Signal transducers and activators of transcription (STAT)1 is the key to the sequential control of Madin-Darby canine kidney tubulogenesis. Loss of STAT1 prevents redifferentiation. Constitutively active STAT1 is sufficient to restore cord formation but not mature lumens. These data suggest that STAT1 is necessary for the redifferentiation phase of tubulogenesis and that mature lumenogenesis requires a distinct signal.
Abstract
Tubule formation in vitro using Madin-Darby canine kidney (MDCK) epithelial cells consists mainly of two processes. First, the cells undergo a partial epithelial–mesenchymal transition (pEMT), losing polarity and migrating. Second, the cells redifferentiate, forming cords and then tubules with continuous lumens. We have shown previously that extracellular signal-regulated kinase activation is required for pEMT. However, the mechanism of how the pEMT phase is turned off and the redifferentiation phase is initiated is largely unknown. To address the central question of the sequential control of these two phases, we used MDCK cells grown as cysts and treated with hepatocyte growth factor to model tubulogenesis. We show that signal transducer and activator of transcription (STAT)1 controls the sequential progression from the pEMT phase to the redifferentiation phase. Loss of STAT1 prevents redifferentiation. Constitutively active STAT1 allows redifferentiation to occur even when cells are otherwise prevented from progressing beyond the pEMT phase by exogenous activation of Raf. Moreover, tyrosine phosphorylation defective STAT1 partially restored cord formation in such cells, suggesting that STAT1 functions in part as nonnuclear protein mediating signal transduction in this process. Constitutively active or inactive forms of STAT1 did not promote lumen maturation, suggesting this requires a distinct signal.
INTRODUCTION
Many internal metazoan organs consist of tubes that are lined by a layer of polarized epithelial cells. How such tubes are formed is a key issue in cell and developmental biology. Tubes in different organs, such as kidney, lung, mammary gland, vasculature, and Drosophila trachea, form by a wide variety of mechanisms. It is likely that common principles underlie this diversity (Hogan and Kolodziej, 2002; O'Brien et al., 2002; Lubarsky and Krasnow, 2003; Bryant and Mostov, 2008). To discover these principles, we are using an organotypic tissue culture model system in which Madin-Darby canine kidney (MDCK) cells are cultured in three-dimensional (3D) gels of collagen I. Treatment of these cysts with hepatocyte growth factor (HGF) causes the cysts to elaborate tubules (Montesano et al., 1991a,b), which resemble tubes normally seen in the kidney and many other organs.
We and others have used this system to dissect the basic cell and molecular mechanisms involved in tubulogenesis. Previously, we defined four morphogenetic stages of HGF-induced MDCK tubulogenesis: First, some cells send long extensions of their basolateral surface into the surrounding extracellular matrix ([ECM]; Pollack et al., 1998). Second, cells move centrifugally out from the cysts to form chains of cells, typically one to three cells long. Because these cells have lost polarity and become migratory, we refer to steps 1 and 2 together as the partial epithelial–mesenchymal transition (pEMT) phase (O'Brien et al., 2002). Third, cells form cords, which are two to three cells thick. Some cells in cords start to repolarize, forming small lumens between cells. Fourth, these lumens enlarge and become continuous with the central lumen of the cyst, thereby forming mature tubules. We refer to steps 3 and 4 collectively as the redifferentiation phase (O'Brien et al., 2002).
HGF acts through c-Met that in turn signals through several intracellular pathways, including the Raf-mitogen-activated protein kinase (MEK)–extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase cascade. We reported previously that we could bypass HGF and c-MET by activating Raf-1 for 24 h (O'Brien et al., 2004). We used an inducible Raf-1 kinase, EGFPΔRaf-1:ER (Raf:ER), in which the estrogen receptor ligand binding domain (ER) and enhanced green fluorescent protein (EGFP) are fused to the Raf-1 kinase domain. Binding of the estrogen analog 4-hydroxytamoxifen (4HT) activates the Raf-1 domain. Activation of Raf-1 for up to 24h causes some cells in cysts to initially send out extensions and then form chains, mimicking the pEMT phase of HGF-induced tubulogenesis. If 4HT is removed at 24 h, inactivating the Raf:ER, cords and then mature tubules form over the next 48 h, indicating that the redifferentiation phase occurs. In contrast, prolonged activation of Raf-1 by treatment with 4HT for >24 h causes the cells to dissociate and migrate.
Signal transducers and activators of transcription (STATs) were originally identified as the critical mediators of cytokine responses and subsequently implicated in diverse external stimuli-initiated cellular signaling. STAT1 has been implicated in antiproliferative and proapoptotic processes. Developmentally, STAT1 has been implicated in the remodeling of the mammary gland during involution (Liu et al., 1996). In endothelial cells, STAT1 was reported to negatively regulate proliferation and tube formation (Battle et al., 2006). An additional role of STAT1 not only as a transcription factor but also as a protein in the cytoplasm has been studied (Stancato et al., 1998). STAT1 and ERK have been shown to coimmunoprecipitate (David et al., 1995) and behave antagonistically (Caraglia et al., 2004; Wang and Koromilas, 2009).
