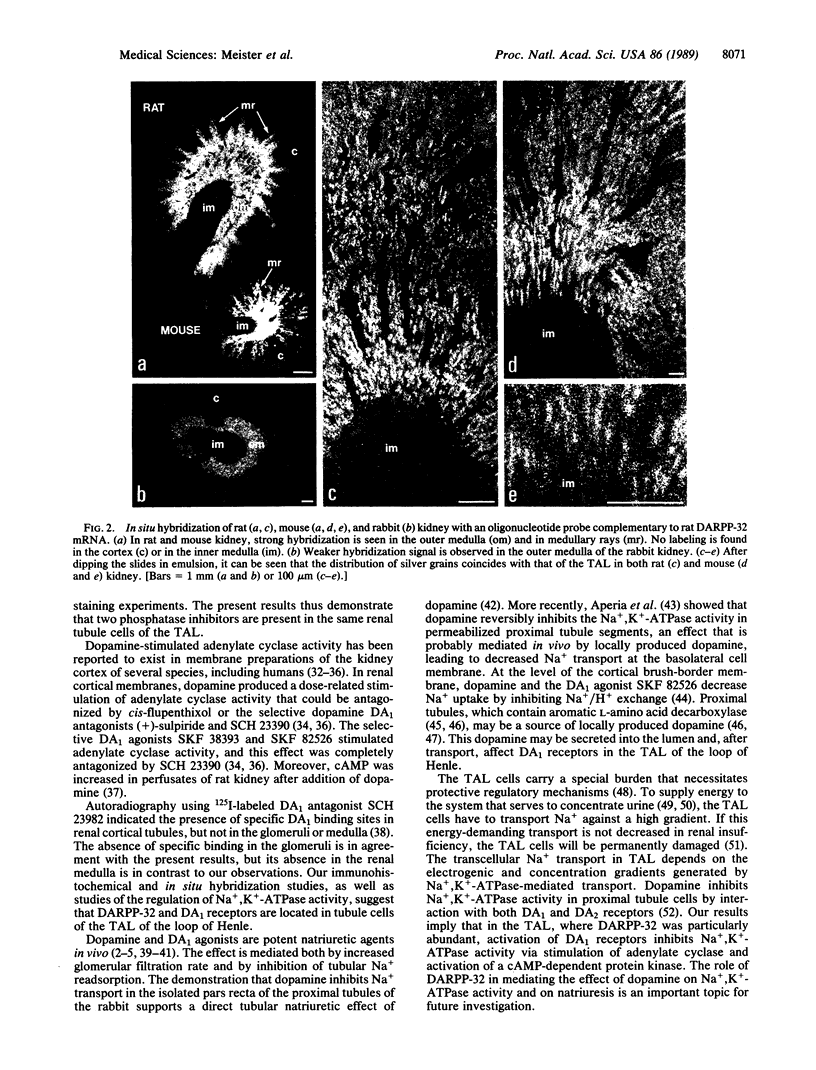
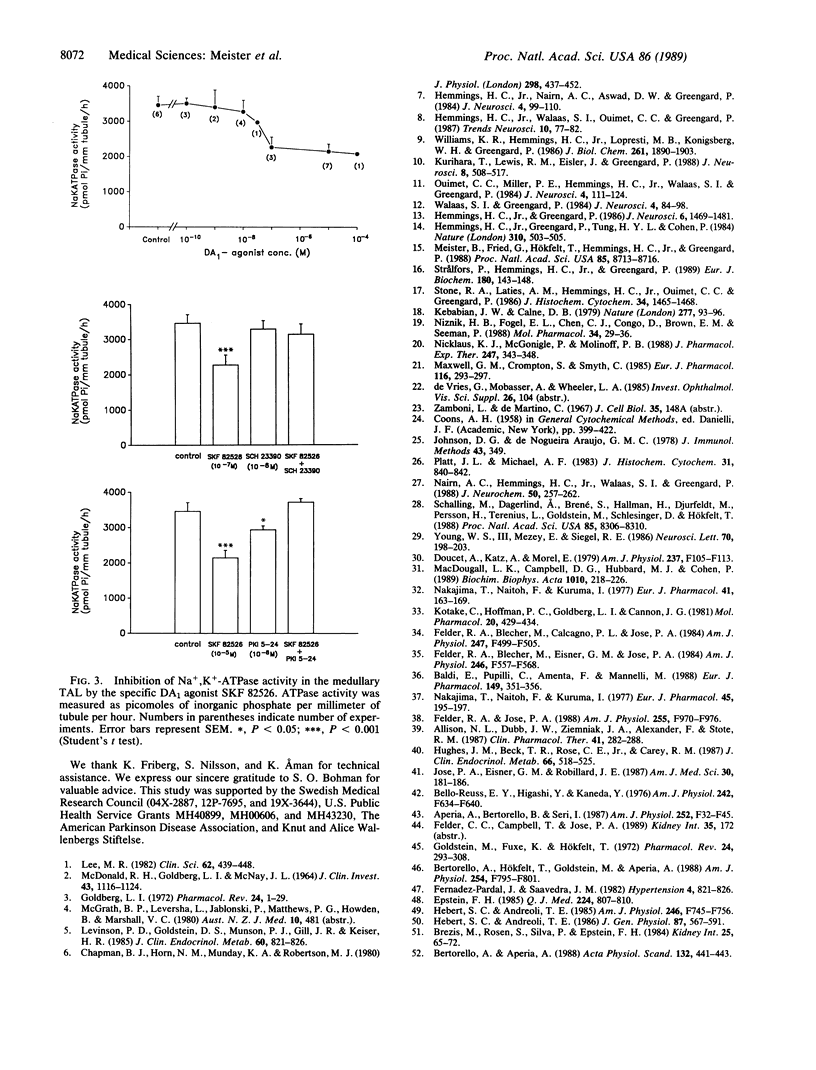
Abstract
The cellular localization of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein of Mr 32,000 that appears to mediate certain actions of dopamine in the mammalian brain by acting as an inhibitor of protein phosphatase 1, was studied in the kidney of several species. DARPP-32 mRNA and DARPP-32-like immunoreactivity were found in the cytoplasm of cells in the thick ascending limb of the loop of Henle. The specific dopamine DA1 agonist SKF 82526 caused a dose-dependent inhibition of Na+,K+-ATPase activity, which could be blocked by SCH 23390, a specific DA1 antagonist, and by PKI-(5-24) amide, a specific inhibitor of cAMP-dependent protein kinase. The results indicate that DA1 dopamine receptors and DARPP-32, an intracellular third messenger for dopamine, are part of the signal-transduction process for dopamine acting on renal tubule cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison N. L., Dubb J. W., Ziemniak J. A., Alexander F., Stote R. M. The effect of fenoldopam, a dopaminergic agonist, on renal hemodynamics. Clin Pharmacol Ther. 1987 Mar;41(3):282–288. doi: 10.1038/clpt.1987.29. [DOI] [PubMed] [Google Scholar]
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Baldi E., Pupilli C., Amenta F., Mannelli M. Presence of dopamine-dependent adenylate cyclase activity in human renal cortex. Eur J Pharmacol. 1988 May 10;149(3):351–356. doi: 10.1016/0014-2999(88)90667-x. [DOI] [PubMed] [Google Scholar]
- Bello-Reuss E., Higashi Y., Kaneda Y. Dopamine decreases fluid reabsorption in straight portions of rabbit proximal tubule. Am J Physiol. 1982 Jun;242(6):F634–F640. doi: 10.1152/ajprenal.1982.242.6.F634. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Both DA1 and DA2 receptor agonists are necessary to inhibit NaKATPase activity in proximal tubules from rat kidney. Acta Physiol Scand. 1988 Mar;132(3):441–443. doi: 10.1111/j.1748-1716.1988.tb08350.x. [DOI] [PubMed] [Google Scholar]
- Bertorello A., Hökfelt T., Goldstein M., Aperia A. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol. 1988 Jun;254(6 Pt 2):F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Transport activity modifies thick ascending limb damage in the isolated perfused kidney. Kidney Int. 1984 Jan;25(1):65–72. doi: 10.1038/ki.1984.9. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chapman B. J., Horn N. M., Munday K. A., Robertson M. J. The actions of dopamine and of sulpiride on regional blood flows in the rat kidney. J Physiol. 1980 Jan;298:437–452. doi: 10.1113/jphysiol.1980.sp013093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet A., Katz A. I., Morel F. Determination of Na-K-ATPase activity in single segments of the mammalian nephron. Am J Physiol. 1979 Aug;237(2):F105–F113. doi: 10.1152/ajprenal.1979.237.2.F105. [DOI] [PubMed] [Google Scholar]
