The PKD2 protein polycystin-2 is a nonselective Ca2+-permeable cation channel whose function is essential for normal kidney morphogenesis. Here, we report that phosphorylation of polycystin-2 by protein kinase D is essential for its function as an ATP-stimulated endoplasmic reticulum Ca2+ release channel and its effects on proliferation.
Abstract
PKD2 is mutated in 15% of patients with autosomal dominant polycystic kidney disease. The PKD2 protein, polycystin-2 or TRPP2, is a nonselective Ca2+-permeable cation channel that has been shown to function at several locations, including primary cilia, basolateral membrane, and at the endoplasmic reticulum (ER). Nevertheless, the factors that regulate the channel activity of polycystin-2 are not well understood. Polycystin-2 has been shown previously to be regulated by phosphorylation at two serine residues (Ser812 and Ser76) with distinct functional consequences. Here, we report the identification of a previously unrecognized phosphorylation site within the polycystin-2 C terminus (Ser801), and we demonstrate that it is phosphorylated by protein kinase D. Phosphorylation at this site was significantly increased in response to serum and epidermal growth factor stimulation. In nonciliated Madin-Darby canine kidney I cells, inducible expression of polycystin-2 inhibited cell proliferation compared with wild-type cells. Mutagenesis at Ser801 abolished these effects and reduced ATP-stimulated Ca2+ release from ER stores. Finally, we show that a pathogenic mutation (S804N) within the consensus kinase recognition sequence abolished Ser801 phosphorylation. These results suggest that growth factor-stimulated, protein kinase D-mediated phosphorylation of polycystin-2 is essential for its ER channel function and links extracellular stimuli to its effects on cell growth and intracellular calcium regulation.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited human renal disease (incidence 1 in 1000 live births) and is caused by mutations in two genes, PKD1 (85%) and PKD2 (15%) (Ong and Harris, 2005; Torres and Harris, 2006). ADPKD is an important cause of end-stage renal failure, accounting for ∼10% of patients on renal replacement therapy. The disease is characterized by the formation of fluid filled cysts in both kidneys of affected individuals, which ultimately result in end-stage renal failure. Other extrarenal manifestations of the disease include hypertension, cardiac valvular abnormalities, and cerebral aneurysms (Calvet and Grantham, 2001; Wilson, 2004).
The ADPKD proteins polycystin (PC)-1 and PC2 have been shown to function as a heterodimeric complex (Hanaoka et al., 2000; Newby et al., 2002), activating several key signaling pathways that in turn regulate diverse cellular functions, including proliferation, apoptosis, tubulogenesis, and fluid secretion. This is consistent with the largely overlapping renal and extrarenal phenotypes of PKD1 and PKD2 patients. PC1 and PC2 are likely to function together in many systems, but there is evidence to suggest they also can function independently (Ong and Harris, 2005).
PC2 is a 968-aa protein with six transmembrane spans, a pore-forming region, and cytoplasmic N- and C-terminal tails. Based on sequence homology, it has been adopted as a member of the transient receptor potential (TRP) channel superfamily (TRPP2), which is highly conserved throughout evolution and whose members function as nonselective cation channels responding to a wide range of stimuli. Although PC1 is believed to act at the plasma membrane (PM), PC2 has been shown to function at multiple locations, including primary cilia (Nauli et al., 2003), basolateral membrane (Ma et al., 2005), and the endoplasmic reticulum (ER) (Koulen et al., 2002). PC1 and PC2 have been shown to reconstitute a likely receptor–ion channel complex at the PM and PM—endoplasmic reticulum (ER) junctions, which regulates epithelial morphogenesis, whereas ER PC2 channels are likely to regulate determination of the left–right body axis (Giamarchi et al., 2010).
Protein phosphorylation is a major posttranslational mechanism for regulating the function of most proteins, including members of the TRP family of ion channels (Yao et al., 2005). In most cases, phosphorylation leads to increased channel activity. For example, PKC phosphorylation on multiple sites increases the activity of TRPC1 (Ahmmed et al., 2004) and TRPM4 (Guinamard et al., 2004; Nilius et al., 2005). Similarly, phosphorylation may regulate PC2 channel activity (Cai et al., 2004; Laycock et al., 2009).
In previous studies, work from our group and others had identified two mammalian PC2 serine residues that are phosphorylated (Cai et al., 2004; Streets et al., 2006). Phosphorylation at Ser812 is mediated by CK2 and is crucial for several functions, including its Ca2+ dependence; its retrograde trafficking via binding to the adaptor proteins, PACS-1 and PACS-2; and the cytoplasmic retention of the cell cycle regulatory protein Id2 (Cai et al., 2004; Kottgen et al., 2005; Li et al., 2005). In addition, glycogen synthase kinase (GSK)-3–dependent phosphorylation at Ser76 has been shown to be critical for PC2 retention at the basolateral PM in vitro and essential for zebrafish pronephric development in vivo (Streets et al., 2006).
In a previous article, we reported that mutation of both Ser76 and Ser812 residues did not abolish PC2 phospholabeling (Streets et al., 2006). This led us to seek other potential phosphorylation sites and to define their functional significance. Using a combination of site-directed mutagenesis, in vitro kinase assays, and phospho-specific antibody mapping, we have identified a new phosphorylation site within the PC2 C terminus at Ser801 that contains an evolutionarily conserved recognition sequence for protein kinase D (PrKD), also known as human protein kinase C-μ (PKCμ). In nonciliated Madin-Darby canine kidney (MDCK) I cells with inducible PC2 expression, we demonstrate that phosphorylation at this site specifically regulates cell growth probably mediated through its permissive effects on Ca2+ transients from ER-located PC2 channels. Finally, a pathogenic mutation (Ser804) within the PrKD recognition consensus sequence abolished Ser801phosphorylation, indicating its likely physiological significance.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma Chemical (Poole, Dorset, United Kingdom), unless otherwise stated. MDCK I and II cells were obtained from N. Simmons (University of Newcastle, Newcastle, United Kingdom) and the MDCK I FRT line was a kind gift of O. Frohlich (Emory University, Atlanta, GA). The human PKCμ plasmid was a gift of K. Pfizenmaier (University of Stuttgartm Stuttgart, Germany), and the pCMUIV-CD8 plasmid was a gift of S. Ponnambalam (University of Leeds, Leeds, United Kingdom). The PC2 antibodies p30 and 1A11 (gift of G. Wu, Vanderbilt University, Nashville, TN), which recognize the C-terminal 258 amino acids of human PC2, have been described previously (Ong et al., 1999; Li et al., 2003). The phospho-serine rabbit polyclonal antibody was obtained from Invitrogen (Paisley, United Kingdom). The PKCμ antibody was obtained from Cell Signaling Technology (Danvers, MA), and the PC2 antibody G20 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Protein kinase inhibitors, ionomycin, hepatocyte growth factor (HGF) and epidermal growth factor (EGF) were from Calbiochem (Nottingham, United Kingdom).
Cell Culture and Transfection
Human embryonic kidney (HEK)-293 and MDCK cells were cultured in DMEM supplemented with 10% fetal bovine serum. Transient transfection was carried out on cells cultured to 90% confluence by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. MDCK I FRT cells (Frohlich et al., 2004) were transfected with the Tet repressor (pcDNA6/TR; Invitrogen) to generate a Flp-In T-Rex line, and several stable clones were selected in media containing blasticidin (5 μg/ml). Clones were screened for transient receptor (TR) activity by transient expression of a firefly luciferase reporter plasmid under the control of a cytomegalovirus/TetO2 promoter and subsequent induction with tetracycline (1 μg/ml). The clone with the highest inducible TR activity was then used to generate tetracycline inducible Flp-In hemagglutinin (HA)-PC2 or HA-PC2 mutant lines by cotransfecting pcDNA5/FRT/TO HA-PC2 and pOG44 Flp recombinase expression plasmids (Invitrogen) followed by selection in media containing hygromycin (100 μg/ml).
Immunoprecipitation and Immunoblotting
Total cell lysates were prepared and processed for immunoprecipitation and Western blotting as described previously (Newby et al., 2002). To assess the effect of serum stimulation on PC2 phosphorylation, stable or transiently transfected cells were seeded into 12-well plates and grown in complete medium to reach 90% confluence. Cells were then cultured in serum-free media for 24 h at 37°C to induce quiescence. Cells were stimulated with EGF (100 ng/ml) for 15 min or serum for 4 h. For inhibitor studies, cells were pretreated with inhibitors (Calbiochem) 4 h before stimulation. Cells were solubilized in detergent lysis buffer (50 mM Tris, 0.14 M NaCl, 1% Triton X-100, and 0.5% NP40) supplemented with Complete protease inhibitors and PhosStop phosphatase inhibitors (Roche Diagnostics, Mannheim, Germany).