In this study, we dissected the two phases of MDCK tubulogenesis and identified STAT1 as a key component that is required specifically for the redifferentiation phase. We showed that the knockdown of STAT1 by short hairpin RNA (shRNA) impairs HGF-induced MDCK tubulogenesis. STAT1 is down-regulated by Raf-1 activation. If Raf is activated for >24 h to cause cell migration and dissociation, the presence of tyrosine phosphorylation defective STAT1 partially restores cord formation, but constitutively active STAT1 is sufficient to restore cord formation, although not mature lumens. Only small, discontinuous lumens form, rather than the large, single lumen characteristic of normal tubules. These data suggest that STAT1 is necessary for the late, redifferentiation phase of tubulogenesis and that mature lumenogenesis requires an even later, distinct signal.
MATERIALS AND METHODS
Antibodies and Reagents
Type I collagen was from Cohesion Technologies. Primary antibodies were mouse anti-STAT1, mouse anti-phospho-STAT1 (Y701), mouse anti-Raf-1 (BD Biosciences, San Jose, CA); mouse anti-STAT1, rabbit anti-phospho-STAT1 (Y701) (Cell Signaling Technology, Danvers, MA); rabbit anti-ERK1/2, mouse anti-phosphoERK1/2, mouse anti-FLAG (Santa Cruz Biotechnology, Santa Cruz, CA); and mouse anti-glyceraldehyde-3-phosphate dehydrogenase ([GAPDH] Millipore Bioscience Research Reagents, Temecula, CA). Secondary antibodies used for immunoblotting was rabbit anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA) and for confocal microscopy: goat anti-chicken Alexa Fluor 488. Actin filaments were stained with Alexa Fluor 546 phalloidin (Invitrogen, Carlsbad, CA). Nuclei were stained with Hoechst 33342 (Invitrogen).
Cell Culture
MDCK cells were maintained in minimal essential medium with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml streptomycin. EGFPΔRaf-1:ER cells (DD32 or YY34) have been maintained as described previously (Hansen et al., 2000). MDCK or Raf:ER cysts were treated with 10 ng/ml HGF (gift of R. Schwall, Genentech, South San Francisco, CA). To induce Raf:ER activity, Raf:ER-transfected cysts were treated with 1 μM 4HT (Calbiochem, San Diego, CA).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNAs were extracted from collagen containing cysts using the RNeasy Mini kit (QIAGEN, Valencia, CA). One microgram of total RNA was reverse transcribed by SuperScript III RT (Invitrogen). The yield of cDNA was normalized by GAPDH as an internal standard. Primers used were as follows: for STAT1: forward primer, 5′-ATGGAAATCAGACAGTACCT-3′ and reverse primer, 5′-TGGTGAAATTGCAAGAGCTG-3′; and for GAPDH: forward primer, 5′-CAGTTGTGGATCTGACCTGC-3′ and reverse primer, 5′-CCTTGGAGGCCATGTAGACC-3′.
Immunoblotting and Immunofluorescence Analyses
For STAT1 experiments, cysts were processed as described previously (Kim et al., 2007). For ERK experiments, total cell lysates were immunoprecipitated with anti-total ERK and then simultaneously immunoblotted for total ERK and phospho-ERK. Blots were analyzed by Odyssey (LI-COR Biosciences, Lincoln, NE). Cysts and tubules were processed for immunofluorescence and analyzed on a LSM 510 confocal fluorescence microscopes (Carl Zeiss, Thornwood, NY) as described previously (Kim et al., 2007).
Overexpession of a Constitutively Active Form of STAT1
CA-STAT1 was FLAG-tagged by introducing Cys residues into Ala-656 and Asn-658 in the SH2 domain, a gift from Dr. T. Ouchi (Mount Sinai School of Medicine, New York, NY). FLAG-tagged Y701F-STAT1 construct, in which Tyr701 was changed to Phe, was obtained from Addgene (Cambridge, MA). After transfection, stably overexpressing cells were cloned.
Inhibition of STAT1 by shRNA
To generate a shRNA of STAT1, a 97mer oligonucleotide sequence containing specifically the canine STAT1, 5′-CACCCAGAAAGCCCTGATTAAT-3′, was synthesized by Sigma-Aldrich (St. Louis, MO) and cloned into GFP-pPRIME. Virus containing media was used to infect MDCK cells. Efficiency of infection was checked by examining expression of green fluorescent protein (GFP). Positive clones were selected.
RESULTS
Depletion of STAT1 Blocks HGF-induced Tubulogenesis
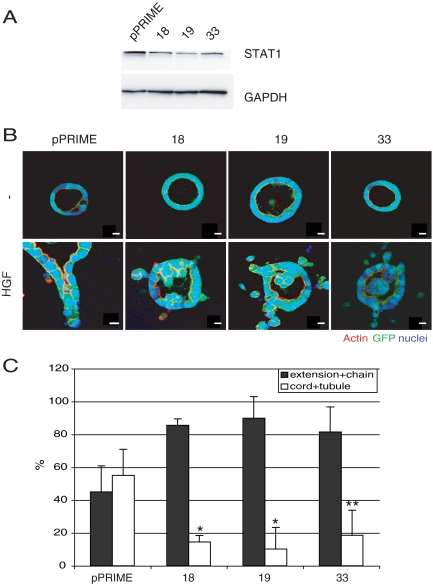
To address the question of the sequential control of MDCK tubulogenesis, we knocked down (KD) STAT1 in MDCK cells by using the pPRIME shRNA vector. Several clones with efficient (∼60%) KD were selected and the levels of STAT1 in the cells were determined by immunoblotting (Figure 1A). A control for nonspecific KD is also shown (pPRIME), as is the level of GAPDH in each clone. Figure 1B shows typical morphological responses of cysts of these clones to treatment with HGF for 72 h. Responses are quantified in Figure 1C, showing the percentage of cysts forming extensions/chains, or cords/tubules for control and the three KD clones. Although ∼40% of the control cells exhibited cords/tubules in response to HGF, only 5–17% of the KD cysts formed cords and tubules and instead had a corresponding increase in extensions and chains. We conclude that STAT1 is required for the redifferentiation phase of HGF-induced tubulogenesis but not for the earlier pEMT phase.
