Transforming growth factor-β receptor recycling is regulated by the clathrin adaptor Dab2 protein. In the absence of Dab2, receptors localize in a perinuclear locale because they are unable to transit from the early endosomal antigen 1-positive early endosome to the Rab11-positive endosomal recycling compartment.
Abstract
Transforming growth factor (TGF)-β family proteins form heteromeric complexes with transmembrane serine/threonine kinases referred to as type I and type II receptors. Ligand binding initiates a signaling cascade that generates a variety of cell type-specific phenotypes. Whereas numerous studies have investigated the regulatory activities controlling TGF-β signaling, there is relatively little information addressing the endocytic and trafficking itinerary of TGF-β receptor subunits. In the current study we have investigated the role of the clathrin-associated sorting protein Disabled-2 (Dab2) in TGF-β receptor endocytosis. Although small interfering RNA-mediated Dab2 knockdown had no affect on the internalization of various clathrin-dependent (i.e., TGF-β, low-density lipoprotein, or transferrin) or -independent (i.e., LacCer) cargo, TGF-β receptor recycling was abrogated. Loss of Dab2 resulted in enlarged early endosomal antigen 1-positive endosomes, reflecting the inability of cargo to traffic from the early endosome to the endosomal recycling compartment and, as documented previously, diminished Smad2 phosphorylation. The results support a model whereby Dab2 acts as a multifunctional adaptor in mesenchymal cells required for TGF-β receptor recycling as well as Smad2 phosphorylation.
INTRODUCTION
Transforming growth factor (TGF)-β is a ubiquitous 25-kDa polypeptide that modulates a variety of cellular processes, such as proliferation, differentiation, and apoptosis. The responses to TGF-β are cell type specific and can be as diverse as growth arrest or stimulation of growth (Sporn et al., 1986; Massagué, 1998; Ikushima and Miyazono, 2010). TGF-β superfamily members signal via a heteromeric complex of type I and type II serine/threonine kinase receptors. Signaling is initiated by TGF-β ligand binding to the constitutively active type II receptor, resulting in hetero-oligomerization and phosphorylation of type I receptors. The activated type I receptor triggers a series of signaling events by phosphorylating Smad2 and Smad3, also known as receptor-regulated Smads or R-Smads, at their C-terminal SSXS motif. The phosphorylated R-Smads oligomerize with Smad4 and translocate to the nucleus where they bind to the promoters of a variety of genes and modulate their transcription (Moustakas et al., 2001; Shi and Massagué, 2003; Feng and Derynck, 2005; Massagué et al., 2005). In addition to Smad-mediated signaling, the receptors also activate non-Smad signaling pathways, resulting in activation of extracellular signal-regulated kinase, p38, and c-Jun NH2-terminal kinase mitogen-activated protein (MAP) kinases, Rho-like GTPases, p21 activated kinase 2 (PAK2), and phosphatidylinositol 3-kinase (PI3K), for example, in various cell types (Derynck and Zhang, 2003; Wilkes et al., 2003; Moustakas and Heldin, 2005; Rahimi et al., 2009). Although these activities are often referred as Smad independent, there is often cross-talk with the Smad proteins to obtain a full biological response (Moustakas et al., 2001; Di Guglielmo et al., 2003; Wilkes et al., 2003; Moustakas and Heldin, 2005).
Phosphorylation of the type I receptor occurs at the plasma membrane, but downstream Smad signaling in many cell types requires TGF-β receptor internalization that occurs via clathrin-mediated endocytosis and leads to accumulation of the receptors in early endosomal antigen (EEA) 1-positive endosomes (Hayes et al., 2002; Di Guglielmo et al., 2003; Mitchell et al., 2004). Although these findings clearly document the interrelation between the endocytic and signaling machineries in regulating TGF-β action, the reality is significantly more complex because 1) clathrin-independent mechanisms also have been reported for Smad activation (Runyan et al., 2005); and 2) non-Smad targets such as PI3K, PAK2, and c-Abl are activated in membrane proximal locales, independently of receptor internalization (Wilkes et al., 2005). In addition to ligand-mediated effects, TGF-β receptors constitutively recycle (Mitchell et al., 2004). This occurs in the presence or absence of ligand and is dependent upon clathrin and Rab11.
Key components of clathrin-dependent receptor internalization include clathrin, adaptor protein (AP) 2, and dynamin (Brodsky et al., 2001). In addition, the endocytic process also involves several adaptor proteins that bind directly or indirectly to cargo and concurrently interact with membrane lipids, coat components, endocytic accessory proteins, and enzymes involved in phospholipid metabolism. These multiple adaptors are families of functionally redundant proteins such that internalization of one class of receptors can use several related adaptors. One such well characterized adaptor protein is Dab2, which, despite the complexities of redundancy, plays an important role in cell surface receptor turnover, endocytosis and cell signaling (Hocevar et al., 2001; Morris and Cooper, 2001; Mishra et al., 2002; Morris et al., 2002). The N terminus of Dab2 includes a phospho-tyrosine binding (PTB) domain (capable of binding NPXY motifs) that is structurally similar to pleckstrin homology (PH) domains and also binds phosphoinositides. In addition, Dab2 contains two DPF (Asp-ProPhe) and five NPF (Asn-ProPhe) motifs capable of binding AP2 and clathrin and Eps-15 homology (EH) domains, respectively. At the C terminus is a proline-rich domain that associates with myosin VI. The importance of Dab2 in endocytic and trafficking pathways has been examined by either increasing expression levels of Dab2 or removal of Dab2 from cells. In one report, overexpression of Dab2 resulted in redistribution of AP2 clusters that do not contain clathrin (Morris and Cooper, 2001), whereas another study demonstrated that overexpression of the PTB domain alone prevented low-density lipoprotein (LDL) receptor uptake (Mishra et al., 2002). Conversely, conditional Dab2 knockout decreases the number of apical coated pits and a decline in the excretion of megalin-dependent proteins (Morris et al., 2002). Thus, Dab2 is a multidomain, multiple protein adaptor that plays a key role in endocytosis and trafficking. In the context of TGF-β biology, most studies have focused on establishing the importance of Dab2 in the growth inhibitory aspects of TGF-β signaling (Hocevar et al., 2001; Hocevar et al., 2005; Prunier and Howe, 2005). Dab2 is a known tumor suppressor and diminished Dab2 expression is observed in many human carcinomas (Mok et al., 1994; Tseng et al., 1998; Fazili et al., 1999). One explanation for growth inhibition by Dab2 can be that overexpression of Dab2 interferes with mitogenic growth factor signal transduction, possibly by inhibiting Ras or MAP kinase activation (Xu et al., 1998; Tseng et al., 1999; Smith et al., 2001; Zhou and Hsieh, 2001). Alternatively, Dab2 may act as a tumor suppressor by promoting TGF-β signaling and subsequent growth inhibition (Hocevar et al., 2001).
Because Dab2 has been shown to associate with the type I and type II TGF-β receptors and modulate Smad activation (Hocevar et al., 2001), we investigated whether this might indicate an additional role in TGF-β receptor trafficking. Surprisingly, our data establish that although knocking down Dab2 does not affect receptor internalization, it has a dramatic impact on TGF-β receptor recycling and endosomal localization. Specifically, we demonstrate that 1) type II receptor recycling is disrupted in Dab2 knockdown (KD) cells; 2) although localization of receptors with EEA1 is transient in wild-type cells, it is prolonged in the absence of Dab2; and 3) although type II receptors colocalize with Rab11 in parental cultures, this is significantly decreased in the Dab2 knockdown cells. Thus, the study establishes a novel role for Dab2 in TGF-β receptor trafficking and recycling, placing Dab2 as a crucial regulator of trafficking between the EEA1-positive early endosome and the Rab11-positive recycling endosome.