λ-Phosphatase Assay
After transfection, cells were cultured for 48 h and lysed in immunoprecipitation (IP) buffer containing Complete protease inhibitors for 1 h at 4°C on a rotator. Dephosphorylation was carried out by incubating 50 μg of total cell lysates with 200 U of λ-protein phosphatase (New England Biolabs, Hitchin, United Kingdom) for 30, 60, and 90 min at 30°C in reaction buffer (50 mM Tris-HCl, 0.1 mM EDTA, 5 mM dithiothreitol, and 0.01% Brij 35 pH 7.5) supplemented with 2 mM MnCl2. Reactions were terminated by addition of an equal volume of 2× sample loading buffer. Lysates were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting as described previously (Newby et al., 2002).
Generation of cDNA Constructs
Site directed mutagenesis was carried out using the QuikChange site-directed mutagenesis kit (Stratagene, Stockport, United Kingdom) according to the manufacturer's instructions. The full-length PKD2 plasmids PKD2Pk and TM4FL have been described previously and were used as templates (Mochizuki et al., 1996; Ong et al., 1999). The PKD2 C-terminal construct (CT2) used in this study was synthesized by cloning the entire PKD2 C terminus (680-968 aa) in frame with the extracellular and membrane-spanning region of pCMUIV-CD8 (Ponnambalam et al., 1994) to generate a CT2-CD8 fusion protein. PAGE-purified mutagenic primers were synthesized by Sigma Genosys (Cambridge, UK); primer sequences are available on request.
Immunofluorescence
Cells were grown on filters or coverslips and fixed with 4% paraformaldehyde for 10 min followed by permeabilization with phosphate-buffered saline (PBS)/0.1% Triton X-100. Blocking was carried out for 1 h with 5% milk powder/PBS, and primary antibodies were incubated overnight at 4°C in 3% bovine serum albumin/PBS. Controls included cells stained with primary antibody omitted, an irrelevant immunoglobulin (Ig)G1 monoclonal antibody (mAb) (Serotec, Kidlington, United Kingdom), or a nonimmune rabbit IgG fraction (Dako UK, Ely, Cambridgeshire, United Kingdom). Antibody binding was visualized using fluorescein isothiocyanate-conjugated goat ant-mouse IgG and Alexa Fluor 568-labeled goat anti-rabbit secondary antibodies. Slides were viewed using an Imaging Systems inverted IX71 microscope (Olympus, Tokyo, Japan) configured for multifluorescence image capture. Images were acquired and analyzed using SimplePCI imaging software (Compix, Hamamatsu, Sewickley, PA).
In Vitro Kinase Assay
A pet32a+ plasmid encoding the PC2 C terminus (700–968 aa) in frame with 6xHis and thioredoxin sequences (Novagen, Nottingham, United Kingdom) was used to generate recombinant fusion protein (Ong et al., 1999). Constructs expressing HIS-tagged fusions were maintained in Escherichia coli strain BL21-DE3 and were induced with 1 mM isopropyl β-d-thiogalactopyranoside for 2 h. The fusion proteins were purified on HIS-Select nickel magnetic beads (Sigma Chemical) as described by the manufacturer, and purified recombinant proteins were visualized on SDS-PAGE gels stained with Coomassie Blue and Western blots stained with antibodies against thioredoxin (Invitrogen). In vitro phosphorylation was carried out on 2 μg of recombinant protein. Proteins were incubated for 10 min at 30°C in protein kinase C (PKC) buffer with [32P]γ-ATP, 3000 Ci/mmol (PerkinElmer Life and Analytical Sciences, Boston, MA) in the presence of rat brain-purified PKC (Millipore, Billerica, MA). Reactions were terminated by the addition of SDS-PAGE loading buffer and separated as described. After electrophoresis, gels were stained with Coomassie Blue, dried, and placed under x-ray film for autoradiography.
2-D Gel Electrophoresis
For two-dimensional electrophoresis, in vitro labeled protein samples were solubilized in IPG rehydration/sample buffer (8 M urea, 2% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate, 50 mM dithiothreitol [DTT], 0.2% Bio-Lyte 3/10 ampholyte, and 0.001% bromphenol blue). The first dimension used ReadyStrip IPG Strips pH 3–10 (Bio-Rad Laboratories, Hemel, Hempstead, United Kingdom), and samples were focused for 50,000 V/h. The strips were removed from the focusing tray and equilibrated in 5 ml of buffer containing 50 mM Tris-HCl, pH 8.8, 6 M urea, 2% SDS, 30% glycerol, and 1% DTT. A second equilibration was carried out in 5-ml volume of the same buffer with 1.5% iodoacetamide substituted for the DTT. After equilibration, the ReadyStrip IPG strip was separated on a 11% SDS-PAGE gel. After second-dimension separation, samples were immunoblotted, and protein was detected using an anti-thio antibody (Invitrogen) as described above.
Small Interfering (siRNA) or Short Hairpin RNA (shRNA) Transfection
Isoform-specific siRNA to human PrKD was chemically synthesized by Ambion (Austin, TX) according to the following sequence: 5′-GGAAGAGAUGUAGCUAUUAtt-3′. A scrambled negative control siRNA (Silencer) was purchased from Ambion. For knockdown of endogenous PC2 in MDCK cells, canine PKD2 targeting and control shRNAs cloned into pSuper vectors were used (gift of M. Kottgen, John Hopkins University, Baltimore, MD) (Wegierski et al., 2009). Transfection of siRNA and shRNA into cells was achieved using Lipofectamine reagent (Invitrogen). Analysis of knockdown was by immunoblotting for PrKD or PC2 48 h posttransfection.
Cell Proliferation Assay
For the proliferation assay, 5000 cells from each cell line were seeded into 12 separate wells of a 96-well plate and cultured for 48 h. Cell proliferation was determined using a colorimetric immunoassay based on the measurement of 5-bromo-2′-deoxyuridine (BrdU) incorporation during DNA synthesis (Roche Diagnostics). The BrdU enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer's instructions. In brief, cells were pulsed with 20 μl/well 100 μM BrdU solution for 2 h. Afterward, the culture medium was removed, and the cells were denatured with FixDenat solution and then incubated for 60 min with 1:100 diluted mouse anti-BrdU mAbs conjugated to peroxidase. After removing antibody conjugate, the substrate solution was added for 10 min. The absorbance was measured within 5 min at 450 nm with a reference wavelength at 690 nm using an ELISA plate reader. The blank corresponded to 200 μl of culture medium with or without BrdU. Each experiment was repeated three times.
Flow Cytometry
MDCK cells were cultured in 12-well dishes overnight, serum starved for 24 h, and then serum stimulated (10% fetal calf serum) for 24 h. Cells were then fixed with cold 70% ethanol, and suspended in propidium iodide (50 μg/ml) containing RNase A (0.1 mg/ml). After incubation at 37°C for 30 min, fluorescence-activated cell sorting (FACS) analysis was performed. Cell cycle stage analysis was carried out to determine the percentage increase in the number of proliferating cells in S/G2 after serum stimulation by using a FlowJo software package (TreeStar, Ashland, OR).
Apoptosis Assays
Apoptosis was induced in MDCK cells by incubating cells for 6 h with 20 μM ceramide or 0.1 μM staurosporine. Dimethyl sulfoxide (DMSO) was used for control treatments. Caspase-3 cleavage was quantified by FACS after incubation with a specific antibody to cleaved caspase-3 (Cell Signaling Technology). Experiments were carried out in triplicate.
Calcium Imaging
Control and stably transfected MDCK I FRT cells were grown in 96-well plates to subconfluent density. Cells were incubated in cell-permeant NW Fluo-4 (Invitrogen) for 30 min at 37°C and 30 min at room temperature according to the manufacturer's instructions in calcium free media. Changes in fluorescence intensity were determined based on observations of 50 randomly selected cells in each experiment. Cells were selected randomly before each experiment and the borders of the entire cell were outlined manually to eliminate any selective contribution from nonuniform distribution of the dye within cells. Dye-loaded cells were excited at 488 nm and increases in intracellular calcium were measured at an emission wavelength of 522 nm. Images were acquired every 10 s. Calcium transients were initiated by the addition of 10 μM ATP. Transient durations were measured from the start of the calcium transients until fluorescence changes returned to the prestimulus background. To confirm that calcium stores in each cell line were equivalent, calcium release from internal stores was measured after ionomycin (1 μM) or after blocking calcium reuptake with thapsigargin (5 μM). Images were acquired using SimplePCI imaging software (Compix, Hamamatsu) and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
Data are presented as mean values ± SEM. Student's t test was used for statistical analysis with a p value of <0.05 indicating statistical significance.