Figure 1.
Inhibition of STAT1 retards HGF-induced MDCK tubulogenesis. (A) Expression level of STAT1 in control (pPRIME) and STAT1 knockdown lines (18, 19, and 33) was compared by immunoblot with anti-STAT1 antibody. (B) Control cells or STAT1 knockdown cells were grown as cysts and either left untreated (top row) or treated with HGF for 72 h (bottom row). Inhibition of STAT1 blocks the HGF-induced redifferentiation phase. The GFP represents GFP-pPRIME transfectant. Bar, 10 μm. (C) Quantification of tubular structures in the presence of HGF. One hundred GFP-positive structures per sample were counted, and values are the mean ± SEM of three independent experiments. *p < 0.01 and **p < 0.05.
Raf:ER Activation Suppresses STAT1
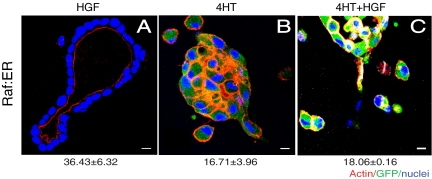
We found previously that treatment of cysts with HGF leads to prolonged activation (≥72 h) of the MAP kinase pathway (O'Brien et al., 2004). Direct activation of this pathway by 4HT in Raf:ER leads to cell dissociation, whereas activation via HGF leads first to pEMT and then to redifferentiation and tubule formation. Why does HGF activation of the MAP kinase pathway eventually lead to redifferentiation, whereas activation over the same time period by Raf:ER leads instead to cell dissociation? One possibility is that HGF activates another pathway(s) that prevents cell dissociation, perhaps by reversing the pEMT after 24 h and thereby allowing redifferentiation to occur. To test this possibility we treated Raf:ER cysts for 72 h with HGF alone, 4HT alone or a combination of HGF and 4HT (Figure 2). As expected, HGF alone caused Raf:ER cells to form tubules, whereas 4HT alone gave scattered cells. The combination of HGF plus 4HT gave scattered cells but not tubules. This result suggested that HGF does not simply activate a pathway(s) that opposes the action of activated Raf-1, as redifferentiation did not occur.
Figure 2.
Redifferentiation is not promoted by (additional) HGF treatment in activated Raf:ER cells. Raf:ER cysts were treated with 4HT alone for 48 h, and HGF was added or not for additional 24 h. (A) In the absence of 4HT, HGF-induced cysts formed tubules with mature lumens. (B) Cysts treated with 4HT alone for 72 h exhibited cell dispersion. GFP fluorescence reflects Raf:ER induction. (C) Treatment with HGF plus 4HT failed to complete tubule development. Quantification of cysts with tubule(s) (bottom) (mean ± SEM, n = 3). Bar, 10 μm.
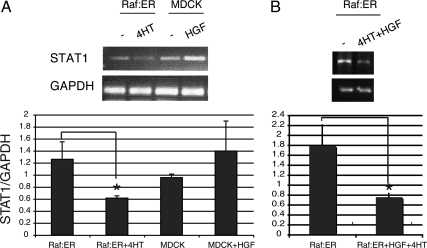
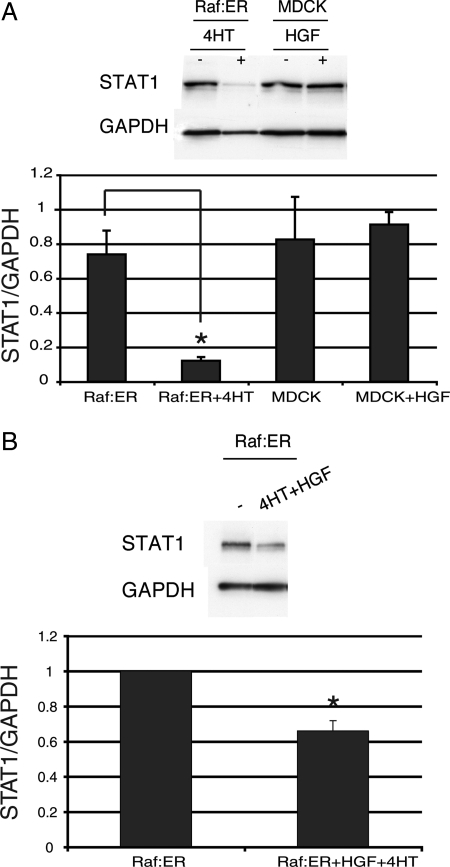
Interestingly, the inability of Raf:ER tubules to proceed into redifferentiation resembles the phenotype of STAT1-knockdown tubules. We thus considered the effect of HGF or 4HT on the level of STAT1. Figure 3A shows the levels of STAT1 mRNA (as indicated by RT-PCR; quantified in Figure 3, bottom). Compared with untreated MDCK cells (Figure 3, lane 3), treatment with HGF alone did not give a statistically significant change in the level of STAT1 mRNA (lane 4). Untreated Raf:ER cells had a similar level of STAT1 mRNA (lane 1). However, in Raf:ER cysts treated with 4HT for 72 h, STAT1 was down-regulated (lane 2). Similarly, the combined treatment of HGF and 4HT results in a statistically significant decrease of STAT1 (Figure 3B), which is consistent with a result in Figure 2. In accordance with this, we observed that the protein level of STAT1 was also significantly decreased when Raf:ER cells were stimulated with 4HT or a combination of 4HT and HGF (Figure 4, A and B, compare lanes 1 and 2, quantified in Figure 4, bottom). These suggest that the down-regulation of STAT1 by activation of Raf:ER may be responsible for the tendency of Raf:ER cells to scatter rather than proceed into redifferentiation and form mature tubules. Furthermore, the level of tyrosine phosphorylated STAT1 was unchanged during HGF-induced tubulogenesis at the time points of 24, 48, and 72 h and no nuclear accumulation of STAT1 was detected (Supplemental Figure S1, A and B). Two plausible interpretations of this result are that the basal activity of STAT1 is sufficient to allow MDCK cells to redifferentiate during tubulogenesis or alternatively that STAT1 functions in a nonnuclear signaling mode, as explained below.
