Abstract
The purpose of this survey is to generate baseline data on the level of HIV infection awareness and willingness to participate (WTP) in hypothetical vaccine trials, ahead of any trial conduct in Nigeria. In a cross-sectional survey, 500 respondents were interviewed, including sex workers, male motorcycle taxi drivers, students, and the general public. About 153 (30.6%) of the respondents did not believe that correct and consistent use of condom can protect people from getting HIV, while about 66 (13.2%) respondents believed it is possible to get HIV by sharing meal with an infected person. Population groups considered at high risk for HIV were less aware of the disease, however, they were more willing to participate in HIV vaccine trials compared those at low risk of the disease. A total of 55% expressed WTP in a hypothetical vaccine trial after they were informed about it. Age, population group, and ethnicity were significantly associated with WTP.
Keywords: HIV awareness, willingness, vaccine trials, Nigeria
Introduction
An estimated 3.1% of adults between ages 15–49 in Nigeria live with HIV and AIDS (UNAIDS 2008 report). With population of 140 million, Nigeria has greater mortality and morbidity resulting from HIV/AIDS than many other African countries with higher prevalence rates. The epidemic in Nigeria is largely driven by circulating recombinant subtypes A, G, and CRF02_AG (McCutchan et al., 1999; Peeters et al., 2000). Although previous research has indicated a number of HIV-1 subtypes and HIV-2 in Nigeria, studies have documented a wide distribution of subtype CRF02_AG in all parts of the country and confirmed the predominance of this clade in some parts of West Africa (Abimiku et al., 1994; Agwale et al., 2002; Howard, Olaylele, & Rasheed, 1994; Zeh et al., 2005). Despite considerable efforts in prevention and the reported decline in the median prevalence, the HIV epidemic in Nigeria is still rampant, justifying the development of HIV vaccines tailored to the local epidemic. The Nigerian national vaccine plan and guidelines for conducting HIV vaccine trials and development has recently been ratified in collaboration with the World Health Organization (WHO).
The disappointing setback from the STEP Merck human phase 2b efficacy trial generated concerns over the future of HIV vaccine development (Buchbinder et al., 2008; Robb, 2008). The trial was designed to elicit a cellular immune response to control viral loads in the HIV-infected vaccinees, however, it resulted in more HIV infections in the intervention group compared to the placebo. This led to the calls for a re-evaluation in strategies and overall approach in the discovery research, clinical research, and the use of animal models (Sekaly, 2008; Watkins, Burton, Kallas, Moore, & Koff, 2008). However, the outcome of the recently concluded Phase III trial in Thailand in which for the first time a substantially less HIV infection was recorded in the intervention group compared to the placebo (Rerks-Ngarm et al., 2009), raised the hope that developing an HIV vaccine was indeed possible and has re-energized the field leading to more trials in the future. It is thus important to conduct a baseline assessment of communities with high burden of HIV infection that might play a critical role in future HIV vaccine trials.
Several studies on different high-risk group conducted outside the continent of Africa indicated declining willingness to participate (WTP) after participants learned about the hazards of HIV vaccine trial participation (Halpern, Metzger, Berlin, & Ubel, 2001). However, previous studies in some African communities showed the more participants know about the trials the more willing they are to volunteer (Kiwanuka et al., 2004; Smit et al., 2006). The vaccine preparedness survey reported here was designed to capture the response of population groups considered at low and high risk of HIV infection in Abuja, Nigeria with respect to awareness related to HIV infection and WTP in a hypothetical vaccine trial.
Methods
Study design
This study was conducted from June to August 2006 using a cross-sectional study design. Stratified random sampling of sex workers (SW), motorcycle taxi drivers (MCTD), students (ST), and general public (GP) was performed. About 500 (90%) out of a total of 555 respondents that provided an oral consent were interviewed, the rest either declined to participate or were ineligible for the study. Eligibility was defined by participant's age (18 years or older), an oral consent for the interview, and belonging to one of the population groups listed above. We further divided the study population into a low- and high-risk groups suitable for Phases I/II and III, respectively, for vaccine trials. The distinction was based on behavioral risk, we defined high-risk individuals as female who trade sex for money (SW) or male MCTD who were usually young, single, less educated, and mobile with disposable income. ST and the GP were in comparison to the former considered to be at lower risk of acquiring the HIV infection.