MATERIALS AND METHODS
Cell Culture and Transfections
Parental (WT) and Dab2 KD NIH-3T3 cells were used to generate lines stably expressing chimeric TGF-β receptors that have been shown to faithfully recapitulate all trafficking and signaling responses of native TGF-β receptors (Anders and Leof, 1996; Anders et al., 1997). They consist of the ligand binding domain of the granulocyte macrophage–colony-stimulating factor (GM-CSF) α or β receptor fused to the transmembrane and cytoplasmic domains of the type I or type II TGF-β receptor (Anders and Leof, 1996). Thus, β2 refers to a chimeric receptor with a GM-CSF receptor-β extracellular domain and a TGF-β type II receptor cytoplasmic domain. Cultures were maintained in DMEM containing 10% fetal bovine serum and supplemented with 50 μg/ml hygromycin and 100 μg/ml geneticin (Anders et al., 1997, 1998; Mitchell et al., 2004). Dab2 KD cells were generated by transfecting NIH-3T3 cells with pSUPER-siDab2 as described previously (Prunier and Howe, 2005). Loss of Dab2 had no effect on the expression of chimeric or native TGF-β receptors or trafficking proteins including EEA1 (1:1000 dilution; BD Biosciences, San Jose, CA), Rab4 (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), or Rab11 (1:1000 dilution; BD Biosciences) (Supplemental Figures 1 and 2). The Dab2 KD cell media also contained 1 μg/ml puromycin to maintain selection for the siRNA construct. All transient transfections were conducted on cells seeded on coverslips at 4 × 105 cells/well of a six-well plate. After overnight incubation, cells were transfected with 2 μg of DNA by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) for 24 h and processed for immunofluorescence.
Western Blot Analyses
To detect Dab2, WT and Dab2 KD cell lysates were prepared in buffer containing 50 mM Tris, pH 7.4, 1% Triton X-100, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, and 1× Complete protease inhibitor (Roche Diagnostics, Indianapolis, IN) and probed with Dab2 antibody (BD Biosciences Transduction Laboratories) at 1:1000 dilution. Phosphorylated Smad2 (Smad2-P) was examined after stimulation of arrested cultures with either 50 ng of GM-CSF/ml to activate the chimeric receptors or 5 ng of TGF-β/ml to activate the endogenous receptors. After ligand treatment, cellular lysates were prepared as described above and probed with a phospho-specific Smad2 antibody (1:2000 dilution; Calbiochem, San Diego, CA). Total Smad2 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to document equal loading using a Smad2/3 (1:1000 dilution; Millipore) or GAPDH antibody (1:15000 dilution; Calbiochem). Each experiment was done at least three times, and representative blots were chosen.
Immunofluorescence and Colocalization
For all immunofluorescence studies, cells were seeded on coverslips at 4 × 105 cells/well in six-well plates. To assess TGF-β receptor trafficking, cultures were preincubated with 5 μg/ml monoclonal anti-GM-CSF receptor-β antibody (SC-457; Santa Cruz Biotechnology) or with mouse anti-hemagglutinin (HA) (clone 12CA5; Roche Diagnostics), used at 1:1500 dilution, for chimeric and HA epitope-tagged native type II receptors, respectively, for 1 h at 10°C in antibody buffer (50 mM HEPES and DMEM, pH 7.2). Coverslips were washed three times with antibody buffer before 37°C transfer for the indicated times. Subsequently, they were washed with DMEM, pH 2.0, fixed in 4% paraformaldehyde, and permeabilized for 2 min in 0.4% Triton X-100 phosphate-buffered saline (PBS). To determine receptor colocalization with markers of endocytosis, after permeabilization cells were incubated in blocking buffer (0.05% saponin, 1 mM glycine, and 2% bovine serum albumin in PBS) with rabbit antibodies to EEA1 (1:1000 dilution; Abcam, Cambridge, MA), Rab11 (1:400 dilution; BD Biosciences), or Rab4 (1:200 dilution; Santa Cruz Biotechnology) for 1 h at room temperature. Internalized receptors and compartments positive for the endocytic marker were detected by subsequent binding with Cy3-labeled (Jackson ImmunoResearch Laboratories, West Grove, PA) anti-mouse (1:200), and Alexa Fluor 488-conjugated (Invitrogen, Carlsbad, CA) anti-mouse antibodies (1:400), respectively, in the saponin blocking buffer. After washing with blocking buffer three times, coverslips were mounted with Vectashield (Vector Laboratories, Burlingame, CA) mounting medium (for experiments with fixed cells) and imaged by standard fluorescence microscopy at room temperature by using an oil immersion 60× or 100× objective with a numerical aperture of 1.40 on an AX70 fluorescence microscope (Olympus, Melville, NY) equipped with a C4742-95 digital camera (Hamamatsu, Bridgewater, NJ). In experiments using double-labeled specimens, control samples were labeled with the individual fluorophores and exposed identically as the dual-labeled samples at each wavelength to verify that there was no crossover among emission channels. Digital images were quantified by image processing using MetaMorph software (Molecular Devices, Sunnyvale, CA) as described previously (Singh et al., 2003).
Internalization Assays
125I-labeled GM-CSF (PerkinElmer Life and Analytical Sciences, Boston, MA) internalization was performed on Dab2 WT stable cells expressing the chimeric receptors and Dab2 KD clones as described previously (Anders et al., 1997). Transferrin (Tfn) internalization was visualized by incubation of cultures in serum-free medium for 2 h, followed by treatment with 25 μg/ml Alexa Fluor 594 (red) Tfn (Invitrogen) at 37°C for 5 min. Cells were fixed in 4% formaldehyde, mounted, and visualized using an AX-70 fluorescence microscope. For studies using fluorescent lactosylceramide (LacCer), cells were incubated with 2.5 μM Bodipy-LacCer at 10°C, washed and internalization determined following 3 min incubation at 37°C (Singh et al., 2003). Low-density lipoprotein internalization was conducted as described previously (Puri et al., 2001). In brief, cells were incubated with 10% lipoprotein deficient serum for 24 h and loaded with 20 μg/ml DiI-LDL (Intracell, Frederick, MD) for 5 min. Fluorescent Tfn, LDL, and LacCer internalization was quantified using MetaMorph.
Ligand-independent internalization was performed essentially as described previously (Mitchell et al., 2004). In brief, anti-GM-CSF receptor β antibody (GMCSF2RB; eBioscience, San Diego, CA) was labeled with 125I to a specific activity of 80 μCi/μg with chloramine T. Cells were incubated for 2 h at 4°C with 8 ng/ml labeled antibody in antibody buffer (25 mM HEPES-DMEM). Buffer with labeled antibody was removed, warmed to 37°C and then re-added for the indicated times at 37°C. After acid stripping with DMEM, pH 2.0, cell-associated radioactivity was assessed as described previously (Anders et al., 1997).
Tfn Recycling
Cos-7 cells were seeded on coverslips as described and transfected with Ds-Red tagged Rab11 wild-type and dominant-negative (S25N) constructs via Lipofectamine 2000. Forty-eight hours after transfection, cells were serum starved for 2 h in phenol-red free DMEM and pulsed with Alexa Fluor 488-conjugated Tfn (25 μg/ml) for 1 h at 16°C to label the early endosomal compartments. After DMEM washing, cultures were subsequently transferred to 37°C for 10 min. After a brief acid wash (PBS, pH 3.0) cells were fixed in 4% formaldehyde, mounted, and visualized using an AX-70 fluorescence microscope.