Figure 3.
STAT1 mRNA is down-regulated in activated Raf:ER cells. (A) MDCK or Raf:ER cysts grown in collagen were treated with either HGF or 4HT. (B) Raf:ER cysts were treated with both HGF and 4HT for 72 h. STAT1 mRNA levels were analyzed by RT-PCR using GAPDH as an internal control (mean ± SEM, n = 3). *p < 0.05.
Figure 4.
STAT1 is down-regulated in activated Raf:ER cells. (A) Protein levels of STAT1 were assayed in either 48-h HGF-treated MDCK or 4HT-treated Raf:ER cysts by immunoblot. (B) Raf:ER cysts treated with the combination of 4HT and HGF were lysed and blotted for STAT1. GAPDH was used as a loading control. The relative decrease of STAT1 is shown (mean ± SEM, n = 3). *p < 0.05.
Constitutively Active STAT1 Rescues the Block in Cord Formation Caused by Raf:ER Activation
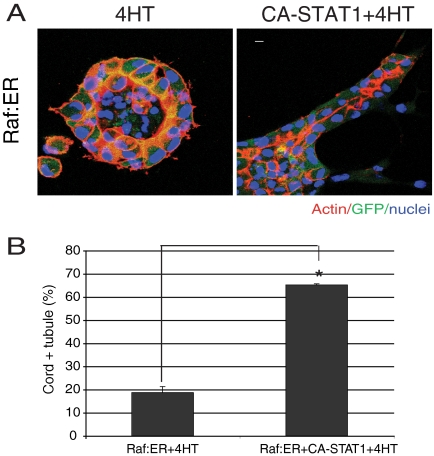
Mutation of Arg-656 and Asn-658 in STAT1 to Cys (CA-STAT1) produces a well-characterized constitutively active (CA) form of STAT1 (Sironi and Ouchi, 2004). To further examine the function of STAT1 in MDCK tubulogenesis, we stably expressed FLAG-tagged CA-STAT in Raf:ER cells. In cysts expressing Raf:ER and treated with 4HT for 72 h, individual cells migrated out from the cysts, as mentioned previously (Figure 5A, left). Cords were largely absent. In contrast, when we coexpressed Raf:ER and CA-STAT1 and treated with 4HT for 72 h, cords of cells protruded from a high percentage of the cysts (Figure 5A, right; quantified in Figure 5B). Notably, the cords formed from the CA-STAT1 cysts often lacked detectable lumens or had only small, discontinuous lumens, i.e., they failed to progress to the mature tubule stage with their characteristic continuous lumens, even at longer times (compare the lumen in Figure 5A, right with control mature tubules in Figure 1B, left or Figure 2A).
Figure 5.
Overexpression of constitutively active form of STAT1 restores redifferentiation of Raf:ER cells in the presence of 4HT. (A) Raf:ER cysts (left) and Raf:ER cysts overexpressing CA-STAT1 were treated with 4HT for 72 h. CA-STAT1 overexpression promotes redifferentiation in activated Raf:ER cysts. Bar, 10 μm. (B) Quantification of cords plus tubules in the presence of 4HT. One hundred GFP-positive structures per sample were counted (mean ± SEM, n = 3). *p < 0.005.
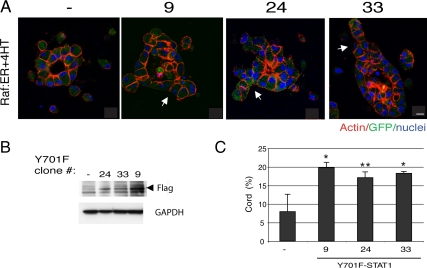
STATs are known to be phosphorylated by various kinases and to be imported to the nucleus. However, recent work has shown that STAT3 is also found in mitochondria, where it is required for Ras-mediated transformation. This is distinct from the classical role of STAT3 in the nucleus as a transcription factor (Gough et al., 2009). To determine whether the activity of STAT1 as a transcription factor is required for tubulogenesis, we introduced into Raf:ER cells a mutant form of STAT1, in which its tyrosine phosphorylation site is defective (Y701F). Tyrosine phosphorylation by extracellular signaling proteins is known to translocate STAT proteins into the nucleus to drive transcription; mutation of Tyr701 to Phe prevents this translocation (Shuai et al., 1993). Interestingly, expression of the Y701F-STAT1 mutant partially promoted cord formation in cysts with activated Raf:ER (Figure 6C). This is consistent with the idea that at least some of the role of STAT1 in this tubulogenesis may be due to a nonnuclear function. However, compared with the abundant cords formed from the CA-STAT1 cysts (∼65%), not only did a low percentage of cysts form cords in Y701F-STAT1 (∼20%) but also the tubules were significantly shorter (compare Figure 5A with Figure 6A). These differences suggest that the transcriptional activity of STAT1 does play a significant role in tubulogenesis.