The SW were camp/brothel-based. Camps were selected at random from a list of camps and brothels already developed for an ongoing HIV seroprevalence survey. The MCTD's stations were similarly selected from an existing list of stations available within Abuja metropolis at that time. The ST interviewed were undergraduates from the University of Abuja. In all of the sites, participants were selected with the toss of a coin. The survey instrument was tested a priori with a pilot study of 50 participants from similar population groups. The survey was administered in privacy by same-sex interviewers and all questions were asked in plain English or the popular Nigerian market English (pidgin) spoken by a majority of the semiliterate population. Respondents who could speak neither were excluded from the study.
Participants were generally questioned on HIV existence (if they had ever heard of the virus HIV or an illness called AIDS), its modes of transmission, prevention, and risks factors for transmission. Other questions covered vaccinations, vaccine trials, WTP in HIV vaccine trials, and availability for an extended follow-up. The average duration for the interview was 18 minutes. The protocol for this survey was reviewed and approved by the institutional review board at Asokoro Hospital and the National Institute for Pharmaceutical Research and Development, Idu-Abuja.
Variables
The outcome variables were HIV awareness and WTP in HIV vaccine trials. HIV awareness was defined by the mean proportion of correct responses to 14-scaled items on HIV transmission and prevention. WTP was defined by a binary response to the question, “If HIV vaccine trials were to be conducted here in Abuja, would you be willing to participate?” The independent variables were gender, age (18–25, 26–32, 33–39, and 40 years or older), education (none, primary school graduate, secondary school graduate, and diploma or higher), population (ST, SW, MCTD, and the GP), religion (traditional, Christianity, and Islam), marital status with levels: married, single, living together, and other (widowed, divorced, and separated), and ethnicity (Igbo, Yoruba, Hausa/Fulani and others-minority tribes).
Statistical methods
Statistical analysis software (SAS, version 9.1) was used to analyze the data. Microsoft Access XP database were used to enter data collected into different tables related by participants' ID. The data were entered once, however, applicable error checking and data validation like the input mask, validation rule, validation text, and referential integrity were set-up and enforced before the start of data entry.
HIV infection awareness was evaluated with a 14-item scale on HIV transmission and prevention. Response to each of the 14 items was dichotomous (yes/no). The software was used to determine the proportion of correct responses for each individual. The mean of the sum of all the proportions for the entire study sample was used to define the mean awareness level. Scores above the mean were considered as high awareness levels, while those below or equal to the mean were considered as low awareness levels. The scale ranged between 0 and 1.0 and its reliability was assessed with Cronbach's alpha (c = 0.69). In general, a coefficient ≥0.70 indicates satisfactory reliability. A T-test was used to assess the difference in the mean awareness between the population groups. WTP was defined by a binary response (yes or no). Pearson Chi-square and Fisher's exact test, where applicable, were used to examine the significance of associations between categorical groups. P-values of 0.05 or less were considered statistically significant. Multiple logistic regressions were used to determine the association between the outcome variable WTP and the relevant independent variables. Levels of variables that were not found to significantly differ from each other within the same variable at the preliminary analyses were merged together in the subsequent analyses.
Results
Study population
Table 1 presents the demographic characteristics of the study population. Of the 500 respondents interviewed, most (n = 372; 74.4%) of them were aged between 18 and 32 years (mean age, 28 ± 6.2). More than half were secondary school graduates (n = 275; 55%). ST constituted about 20% (n = 103) of the participants while there were equal proportions (n = 197) of SW/MCTD and GP. The majority (n = 379; 75.8%) of the respondents were of Christian faith.
Table 1.