Direct Recycling Assay
Direct recycling was assayed based on a protocol by Fraile-Ramos et al. (2001) whereby an antibody recognizing the extracellular receptor domain is visualized through 1.5 cycles of recycling (Mitchell et al., 2004). Cells were seeded on coverslips as outlined above and incubated with anti-GM-CSF receptor-β antibody at 4 or 37°C for 1 h. Any remaining surface antibody was removed by washing in DMEM, pH 2.0. Cells were then incubated with Alexa Fluor 488-conjugated anti-mouse antibody (diluted 1:200) at 37°C for 1 h followed by a second round of washing and acid stripping. Cultures were fixed and imaged with fluorescence microscopy as described above. Our previous work documented that whole antibody gave the identical findings as Fab fragments (Mitchell et al., 2004).
RESULTS
Dab2 Acts Downstream of TGF-β Receptor Internalization
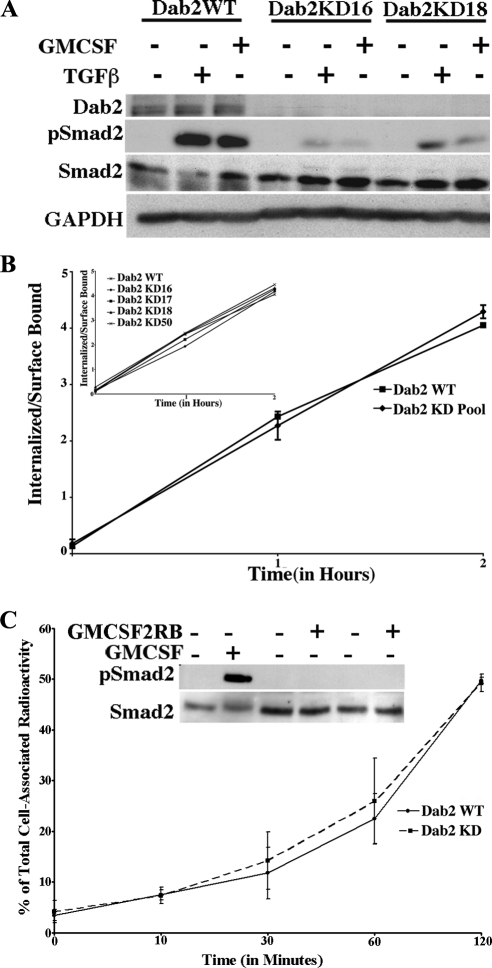
Because the regulatory components mediating TGF-β receptor endocytosis have not been well defined, and Dab2 has been proposed as an adaptor protein that regulates protein trafficking (Morris and Cooper, 2001), we initiated studies to determine whether Dab2 had a similar role in TGF-β receptor internalization. To address this question and to allow for accurate determination of internalized versus surface receptors (Anders et al., 1997, 1998), we knocked down Dab2 in NIH-3T3 cells and generated stable clones expressing similar levels of the chimeric type I and type II TGF-β receptors in the parent (Dab2 WT) and Dab2 KD NIH-3T3 cultures (Supplemental Figure 1, A–D). The use of chimeric receptors circumvents the problems associated with nonspecific binding of TGF-β ligand (GM-CSF is very specific) and uses the highly specific antibodies available for extracellular domains of GM-CSFRs, that are currently not available for TGF-β receptors. Western blot analysis of these cell lines using antibodies against Dab2 indicated significantly lower levels in the KD clones versus WT cells (Figure 1A). In agreement with previous findings (Hocevar et al., 2001), loss of Dab2 significantly reduced Smad2 phosphorylation stimulated through endogenous or chimeric TGF-β receptors treated with TGF-β or GM-CSF, respectively (Figure 1A).
Figure 1.
Dab2 is not essential for TGF-β receptor internalization, but it impacts TGF-β Smad signaling. (A) Serum-starved Dab2 WT cells or Dab2 KD clones 16 and 18 (the numbers represent different clones of NIH-3T3 cell lines expressing stably integrated chimeric GM-CSF TGF-β receptors) were either left untreated (−) or stimulated with 50 ng/ml GM-CSF (activates the chimeric receptors) or 5 ng/ml TGF-ß (activates the native receptors) for 45 min. Lysates were prepared from parallel plates and equivalent protein (30 μg) immunoblotted with a Dab2 and a phospho-specific Smad2 antibody (pSmad2). The blot was stripped and probed for total Smad2 and GAPDH to confirm loading. (B) Internalization of 125I-labeled GM-CSF through chimeric TGF-β receptors was performed in WT and Dab2 KD clones (KD 16, 17, 18, and 50). The ratio of internalized versus cell surface receptors was determined at 1 and 2 h. The graph shows average pooled data of the four KD clones, whereas individual values of the KD clones are shown in the inset. The results represent the mean ± SD of three different experiments performed in duplicate. (C) Dab2 WT and Dab2 KD (clone 16) cells were prebound with radiolabeled antibody to the chimeric type II receptor (GMCSF2RB) and then incubated at 37°C for the indicated times. Surface antibody was removed by acid treatment at 4°C, after which cells were processed to determine internalized radioactivity (as described in methods). Internalized counts are expressed as percentage of total cell-associated radioactivity at the indicated times and represent the mean ± SD of two experiments done in duplicate. Inset. serum-starved Dab2 WT cells were either left untreated (−) or stimulated (+) with 50 ng/ml GM-CSF (lane 2) or anti-GM-CSF receptor β antibody (GMCSF2RB; 1 μg/ml lane 4; 10 μg/ml lane 6). Lysates were prepared and equivalent protein (25 μg) immunoblotted with a phospho-specific Smad2 antibody (pSmad2). The blot was stripped and probed for total Smad2.
We and others have shown that Smad phosphorylation is coupled to TGF-β receptor internalization in a number of cell types (Hayes et al., 2002; Penheiter et al., 2002; Di Guglielmo et al., 2003). Because Dab2 has been reported to associate with the type I and type II receptors (Hocevar et al., 2005) and functions as an endocytic adaptor, it seemed likely that the decreased Smad2 phosphorylation observed in Dab2 KD cells resulted from a defect in receptor trafficking. Surprisingly, when radioligand binding assays were performed, no difference in the degree or rate of internalization was detected in the WT and Dab2 KD clones (Figure 1B). A similar finding was observed when ligand-independent internalization was examined using 125I-labeled antibody to the chimeric type II receptor (Figure 1C). Smad2 phosphorylation was assessed to confirm that the receptors were not being activated by the antibody (Figure 1C, inset).
Dab2 Is Not Required for Internalization of Other Endocytic Markers
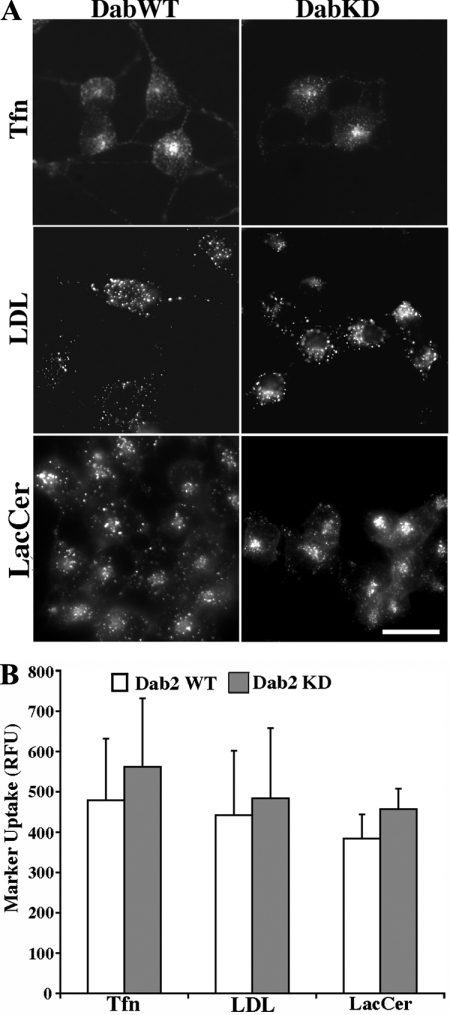
Since Dab2 KD did not affect TGF-β receptor internalization (either ligand dependent or independent; Figure 1, B and C), which occurs primarily via clathrin-mediated endocytosis (Mitchell et al., 2004), we investigated whether it was required for other clathrin-dependent as well as -independent cargo. Because Dab2 has been reported to associate with the LDL receptor (LDLR) and facilitate LDLR movement into clathrin-coated pits (Maurer and Cooper, 2006), we examined LDL internalization in WT and Dab2 KD cells. We found that loss of Dab2 had no impact on LDL internalization as the receptor internalized similarly in both cell types (Figure 2A, middle, and B). This is consistent with previous reports suggesting functional redundancy between Dab2 and other endocytic adaptors (Mishra et al., 2002; Traub, 2003). Analogous findings were observed when Tfn (clathrin-dependent) or LacCer (clathrin-independent) endocytosis was examined (Figure 2A, top and bottom, respectively; and B). Thus, similar to TGF-β endocytosis (Figure 1), loss of Dab2 has no discernible impact on LDL, Tfn, or LacCer internalization (Figures 1 and 2). Because these results are consistent with studies documenting redundancy between proteins affecting the cellular endocytic machinery (Morris et al., 2002; Traub, 2003), and internalized LDL and LacCer displayed a more perinuclear distribution after Dab2 knockdown (Figure 2), we next addressed the alternative possibility that a locale where Dab2 had an obligate function is postendocytic and remained to be identified.