Figure 6.
Overexpression of tyrosine phosphorylation defective STAT1 partially rescues cord formation of activated Raf:ER cysts. (A) Raf:ER cysts (left) and three clones overexpressing Y701F-STAT1 were treated with 4HT for 72 h. Bar, 10 μm. (B) Total lysate from Raf:ER cells and three Y701F-STAT1 transfectants were immunoblotted for FLAG tag on Y701F-STAT1. (C) Quantification of cords in the presence of 4HT (mean ± SEM, n = 3). *p < 0.05 and **p = 0.06).
Raf-1 Activation Reduces Interaction of Raf-1 with STAT1
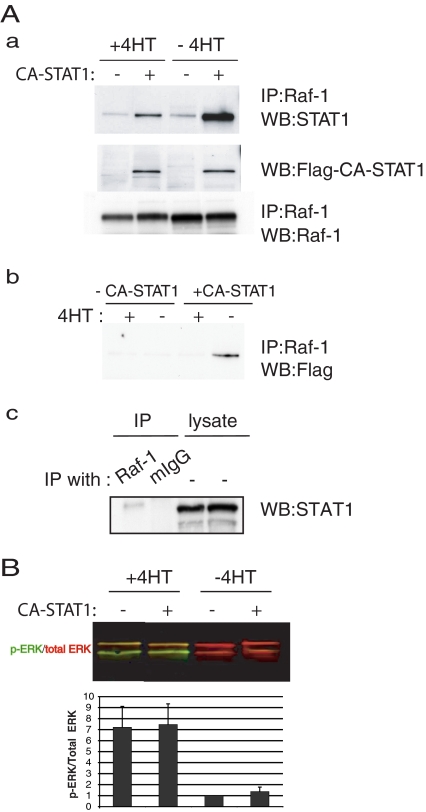
In lymphocytes, Raf-1 is known to be physically associated with STAT1 but not with either STAT3 or STAT5 (Stancato et al., 1998). We used Raf:ER cells, transfected or not with CA-STAT1 and that were treated or not with 4HT for 48 h. We solubilized the cells and immunoprecipitated with antibodies to Raf-1. We western blotted the immunoprecipitates with antibodies to STAT1. As shown in Figure 7Aa, STAT1 coimmunoprecipitated with Raf-1; this was not observed in the immunoprecipitate with control immunoglobulin (Ig)G (Figure 7Ac). The STAT1 signal was stronger in cells expressing CA-STAT1, due to coimmunoprecipitation of the CA-STAT1 with the Raf-1. Notably, when Raf:ER is activated by 4HT, the amount of coimmunoprecipitated STAT1 is reduced (Figure 7Aa, top). The middle panel is a loading control, where an aliquot of the total lysate is blotted for the FLAG tag on the CA-STAT1. The bottom panel shows the amount of Raf-1 that was immunoprecipitated. This demonstrates that even though the levels of FLAG-tagged CA-STAT1 and immunoprecipitated Raf-1 were unchanged by treatment with 4HT, the amount of CA-STAT1 that coimmunoprecipitated with Raf-1 is reduced by 4HT. Figure 7Ab shows cells immunoprecipitated with antibodies to Raf-1 and then blotted for the FLAG tag on the CA-STAT1, so only the CA-STAT1 that coimmunoprecipitated with the Raf-1 is detected. Again, activation of Raf:ER by 4HT reduces the interaction of Raf-1 and STAT1.
Figure 7.
Activation of Raf-1 represses the association between Raf-1 and STAT1. (A) CA-STAT1 overexpressing or not Raf:ER cells were cultured either in the presence or absence 4HT for 48 h. Cell lysates were coimmunoprecipitated with the antibody to Raf-1 and then blotted for STAT1 or Raf-1 or FLAG tag on CA-STAT1 (a and b). (c) Raf:ER cell lysates in the absence of 4-HT were immunoprecipitated with Raf-1 or mouse IgG and analyzed by immunoblotting with STAT1 (lanes 1 and 2). Total lysates were immunoblotted for STAT1 as loading controls (lane 3 and 4). (B) Quantification of ERK activation. The ratios of phospho-ERK (green) to total ERK (red) were quantified (bottom) (mean ± SEM, n = 3). Activation of Raf-1 by 4HT induces ERK activation, yielding green and yellow bands. but CA-STAT1 overexpression has no effect on it.
Raf-1 acts through the MAP kinase cascade to cause the activation of ERK. To test whether the effects of CA-STAT1 on cord formation are due to an effect of CA-STAT1 on the MAP kinase pathway, we examined the level of phosphorylation of ERK. In cells treated with 4HT, phospho-ERK (green) is detected (Figure 7B, top). The ratio of phospho-ERK to total ERK is quantitated in Figure 7B (bottom). Notably, the expression of CA-STAT1 has no impact on the phosphorylation of ERK, suggesting that the effects of CA-STAT1 are independent of the MAP kinase cascade.