- Epstein F. H. Hypoxia of the renal medulla. Q J Med. 1985 Dec;57(224):807–810. [PubMed] [Google Scholar]
- Felder R. A., Blecher M., Calcagno P. L., Jose P. A. Dopamine receptors in the proximal tubule of the rabbit. Am J Physiol. 1984 Sep;247(3 Pt 2):F499–F505. doi: 10.1152/ajprenal.1984.247.3.F499. [DOI] [PubMed] [Google Scholar]
- Felder R. A., Blecher M., Eisner G. M., Jose P. A. Cortical tubular and glomerular dopamine receptors in the rat kidney. Am J Physiol. 1984 May;246(5 Pt 2):F557–F568. doi: 10.1152/ajprenal.1984.246.5.F557. [DOI] [PubMed] [Google Scholar]
- Felder R. A., Jose P. A. Dopamine1 receptors in rat kidneys identified with 125I-Sch 23982. Am J Physiol. 1988 Nov;255(5 Pt 2):F970–F976. doi: 10.1152/ajprenal.1988.255.5.F970. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pardal J., Saavedra J. M. Catecholamines in discrete kidney regions. Changes in salt-sensitive Dahl hypertensive rats. Hypertension. 1982 Nov-Dec;4(6):821–826. doi: 10.1161/01.hyp.4.6.821. [DOI] [PubMed] [Google Scholar]
- Goldberg L. I. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev. 1972 Mar;24(1):1–29. [PubMed] [Google Scholar]
- Goldstein M., Fuxe K., Hökfelt T. Characterization and tissue localization of catecholamine synthesizing enzymes. Pharmacol Rev. 1972 Jun;24(2):293–309. [PubMed] [Google Scholar]
- Hebert S. C., Andreoli T. E. Control of NaCl transport in the thick ascending limb. Am J Physiol. 1984 Jun;246(6 Pt 2):F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- Hebert S. C., Andreoli T. E. Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl- absorption. J Gen Physiol. 1986 Apr;87(4):567–590. doi: 10.1085/jgp.87.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986 May;6(5):1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P., Tung H. Y., Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984 Aug 9;310(5977):503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., Aswad D. W., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. II. Purification and characterization of the phosphoprotein from bovine caudate nucleus. J Neurosci. 1984 Jan;4(1):99–110. doi: 10.1523/JNEUROSCI.04-01-00099.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Beck T. R., Rose C. E., Jr, Carey R. M. The effect of selective dopamine-1 receptor stimulation on renal and adrenal function in man. J Clin Endocrinol Metab. 1988 Mar;66(3):518–525. doi: 10.1210/jcem-66-3-518. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jose P. A., Eisner G. M., Robillard J. E. Renal hemodynamics and natriuresis induced by the dopamine-1 agonist, SKF 82526. Am J Med Sci. 1987 Sep;294(3):181–186. doi: 10.1097/00000441-198709000-00009. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kotake C., Hoffmann P. C., Goldberg L. I., Cannon J. G. Comparison of the effects of dopamine and beta-adrenergic agonists on adenylate cyclase of renal glomeruli and striatum. Mol Pharmacol. 1981 Sep;20(2):429–434. [PubMed] [Google Scholar]
- Kurihara T., Lewis R. M., Eisler J., Greengard P. Cloning of cDNA for DARPP-32, a dopamine- and cyclic AMP-regulated neuronal phosphoprotein. J Neurosci. 1988 Feb;8(2):508–517. doi: 10.1523/JNEUROSCI.08-02-00508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. R. Dopamine and the kidney. Clin Sci (Lond) 1982 May;62(5):439–448. doi: 10.1042/cs0620439. [DOI] [PubMed] [Google Scholar]
- Levinson P. D., Goldstein D. S., Munson P. J., Gill J. R., Jr, Keiser H. R. Endocrine, renal, and hemodynamic responses to graded dopamine infusions in normal men. J Clin Endocrinol Metab. 1985 May;60(5):821–826. doi: 10.1210/jcem-60-5-821. [DOI] [PubMed] [Google Scholar]
- MCDONALD R. H., Jr, GOLDBERG L. I., MCNAY J. L., TUTTLE E. P., Jr EFFECT OF DOPAMINE IN MAN: AUGMENTATION OF SODIUM EXCRETION, GLOMERULAR FILTRATION RATE, AND RENAL PLASMA FLOW. J Clin Invest. 1964 Jun;43:1116–1124. doi: 10.1172/JCI104996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall L. K., Campbell D. G., Hubbard M. J., Cohen P. Partial structure and hormonal regulation of rabbit liver inhibitor-1; distribution of inhibitor-1 and inhibitor-2 in rabbit and rat tissues. Biochim Biophys Acta. 1989 Feb 9;1010(2):218–226. doi: 10.1016/0167-4889(89)90164-x. [DOI] [PubMed] [Google Scholar]
- Maxwell G., Crompton S., Smyth C. The effect of dopamine upon oxidative metabolism of brown fat adipocytes. Eur J Pharmacol. 1985 Oct 22;116(3):293–297. doi: 10.1016/0014-2999(85)90165-7. [DOI] [PubMed] [Google Scholar]
- Meister B., Fried G., Hökfelt T., Hemmings H. C., Jr, Greengard P. Immunohistochemical evidence for the existence of a dopamine- and cyclic AMP-regulated phosphoprotein (DARPP-32) in brown adipose tissue of pigs. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8713–8716. doi: 10.1073/pnas.85.22.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Walaas S. I., Greengard P. DARPP-32 and phosphatase inhibitor-1, two structurally related inhibitors of protein phosphatase-1, are both present in striatonigral neurons. J Neurochem. 1988 Jan;50(1):257–262. doi: 10.1111/j.1471-4159.1988.tb13258.x. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Naitoh F., Kuruma I. Dopamine-sensitive adenylate cyclase in the rat kidney particulate preparation. Eur J Pharmacol. 1977 Jan 21;41(2):163–169. doi: 10.1016/0014-2999(77)90205-9. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Naitoh F., Kuruma I. Elevation of adenosine 3',5'-monophosphate in the perfusate of rat kidney after addition of dopamine. Eur J Pharmacol. 1977 Sep 15;45(2):195–197. doi: 10.1016/0014-2999(77)90089-9. [DOI] [PubMed] [Google Scholar]
- Nicklaus K. J., McGonigle P., Molinoff P. B. [3H]SCH 23390 labels both dopamine-1 and 5-hydroxytryptamine1c receptors in the choroid plexus. J Pharmacol Exp Ther. 1988 Oct;247(1):343–348. [PubMed] [Google Scholar]
- Niznik H. B., Fogel E. L., Chen C. J., Congo D., Brown E. M., Seeman P. Dopamine D1 receptors of the calf parathyroid gland: identification and characterization. Mol Pharmacol. 1988 Jul;34(1):29–36. [PubMed] [Google Scholar]
- Ouimet C. C., Miller P. E., Hemmings H. C., Jr, Walaas S. I., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984 Jan;4(1):111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Schalling M., Dagerlind A., Brené S., Hallman H., Djurfeldt M., Persson H., Terenius L., Goldstein M., Schlesinger D., Hökfelt T. Coexistence and gene expression of phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and neuropeptide tyrosine in the rat and bovine adrenal gland: effects of reserpine. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8306–8310. doi: 10.1073/pnas.85.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone R. A., Laties A. M., Hemmings H. C., Jr, Ouimet C. C., Greengard P. DARPP-32 in the ciliary epithelium of the eye: a neurotransmitter-regulated phosphoprotein of brain localizes to secretory cells. J Histochem Cytochem. 1986 Nov;34(11):1465–1468. doi: 10.1177/34.11.2877023. [DOI] [PubMed] [Google Scholar]
- Strålfors P., Hemmings H. C., Jr, Greengard P. Inhibitors of protein phosphatase-1. Inhibitor-1 of bovine adipose tissue and a dopamine- and cAMP-regulated phosphoprotein of bovine brain are identical. Eur J Biochem. 1989 Mar 1;180(1):143–148. doi: 10.1111/j.1432-1033.1989.tb14624.x. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in the rat brain. J Neurosci. 1984 Jan;4(1):84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. R., Hemmings H. C., Jr, LoPresti M. B., Konigsberg W. H., Greengard P. DARPP-32, a dopamine- and cyclic AMP-regulated neuronal phosphoprotein. Primary structure and homology with protein phosphatase inhibitor-1. J Biol Chem. 1986 Feb 5;261(4):1890–1903. [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986 Oct 8;70(2):198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]