Figure 2.
Dab2 knockdown does not impact internalization of transferrin, DiI-LDL, or Bodipy-LacCer. (A) Tfn (top), DiI-LDL (LDL, middle), and LacCer (bottom) were visualized via fluorescence microscopy in Dab2 WT and Dab2 KD16 cells as described in Materials and Methods. (B) Quantification of Tfn, LDL, and Lac-Cer internalization was determined in Dab2 WT and KD16 cultures by using MetaMorph and is expressed as relative fluorescence units (RFU) for each marker. Data represent the mean ± SD from at least 30 cells in each of three independent experiments. Bar, 10 μm.
Dab2 Regulates TGF-β Receptor Recycling
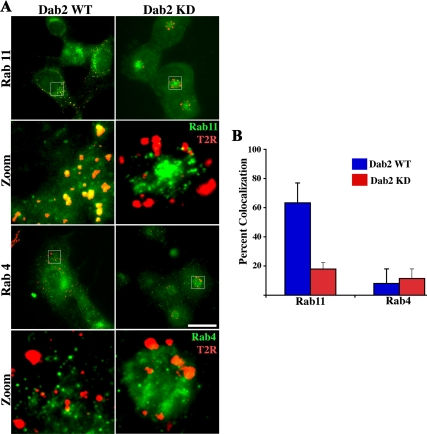
It has been documented previously that TGF-β receptors recycle via a clathrin-and Rab11-dependent pathway(s) (Mitchell et al., 2004). As such, we next examined whether this was maintained in the absence of Dab2. To address that issue, the cellular localization of type II TGF-β receptors and Rab11 were assessed by immunofluorescence in WT and Dab2 KD cells (Figure 3). Consistent with other studies, the data show that ∼60% of type II receptors colocalize with Rab11 in the WT cells 1 h after receptor internalization (Figure 3A, top left; and B). This is in contrast to the Dab2 KD cultures where <20% colocalization is observed (Figure 3A, top right; and B). Decreased colocalization in the KD cultures, however, does not reflect a shunting to an alternative pathway because there was a similar absence of type II receptor/Rab4 colocalization in both WT and Dab2 KD cells (Figure 3A, bottom; and B) nor is it a result of altered Rab4 or Rab11 expression (Supplemental Figure 2). In addition, although the endosome containing receptors in the WT cells were diffuse in appearance, in the KD cells they seemed enlarged and concentrated primarily around the perinuclear region.
Figure 3.
TGF-β receptors localize to a Rab11-positive compartment in Dab2 WT cells but not in Dab2 KD cells. (A) Chimeric TGF-β receptors stably expressed in Dab2 WT (left) and Dab2 KD16 cells (right) were bound to anti-GM-CSFR-β antibody (recognizes the extracellular domain) at 10°C and internalized at 37°C for 45 min. Cells were then acid washed to remove remaining cell surface antibodies, fixed, permeabilized, and incubated with rabbit anti-Rab11 (top) and Rab4 antibody (bottom). The Rab11/Rab4 compartment was detected by incubation with Alexa Fluor 488-labeled (green) anti-rabbit, whereas internalized receptors (red) were visualized after treatment with Cy3-conjugated anti-mouse secondary antibodies, respectively. Separate images were acquired for each fluorophore via fluorescence microscopy. Images were collected in pseudocolor and are presented as overlays. (B) Percentage of colocalization of receptors with Rab11 and Rab4 and represents the mean ± SD for 50 cells from each of three independent experiments. Bar, 10 μm.
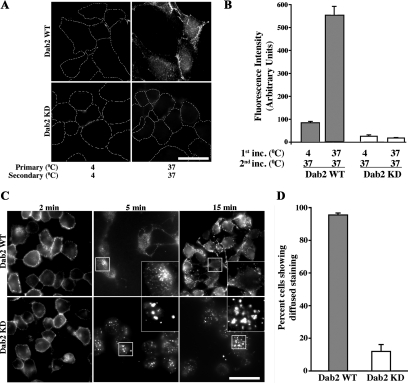
The data in Figure 3 suggest that in the absence of ligand and 1 h postinternalization, Rab11-dependent TGF-β receptor recycling is altered in Dab2 KD cells. To investigate this further, we directly examined TGF-β receptor recycling by using a modification of a procedure by Fraile-Ramos et al. (2001) where an antibody to the receptor's extracellular domain is visualized following 1.5 cycles of receptor recycling (Mitchell et al., 2004). In this assay, cells incubated at 4°C with the primary antibody (with no vesicular movement) give a similar background staining to cells that have never been treated with primary antibody due to the acid strip. Moreover, because the fluorescent secondary antibody only binds those receptors that return to the cell surface with attached primary antibody, subsequent intracellular staining is only observed after an additional internalization event. When recycling assays were performed on WT and Dab2 KD chimeric receptor expressing cells, significant differences were observed. Although TGF-β receptors recycled in the WT cells (Figure 4A, top right), there was essentially a complete absence of intracellular receptor staining in the Dab2 KD cells (Figure 4A, bottom right). As expected, staining was also absent in WT cells that were incubated at 4°C, documenting the efficacy of the acid stripping (Figure 4A, top left). The fluorescent intensity for WT and two independent Dab2 KD clones were quantified and the averages are shown in Figure 4B. Thus, although decreased Dab2 expression has no affect on either the rate or extent of chimeric TGF-β receptor internalization (Figure 1B), it completely blocks their exit and return to the cell surface.
Figure 4.