STAT3 has been shown previously to be involved in tubulogenesis in the HGF–MDCK system (Boccaccio et al., 1998). Given that STAT1 and STAT3 are known to heterodimerize, we considered the possibility that CA-STAT1 could be acting through STAT3. We determined the activation state of STAT3 and found no evidence of STAT3 activation in CA-STAT1–overexpressing cells in response to 4HT by immunoblot (Supplemental Figure S2). These data suggest that the effect of CA-STAT1 in promoting cord formation probably does not involve coactivation of STAT3.
DISCUSSION
We report that STAT1 is needed for the redifferentiation phase of tubulogenesis. Loss of STAT1 prevents redifferentiation. Tellingly, CA-STAT1 can even overcome the effect of prolonged activation of exogenous Raf that otherwise maintains pEMT and prevents redifferentiation. Essentially, CA-STAT1 is able to allow redifferentiation under these conditions, supporting the notion that STAT1 plays a central role in the redifferentiation phase of tubulogenesis.
The Janus tyrosine kinase (JAK)–STAT pathway has been previously implicated in tubular morphogenesis. STAT3, which associates with c-Met, was found previously to be necessary for HGF–MDCK tubulogenesis (Boccaccio et al., 1998), although the exact step of tubulogenesis involved was not analyzed. Several other lines of evidence supporting a role of JAK-STAT in tubulogenesis have been reported. The JAK–STAT pathway is negatively regulated by the suppressor of cytokine signaling (SOCS) (Krebs and Hilton, 2001). HNF-1β regulates tubulogenesis by controlling SOCS3 expression via the JAK—STAT pathway (Ma et al., 2007). Protein tyrosine phosphatases, such as SHP-2, limit STAT tyrosine phosphorylation via dephosphorylation of cytokine receptor (Yu et al., 2000). SHP-2 controls STAT1 activation (Wu et al., 2002) and is involved together with STAT1 and STAT3 in HGF–MDCK tubulogenesis (Wang et al., 2006). SHP-2 also has been implicated in tubule branching in early lung development (Tefft et al., 2005).
Most intriguingly, STAT1 may be involved in the formation of pathologically large cysts in autosomal dominant polycystic kidney disease, which is caused by mutations in either polycystin-1 or polycystin-2, which form a complex. Polycystin-1 activates the JAK–STAT pathway and up-regulates the cell cycle inhibitor p21waf1, thus inducing cell cycle arrest (Bhunia et al., 2002). Mice mutant in polycystin-1 have a defect in STAT1 phosphorylation and induction of p21waf1. However, this result is controversial, because it has been claimed recently that the abnormal proliferation in cells expressing mutant polycystin-2 is independent of STAT1 and p21waf1 (Felekkis et al., 2008).
Cell migration is necessary for tubule development. Focal adhesion kinase (FAK) is known to integrate HGF and integrin signals for promoting cell migration (Sieg et al., 2000). Interestingly, a previous study has shown that FAK activates STAT1 with its interaction through its C-terminal domain, but not STAT3 (Xie et al., 2001). Thus, it would be interesting to investigate the role of FAK in STAT1-regulated tubulogenesis, although a recent study reveals that FAK is required but not sufficient for MDCK tubulogenesis (Wei et al., 2009). This is consistent with our observation that CA-STAT1 overexpression itself in the absence of 4HT has no morphological changes even though STAT1 is highly activated (data not shown).
When tubulogenesis is driven by HGF alone, the MAP kinase cascade remains active for at least 72 h. This presents a paradox in that continued activation of the MAP kinase cascade by Raf:ER for >24 h causes cell scattering and not tubulogenesis. We have now resolved this paradox by finding that Raf:ER activation causes the down-regulation of STAT1, whereas HGF activation does not down-regulate STAT1. Interestingly, a negative cross-talk of STAT1 and ERK has been suggested previously. The inhibition of all JAKs induced not only a complete block of STAT1 but also an increased baseline phospho-ERK (Reiterer and Yen, 2006). It also has been reported that the specific inhibition of MEK triggers JAK-dependent STAT1 expression and exposure to Clostridium difficile toxin B, which specifically inhibits Rho GTPases, induces ERK dephosphorylation and STAT1 up-regulation (Loucks et al., 2006). However, CA-STAT1 overexpression induces no decrease in ERK activation (Figure 7B), and we conclude that STAT1 activation does not result in deactivation of ERK in our system.