RESULTS
A PC2 Doublet Band Is Due to Phosphorylation at Ser801
Sequence analysis of human PC2 by prediction programs PhosphoBase, DISPHOS 1.3, and NetPhos suggested that it could contain in excess of 100 predicted phosphorylation sites (data not shown). It had been shown previously that PC2 can be constitutively phosphorylated at two residues: Ser76 and Ser812 (Cai et al., 2004; Streets et al., 2006). Phosphorylation analysis was performed by 32P labeling PC2 in transiently transfected HEK-293 cells. Nevertheless, we were unable to eliminate PC2 phosphorylation by mutation of both sites (Streets et al., 2006). These findings led us to hypothesize that PC2 could be phosphorylated on alternative residues.
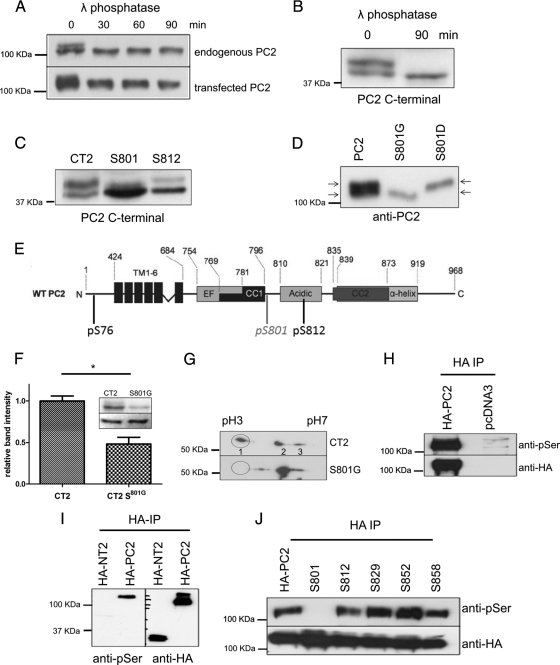
In multiple experiments, we observed that native or exogenous PC2 was often detectable as a doublet band by immunoblotting and hypothesized that the upper band might represent a specific phosphorylated form of PC2 (Figure 1A). Dephosphorylation with the enzyme, λ phosphatase (which removes all phosphate groups from serine, threonine, or tyrosine residues) resulted in loss of the upper band in a time-dependent manner, confirming that the reduction in mobility on SDS-PAGE was indeed the result of phosphorylation (Figure 1A). Phosphorylation at Ser76 has been visualized previously as a doublet band in N-terminal PC2 constructs (Streets et al., 2006). We found that a C-terminal PC2 construct was similarly detectable as a doublet band that could be reduced to a single band after treatment with λ-phosphatase (Figure 1B).
Figure 1.
PC2 is phosphorylated in situ at Ser801. (A) Cell lysates from native mouse kidney cells (M8) and HEK-293 cells transfected with epitope-tagged PC2 (pkTag) were treated with λ-phosphatase for 0 and 60 min and analyzed by Western blotting with PC2- and pkTag-specific antibodies. A clear doublet band is seen in both native and transfected cells (see arrows). The top band disappears after incubation with the enzyme, demonstrating that it is due to phosphorylation. (B) Cell lysates from HEK-293 cells transfected with a C-terminal PKD2 construct were treated with λ-phosphatase for 0 and 90 min and analyzed by Western blotting with PC2-specific antibodies. A clear doublet band is seen. The top band disappears after incubation with the enzyme, demonstrating that it is due to phosphorylation. (C) To confirm the site responsible for the doublet band, single site mutants of CT2 were immunoblotted with a PC2-specific antibody to detect the presence of a phosphorylated banding pattern. Loss of the top phosphorylated band was only seen in cells transfected with the CT2 Ser801 mutant construct and not the Ser812 mutant construct. (D) To confirm the site responsible for the doublet band, single site mutants of PC2 were immunoblotted with an antibody directed to the pkTag epitope tag to detect the presence of a phosphorylated banding pattern. Loss of the top phosphorylated band was only seen in cells transfected with the PC2 Ser801G mutant construct. Conversely, a phosphomimic S801D mutant reduces the electrophoretic mobility of PC2, demonstrating that phosphorylation of Ser801 is sufficient to change the mobility of PC2. (E) Diagram showing the main structural motifs of PC2 relative to the position of previously described phosphorylation sites at Ser76 and Ser812 and the position of the new site at Ser801 reported in this study. (F) Active purified PKC was able to phosphorylate recombinant PC2 C terminus (CT2) in an in vitro kinase assay. The histogram shows that in vitro phosphorylation of the C-terminal of PC2 (CT2) by PKC is reduced by 53% (n = 3) when Ser801 is mutated to Gly (n = 3). Top, autoradiography. Bottom, Coomassie staining showing equal loading of recombinant protein used in the assay. An asterisk indicates a statistically significant difference (p < 0.0065, Student's t test). (G) Two-dimensional gel analysis of phosphorylation of CT2 and CT2 S801G. After an in vitro kinase assay with purified PKC, samples were electrofocused under a pH gradient of 3–10 followed by separation by molecular weight on an 11% SDS-PAGE gel. Proteins were then immunoblotted with an anti-thio antibody. Phosphorylation results in a change in the isoelectric point of CT2. Multiple spots indicate multiple phosphorylation events. Wild-type CT2 separates isoelectrically into three distinct spots. CT2 containing a mutation at Ser801 shows a different pattern of isoelectric separation and has lost the most acidic spot indicating a reduction in overall phosphorylation. (H) A phospho-serine specific antibody (Invitrogen) recognizes HA-PC2 immunoprecipitated from HEK cells. Cell lysates prepared from HA-tagged PC2-expressing HEK cells were immunoprecipitated with an HA antibody. Samples were immunoblotted with an antibody to HA (top) and a phospho-serine antibody (bottom) to detect the presence of a phosphorylated isoform. A clear band was seen with the phospho-serine antibody indicating that PC2 is phosphorylated on serine residues. No signal was seen in mock transfected cells. (I) Immunoblot showing that an anti-phospho-serine antibody (Invitrogen) recognizes the full-length phosphorylated form of PC2 but not serine phosphorylation of the N-terminal domain of PC2 (NT2). (J) To confirm the site responsible for C-terminal phosphorylation, HA-tagged single serine site mutants of PC2 were immunoprecipitated from HEK cell lysates followed by detection with a phospho-serine antibody. Loss of the serine phosphorylated band was only seen in cells transfected with the Ser801 mutant construct. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody.
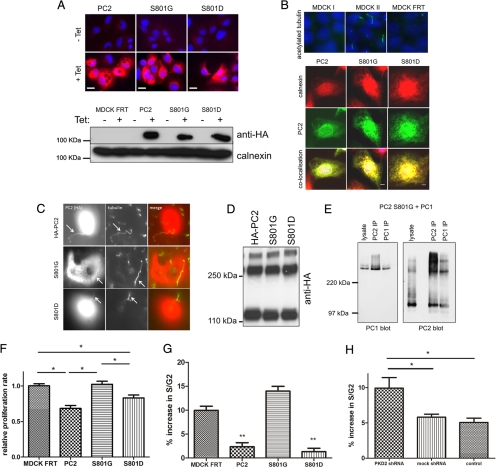
To determine the specific residues responsible for the mobility shift on Western blots, we substituted alanine or glycine at various combinations of five of the highest scoring predicted phosphorylation sites within the C terminus, i.e., Thr721, Ser801, Ser812, Ser831, and Ser943. Mutation of Thr721, Ser812, Ser831, and Ser943 did not alter doublet band formation (data not shown), whereas mutation of Ser801 to Gly801 resulted in complete loss of the upper band in both C-terminal (Figure 1C) and full-length PC2 constructs (Figure 1D). Confirming that phosphorylation at Ser801 was responsible for the mobility shift, a phosphomimic S801D mutation resulted in the complete shift of PC2 to the upper band (Figure 1D). These data suggest that PC2 is constitutively phosphorylated in situ on at least three serine residues (Figure 1E).
Recombinant PC2 C Terminus Can Be Phosphorylated by PKC In Vitro
Ser801 in human PC2 is highly conserved in PC2 orthologues from higher and lower vertebrates, forming part of a consensus PKC recognition sequence and indicating its likely functional significance. To determine whether the C terminus of PC2 could act as a substrate for PKC, an in vitro kinase assay was performed using a recombinant C-terminal PC2 fusion protein (700–968 aa) and rat brain purified PKC, which contains a mixture of PKC isoforms (Figure 1F). Analysis of three separate experiments confirmed that it could be phosphorylated by purified PKC. Mutation of Ser801 decreased (although it did not abolish) phosphorylation by PKC (p < 0.05), implying the existence of other PKC sites.