Demographic characteristics of participants in HIV awareness and vaccine trial feasibility survey in Abuja, Nigeria.
| Variable | N (500) | % |
|---|---|---|
| Age | ||
| 18–25 years | 190 | 38 |
| 26–32 years | 182 | 36.4 |
| 33–39 years | 79 | 15.8 |
| >40 years | 49 | 9.8 |
| Education | ||
| None | 12 | 2.4 |
| 6 years of education | 58 | 11.6 |
| 12 years of education | 275 | 55 |
| > 12 years of education | 155 | 31 |
| Population | ||
| Student | 103 | 20.8 |
| SW + MCTD | 197 | 39.7 |
| General public | 196 | 39.5 |
| Religion | ||
| Traditional | 11 | 2.2 |
| Christianity | 379 | 75.8 |
| Islam | 110 | 22 |
| Marital status | ||
| Married | 155 | 31 |
| Living together | 16 | 3.2 |
| Others | 46 | 9.2 |
| Single | 283 | 56.6 |
| Gender | ||
| Male | 282 | 57.4 |
| Female | 209 | 42.6 |
| Ethnic group | ||
| Others | 217 | 43.4 |
| Hausa/Fulani | 67 | 13.4 |
| Yoruba | 51 | 10.2 |
| Igbo | 165 | 33 |
Note: SW, sex worker; MCTD, motorcycle taxi driver.
HIV infection awareness
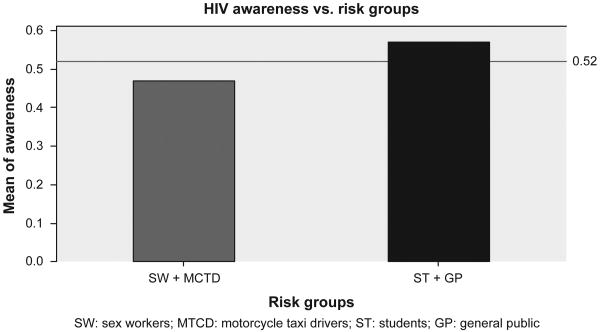
All the respondents approached had heard of HIV or the illness called AIDS before the survey, so the analysis focused mainly on differentiating high from low awareness. The mean HIV awareness score of the study sample was 0.52 (SD = 0.17). About 252 (50.4%) had an awareness level above the mean (high). The mean awareness level of the group at higher risk of HIV was 0.47 (SD = 0.15), while the group at lower risk had a mean awareness level of 0.57 (SD = 0.17) as summarized in Figure 1. Among the 14 items, we used to measure HIV awareness was the respondent's belief on correct and consistent use of condom in preventing contraction of HIV. A substantial proportion (30.6%) of the respondents did not believe that correct and consistent use of condom could protect people from getting HIV, while some 13.2% believed it was possible to get HIV by sharing a meal with a person who has the virus. About 285 (57%) claimed to had ever been tested for HIV, and of these, 48 (17%) admitted being requested to undergo additional testing to confirm their status. The high-risk group was more likely to have a low awareness level compared to the low-risk group (adjusted OR = 1.8; 95% CI = 1.1, 2.9). HIV awareness was also strongly associated with respondents' level of education. Individuals at the 6 years or less education level were more likely to have a low level of awareness compared to individuals at the secondary or above level of education (adjusted OR = 4.1; 95% CI = 1.9, 8.5). However, no other variable was found to be significantly associated with respondents' level of awareness.
Figure 1.
Awareness across population groups at “high” and “low” risk of HIV infection.
Willingness to participate (WTP) in future HIV vaccine trials
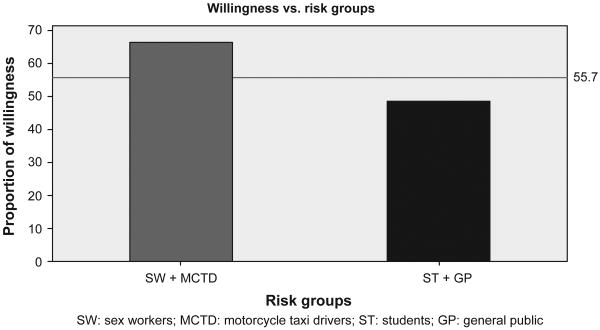
In all, 445 participants (91%) admitted having ever received vaccination in the past for prevention of other illnesses before the survey. About 443 (88.6%) of the participants believed it was possible to develop a vaccine that can prevent against HIV infection. A total of 55.4% indicated their WTP in future HIV vaccine trials after they were informed about it. The high-risk group expressed more WTP compared to the low-risk group (Figure 2). Some correlates of WTP in this population were also identified. Table 2 presents the proportion, unadjusted and adjusted associations of WTP with selected covariates.