Consistent with the finding that STAT1 is needed for the redifferentiation phase, but not the pEMT phase, we found that expression of CA-STAT1 in cysts with activated Raf:ER allowed the formation of cords. Moreover, Y701F-STAT1, which is not imported into the nucleus, partially rescued cord formation, suggesting that STAT1 functions in a nonnuclear role in addition to its transcriptional role in tubulogenesis. Importantly, these cords either lacked lumens or at best formed small, discontinuous lumens; they did not progress to the single, continuous lumen that defines a mature tubule. Cords are two or more cells thick and contain extensive cell–cell contacts that are distant from the ECM. We had proposed previously that these cell–cell contacts would provide a signal for lumen formation and that tubular lumen formation was a recapitulation of the process by which lumens form in cysts before HGF stimulation (O'Brien et al., 2002). Our new data indicate that tubulogenesis is more complex. Rather, the redifferentiation phase can be divided into separate steps of lumen initiation (which occurs in cords) and subsequent maturation (which involves coalescence of small, discontinuous lumens and expansion of lumen diameter and length), the latter leading to mature tubules. Several other molecular pathways are known to underlie lumen formation (Bagnat et al., 2007; Ferrari et al., 2008; Martin-Belmonte et al., 2008; Delous et al., 2009; Hurd and Margolis, 2009; Kim et al., 2009; Schluter et al., 2009). Distinct mechanisms have been uncovered that control tubule size and diameter (Affolter et al., 2009; Nelson et al., 2010). Expression of CA-STAT1 in cysts with activated Raf:ER rescues lumen initiation, but may be dysregulated in a pathway(s) that controls formation of mature tubules, such as enlargement and coalescence of lumens or other processes. Our results may help us understand the regulation of mammalian tubule diameter, which is central to several important disorders, such as vascular stenosis and cystic diseases.
In conclusion, STAT1 is required for the redifferentiation phase of tubulogenesis. Constitutively active or phosphorylation defective rescues the block in cord formation caused by prolonged activation of Raf:ER but does not restore formation of the single, continuous lumens characteristic of mature tubules. This suggests that the complex processes of lumen formation and maturation may be under the control of a distinct signal(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. T. Ouchi for STAT1 constructs and the late R. Schwall for HGF. We also thank Dr. J. Lipschutz for critical reading of the manuscript and members of Mostov laboratory for advice and stimulating discussion. This work was supported by National Institutes of Health grants R01 DK-074398 and P01 AI-53194 (to K. M.). M. K. was supported by13FT-0159.
Abbreviations used:
- 4HT
4-hydroxytamoxifen
- HGF
hepatocyte growth factor
- MAP
mitogen-activated protein
- MDCK
Madin-Darby canine kidney
- STAT
signal transducers and activators of transcription.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-02-0112) on September 22, 2010.
REFERENCES
- Affolter M., Zeller R., Caussinus E. Tissue remodelling through branching morphogenesis. Nat. Rev. Mol. Cell Biol. 2009;10:831–842. doi: 10.1038/nrm2797. [DOI] [PubMed] [Google Scholar]
- Bagnat M., Cheung I. D., Mostov K. E., Stainier D. Y. Genetic control of single lumen formation in the zebrafish gut. Nat. Cell Biol. 2007;9:954–960. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- Battle T. E., Lynch R. A., Frank D. A. Signal transducer and activator of transcription 1 activation in endothelial cells is a negative regulator of angiogenesis. Cancer Res. 2006;66:3649–3657. doi: 10.1158/0008-5472.CAN-05-3612. [DOI] [PubMed] [Google Scholar]
- Bhunia A. K., Piontek K., Boletta A., Liu L., Qian F., Xu P. N., Germino F. J., Germino G. G. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Boccaccio C., Ando M., Tamagnone L., Bardelli A., Michieli P., Battistini C., Comoglio P. M. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Mostov K. E. From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraglia M., Vitale G., Marra M., Budillon A., Tagliaferri P., Abbruzzese A. Alpha-interferon and its effects on signalling pathways within cells. Curr. Protein Pept. Sci. 2004;5:475–485. doi: 10.2174/1389203043379378. [DOI] [PubMed] [Google Scholar]
- David M., Petricoin E., 3rd, Benjamin C., Pine R., Weber M. J., Larner A. C. Requirement for MAP kinase (ERK2) activity in interferon alpha- and interferon beta-stimulated gene expression through STAT proteins. Science. 1995;269:1721–1723. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- Delous M., Hellman N. E., Gaude H. M., Silbermann F., Le Bivic A., Salomon R., Antignac C., Saunier S. Nephrocystin-1 and nephrocystin-4 are required for epithelial morphogenesis and associate with PALS1/PATJ and Par6. Hum. Mol. Genet. 2009;18:4711–4723. doi: 10.1093/hmg/ddp434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felekkis K. N., Koupepidou P., Kastanos E., Witzgall R., Bai C. X., Li L., Tsiokas L., Gretz N., Deltas C. Mutant polycystin-2 induces proliferation in primary rat tubular epithelial cells in a STAT-1/p21-independent fashion accompanied instead by alterations in expression of p57KIP2 and Cdk2. BMC Nephrol. 2008;9:10. doi: 10.1186/1471-2369-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A., Veligodskiy A., Berge U., Lucas M. S., Kroschewski R. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J. Cell Sci. 2008;121:3649–3663. doi: 10.1242/jcs.018648. [DOI] [PubMed] [Google Scholar]