We confirmed these results by two-dimensional (2-D) gel electrophoresis (Figure 1G). Two-dimensional gel electrophoresis has been successfully used to identify many other phosphorylated proteins. The proteins are first separated in the first dimension by their isoelectric point and then by molecular weight in the second dimension. The 80-Da phosphate does not resolve phosphoproteins from their unphosphorylated counterparts in the molecular weight dimension. However, the negative charge of the phosphate does change the isoelectric point. The result is a “phosphorylation train”—a series of spots on the gel that correspond to the molecular weight of the protein and that are separated in the isoelectric focusing dimension according to the number of associated phosphate groups. Thus, the train also can reflect the existence of multiple phosphorylation events (Duncan and Song, 1999; Zhang et al., 2005). The predicted pI of nonphosphorylated CT2 is pH 5.31. Phosphorylation of CT2 by PKC resulted in the appearance of three distinct spots on 2-D gels. The more acidic, hence more highly phosphorylated, forms are to the left of the gel. Mutation of Ser801 resulted in loss of the most negatively charged spot (highlighted circle) that also can be seen to be at a slightly higher molecular weight, mirroring what we had seen on a one-dimensional (1-D) gel. The finding that mutation of Ser801 does not completely eliminate in vitro phospholabeling of CT2 indicates that additional PKC sites are present.
Phospho-specific Antibody Mapping Identifies PC2 Phosphorylation at Ser801
Although the PC2 doublet band was often detected, it was difficult to reproduce the SDS-PAGE and Western blotting conditions consistently to resolve the small mobility shift on 1-D gels. Taking a parallel approach, we screened FIVE commercial phospho-serine antibodies generated to general phospho-serine consensus sequences for their ability to recognize phosphorylated PC2 after immunoprecipitation. Of the antibodies tested, one (Invitrogen) recognized phosphorylated PC2 (∼110 kDa) from transfected HEK cells (Figure 1H) and native mouse collecting duct cells (see Figure 3A).
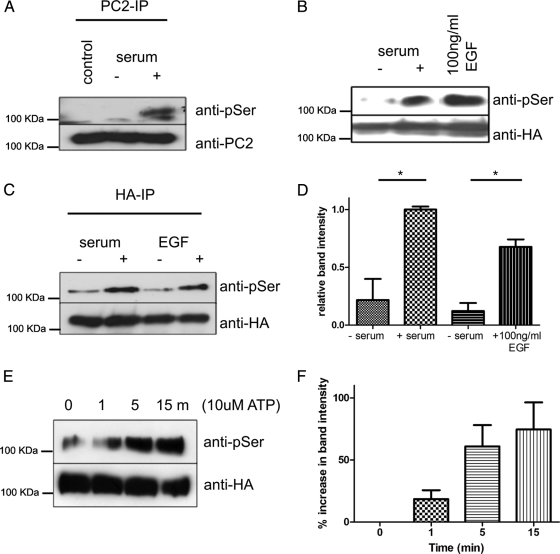
Figure 3.
Stimulation of Ser801phosphorylation in kidney epithelial cells. (A) Phosphorylation of endogenous PC2 at Ser801 after 4-h serum stimulation of serum starved M8 cells. PC2 was immunoprecipitated with an antibody directed to the C-terminal (G20) followed by detection with the phospho-serine antibody. The same blots were reprobed with the PC2 mAb 1A11 and show equal loading. (B) Serum and EGF stimulates Ser801 phosphorylation. HEK cells transiently expressing HA-tagged PC2 were serum starved for 24 h followed by stimulation with serum for 4 h or 100 ng/ml EGF for 15 min. PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. Significant increases in Ser801 phosphorylation were seen in stimulated cells (n = 3). Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (C) MDCK cells stably expressing HA-tagged PC2 were serum starved for 24 h followed by 4-h serum stimulation or 100 ng/ml EGF for 15 min. PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. (D) Significant increases in Ser801 phosphorylation were seen in stimulated cells (n = 3). Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. An asterisk indicates a statistically significant difference (p < 0.02, Student's t test). (E) ATP stimulates Ser801 phosphorylation. HEK cells transiently expressing HA-tagged PC2 were serum starved for 24 h followed by stimulation with 10 μM ATP for 0, 1, 5, and 15 min. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (F) Significant increases in Ser801 phosphorylation were seen in stimulated cells (n = 3) after densitometry of Western blots.
To identify the specific phosphorylated PC2 serine residue detected by this antibody, we first tested an N-terminal PC2 construct (1-223 aa) that can be phosphorylated at Ser76 by GSK3 (Streets et al., 2006). This phospho-serine antibody did not recognize the PC2 N-terminal domain (Figure 1I), suggesting that it might recognize a C-terminal serine residue instead. Using full-length PKD2 cDNAs with specific mutations at the highest predicted C-terminal phosphorylation sites, we found that a Ser801 mutation abolished antibody binding (Figure 1J).
PC2 Is Phosphorylated at Ser801 In Situ by Protein Kinase D
To confirm our in vitro kinase findings suggesting that PC2 Ser801 could be phosphorylated by purified PKC, we examined Ser801 phosphorylation in MDCK cells stably expressing PC2 after pretreatment with a series of PKC inhibitors. Surprisingly, although known PKC inhibitors such as staurosporine and Gö6976 resulted in complete loss of Ser801 phosphorylation, treatment with another common PKC inhibitor, bisindolylmalemide I, had no effect. Similarly, peptide inhibitors to PKCζ, PKA, and calmodulin-dependent protein kinase II (CAMKII; KN93) did not significantly reduce Ser801 phosphorylation (Figure 2B).
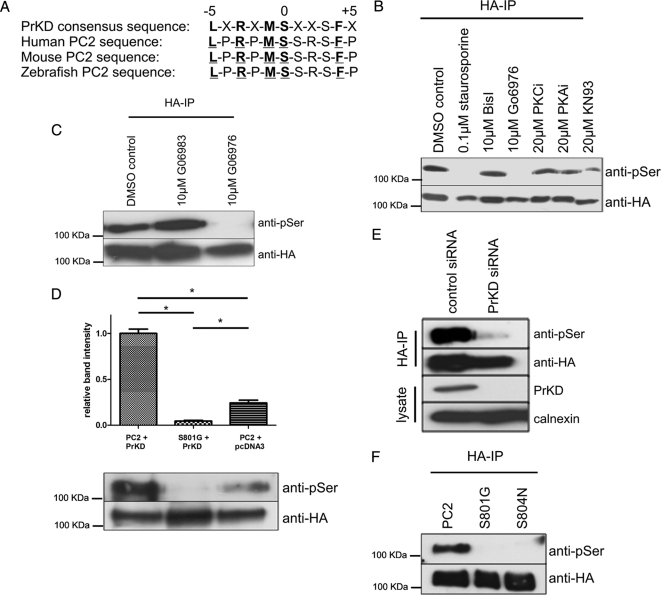
Figure 2.
Protein kinase D phosphorylates PC2 at Ser801. (A) The consensus PrKD phosphorylation motif (bold residues) and the corresponding motif in human (Q13563), mouse (O35245), and zebrafish (Q2VF27) PC2 are shown with the critical amino acids underlined. (B) To identify the kinase responsible for Ser801 phosphorylation, MDCK cells stably expressing HA-tagged PC2 were treated with specific kinase inhibitors. These included 0.1 μM staurosporine (broad-spectrum kinase inhibitor), 10 μM bisindolylmalemide I (BisI; PKC inhibitor), 10 μM Gö6976 (PKC inhibitor), 20 μM PKC peptide inhibitor (PKC inhibitor), 20 μM PKA peptide inhibitor (PKA inhibitor), and 20 μM KN93 (CAMKII inhibitor). PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. Loss of Ser801 phosphorylation was only seen in cells incubated with staurosporine and Gö6976. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (C) MDCK cells stably expressing HA-tagged PC2 were pretreated with DMSO, 10 μM Gö6976, or 10 μM Gö6983 followed by 4-h serum stimulation. PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. Loss of Ser801 phosphorylation was seen only in cells incubated with Gö6976, suggesting that Ser801 could be phosphorylated by PrKD. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (D) Expression of PrKD increases PC2 phosphorylation at Ser801. Cell lysates prepared from HEK cells coexpressing PrKD with HA-tagged PC2 or PC2 Ser801G were immunoprecipitated with an HA antibody. Samples were immunoblotted with an antibody to PC2 (bottom) and a phospho-serine antibody (top) to detect the presence of Ser801 phosphorylation. A significant increase in phosphorylation was seen when PC2 was coexpressed with PrKD compared with PC2 alone. No signal was seen when PC2 Ser801G was coexpressed with PrKD. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. An asterisk indicates a statistically significant difference (p < 0.0001, Student's t test). (E) HEK cells were treated with control siRNAs or with validated siRNA specific to PrKD as described in Materials and Methods. PrKD knockdown was detected after immunoblotting of cell lysates with a PrKD-specific antibody (Santa Cruz Biotechnology). No reduction was seen in a control protein (calnexin). PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. A significant reduction in Ser801 phosphorylation was only seen in cells treated with PrKD-specific siRNA. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (F) Loss of the Ser801-phosphorylated band was seen both in cells transfected with the Ser801 mutant construct as well as a pathogenic mutant S804N. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody.