Figure 2.
Proportion of expressed willingness to participate in HIV vaccine trials by population groups at “high” and “low” risk of HIV infection.
Table 2.
Selected covariates and WTP, unadjusted and adjusted associations.
| Variable | N (500) | Willingness to participate | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
|
|---|---|---|---|---|---|
| Yes, N (277)% |
No, N (223)% |
||||
| Population | |||||
| SW + MCTD | 197 | 131 (66.5) | 66 (33.5) | 2.1 (1.5, 3.1) | 1.9 (1.2, 3.1) |
| Students + GP | 299 | 145 (48.5) | 154 (51.5) | Reference | |
| Age | |||||
| 18–32 years | 372 | 223 (59.9) | 149 (40.1) | 2.1 (1.4, 3.1) | 2.0 (1.1, 3.6) |
| ≥ 33 years | 128 | 54 (42.2) | 74 (57.8) | Reference | |
| Education | |||||
| ≤ 6 years | 70 | 38 (54.3) | 32 (45.7) | 0.9 (0.6, 1.6) | 1.2 (0.6, 2.2) |
| ≥ 12 years | 430 | 239 (55.6) | 191 (44.4) | Reference | |
| Marital status | |||||
| Married | 155 | 79 (51.0) | 76 (49.0) | 0.8 (0.5, 1.2) | 1.1 (0.6, 2.0) |
| Living together | 16 | 4 (25.0) | 12 (75.0) | 0.3 (0.1, 0.8) | 0.4 (0.1, 1.4) |
| Others | 46 | 33 (71.7) | 13 (28.3) | 1.9 (1.0, 3.8) | 0.9 (0.3, 2.4) |
| Single | 283 | 161 (56.9) | 122 (43.1) | Reference | |
| Gender | |||||
| Male | 282 | 150 (53.2) | 132 (46.8) | 0.8 (0.6, 1.2) | 0.8 (0.5, 1.3) |
| Female | 209 | 122 (58.4) | 87 (41.6) | Reference | |
| Awareness | |||||
| Low | 248 | 136 (54.8) | 112 (45.2) | 1.0 (0.7, 1.4) | 1.2 (0.8, 1.9) |
| High | 252 | 141 (55.9) | 110 (44.1) | Reference | |
| Ethnic group | |||||
| Tiv, Idoma, and Igala | 80 | 57 (71.3) | 23 (28.7) | 2.8 (1.6, 4.7) | 2.6 (1.5, 4.6) |
| H/F, Yoruba, and Igbo | 283 | 134 (47.4) | 149 (52.7) | Reference | |
Note: OR, odds ratio; CI, confidence interval.
Compared to ST and GP, SW and MCTD (high-risk group) were more likely to be willing to participate (OR = 1.9; 95% CI = 1.2, 3.1) in HIV vaccine trials. The association between gender and WTP was, however, not significant. Participants aged between 18 and 32 years were also more likely to express WTP compared to those aged 33 years or older (OR = 2.0; 95% CI = 1.1, 3.6). In addition, minority ethnic groups (Tiv, Igala, and Idoma) were more likely to be willing to participate compared to the three major groups (Igbo, Yoruba, and Hausa-Fulani) (OR = 2.6; 95% CI = 1.5, 4.6). About 48% of the minority ethnic groups interviewed fell within the high-risk group compared to the 35% in the major ethnic groups. However, the association between HIV awareness and WTP was not significant in this model.
Characteristics of potential vaccine participants
High-risk population group, younger age, and ethnicity not from the major linguistic/ethnic group remained significantly associated with WTP in vaccine trials. To further characterize which group would be most suitable for vaccine trial participation and provide a better target for recruitment efforts, we looked across all subjects to identify those who would be reliable volunteers. We created and defined “reliable volunteers” as having a high level of HIV awareness, WTP, and ever tested for HIV. We also defined “feasibility for participation” as availability for long-term follow-up, WTP, and ever tested for HIV. Only 15 (7.6%) of the 197 high-risk individuals interviewed were likely to be reliable volunteers; the proportion of this group who might feasibly participate was equally low; 35 (17.8%) out of 197.