TGF-β receptor recycling is dependent on Dab2. (A) Direct recycling assay: Dab2 WT (top) and Dab2 KD16 cells stably expressing chimeric TGF-β receptors were incubated with mouse anti-GM-CSFR-β antibody for 1 h at 4 or 37°C (first incubation, left, top and bottom). Cells were subsequently washed and treated with acid to strip any surface, non-internalized antibody. Subsequently, cells were incubated with fluorescent Cy3-labeled anti-mouse secondary antibody for 1 h at 37°C (second incubation, right, top and bottom), washed, and acid treated again. This process allows only the receptors that have undergone 1.5 cycles of recycling to be fluorescently labeled (Mitchell et al., 2004). Cells were fixed, mounted, and quantified for cell associated fluorescence as described in Materials and Methods. (B) Data are represented as arbitrary units of fluorescence from 50 cells in each of three independent experiments from each clone. Images in A are from Dab2 KD16 and quantitation in B is an average of Dab2 KD16 and Dab2 KD18. (C) Transferrin recycling: Dab2 WT (top 3 panels) and Dab2 KD cells (bottom 3 panels) were incubated with Alexa Fluor 594 Tfn as described in Figure 2 for the indicated times. Cells were fixed and imaged as described in Figure 2. (D) Cells that sequestered Tfn internally in the perinuclear compartment and cells that showed diffused staining (indicative of Tfn recycling) at 15 min were counted manually. Data presented are expressed as percentage of diffused staining at 15 min seen in the WT cells and indicate the mean ± SD of 50 cells in each of three independent experiments. Images in C are from Dab2 KD16 and quantitation in D is an average of Dab2 KD16 and Dab2 KD18. Bar, 10 μm.
These findings raise three immediate questions. First, is the recycling role for Dab2 unique to the TGF-β receptor system; second, is there a defined subcellular locale in the endocytic pathway where Dab2 functions; and third, do native TGF-β receptors show a similar dependence on Dab2 for recycling as chimeric receptors? Studies were performed to address each of these questions.
A commonly used marker for examining recycling is Tfn. After endocytosis, Tfn is recycled back to the plasma membrane either through a rapid recycling pathway directly from the early/sorting endosome that uses Rab4 and Rab35 or via the endocytic recycling compartment (ERC) that requires Rab11 (Maxfield and McGraw, 2004; Grant and Donaldson, 2009). Similar to what we observed for recycling of chimeric TGF-β receptors, decreased Dab2 expression reduced diffuse Tfn staining at the plasma membrane (i.e., indicative of recycling) by ∼85% (Figure 4, C and D). Moreover, analogous to that seen previously for the Rab11 compartment (Figure 3A), the internalized Tfn accumulated in enlarged or swollen perinuclear endocytic structures that persisted (i.e., 1 h) for an extended period (Figure 4C; data not shown). Abrogation of recycling and accumulation of Tfn in similar-appearing structures also have been reported in EHD4 and myosin VI-deficient cells (Chibalina et al., 2007; Sharma et al., 2008) (see Discussion).
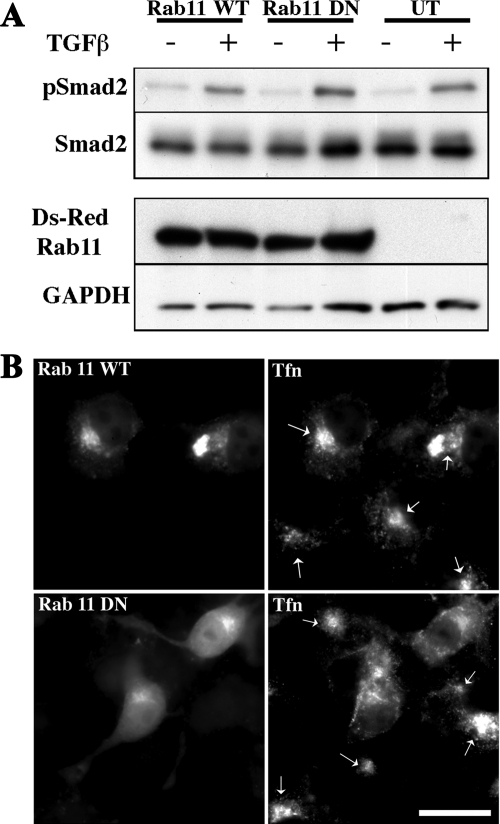
The relation of TGF-β receptor endocytic activity and signaling is controversial. Although we and others have provided evidence documenting that Smad phosphorylation is directly coupled to receptor internalization and occurs downstream of dynamin action (Hayes et al., 2002; Penheiter et al., 2002; Di Guglielmo et al., 2003), analogous studies have observed alternative (Runyan et al., 2005) or little requirement (Chen, 2009) for clathrin-dependent endocytosis and Smad signaling. Because Dab2 is required for Smad2 phosphorylation and TGF-β receptor recycling (Figures 1 and 4), we investigated whether these processes were linked. To address this question, Cos-7 cells were transiently transfected with WT or DN Rab11, and the effect of TGF-β on Smad2 phosphorylation was determined. Although Tfn recycling, as indicated by diffuse cytoplasmic and decreased pericentriolar Tfn staining (Ullrich et al., 1996; Ren et al., 1998; Choudhury et al., 2002), was prevented by the S25N DN Rab11 construct (Figure 5B), WT and DN Rab11 similarly activated Smad2 (Figure 5A).
Figure 5.
Smad2 phosphorylation occurs independent of TGF-β receptor recycling. (A) Cos-7 cells were transfected with plasmids expressing Ds-Red tagged wild type (WT; lanes 1 and 2), dominant negative (DN; lanes 3 and 4) Rab11, or no Rab construct (UT; lanes 5 and 6) together with FLAG-tagged Smad2, FLAG-tagged TGF-β type I receptor, and HA-tagged TGF-β type II receptor (lanes 1–6). To ensure that only transfected cells were examined for phosphorylation, FLAG-Smad2 was immunoprecipitated from cell lysates (900 μg) obtained from either unstimulated (−) cells or cells treated with 10 ng/ml TGF-β (+) for 1 h and examined by Western analysis with a phospho-specific Smad2 antibody (pSmad2). The blot was stripped and reprobed with an antibody to total Smad2 to confirm equivalent FLAG-Smad2 in the immunoprecipitates (second panel). Expression of the transfected Ds-Red–tagged Rab11 constructs (third panel) was confirmed on a separate gel by immunoblotting (30 μg), and equal loading was documented after stripping and reprobing with GAPDH (bottom). Endogenous Rab11 is not observed in the UT lanes as the Ds-Red construct runs at ∼50 kDa (vs. ∼21 kDa for endogenous Rabs). (B) After transient transfection with WT or DN (S25N) Rab11 constructs as described in A, parallel plates of Cos-7 cells were incubated with AF-488 transferrin (Tfn) and processed for immunofluorescence as described in Materials and Methods. The panels on the left illustrate the distribution of Ds-Red–tagged Rab11 WT (concentrated in the pericentriolar compartment) and DN (dispersed throughout the cytoplasm) in transfected cells. Right (top and bottom), Tfn distribution in Rab11-transfected and untransfected cells. Arrows indicate the pericentriolar recycling compartment in the Rab11 WT-transfected and untransfected cells (top right) and only in the untransfected Rab11 DN cells (bottom right). Cells transfected with DN Rab11 (bottom right, no arrows) show diminished pericentriolar labeling and a more dispersed cytoplasmic Tfn staining. Bar, 10 μm.
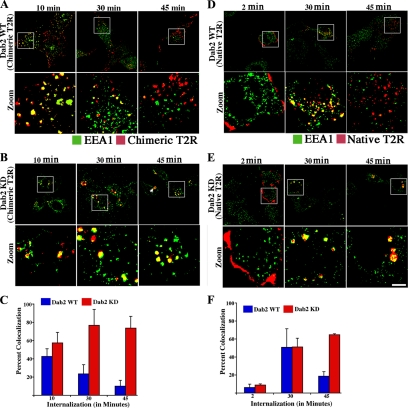
The finding that diminished Dab2 levels results in the accumulation of chimeric TGF-β receptors as well as Tfn in morphologically similar endosomal structures suggests a defined locale for Dab2 action. Because previous studies (Hayes et al., 2002; Di Guglielmo et al., 2003; To et al., 2008) have shown that TGF-β receptors colocalize with the early endocytic compartment marker EEA1, we examined the kinetics of EEA1 immunostaining in WT and Dab2 KD cells. Although ∼40% of receptors are in an EEA1 associated compartment in WT cells 10 min postinternalization, the accumulation is transient, such that by 45 min it decreases to 10% (Figure 6, A and C). In contrast, although similar colocalization of EEA1 and TGF-β receptors is initially observed in the Dab2 KD cultures (Figure 6B, first panel; and C), rather than diminishing over time as in the WT cells, the percentage of receptors in this compartment increased at 30 and 45 min (Figure 6B, middle and third panel; and C). Moreover, no receptor efflux was observed even at 2 h (data not shown). These results are consistent with a model whereby Dab2 functions in the trafficking of internalized cargo from an EEA1- to Rab11-positive compartment(s) in the recycling of plasma membrane constituents.
