cold Spring Harbor Laboratory; 1974. Methods in Yeast Genetics, Laboratory Manual. [Google Scholar]
- Soares-Silva I., Paiva S., Diallinas G., Casal M. The conserved sequence NXX[S/T]HX[S/T]QDXXXT of the lactate/pyruvate:H(+) symporter subfamily defines the function of the substrate translocation pathway. Mol. Membr Biol. 2007;24:464–474. doi: 10.1080/09687680701342669. [DOI] [PubMed] [Google Scholar]
- Soares-Silva I., Schuller D., Andrade R. P., Baltazar F., Cassio F., Casal M. Functional expression of the lactate permease Jen1p of Saccharomyces cerevisiae in Pichia pastoris. Biochem J. 2003;376:781–787. doi: 10.1042/BJ20031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Annu. Rev. Biochem. 1990;59:111–127. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. A gold mine of fascinating enzymes: those remarkable, strictly anaerobic bacteria, Methanococcus vannielii and Clostridium sticklandii. J. Biol. Chem. 2002;277:49091–49100. doi: 10.1074/jbc.X200005200. [DOI] [PubMed] [Google Scholar]
- Tarze A., Dauplais M., Grigoras I., Lazard M., Ha-Duong N. T., Barbier F., Blanquet S., Plateau P. Extracellular production of hydrogen selenide accounts for thiol-assisted toxicity of selenite against Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:8759–8767. doi: 10.1074/jbc.M610078200. [DOI] [PubMed] [Google Scholar]
- Vadhanavikit S., Ip C., Ganther H. E. Metabolites of sodium selenite and methylated selenium compounds administered at cancer chemoprevention levels in the rat. Xenobiotica. 1993;23:731–745. doi: 10.3109/00498259309166780. [DOI] [PubMed] [Google Scholar]
- Veres Z., Tsai L., Politino M., Stadtman T. C. In vitro incorporation of selenium into tRNAs of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA. 1990;87:6341–6344. doi: 10.1073/pnas.87.16.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki R., Chery C. C., Wawrzycka D., Van Hulle M., Cornelis R., Thevelein J. M., Tamas M. J. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 2001;40:1391–1401. doi: 10.1046/j.1365-2958.2001.02485.x. [DOI] [PubMed] [Google Scholar]
- Wysocki R., Tamas M. J. How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol Rev. 2010 doi: 10.1111/j.1574-6976.2010.00217.x. (in press) [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Yamashita M. Identification of a novel selenium-containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J. Biol. Chem. 2010;285:18134–18138. doi: 10.1074/jbc.C110.106377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasokawa D., Murata S., Iwahashi Y., Kitagawa E., Nakagawa R., Hashido T., Iwahashi H. Toxicity of methanol and formaldehyde towards Saccharomyces cerevisiae as assessed by DNA microarray analysis. Appl. Biochem. Biotechnol. 2010;160:1685–1698. doi: 10.1007/s12010-009-8684-y. [DOI] [PubMed] [Google Scholar]
- Zeng H., Uthus E. O., Combs G. F., Jr Mechanistic aspects of the interaction between selenium and arsenic. J. Inorg. Biochem. 2005;99:1269–1274. doi: 10.1016/j.jinorgbio.2005.03.006. [DOI] [PubMed] [Google Scholar]