Discussion
This survey, to our knowledge, was the first to look at HIV infection awareness and WTP in HIV vaccine trials in different Nigerian sub-populations. The level of HIV awareness for this study population was low even though the entire sample of respondents knew of HIV or the illness called AIDS. This is consistent with the findings of similar surveys in Nigeria (Ekabua, Oyo-Ita, Ogaji, & Omuemu, 2006; Iliyasu, Kabir, Galadanci, Abubakar, & Aliyu, 2005), in which nearly all the respondents knew of HIV but less than half of the people interviewed had a good knowledge of HIV transmission. Awareness was lower among the most vulnerable sub-populations (high-risk groups) that are critical for stemming the tide of the epidemic and who are also potential targets for future vaccine trials. The relative high level of awareness among the group consisting of the undergraduate ST and GP is not unexpected. Several previous studies reported similar findings (Harding, Anadu, Gray, & Champeau, 1999; Onah, Mbah, Chukwuka, & Ikeme, 2004). High level of education promotes access to information and better understanding of the modes of transmission and prevention. This increases individual's risk perception and its possible impact on behavioral change toward safe sex practices. Previous studies indicated HIV awareness relates directly to the individual's level of education underscoring the importance of education in creating HIV awareness (Chauhan, Lal, Kumar, Malhotra, & Ingle, 2008; Sudha, Vijay, & Lakshmi, 2005). However, awareness alone without interventions for behavioral modifications does not always translate to risk behavior reduction (Hartung, Nash, Ngubane, & Fredlund, 2002; James, Reddy, Taylor, & Jinabhai, 2004).
In contrast to the study population's low knowledge of HIV transmission and prevention, its WTP in a hypothetical HIV vaccine was considerably high, in addition to the optimism for the prospect of getting a vaccine against HIV. This is consistent with similar studies in some parts of Africa where respondents expressed a positive attitude toward an HIV vaccine and WTP (Kiwanuka et al., 2004; Lindegger, Quayle, & Ndlovu, 2007). The high proportion of WTP expressed by the population groups at greater risks indicates their desire for protection against HIV and explains their level of at risk perception for the disease to which they were lacking sufficient knowledge on its modes of transmission and prevention. These groups would likely be among the targets for a future HIV prevention vaccine trial in Nigeria. It is important that such groups are approached early enough for vaccine preparedness education emphasizing that a candidate vaccine does not guarantee protection against HIV infection. These findings were similar to those reported in previous studies involving different population groups at a high risk of HIV infection (Koblin et al., 1998; Suhadev et al., 2006; Van de Ven et al., 2005). The desire for protection from the candidate vaccine was identified as one of the reasons for the expressed WTP among high-risk groups (Koblin, Holte, Lenderking, & Heagerty, 2000; McGrath et al., 2001).
Studies have shown that the more respondents knew about HIV vaccine trials, the less willing they were to participate (Bartholow et al., 1997; Koblin et al., 2000; Moodley, Barnes, van Rensburg, & Myer, 2002; O'Connell et al., 2002). Our study shows that the more participants knew about HIV modes of transmission and prevention, the less likely they were to express WTP in hypothetical vaccine trials. Respondents, who declined to participate in actual trials, cited several reasons for their decisions including fears of discrimination from post-vaccination-induced HIV seropositivity and vaccine safety (Allen et al., 2001; Celentano et al., 1995; Newman et al., 2007; Sahay et al., 2005; Thapinta et al., 2002). Whether this population will express concerns on issues like vaccine safety following HIV vaccine specific education or when the actual trial is being conducted may be hard to predict. However, the implications of false-positive HIV status with respect to employment and spousal choice could adversely affect WTP in this population. Unlike other studies that found positive association between increasing age, male gender, and low literacy level with WTP (Smit et al., 2006; Van de Ven et al., 2005), our study found positive association with younger age, population group, and ethnicity.