Figure 6.
Native and chimeric TGF-β receptors are similarly sequestered in an EEA1-positive compartment in Dab2 KD cells. (A and B) Dab2 WT and Dab2 KD16 cells stably expressing chimeric TGF-β receptors (Chimeric T2R) were incubated with GM-CSFR-β antibody for 1 h at 10°C. Receptors were internalized at 37°C for 10, 30, and 45 min. Cultures were subsequently acid washed, fixed, permeabilized, and incubated with rabbit anti-EEA1 antibody for 1 h. Internalized receptors and EEA1-positive compartments were detected by binding Cy3-labeled (red) and Alexa Fluor 488-conjugated (green) antibodies, respectively. Separate images were acquired for each fluorophore collected in pseudocolor and are presented as overlays as described in Figure 3. The boxed area is enlarged in the panel below (Zoom), whereas the arrow (B; top, third panel) shows the enlarged endosomes. (C) Percentage of colocalization of TGF-β receptors and EEA1 in Dab2 WT cells and Dab2 KD cells was determined and represents the mean ± SD of 50 cells in each of three independent experiments. Quantitation in C is an average of Dab2 KD16 and Dab2 KD17. (D and E) Dab2 WT and Dab2 KD16 cells were transiently transfected with N-terminal HA-tagged TGF-β type II receptors. Receptors were bound to mouse anti-HA antibody and processed for internalization as described in A and B for the indicated times. (F) Percentage of colocalization of native TGF-β receptors and EEA1 in Dab2 WT and Dab2 KD16 cells represents the mean ± SD of 30 cells in each of three independent experiments. Bar, 10 μm.
Last, although we have previously shown that chimeric and native TGF-β receptors have identical trafficking and signaling actions (Anders and Leof, 1996; Penheiter et al., 2002; Mitchell et al., 2004), a recent study reported differences in initial membrane compartmentalization dependent upon the extracellular ligand binding domain (Luga et al., 2009). To determine whether these findings impacted our conclusions on TGF-β receptor recycling, we investigated the role of Dab2 in recycling of endogenous type II receptors. Because reagents are not available to clearly analyze endogenous receptors, WT and Dab2 KD cells were transfected with epitope-tagged receptors and ligand-independent internalization assessed at 2, 30, and 45 min (Figure 6, D–F). Similar to what we observed with the chimeric receptors, although significant association occurred in both WT and Dab2 KD cultures by 30 min (compare Figure 6, D and E, middle), endogenous type II receptors traversed out of the EEA1-positive compartment by 45 min in WT cultures (Figure 6D, bottom right). However, in the absence of Dab2 this efflux was prevented (Figure 6E, bottom right) and enlarged perinuclear endosomal structures, indicative of receptors trapped and accumulating in the compartment and similar to structures seen with the chimeric receptors and Tfn staining (Figures 3A, 4C, and 6B; and Supplemental Figure 3), were apparent. Thus, Dab2 provides an analogous role in the recycling of native and chimeric TGF-β receptors. It should be noted that the percentage of colocalization of the native (WT) receptor with EEA1 (Figure 6F) is greater than that observed with the chimeric receptor (Figure 6C) at 30 min; however, the trend of efflux from the EEA1-positive compartment is identical. Although this may simply reflect overexpression of the native receptors (due to transient transfections) leading to their accumulation in the early endosome compartment, at 45 min both native and chimeric receptors have traversed out of the EEA1 compartment in WT cells (Figure 6, C and F).
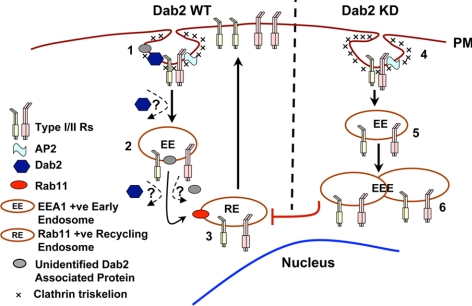
A schematic depicting these considerations is presented in Figure 7. On clathrin-dependent internalization native and/or chimeric TGF-β receptors enter an EEA1-positive compartment. Dab2 (and potentially other unidentified associated proteins) functions to promote exit and subsequent recycling to the cell surface via a Rab11-regulated process. In the absence of Dab2, however, the appearance of enlarged endosomes reflects the inability of internalized TGF-β receptors to depart the EEA1 compartment and enter the Rab11-positive recycling compartment.
Figure 7.
Proposed itinerary of TGF-β receptors in Dab2 WT and Dab2 KD cells. 1) TGF-β receptors internalize via clathrin-coated pits where AP2, Dab2, and other adaptors may be present. Dab2 is not required for initial endocytosis. 2) In the absence of ligand, receptors travel to an EEA1-positive early endosome compartment (EE) and travel back to the cell surface via a Rab11-positive recycling endosome (RE) (3). Because Dab2 binds the receptors constitutively (Hocevar et al., 2001), it is currently unknown whether it accompanies the receptor complex to the early endosome, or alternatively, dissociates after internalization. Dab2 or an unidentified associated adaptor protein recruited by Dab2, may assist in translocation of receptors to the Rab11-positive recycling compartment (3). In the absence of Dab2 and/or Dab2-associated proteins, endocytosed receptors (4) are sequestered in a perinuclear EEA1 associated compartment (5), some of which become enlarged early endosomes (EEE). Although the type I receptor was not directly assessed, our previous work has established that both type I and type II receptors display similar trafficking behavior (Mitchell et al., 2004).
DISCUSSION
Dab2 is a cargo specific adaptor protein that functions independently (Traub, 2003; Maurer and Cooper, 2006) or cooperatively with other clathrin adaptors (Morris and Cooper, 2001) to facilitate clathrin-coat assembly by coordinating cargo selection and lattice polymerization. Dab2 is also part of a multiprotein signaling complex that associates with TGF-β receptors and with the downstream signaling intermediaries Smad2 and Smad3. Given the importance of Dab2 as a clathrin adaptor, and the significance of clathrin-mediated endocytosis in receptor function (Doré et al., 1998; Penheiter et al., 2002; Mitchell et al., 2004), we examined the effect of Dab2 knockdown on TGF-β receptor trafficking. Although the loss of Dab2 diminished Smad2 phosphorylation by ∼70–90%, it had no effect on TGF-β receptor, LDLR, transferrin or LacCer internalization (Figures 1 and 2). Although the observations with LDL and Tfn agree with reports documenting that in the absence of Dab2 other PTB domain proteins or endocytic adaptors can act in a compensatory manner to mediate clathrin-dependent endocytosis (Mishra et al., 2002; Keyel et al., 2006; Maurer and Cooper, 2006), caveolar uptake (i.e., LacCer; Singh et al., 2003) had not been examined. In contrast to the LDL and Tfn findings, the absence of any discernible role for Dab2 on TGF-β receptor internalization was surprising as it has been shown previously that Smad2/3 phosphorylation occurs in a compartment downstream of dynamin action (Hayes et al., 2002; Penheiter et al., 2002; Di Guglielmo et al., 2003). As such, the possibility that Dab2 had an additional role in the endocytic activity of the TGF-β receptor complex was investigated.