In view of these findings, we reanalyzed the PKC consensus sequence around Ser801. The consensus recognition sequence of PrKD or PKCμ shows extreme selectivity for Leu at position −5 as well as selectivity for Arg at −3, Met at −1, and Phe at +4 (Nishikawa et al., 1997). This recognition sequence is perfectly preserved around Ser801 in PC2 orthologues from higher and lower vertebrates (Figure 2A). The initial description of PrKD as an atypical isoform of PKC and the inclusion of PrKD/PKCμ in reviews concerning the PKC family, which belongs to the AGC group (named for PKA, PKG, and PKC), contributed to a perception that PrKD belongs to the PKC family. However, it was noted from the outset that the catalytic domain of PrKD has highest sequence homology with myosin light chain kinase and CAMKs. Indeed, the three isoforms of PrKD are now classified as a new protein kinase family within the CAMK group, separate from the AGC group (Wang, 2006).
Because there are no known specific PrKD inhibitors, we compared the differential ability of two staurosporine derivatives, Gö6976 and Gö6983, to inhibit Ser801 phosphorylation. Gö6976 selectively inhibits classical PKC isoforms as well as PrKD, whereas Gö6983 suppresses the activity of all three PKC subgroups (classical, novel, and atypical) but not PrKD (Gschwendt et al., 1996; Ivison et al., 2007). As shown in Figure 2C, treatment with Gö6976 but not Gö6983 inhibited Ser801 phosphorylation, suggesting that Ser801 was phosphorylated by PrKD. Consistent with this notion, bisindolylmalemide I is known to be a potent inhibitor of several PKC isoforms but not of PrKD (Gschwendt et al., 1996).
We further confirmed this result by investigating Ser801 phosphorylation under conditions of PrKD overexpression and knockdown. As shown in Figure 2D, enhanced Ser801 phosphorylation was observed in wild-type PC2 when cotransfected with PrKD; this was not seen in the PC2 Ser801 mutant. Efficient siRNA knockdown of endogenous PrKD in HEK cells also led to the abolition of PC2 Ser801 phosphorylation (Figure 2E). These results indicate that PrKD is the physiological kinase for PC2 Ser801.
Although there are no known pathogenic mutations of PC2 at Ser801, a pathogenic mutation in a PKD2 pedigree resulting in an amino acid substitution of Ser804 to Asn804 has been reported previously (Rossetti et al., 2007). This residue forms part of the consensus binding domain for PrKD. Using full-length PKD2 cDNAs with specific mutations at either Ser801 or S804, we found that a Ser804N mutation also abolished phosphorylation at Ser801 as detected by the phosphoserine antibody (Figure 2F).
PC2 Is Phosphorylated at Ser801 in Response to Growth Factors
PrKD has been shown to be activated after growth factor stimulation in many different studies (Rozengurt et al., 2005; Wang, 2006). We therefore tested whether Ser801 phosphorylation could be altered by serum or EGF stimulation. As shown in Figure 3A, a significant increase in endogenous PC2 Ser801 phosphorylation was detected after serum stimulation in mouse collecting duct kidney epithelial cells (M8) (Newby et al., 2002; Figure 3A). The stimulatory effect of serum and EGF was similarly observed in both transiently transfected HEK cells (Figure 3B) and stably transfected MDCK cells (Figure 3C). ATP has been demonstrated to stimulate calcium induced calcium release through PC2 channels in this study (see Figure 5) as well as others (Giamarchi et al., 2010; Sammels et al., 2010). To determine whether ATP also was able to stimulate phosphorylation of PC2, transiently transfected HEK cells were stimulated with 10 μM ATP for different time intervals from 0 to 60 min (Figure 3E). Immunoblotting with the anti-phospho-serine antibody revealed low levels of phosphorylation of Ser801 at 0 min, which increased by 1 min and reached a peak 15 min after treatment with ATP (Figure 3F) before decreasing to baseline (data not shown).
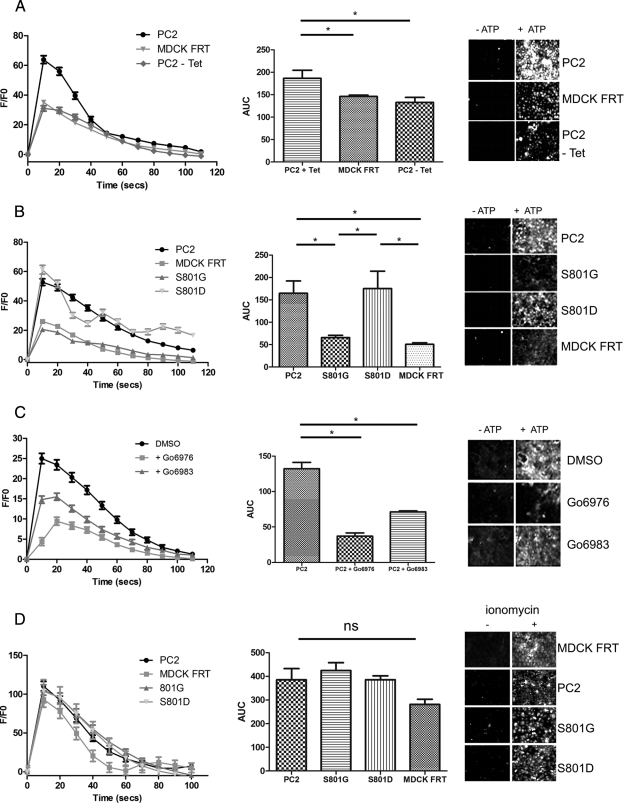
Figure 5.
Ser801 phosphorylation regulates the function of ATP-stimulated PC2-mediated ER Ca2+-release channels. (A) Representative traces of Ca2+ transients elicited by ATP (10 μM) in control MDCK cells (MDCK FRT) and MDCK cells overexpressing PC2 with or without overnight incubation with 1 μg/ml tetracycline shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/F0) (left). The area under the curve (AUC) (middle) of ATP-induced Ca2+ transients was measured in each cell line. Bars represent mean ± SEM for 50 cells. PC2-overexpressing cells differed significantly compared with mock or uninduced cells. The increases in Fluo-4 fluorescence (right) can be seen after ATP stimulation. MDCK cells were loaded with the Fluo-4 dye. All cells respond to ATP stimulus (10 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right). *p < 0.05. (B) Representative traces of Ca2+ transients elicited by ATP (10 μM) in control MDCK cells (MDCK FRT), and MDCK cells overexpressing either PC2, S801G, or S801D shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/F0) (left). The AUC (middle) of ATP-induced Ca2+ transients measured in each cell line. Bars represent mean ± SEM for 50 cells. PC2- and S801D-overexpressing cells differed significantly compared with control and S801G cells (p < 0.05). MDCK cells were loaded with the Fluo-4 dye. All cells respond to ATP stimulus (10 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right). *p < 0.05. (C) Representative traces of Ca2+ transients elicited by ATP (10 μM) in MDCK cells overexpressing PC2 after 2-h pretreatment with PKC inhibitors (10 μM Gö6976/Gö6983) shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/F0) (left). The AUC (middle) of ATP-induced Ca2+ transients measured in each cell line. Bars represent mean ± SEM for 50 cells. Treatment of cells with Gö6976 significantly reduced the Ca2+ transients elicited by ATP compared with control (DMSO-treated) cells. PC2-expressing cells treated with G06983 showed a much smaller decrease in Ca2+-dependent Fluo-4 fluorescence. The increases in Fluo-4 fluorescence (right) can be seen after ATP stimulation. MDCK cells were loaded with the Fluo-4 dye. All cells respond to ATP stimulus (10 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right). *p < 0.05. (D) Representative traces of Ca2+ transients elicited by ionomycin (1 μm) in control MDCK cells (MDCK FRT), and MDCK cells overexpressing either PC2, S801G, or S801D shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/F0) (left). The AUC (middle) of ionomycin-induced Ca2+ transients measured in each cell line. Bars represent mean ± SEM for 50 cells. There was no significant difference between the cell lines. MDCK cells were loaded with the Fluo-4 dye. All cells responded to ionomycin stimulus (1 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right).