- Gough D. J., Corlett A., Schlessinger K., Wegrzyn J., Larner A. C., Levy D. E. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. H., Zegers M. M., Woodrow M., Rodriguez-Viciana P., Chardin P., Mostov K. E., McMahon M. Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signalregulated kinase pathway. Mol. Cell Biol. 2000;20:9364–9375. doi: 10.1128/mcb.20.24.9364-9375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L., Kolodziej P. A. Organogenesis: molecular mechanisms of tubulogenesis. Nat. Rev. Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Hurd T. W., Margolis B. Cystin, cilia, and cysts: unraveling trafficking determinants. J. Am. Soc. Nephrol. 2009;20:2485–2486. doi: 10.1681/ASN.2009090996. [DOI] [PubMed] [Google Scholar]
- Kim M., Datta A., Brakeman P., Yu W., Mostov K. E. Polarity proteins PAR6 and aPKC regulate cell death through GSK-3beta in 3D epithelial morphogenesis. J. Cell Sci. 2007;120:2309–2317. doi: 10.1242/jcs.007443. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Park S., Mostov K., Debnath J., Hunt C. A. Computational investigation of epithelial cell dynamic phenotype in vitro. Theor. Biol. Med. Model. 2009;6:8. doi: 10.1186/1742-4682-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs D. L., Hilton D. J. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- Liu X., Robinson G. W., Hennighausen L. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 1996;10:1496–1506. doi: 10.1210/mend.10.12.8961260. [DOI] [PubMed] [Google Scholar]
- Loucks F. A., Le S. S., Zimmermann A. K., Ryan K. R., Barth H., Aktories K., Linseman D. A. Rho family GTPase inhibition reveals opposing effects of mitogen-activated protein kinase kinase/extracellular signal-regulated kinase and Janus kinase/signal transducer and activator of transcription signaling cascades on neuronal survival. J. Neurochem. 2006;97:957–967. doi: 10.1111/j.1471-4159.2006.03802.x. [DOI] [PubMed] [Google Scholar]
- Lubarsky B., Krasnow M. A. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Ma Z., Gong Y., Patel V., Karner C. M., Fischer E., Hiesberger T., Carroll T. J., Pontoglio M., Igarashi P. Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc. Natl. Acad. Sci. USA. 2007;104:20386–20391. doi: 10.1073/pnas.0705957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F., Yu W., Rodriguez-Fraticelli A. E., Ewald A. J., Werb Z., Alonso M. A., Mostov K. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr. Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991a;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Montesano R., Schaller G., Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991b;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- Nelson K. S., Furuse M., Beitel G. J. The Drosophila claudin Kune-kune is required for septate junction organization and tracheal tube size control. Genetics. 2010;185:831–839. doi: 10.1534/genetics.110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Tang K., Kats E. S., Schutz-Geschwender A., Lipschutz J. H., Mostov K. E. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell. 2004;7:21–32. doi: 10.1016/j.devcel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Zegers M. M., Mostov K. E. Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Pollack A. L., Runyan R. B., Mostov K. E. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- Reiterer G., Yen A. Inhibition of the janus kinase family increases extracellular signal-regulated kinase 1/2 phosphorylation and causes endoreduplication. Cancer Res. 2006;66:9083–9089. doi: 10.1158/0008-5472.CAN-06-0972. [DOI] [PubMed] [Google Scholar]
- Schluter M. A., Pfarr C. S., Pieczynski J., Whiteman E. L., Hurd T. W., Fan S., Liu C. J., Margolis B. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol. Biol. Cell. 2009;20:4652–4663. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K., Stark G. R., Kerr I. M., Darnell J. E., Jr A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Sironi J. J., Ouchi T. STAT1-induced apoptosis is mediated by caspases 2, 3, and 7. J. Biol. Chem. 2004;279:4066–4074. doi: 10.1074/jbc.M307774200. [DOI] [PubMed] [Google Scholar]
- Stancato L. F., Yu C. R., Petricoin E. F., 3rd, Larner A. C. Activation of Raf-1 by interferon gamma and oncostatin M requires expression of the Stat1 transcription factor. J. Biol. Chem. 1998;273:18701–18704. doi: 10.1074/jbc.273.30.18701. [DOI] [PubMed] [Google Scholar]
- Tefft D., De Langhe S. P., Del Moral P. M., Sala F., Shi W., Bellusci S., Warburton D. A novel function for the protein tyrosine phosphatase Shp2 during lung branching morphogenesis. Dev. Biol. 2005;282:422–431. doi: 10.1016/j.ydbio.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Wang C. Z., Su H. W., Hsu Y. C., Shen M. R., Tang M. J. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol. Biol. Cell. 2006;17:2839–2852. doi: 10.1091/mbc.E05-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Koromilas A. E. Stat1 is an inhibitor of Ras-MAPK signaling and Rho small GTPase expression with implications in the transcriptional signature of Ras transformed cells. Cell Cycle. 2009;8:2070–2079. doi: 10.4161/cc.8.13.8891. [DOI] [PubMed] [Google Scholar]
- Wei W. C., Kopec A. K., Tang M. J. Requirement of focal adhesion kinase in branching tubulogenesis. J. Biomed. Sci. 2009;16:5. doi: 10.1186/1423-0127-16-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. R., Hong Y. K., Wang X. D., Ling M. Y., Dragoi A. M., Chung A. S., Campbell A. G., Han Z. Y., Feng G. S., Chin Y. E. SHP-2 is a dual-specificity phosphatase involved in Stat1 dephosphorylation at both tyrosine and serine residues in nuclei. J. Biol. Chem. 2002;277:47572–47580. doi: 10.1074/jbc.M207536200. [DOI] [PubMed] [Google Scholar]
- Xie B., Zhao J., Kitagawa M., Durbin J., Madri J. A., Guan J. L., Fu X. Y. Focal adhesion kinase activates Stat1 in integrin-mediated cell migration and adhesion. J. Biol. Chem. 2001;276:19512–19523. doi: 10.1074/jbc.M009063200. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Jin Y. J., Burakoff S. J. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J. Biol. Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.