Plasma membrane composition is determined by the balance between endocytic uptake and recycling of macromolecules (Maxfield and McGraw, 2004; Grant and Donaldson, 2009). Recycling, defined as the return of a particular membrane molecule to the cell surface after internalization, is a fundamental process that can occur via clathrin-dependent or -independent mechanisms (Maxfield and McGraw, 2004; Grant and Donaldson, 2009). Although constitutive recycling of Tfn and LDL receptors occurs to transport critical nutrients, an analogous rationale for other cell surface receptors has not been as apparent. One possibility, however, is that it might reflect a cellular adaptation to the highly oxidizing extracellular environment that these proteins are exposed. As such, the endocytic Rab 11-positive recycling compartment might provide a “quick-fix” of the damage needed to maintain receptor integrity and functionality. Alternatively, recent computational models indicate 1) that the particular biological response to TGF-β can be modified by the pattern of receptor trafficking (Vilar et al., 2006); and 2) the kinetics of Smad signaling are regulated by depleting TGF-β via constitutive TGF-β type II receptor recycling (Clarke et al., 2009). These findings provide a new urgency to understanding the mechanisms controlling the endocytic activity of the TGF-β receptor complex as they not only address the concept of context-specific TGF-β signaling, but suggest that cells displaying an absence of/alteration in recycling (i.e., tumors with loss of the type II receptor or Dab2) would show enhanced levels of TGF-β that might contribute to an aberrant stromal desmoplastic response and/or protection from immune cell killing.
TGF-β receptor recycling was initially proposed/reported >20 y ago (Massagué and Like, 1985; Sathre et al., 1991). Although there have been relatively few subsequent studies, we reported previously that ligand-dependent and -independent TGF-β receptor recycling was dependent upon clathrin-mediated endocytosis and Rab11 (Mitchell et al., 2004). Because Dab2 is a known clathrin adaptor that bound the TGF-β receptor complex and had recently been suggested to have a role in recycling (Teckchandani et al., 2009), we investigated whether Dab2 functioned in an endocytic compartment downstream of initial receptor internalization. In support of that hypothesis there was an ∼70% decrease in the colocalization of Rab11 and type II TGF-β receptors in Dab2 knockdown cells (Figure 3). Because this observation strongly suggested a defect or alteration in the recycling pathway, we directly examined the role of Dab2 in ligand-independent, constitutive TGF-β receptor recycling. These studies demonstrated that in the absence of Dab2 there was essentially a complete abrogation of chimeric or native TGF-β receptor recycling and a block of receptor trafficking in an EEA1-positive, Rab11-negative compartment (Figures 3, 4, and 6). Furthermore, loss of Dab2 led to the accumulation of receptors (as well as Tfn) in aberrantly enlarged perinuclear endosomes surrounded by, and associated with, structures that stain positive for EEA1 (Figures 4 and 6 and Supplemental Figure 3). Similar structures have been observed in previous publications and it has been documented that depletion of EHD4, a C-terminal EH domain protein that regulates traffic from early endosomes to the recycling compartment, leads to the formation of enlarged endosomes that contain Tfn and EEA1 (Sharma et al., 2008). Because Dab2 contains five NPF (Asn-ProPhe) motifs that bind EH domains, loss of Dab2 might impact the role of EH domain proteins in regulating endosomal traffic and generate a phenotype similar to that seen with EHD4 knockdown (Sharma et al., 2008).
Another protein whose loss mimics the Dab2 KD phenotype at the endosomal level is myosin VI. Dab2 has a myosin VI binding domain (Hasson, 2003), and it associates with the C-terminal tail of myosin via its C-terminal serine- and proline-rich region. It is also known to recruit myosin VI to clathrin-coated structures in the early endocytic pathway (Morris et al., 2002; Spudich et al., 2007), and depletion of myosin VI similarly leads to the formation of EEA1-positive–enlarged endocytic vesicles where Tfn is trapped (Chibalina et al., 2007). Although the exact mechanism(s) regulating the phenotype seen in our study is unknown, it probably reflects the inability to recruit analogous Dab2-interacting proteins that assist receptors from the early to recycling endosome. A model where these considerations are discussed is presented in Figure 7. Although how or where the endocytic and signaling function(s) of Dab2 converge is currently unknown, the effect of Dab2 on recycling and Smad2 phosphorylation is independent and Smad activation occurs upstream (or in an alternative pathway) of the transition from the early to the recycling endosome. Whether Dab2 has a role in non-Smad signaling is not known; however, our preliminary evidence suggests that it is affected, but not to a similar extent as that seen for Smad phosphorylation.
In summary, we present evidence that 1) Smad2 phosphorylation can be inhibited downstream of receptor internalization; 2) loss of Dab2 is not sufficient to inhibit uptake of clathrin-dependent or -independent cargo; and, most importantly, 3) Dab2 has an obligate role in constitutive TGF-β receptor recycling because it is required for receptor trafficking from an EEA1-positive compartment to the Rab11-positive recycling compartment. Current studies are focusing on identifying additional components of the endocytic machinery and how they might integrate with Dab2 and impact recycling and turnover of TGF-β receptors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. David Marks and Alan Penheiter for helpful discussions regarding experimental ideas and planning of this manuscript. This work was supported by funds from National Institutes of Health grants GM-54200 and GM-55816 and the Mayo Foundation (to E.B.L.) and GM-22942 (to R.E.P.).
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-12-1019) on September 29, 2010.
REFERENCES
- Anders R. A., Arline S. L., Doré J.J.E., Leof E. B. Distinct endocytic responses of heteromeric and homomeric transforming growth factor ß receptors. Mol. Biol. Cell. 1997;8:2133–2143. doi: 10.1091/mbc.8.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders R. A., Doré J.E.J., Arline S. A., Garamszegi N., Leof E. B. Differential requirements for type I and type II TGFß receptor kinase activity in ligand-mediated receptor endocytosis. J. Biol. Chem. 1998;273:23118–23125. doi: 10.1074/jbc.273.36.23118. [DOI] [PubMed] [Google Scholar]
- Anders R. A., Leof E. B. Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-ß (TGF-ß) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-ß signaling. J. Biol. Chem. 1996;271:21758–21766. doi: 10.1074/jbc.271.36.21758. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Chen C.-Y., Knuehl C., Towler M. C., Wakeham D. E. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Chen Y. G. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- Chibalina M. V., Seaman M. N., Miller C. C., Kendrick-Jones J., Buss F. Myosin VI and its interacting protein LMTK2 regulate tubule formation and transport to the endocytic recycling compartment. J. Cell Sci. 2007;120:4278–4288. doi: 10.1242/jcs.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A., Dominguez M., Puri V., Sharma D. K., Narita K., Wheatley C. L., Marks D. L., Pagano R. E. Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 2002;109:1541–1550. doi: 10.1172/JCI15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D. C., Brown M. L., Erickson R. A., Shi Y., Liu X. Transforming growth factor beta depletion is the primary determinant of Smad signaling kinetics. Mol. Cell. Biol. 2009;29:2443–2455. doi: 10.1128/MCB.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Zhang Y. E. Smad-dependent and Smad-independent pathways in TGF-ß family member signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G. M., Leroy C., Goodfellow A. F., Wrana J. L. Distinct endocytic pathways regulate TGF-β receptor signaling and turnover. Nat. Cell. Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Doré J.J.E., Jr, Edens M., Garamszegi N., Leof E. B. Heteromeric and homomeric transforming growth factor-ß receptors show distinct signaling and endocytic responses in epithelial cells. J. Biol. Chem. 1998;273:31770–31777. doi: 10.1074/jbc.273.48.31770. [DOI] [PubMed] [Google Scholar]