The ability to conduct baseline assessment of HIV infection awareness and WTP in HIV vaccine trials across different population groups using a single instrument was part of the strength of this study. The non-confirmation of the negative sero-status of the willing high-risk individuals was equally a limitation. The lack of association between WTP and level of HIV awareness after adjustment may be due to a small sample size or unmeasured confounding. The findings of this study may only be generalized to the a priori selected sub-populations, therefore not representing all existing possibilities in Abuja. The findings of this study will prove valuable in the planning and implementation of vaccine trials in Nigeria in the near future.
In conclusion, we found respondents at high risk of HIV infection were more willing to participate in hypothetical HIV vaccine trials – even though their level of HIV infection awareness was lower – compared to respondents at low risk of the infection in Abuja, Nigeria. Intense vaccine preparedness programs for HIV vaccine development and trials in this population may change completely the pattern of WTP among the different risk groups. However, at this moment, educational strategies to improve the knowledge of HIV transmission and prevention among the high-risk groups are needed.
Acknowledgments
This study was sponsored by the WHO and African AIDS Vaccine Program (AAVP). Gambo Aliyu thanks Fogarty International Research Program (Grant no. 5D43TW001041-09) for the training support that provided the necessary skills and expertise for the data analysis.
References
- Abimiku AG, Stern TL, Zwandor A, Markham PD, Calef C, Kyari S, Reitz MS. Subgroup G HIV type 1 isolates from Nigeria. AIDS Research and Human Retroviruses. 1994;10(11):1581–1583. doi: 10.1089/aid.1994.10.1581. [DOI] [PubMed] [Google Scholar]
- Agwale SM, Zeh C, Robbins KE, Odama L, Saekhou A, Edubio A, Kalish ML. Molecular surveillance of HIV-1 field strains in Nigeria in preparation for vaccine trials. Vaccine. 2002;20(16):2131–2139. doi: 10.1016/s0264-410x(02)00059-2. [DOI] [PubMed] [Google Scholar]
- Allen M, Israel H, Rybczyk K, Pugliese MA, Loughran K, Wagner L, Erb S. Trial-related discrimination in HIV vaccine clinical trials. AIDS Research and Human Retroviruses. 2001;17(8):667–674. doi: 10.1089/088922201750236942. [DOI] [PubMed] [Google Scholar]
- Bartholow BN, MacQueen KM, Douglas JM, Jr, Buchbinder S, McKirnan D, Judson FN. Assessment of the changing willingness to participate in phase III HIV vaccine trials among men who have sex with men. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;16(2):108–115. doi: 10.1097/00042560-199710010-00006. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano DD, Beyrer C, Natpratan C, Eiumtrakul S, Sussman L, Renzullo PO, Nelson KE. Willingness to participate in AIDS vaccine trials among high-risk populations in northern Thailand. AIDS. 1995;9(9):1079–1083. doi: 10.1097/00002030-199509000-00015. [DOI] [PubMed] [Google Scholar]
- Chauhan H, Lal P, Kumar V, Malhotra R, Ingle GK. Awareness status about HIV/AIDS among Indian railway's employees and their family members. Journal of Communicable Diseases. 2008;40(4):295–299. [PubMed] [Google Scholar]
- Ekabua JE, Oyo-Ita AE, Ogaji DS, Omuemu VO. KAP of HIV prevention and screening among pregnant women attending specialist antenatal clinics in Calabar, Nigeria. Nigerian Journal of Medicine. 2006;15(4):409–412. doi: 10.4314/njm.v15i4.37256. [DOI] [PubMed] [Google Scholar]
- Halpern SD, Metzger DS, Berlin JA, Ubel PA. Who will enroll? Predicting participation in a phase II AIDS vaccine trial. Journal of Acquired Immune Deficiency Syndromes. 2001;27(3):281–288. doi: 10.1097/00126334-200107010-00011. [DOI] [PubMed] [Google Scholar]