Phosphorylation of Ser801 Does Not Alter PC2 Localization in MDCK I Cells
PC2 has been shown to function in several subcellular compartments, and its localization can be dynamically regulated by phosphorylation at two residues, Ser812 and Ser76 (Kottgen et al., 2005; Streets et al., 2006). Of interest, Ser801 lies within a putative sequence important for ER retention (E787-S820) (Cai et al., 1999).
To investigate the effect of Ser801 phosphorylation on PC2 localization in polarized epithelia, we generated MDCK I clones stably expressing epitope-tagged wild-type or Ser801 PC2 under a tetracycline-inducible promoter (Figure 4A). These cells showed low to absent basal HA-PC2 expression that was significantly induced by tetracycline. Staining for a variety of junctional proteins revealed no alterations in desmosomal (desmoplakin), adherens junction (E-cadherin), or tight junction (zona occludens-1) protein localization in the MDCK I FRT parent line or in the TR expression clones (data not shown). Unexpectedly, we could not detect primary cilia in these cells using an antibody to acetylated α-tubulin (Figure 4B). The lack of cilia expression clearly related to its parental origin (MDCK I) rather than further selection because MDCK II cells clearly expressed primary cilia whereas MDCK I cells do not (Figure 4B). In these cells, wild-type HA-PC2 localized completely with the ER protein calnexin with no detectable basolateral surface staining (Figure 4B). Mutagenesis of Ser801 to glycine or aspartate did not alter PC2 ER localization in these cells. In transiently transfected MDCK II cells, wild-type HA-PC2 localized to primary cilia (Figure 4C). Mutagenesis of Ser801 to glycine or aspartate did not alter PC2 cilial localization in these cells.
Figure 4.
Effect of Ser801 phosphorylation on the subcellular localization of PC2, protein–protein interactions, and proliferation. (A) Top, immunofluorescence of stably transfected MDCK cells. Expression of HA-tagged PC2 (red) was induced by the addition of 1 μg/ml tetracycline for 24 h. All lines showed clear inducible expression of PC2. Bar, 25 μm). Bottom, Western blotting demonstrates that protein expression of PC2 and Ser801 phosphorylation mutants were equally induced in the presence of 1 μg/ml tetracycline for 24 h. Equal loading was confirmed using calnexin as a control. (B) Immunofluorescence was carried out on 4% paraformaldehyde-fixed MDCK cells. Top, staining for primary cilia with an antibody to acetylated tubulin in cells grown to 5 d postconfluence on filters. MDCK II expressed well-developed primary cilia but MDCK I and MDCK I FRT showed no detectable primary cilia. Bottom, subcellular localization of PC2 in MDCK cells was not altered after mutation of Ser801 to either glycine (phospho-deficient) or aspartic acid (phospho-mimic). PC2 (green) was predominantly localized to the ER where it colocalized with calnexin (red). (C) Immunofluorescence was carried out on 4% paraformaldehyde-fixed MDCK II cells transiently transfected with either wild-type PC2, S801G, or S801D mutants. Staining for primary cilia with an antibody to acetylated tubulin in cells grown to 5 d postconfluence on filters showed that MDCK II expressed well-developed primary cilia. Cilial localization of PC2 in MDCK II cells was not altered after mutation of Ser801 to either glycine (phospho-deficient) or aspartic acid (phospho-mimic). (D) Immunoblot detection with an antibody to HA-PC2 showed that under nonreducing conditions, PC2 forms two higher molecular weight oligomers. Mutation of Ser801 had no effect on PC2 oligomerization. (E) HEK cells were cotransfected with PC1-FLAG and PC2-Ser801G-pkTag. The blots represent protein immunoprecipitated from 0.5 mg of transient transfected HEK-293 total cell lysate by epitope-tagged (FLAG, or Pk) antibodies. Immunoblot detection with 7e12 (for PC1) or pkTag (for PC2) shows that polycystin complex formation was not disrupted by the S801G mutation. (F) The proliferation rate of mock transfected MDCK cells or cells expressing PC2 or a Ser801 phospho-deficient or phospho-mimic mutation was compared using a BrdU ELISA assay (Roche Diagnostics). There was a significant decrease in proliferation in cells expressing PC2 (n = 9). An asterisk indicates a statistically significant difference (p < 0.02). (G) The percentage increase in cells entering S/G2 phase of the cell cycle was significantly reduced after 24-h serum stimulation in cells expressing both PC2 and a Ser801 phospho-mimic mutation (n = 9). An asterisk indicates a statistically significant difference (p < 0.05). (H) The percentage increase in cells entering S/G2 phase of the cell cycle was significantly increased after knockdown of PKD2 in MDCK cells compared with control cells (n = 6). An asterisk indicates a statistically significant difference (p < 0.05).
Phosphorylation of Ser801 Does Not Alter Homomeric and Heteromeric PC2 Interactions
PC2 has been shown to interact with itself and with PC1 (Newby et al., 2002). Homophilic PC2 interactions have been shown to be important for its function as a homomeric channel as well as a heteromeric channel with PC1 (Feng et al., 2008; Giamarchi et al., 2010). Mutagenesis of Ser801 did not alter the formation of PC2 oligomers (Figure 4D) nor its ability to interact with PC1 by immunoprecipitation (Figure 4E).
Effect of PC2 on Cell Proliferation Is Dependent on Ser801 Phosphorylation
PC2 has been shown previously to regulate cell proliferation by several pathways, including Id2, ERK and eIF2α (Li et al., 2005; Grimm et al., 2006; Liang et al., 2008). In agreement with previous studies, we observed that MDCK I cells expressing PC2 or a phosphomimic Ser801 mutant proliferated more slowly than control cells using a BrDU incorporation assay (Figure 4F). This effect was lost in cells expressing the S801G mutant protein. These findings were complimented by FACS cell cycle analysis. We observed a significant decrease in cells entering the S/G2 phase of the cell cycle after serum stimulation in MDCK I cells expressing PC2 or a phosphomimic Ser801 mutant (Figure 4G). In contrast, MDCK I cells transfected with a PKD2 shRNA to knockdown endogenous PC2 showed a significant increase in the number of cells entering S/G2 compared with mock shRNA or control cells (Figure 4H). Unlike the effect on proliferation, PC2 expression had no effect on basal apoptosis as measured by an FACS-based assay. Similarly, no difference in apoptosis induced by ceramide or staurosporine was observed among PC2, Ser801 mutant, and control cells (data not shown).
Polycystin-2 Phosphorylation at Ser801 Regulates ATP-induced Calcium Release
Phosphorylation of PC2 at Ser812 by CK2 has been reported to regulate the Ca2+ dependence of its channel activity (Cai et al., 2004). Previous studies have shown that the activation of purinergic receptors by ATP is able to stimulate ER Ca2+ release through PC2 channels in an inositol trisphosphate (IP3)-dependent manner (Gallagher et al., 2006; Giamarchi et al., 2010). This has been confirmed in a recent study that reported a functional interaction between the IP3 receptor and PC2 in regulating Ca2+-induced Ca2+ release (Sammels et al., 2010). We therefore used Ca2+ imaging assays to investigate whether Ser801 phosphorylation could alter channel activity in our MDCK I cell lines.
Induction of wild-type PC2 resulted in enhanced amplitude and duration of ATP-induced Ca2+ release from intracellular stores compared with uninduced or control cells (Figure 5A). Mutation of Ser801 to glycine resulted in loss of this enhanced Ca2+ response, whereas the phosphomimic S801D mutation had the same ATP response as wild-type PC2 (Figure 5B).
To determine whether inhibition of PrKD could alter ATP-mediated Ca2+ transients, we pretreated cells expressing wild-type PC2 with PKC inhibitors Gö6976 and Gö6983 (Figure 5C). In agreement with our results showing that Gö6976 but not Gö6983 could block phosphorylation at Ser801 (Figure 2C), pretreatment with Gö6976 resulted in a greater decrease in amplitude of the ATP induced Ca2+ release relative to Gö6983, compared with DMSO-treated controls. The smaller reduction observed with Gö6983 could indicate the importance of other unidentified PKC sites (Figure 1, D and E). These data are consistent with the hypothesis that phosphorylation at Ser801 is essential for the normal function of PC2 as an ER Ca2+ release channel.
Calcium release after 1 μM ionomycin treatment was not significantly different between the cell lines tested confirming the integrity and equivalence of intracellular stores (Figure 4D). Similar results were obtained with thapsigargin (data not shown).
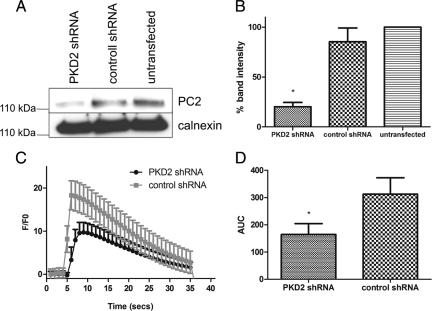
The role of PC2 as an intracellular calcium release channel in these cells was confirmed by knockdown of endogenous PC2 levels in MDCK I cells (Figure 6A). After 48 h, expression of PC2 was reduced by 80% (Figure 6B) and resulted in a significant reduction in the amplitude and duration of ATP induced Ca2+ release from intracellular stores compared with control shRNA-transfected cells (Figure 6, C and D).