- Fazili Z., Sun W., Mittelstaedt S., Cohen C., Xu X. X. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene. 1999;18:3104–3113. doi: 10.1038/sj.onc.1202649. [DOI] [PubMed] [Google Scholar]
- Feng X.-H., Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos A., Kledal T. N., Pelchen-Matthews A., Bowers K., Schwartz T. W., Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell. 2001;12:1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. D., Donaldson J. G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T. Myosin VI: two distinct roles in endocytosis. J. Cell Sci. 2003;116:3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- Hayes S., Chawla A., Corvera S. TGFß receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar B. A., Prunier C., Howe P. H. Disabled-2 (Dab2) mediates transforming growth factor beta (TGFbeta)-stimulated fibronectin synthesis through TGFbeta-activated kinase 1 and activation of the JNK pathway. J. Biol. Chem. 2005;280:25920–25927. doi: 10.1074/jbc.M501150200. [DOI] [PubMed] [Google Scholar]
- Hocevar B. A., Smine A., Xu X.-X., Howe P. H. The adaptor molecule disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikushima H., Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat. Rev. Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- Keyel P. A., Mishra S. K., Roth R., Heuser J. E., Watkins S. C., Traub L. M. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V., McLean S., Le Roy C., O'Connor-McCourt M., Wrana J. L., Di Guglielmo G. M. The extracellular domain of the TGFbeta type II receptor regulates membrane raft partitioning. Biochem. J. 2009;421:119–131. doi: 10.1042/BJ20081131. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-ß signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massagué J., Like B. Cellular receptors for type β transforming growth factor. J. Biol. Chem. 1985;260:2636–2645. [PubMed] [Google Scholar]
- Massagué J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Maurer M. E., Cooper J. A. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J. Cell Sci. 2006;119:4235–4246. doi: 10.1242/jcs.03217. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Keyel P. A., Hawryluk M. J., Agostinelli N. R., Watkins S. C., Traub L. M. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H., Choudhury A., Pagano R. E., Leof E. B. Ligand-dependent and -independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol. Biol. Cell. 2004;15:4166–4178. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S. C., Wong K. K., Chan R. K., Lau C. C., Tsao S. W., Knapp R. C., Berkowitz R. S. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol. Oncol. 1994;52:247–252. doi: 10.1006/gyno.1994.1040. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Arden S. D., Roberts R. C., Kendrick-Jones J., Cooper J. A., Luzio J. P., Buss F. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Cooper J. A. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2 [erratum appears in Traffic 3, 236, 2002] Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Heldin C.-H. Non-Smad TGF-β signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Souchelnytskyi S., Heldin C. H. Smad regulation in TGF-beta signal transduction J. Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Penheiter S. G., Mitchell H., Garamszegi N., Edens M., Doré J.J.E., Jr, Leof E. B. Internalization-dependent and -independent requirements for transforming growth factor ß receptor signaling via the Smad pathway. Mol. Cell. Biol. 2002;22:4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier C., Howe P. H. Disabled-2 (Dab2) is required for transforming growth factor beta-induced epithelial to mesenchymal transition (EMT) J. Biol. Chem. 2005;280:17540–17548. doi: 10.1074/jbc.M500974200. [DOI] [PubMed] [Google Scholar]
- Puri V., Watanabe R., Singh R. D., Dominguez M., Brown J. C., Wheatley C. L., Marks D. L., Pagano R. E. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 2001;154:535–547. doi: 10.1083/jcb.200102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R. A., Andrianifahanana M., Wilkes M. C., Edens M., Kottom T. J., Blenis J., Leof E. B. Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-beta. Cancer Res. 2009;69:84–93. doi: 10.1158/0008-5472.CAN-08-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Xu G., Zeng J., De Lemos-Chiarandini C., Adesnik M., Sabatini D. D. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan C. E., Schnaper H. W., Poncelet A. C. The role of internalization in TGF-β1-induced Smad2 association with SARA and Smad2-dependent signaling in human mesangial cells. J. Biol. Chem. 2005;280:8300–8308. doi: 10.1074/jbc.M407939200. [DOI] [PubMed] [Google Scholar]
- Sathre K. A., Tsang M.L.-S., Weatherbee J. A., Steer C. J. Binding and internalization of transforming growth factor-β1 by human hepatoma cells: evidence for receptor recycling. Hepatology. 1991;14:287–295. [PubMed] [Google Scholar]
- Sharma M., Naslavsky N., Caplan S. A role for EHD4 in the regulation of early endosomal transport. Traffic. 2008;9:995–1018. doi: 10.1111/j.1600-0854.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Singh R. D., Puri V., Valiyaveettil J. T., Marks D. L., Bittman R., Pagano R. E. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell. 2003;14:3254–3265. doi: 10.1091/mbc.E02-12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. R., Capo-chichi C. D., He J., Smedberg J. L., Yang D. H., Prowse A. H., Godwin A. K., Hamilton T. C., Xu X. X. Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J. Biol. Chem. 2001;276:47303–47310. doi: 10.1074/jbc.M106158200. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986;233:532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Spudich G., Chibalina M. V., Au J. S., Arden S. D., Buss F., Kendrick-Jones J. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat. Cell Biol. 2007;9:176–183. doi: 10.1038/ncb1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teckchandani A., Toida N., Goodchild J., Henderson C., Watts J., Wollscheid B., Cooper J. A. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J. Cell Biol. 2009;186:99–111. doi: 10.1083/jcb.200812160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To C., Kulkarni S., Pawson T., Honda T., Gribble G. W., Sporn M. B., Wrana J. L., Di Guglielmo G. M. The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-imidazolide alters transforming growth factor beta-dependent signaling and cell migration by affecting the cytoskeleton and the polarity complex. J. Biol. Chem. 2008;283:11700–11713. doi: 10.1074/jbc.M704064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub L. M. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. P., Ely B. D., Li Y., Pong R. C., Hsieh J. T. Regulation of rat DOC-2 gene during castration-induced rat ventral prostate degeneration and its growth inhibitory function in human prostatic carcinoma cells. Endocrinology. 1998;139:3542–3553. doi: 10.1210/endo.139.8.6159. [DOI] [PubMed] [Google Scholar]
- Tseng C. P., Ely B. D., Pong R. C., Wang Z., Zhou J., Hsieh J. T. The role of DOC-2/DAB2 protein phosphorylation in the inhibition of AP-1 activity. An underlying mechanism of its tumor-suppressive function in prostate cancer. J. Biol. Chem. 1999;274:31981–31986. doi: 10.1074/jbc.274.45.31981. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbé S., Zerial M., Parton R. G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar J. M., Jansen R., Sander C. Signal processing in the TGF-beta superfamily ligand-receptor network. PLoS Comput. Biol. 2006;2:e3. doi: 10.1371/journal.pcbi.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes M. C., Mitchell H., Gulati-Penheiter S., Doré J. J., Suzuki K., Edens M., Sharma D. K., Pagano R. E., Leof E. B. Transforming growth factor-β activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 2005;65:10431–10440. doi: 10.1158/0008-5472.CAN-05-1522. [DOI] [PubMed] [Google Scholar]
- Wilkes M. C., Murphy S. J., Garamszegi N., Leof E. B. Cell-type-specific activation of PAK2 by transforming growth factor β independent of Smad2 and Smad3. Mol. Cell. Biol. 2003;23:8878–8889. doi: 10.1128/MCB.23.23.8878-8889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X. X., Yi T., Tang B., Lambeth J. D. Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene. 1998;16:1561–1569. doi: 10.1038/sj.onc.1201678. [DOI] [PubMed] [Google Scholar]
- Zhou J., Hsieh J.-T. The inhibitory role of DOC-2/DAB2 in growth factor receptor-mediated signal cascade. J. Biol. Chem. 2001;276:27793–27798. doi: 10.1074/jbc.M102803200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.