- Harding AK, Anadu EC, Gray LA, Champeau DA. Nigerian university students' knowledge, perceptions, and behaviours about HIV/AIDS: Are these students at risk? Journal of the Royal Society for the Promotion of Health. 1999;119(1):23–31. doi: 10.1177/146642409911900105. [DOI] [PubMed] [Google Scholar]
- Hartung TK, Nash J, Ngubane N, Fredlund VG. AIDS awareness and sexual behaviour in a high HIV prevalence area in rural northern Kwazulu-Natal, South Africa. International Journal of STD & AIDS. 2002;13(12):829–832. doi: 10.1258/095646202321020107. [DOI] [PubMed] [Google Scholar]
- Howard TM, Olaylele DO, Rasheed S. Sequence analysis of the glycoprotein 120 coding region of a new HIV type 1 subtype A strain (HIV-1IbNg) from Nigeria. AIDS Research and Human Retroviruses. 1994;10(12):1755–1757. doi: 10.1089/aid.1994.10.1755. [DOI] [PubMed] [Google Scholar]
- Iliyasu Z, Kabir M, Galadanci HS, Abubakar IS, Aliyu MH. Awareness and attitude of antenatal clients towards HIV voluntary counselling and testing in Aminu Kano Teaching Hospital, Kano, Nigeria. Nigerian Journal of Medicine. 2005;14(1):27–32. doi: 10.4314/njm.v14i1.37131. [DOI] [PubMed] [Google Scholar]
- James S, Reddy SP, Taylor M, Jinabhai CC. Young people, HIV/AIDS/STIs and sexuality in South Africa: The gap between awareness and behaviour. Acta Paediatrica. 2004;93(2):264–269. [PubMed] [Google Scholar]
- Joint United Nation Program on HIV/AIDS (UNAIDS) Global AIDS epidemic released in July 2008. 2008 Retrieved from http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008-gr-mediakit.asp.
- Kiwanuka N, Robb M, Kigozi G, Birx D, Philips J, Wabwire-Mangen F, Gray RH. Knowledge about vaccines and willingness to participate in preventive HIV vaccine trials: A population-based study, Rakai, Uganda. Journal of Acquired Immune Deficiency Syndromes. 2004;36(2):721–725. doi: 10.1097/00126334-200406010-00009. [DOI] [PubMed] [Google Scholar]
- Koblin BA, Heagerty P, Sheon A, Buchbinder S, Celum C, Douglas JM, Seage G. Readiness of high-risk populations in the HIV network for prevention trials to participate in HIV vaccine efficacy trials in the United States. AIDS. 1998;12(7):785–793. doi: 10.1097/00002030-199807000-00015. [DOI] [PubMed] [Google Scholar]
- Koblin BA, Holte S, Lenderking B, Heagerty P. Readiness for HIV vaccine trials: Changes in willingness and knowledge among high-risk populations in the HIV network for prevention trials. The HIVNET Vaccine Preparedness Study Protocol Team. Journal of Acquired Immune Deficiency Syndromes. 2000;24(5):451–457. doi: 10.1097/00126334-200008150-00010. [DOI] [PubMed] [Google Scholar]
- Lindegger G, Quayle M, Ndlovu M. Local knowledge and experiences of vaccination: Implications for HIV-preventive vaccine trials in South Africa. Health Education & Behavior. 2007;34(1):108–123. doi: 10.1177/1090198105277852. [DOI] [PubMed] [Google Scholar]
- McCutchan FE, Carr JK, Bajani M, Sanders-Buell E, Harry TO, Stoeckli TC, Kalish ML. Subtype G and multiple forms of A/G intersubtype recombinant human immunodeficiency virus type 1 in Nigeria. Virology. 1999;254(2):226–234. doi: 10.1006/viro.1998.9505. [DOI] [PubMed] [Google Scholar]
- McGrath JW, George K, Svilar G, Ihler E, Mafigiri D, Kabugo M, Mugisha E. Knowledge about vaccine trials and willingness to participate in an HIV/AIDS vaccine study in the Ugandan military. Journal of Acquired Immune Deficiency Syndromes. 2001;27(4):381–388. doi: 10.1097/00126334-200108010-00009. [DOI] [PubMed] [Google Scholar]
- Moodley K, Barnes J, van Rensburg EJ, Myer L. Willingness to participate in South African HIV vaccine trials – concerns of medical professionals in the Western Cape. South African Medical Journal. 2002;92(11):904–906. [PubMed] [Google Scholar]