Figure 6.
PKD2 knockdown reduces ATP-stimulated PC2 mediated ER Ca2+-release. (A) MDCK I cells were treated with control or PKD2 shRNA as described in Materials and Methods. PKD2 knockdown was detected after immunoblotting of cell lysates with a PC2-specific antibody. Calnexin was used as a loading control. (B) Significant decreases in PC2 expression were seen in PKD2 shRN-transfected cells after densitometry of western blots (n = 3). (C) Representative traces of Ca2+ transients elicited by ATP (10 μM) in MDCK I cells transfected with PKD2 or control shRNA is shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/F0). (D) The area under the curve (AUC) of ATP-induced Ca2+ transients was measured in cell lines transfected with PKD2 or control shRNA. Bars represent mean ± SEM for 50 cells. Cells expressing PKD2 shRNA differed significantly from control cells.
DISCUSSION
In this study, we have identified Ser801 as a previously unrecognized PrKD phosphorylation site in PC2/TRPP2 that is critical for its function as an ER Ca2+ release channel and in mediating downstream effects on cell proliferation. Previous studies had identified functional phosphorylation sites on PC2 at Ser76 (Streets et al., 2006) and Ser812 (Cai et al., 2004). Ser801 represents the third functional residue to be identified in PC2.
Phosphorylation of Ser801 was shown experimentally to be mediated by PrKD or PKCμ. In silico analysis of the sequence inclusive of and surrounding Ser801 show that it perfectly conforms to a PrKD recognition consensus sequence (Nishikawa et al., 1997). Its evolutionary conservation to teleosts further indicates its likely functional importance in kidney morphogenesis. We have also demonstrated that a pathogenic PKD2 mutation (S804N) within the PrKD recognition sequence abolishes Ser801 phosphorylation, indicating its likely physiological significance.
PrKD is a highly versatile protein kinase that is predominantly located in the cytoplasm but can be recruited to perform distinct functions in various cellular compartments (e.g., plasma membrane, nucleus, Golgi, mitochondria) (Rykx et al., 2003). PrKD is recruited to the plasma membrane by locally generated diacylglycerol where it is activated by PKC-mediated phosphorylation (mainly novel isoforms). In other organelles, alternative activation pathways (such as nonreceptor tyrosine kinases, Gβγ subunit binding and caspase cleavage) have been described previously (Wang, 2006).
PrKD has low constitutive activity but is known to be activated after growth factor stimulation in many different cell types (Rozengurt et al., 2005; Wang, 2006). Consistent with this, phosphorylation of Ser801 was significantly increased by serum and EGF stimulation in our study. In a previous article, EGF was reported to inhibit plasma membrane PC2 channel activity through a phosphatidylinositol bisphosphate-dependent mechanism independent of PC2 phosphorylation (Ma et al., 2005). The phosphorylation-dependent regulation of channel activity that we describe thus represents a distinct mechanism for EGF in regulating function of the PC2 ER channel. EGF itself did not directly stimulate ER Ca2+ release in our assays (data not shown). Nonetheless, the time course of these events is very different (seconds vs. minutes to hours), suggesting that EGF or serum stimulation is permissive for PC2 channel activity. Similarly, ATP stimulated Ser801 phosphorylation over a similar time course to EGF, but this effect is clearly different to its acute effect on Ca2+ release.
Activation of PrKD by PKC-dependent phosphorylation of activation loop serines within the kinase domain is its major mode of activation, although PKC-independent activation also has been described (Brandlin et al., 2002; Lemonnier et al., 2004; Chen et al., 2005). The inability of the PKC inhibitor Gö6983 to block Ser801 phosphorylation in our study may indicate that PrKD is activated independently of PKC. Future work will seek to examine the regulation of this pathway in more detail.
Our results also provide an alternative explanation for the link between growth factor stimulation and PC2 activity in the regulation of cell proliferation in MDCK I cells. Pkd2 heterozygous kidneys display a significantly increased basal and stimulated tubular proliferative index (Chang et al., 2006; Prasad et al., 2009), and PC2 overexpression has been shown to inhibit cell proliferation and morphogenesis in vitro (Li et al., 2005; Grimm et al., 2006; Liang et al., 2008). Our results are consistent with these observations but extend them by showing the dependence of these effects on Ser801 phosphorylation. The effect seemed to be specific because there was no alteration in basal or stimulated apoptosis. A recent article has suggested that PC2 mediates a passive ER channel leak and that PC2 dosage could regulate sensitivity to certain apoptotic stimuli (Wegierski et al., 2009). We were unable to reproduce these findings in MDCK I cells. An unexpected finding was that MDCK I cells do not express primary cilia. It remains to be determined whether Ser801 phosphorylation of PC2 similarly controls cell growth in ciliated cells. Certainly, it does not alter the cilia localization of exogenous PC2 in MDCK II cells.
Previous studies have shown that PC2 can function as an ER Ca2+ release channel but did not distinguish between cilia-dependent and independent Ca2+ release (Koulen et al., 2002). By using MDCK I cells that lack cilia, we were able to study ER channel activity in isolation from cilia-dependent Ca2+ release. MDCK I cells are thought to be of cortical collecting duct cell origin based on their high electrical resistance (Barker and Simmons, 1981; Richardson et al., 1981). In addition, they also can spontaneously initiate tubulogenesis in 3-D culture, unlike MDCK II cells (Hellman et al., 2005). However, this is the first report of a difference in primary cilia expression between MDCK I and II cells.
We confirmed previous studies showing that PC2 mediated Ca2+ release from the ER can be stimulated by ATP (Gallagher et al., 2006; Giamarchi et al., 2010). Ser801 lies in the linker region between a predicted EF-hand domain and the recently reported coiled-coil domain essential for C-terminal homodimerization (Giamarchi et al., 2010). Phosphorylation at Ser801 seems to be permissive for this activity without altering the subcellular localization nor homophilic and heterophilic (with PC1) interactions of wild-type PC2. This function seems to be distinct from that of Ser812 phosphorylation that regulates the Ca2+ sensitivity of PC2 Ca2+ channels, possibly through regulating PC2–IP3 receptor interactions (Koulen et al., 2002; Sammels et al., 2010). Perhaps Ser801 phosphorylation may alter the conformation of this region to bind other regulatory proteins necessary for PC2 function. Future work will seek to investigate this possibility.
ACKNOWLEDGMENTS
We thank Linda Newby for technical assistance and M. Kottgen, N. Simmons, S. Ponnambalam, S. Somlo, G. Wu, O. Frohlich, and K. Pfizenmaier for kind gifts of reagents. This work was funded by the Wellcome Trust, Research Councils (UK), the Medical Research Council and the Sheffield Kidney Research Foundation. A.J.S. is a Research Councils UK Academic Fellow, A.J.N. is in receipt of a Medical Research Council Ph.D. studentship, and A.C.M.O. is a Wellcome Trust Research Leave Senior Fellow.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-04-0377) on September 29, 2010.