- Newman PA, Duan N, Lee SJ, Rudy E, Seiden D, Kakinami L, Cunningham W. Willingness to participate in HIV vaccine trials: The impact of trial attributes. Preventive Medicine. 2007;44(6):554–557. doi: 10.1016/j.ypmed.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell JM, Hogg RS, Chan K, Strathdee SA, McLean N, Martindale SL, Remis R. Willingness to participate and enroll in a phase 3 preventive HIV-1 vaccine trial. Journal of Acquired Immune Deficiency Syndromes. 2002;31(5):521–528. doi: 10.1097/00126334-200212150-00010. [DOI] [PubMed] [Google Scholar]
- Onah HE, Mbah AU, Chukwuka JC, Ikeme AC. HIV/AIDS awareness and sexual practices among undergraduates in Enugu, Nigeria. Nigerian Postgraduate Medical Journal. 2004;11(2):121–125. [PubMed] [Google Scholar]
- Peeters M, Esu-Williams E, Vergne L, Montavon C, Mulanga-Kabeya C, Harry T, Delaporte E. Predominance of subtype A and G HIV type 1 in Nigeria, with geographical differences in their distribution. AIDS Research and Human Retroviruses. 2000;16(4):315–325. doi: 10.1089/088922200309197. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. New England Journal of Medicine. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Robb ML. Failure of the Merck HIV vaccine: An uncertain step forward. Lancet. 2008;372(9653):1857–1858. doi: 10.1016/S0140-6736(08)61593-7. [DOI] [PubMed] [Google Scholar]
- Sahay S, Mehendale S, Sane S, Brahme R, Brown A, Charron K, Paranjape S. Correlates of HIV vaccine trial participation: An Indian perspective. Vaccine. 2005;23(11):1351–1358. doi: 10.1016/j.vaccine.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Sekaly RP. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? Journal of Experimental Medicine. 2008;205(1):7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J, Middelkoop K, Myer L, Seedat S, Bekker LG, Stein DJ. Willingness to participate in HIV vaccine research in a peri-urban South African community. International Journal of STD & AIDS. 2006;17(3):176–179. doi: 10.1258/095646206775809259. [DOI] [PubMed] [Google Scholar]
- Sudha RT, Vijay DT, Lakshmi V. Awareness, attitudes, and beliefs of the general public towards HIV/AIDS in Hyderabad, a capital city from South India. Indian Journal of Medical Sciences. 2005;59(7):307–316. [PubMed] [Google Scholar]
- Suhadev M, Nyamathi AM, Swaminathan S, Venkatesan P, Raja Sakthivel M, Shenbagavalli R, Fahey JL. A pilot study on willingness to participate in future preventive HIV vaccine trials. Indian Journal of Medical Research. 2006;124(6):631–640. [PubMed] [Google Scholar]
- Thapinta D, Jenkins RA, Morgan PA, Chiu J, Boenim W, Bussaratid V, Thongchareon P. Recruiting volunteers for a multisite phase I/II HIV preventive vaccine trial in Thailand. Journal of Acquired Immune Deficiency Syndromes. 2002;30(5):503–513. doi: 10.1097/00126334-200208150-00006. [DOI] [PubMed] [Google Scholar]
- Van de Ven P, Mao L, Crawford J, Prestage G, Grulich A, Kaldor J, Kippax S. Willingness to participate in HIV vaccine trials among HIV-negative gay men in Sydney, Australia. International Journal of STD & AIDS. 2005;16(4):314–317. doi: 10.1258/0956462053654212. [DOI] [PubMed] [Google Scholar]
- Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nature Medicine. 2008;14(6):617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh C, Pieniazek D, Agwale SM, Robbins KE, Odama L, Sani-Gwarzo N, Kalish Marcia L. Nigerian HIV type 2 subtype A and B from heterotypic HIV type 1 and HIV type 2 or monotypic HIV type 2 infections. AIDS Research and Human Retroviruses. 2005;21(1):17–27. doi: 10.1089/aid.2005.21.17. [DOI] [PubMed] [Google Scholar]