REFERENCES
- Ahmmed G. U., Mehta D., Vogel S., Holinstat M., Paria B. C., Tiruppathi C., Malik A. B. Protein kinase Cα phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J. Biol. Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- Barker G., Simmons N. L. Identification of two strains of cultured canine renal epithelial cells (MDCK cells) which display entirely different physiological properties. Q. J. Exp. Physiol. 1981;66:61–72. doi: 10.1113/expphysiol.1981.sp002529. [DOI] [PubMed] [Google Scholar]
- Brandlin I., et al. Protein kinase C (PKC)η-mediated PKCμ activation modulates ERK and JNK signal pathways. J. Biol. Chem. 2002;277:6490–6496. doi: 10.1074/jbc.M106083200. [DOI] [PubMed] [Google Scholar]
- Cai Y., et al. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J. Biol. Chem. 2004;279:19987–19995. doi: 10.1074/jbc.M312031200. [DOI] [PubMed] [Google Scholar]
- Cai Y., Maeda Y., Cedzich A., Torres V. E., Wu G., Hayashi T., Mochizuki T., Park J. H., Witzgall R., Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J. Biol. Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Grantham J. J. The genetics and physiology of polycystic kidney disease. Semin. Nephrol. 2001;21:107–123. doi: 10.1053/snep.2001.20929. [DOI] [PubMed] [Google Scholar]
- Chang M. Y., Parker E., Ibrahim S., Shortland J. R., Nahas M. E., Haylor J. L., Ong A. C. Haploinsufficiency of Pkd2 is associated with increased tubular cell proliferation and interstitial fibrosis in two murine Pkd2 models. Nephrol. Dial. Transplant. 2006;21:2078–2084. doi: 10.1093/ndt/gfl150. [DOI] [PubMed] [Google Scholar]
- Chen J., Lu G., Wang Q. J. Protein kinase C-independent effects of protein kinase D3 in glucose transport in L6 myotubes. Mol. Pharmacol. 2005;67:152–162. doi: 10.1124/mol.104.004200. [DOI] [PubMed] [Google Scholar]
- Duncan R. F., Song H. J. Striking multiplicity of eIF4E-BP1 phosphorylated isoforms identified by 2D gel electrophoresis regulation by heat shock. Eur. J. Biochem. 1999;265:728–743. doi: 10.1046/j.1432-1327.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- Feng S., Okenka G. M., Bai C. X., Streets A. J., Newby L. J., DeChant B. T., Tsiokas L., Obara T., Ong A. C. Identification and functional characterization of an N-terminal oligomerization domain for polycystin-2. J. Biol. Chem. 2008;283:28471–28479. doi: 10.1074/jbc.M803834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich O., Klein J. D., Smith P. M., Sands J. M., Gunn R. B. Urea transport in MDCK cells that are stably transfected with UT-A1. Am. J. Physiol. Cell Physiol. 2004;286:C1264–C1270. doi: 10.1152/ajpcell.00499.2003. [DOI] [PubMed] [Google Scholar]
- Gallagher A. R., et al. A truncated polycystin-2 protein causes polycystic kidney disease and retinal degeneration in transgenic rats. J. Am. Soc. Nephrol. 2006;17:2719–2730. doi: 10.1681/ASN.2005090979. [DOI] [PubMed] [Google Scholar]
- Giamarchi A., et al. A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J. 2010;29:1176–1191. doi: 10.1038/emboj.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D. H., Karihaloo A., Cai Y., Somlo S., Cantley L. G., Caplan M. J. Polycystin-2 regulates proliferation and branching morphogenesis in kidney epithelial cells. J. Biol. Chem. 2006;281:137–144. doi: 10.1074/jbc.M507845200. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Dieterich S., Rennecke J., Kittstein W., Mueller H. J., Johannes F. J. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- Guinamard R., Chatelier A., Lenfant J., Bois P. Activation of the Ca(2+)-activated nonselective cation channel by diacylglycerol analogues in rat cardiomyocytes. J. Cardiovasc. Electrophysiol. 2004;15:342–348. doi: 10.1046/j.1540-8167.2004.03477.x. [DOI] [PubMed] [Google Scholar]
- Hanaoka K., Qian F., Boletta A., Bhunia A. K., Piontek K., Tsiokas L., Sukhatme V. P., Guggino W. B., Germino G. G. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Hellman N. E., Greco A. J., Rogers K. K., Kanchagar C., Balkovetz D. F., Lipschutz J. H. Activated extracellular signal-regulated kinases are necessary and sufficient to initiate tubulogenesis in renal tubular MDCK strain I cell cysts. Am. J. Physiol. Renal Physiol. 2005;289:F777–F785. doi: 10.1152/ajprenal.00429.2004. [DOI] [PubMed] [Google Scholar]
- Ivison S. M., Graham N. R., Bernales C. Q., Kifayet A., Ng N., Shobab L. A., Steiner T. S. Protein kinase D interaction with TLR5 is required for inflammatory signaling in response to bacterial flagellin. J. Immunol. 2007;178:5735–5743. doi: 10.4049/jimmunol.178.9.5735. [DOI] [PubMed] [Google Scholar]
- Kottgen M., et al. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P., Cai Y., Geng L., Maeda Y., Nishimura S., Witzgall R., Ehrlich B. E., Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Laycock S., Taylor H. C., Haigh C., Lee A. T., Cooper G. J., Ong A. C., Robson L. A novel dephosphorylation-activated conductance in a mouse renal collecting duct cell line. Exp. Physiol. 2009;94:914–927. doi: 10.1113/expphysiol.2009.047753. [DOI] [PubMed] [Google Scholar]
- Lemonnier J., Ghayor C., Guicheux J., Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J. Biol. Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- Li Q., Dai Y., Guo L., Liu Y., Hao C., Wu G., Basora N., Michalak M., Chen X. Z. Polycystin-2 associates with tropomyosin-1, an actin microfilament component. J. Mol. Biol. 2003;325:949–962. doi: 10.1016/s0022-2836(02)01333-5. [DOI] [PubMed] [Google Scholar]
- Li X., Luo Y., Starremans P. G., McNamara C. A., Pei Y., Zhou J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat. Cell Biol. 2005;7:1102–1112. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
- Liang G., Yang J., Wang Z., Li Q., Tang Y., Chen X. Z. Polycystin-2 down-regulates cell proliferation via promoting PERK-dependent phosphorylation of eIF2alpha. Hum. Mol. Genet. 2008;17:3254–3262. doi: 10.1093/hmg/ddn221. [DOI] [PubMed] [Google Scholar]
- Ma R., Li W. P., Rundle D., Kong J., Akbarali H. I., Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol. Cell Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- Nauli S. M., et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Newby L. J., Streets A. J., Zhao Y., Harris P. C., Ward C. J., Ong A. C. Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J. Biol. Chem. 2002;277:20763–20773. doi: 10.1074/jbc.M107788200. [DOI] [PubMed] [Google Scholar]
- Nilius B., Prenen J., Tang J., Wang C., Owsianik G., Janssens A., Voets T., Zhu M. X. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J. Biol. Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Toker A., Johannes F. J., Songyang Z., Cantley L. C. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J. Biol. Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- Ong A. C., Harris P. C. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005;67:1234–1247. doi: 10.1111/j.1523-1755.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- Ong A. C., Ward C. J., Butler R. J., Biddolph S., Bowker C., Torra R., Pei Y., Harris P. C. Coordinate expression of the autosomal dominant polycystic kidney disease proteins, polycystin-2 and polycystin-1, in normal and cystic tissue. Am. J. Pathol. 1999;154:1721–1729. doi: 10.1016/S0002-9440(10)65428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnambalam S., Rabouille C., Luzio J. P., Nilsson T., Warren G. The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J. Cell Biol. 1994;125:253–268. doi: 10.1083/jcb.125.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., McDaid J. P., Tam F. W., Haylor J. L., Ong A. C. Pkd2 dosage influences cellular repair responses following ischemia-reperfusion injury. Am. J. Pathol. 2009;175:1493–1503. doi: 10.2353/ajpath.2009.090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. C., Scalera V., Simmons N. L. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim. Biophys. Acta. 1981;673:26–36. [PubMed] [Google Scholar]
- Rossetti S., et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rey O., Waldron R. T. Protein kinase D signaling. J. Biol. Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- Rykx A., De Kimpe L., Mikhalap S., Vantus T., Seufferlein T., Vandenheede J. R., Van Lint J. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–86. doi: 10.1016/s0014-5793(03)00487-3. [DOI] [PubMed] [Google Scholar]
- Sammels E., Devogelaere B., Mekahli D., Bultynck G., Missiaen L., Parys J. B., Cai Y., Somlo S., De Smedt H. Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J. Biol. Chem. 2010;285:18794–18805. doi: 10.1074/jbc.M109.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streets A. J., Moon D. J., Kane M. E., Obara T., Ong A. C. Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum. Mol. Genet. 2006;15:1465–1473. doi: 10.1093/hmg/ddl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres V. E., Harris P. C. Mechanisms of Disease: autosomal dominant and recessive polycystic kidney diseases. Nat. Clin. Pract. Nephrol. 2006;2:40–55. doi: 10.1038/ncpneph0070. quiz 55. [DOI] [PubMed] [Google Scholar]
- Wang Q. J. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol. Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wegierski T., Steffl D., Kopp C., Tauber R., Buchholz B., Nitschke R., Kuehn E. W., Walz G., Kottgen M. TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 2009;28:490–499. doi: 10.1038/emboj.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. D. Polycystic kidney disease: new understanding in the pathogenesis. Int. J. Biochem. Cell Biol. 2004;36:1868–1873. doi: 10.1016/j.biocel.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Yao X., Kwan H. Y., Huang Y. Regulation of TRP channels by phosphorylation. Neurosignals. 2005;14:273–280. doi: 10.1159/000093042. [DOI] [PubMed] [Google Scholar]
- Zhang W., Bergamaschi D., Jin B., Lu X. Posttranslational modifications of p27kip1 determine its binding specificity to different cyclins and cyclin-dependent kinases in vivo. Blood. 2005;105:3691–3698. doi: 10.1182/blood-2003-07-2558. [DOI] [PubMed] [Google Scholar]