Abstract
Integrins bind extracellular matrix fibrils and associate with intracellular actin filaments through a variety of cytoskeletal linker proteins to mechanically connect intracellular and extracellular structures. Each component of the linkage from the cytoskeleton through the integrin-mediated adhesions to the extracellular matrix therefore transmits forces that may derive from both intracellular, myosin-generated contractile forces and forces from outside the cell. These forces activate a wide range of signaling pathways and genetic programs to control cell survival, fate, and behavior. Additionally, cells sense the physical properties of their surrounding environment through forces exerted on integrin-mediated adhesions. This article first summarizes current knowledge about regulation of cell function by mechanical forces acting through integrin-mediated adhesions and then discusses models for mechanotransduction and sensing of environmental forces.
A clutch mechanism involving proteins such as talin and vinculin may allow force transmitted through cell adhesions to modulate intracellular signaling pathways by controlling the timing of protein-protein interactions.
The fact that multicellular organisms can resist the wide range of physical forces encountered in nature requires appropriate mechanical connectivity within each tissue. For example, skin and other epithelial tissues have keratin networks that connect across desmosomes, so that the entire cell layer is mechanically integrated (Uitto 2009). Their adhesion to the basement membrane also involves connection of cytoplasmic filaments (both actin and intermediate filaments) across the membrane to the extracellular matrix (ECM). Genetic defects in any of the key components lead to mechanical fragility and defects such as blistering of these tissues, severe versions of which are lethal (Aumailley et al. 2006).
A key design feature inherent in all complex organisms is their ability to modulate mechanical strength of tissues to match the forces encountered. Artery wall thickness is determined by blood pressure and vessel diameter: The vessel wall thickens or thins so that the force per unit of wall is constant (so called, LaPlace’s Law) (Schiffrin 1992). Arteries also remodel in response to changes in fluid flow so that vessel diameters are well matched to the volume of flowing blood (Schaper 2009). Bone formation and turnover are regulated by weight-bearing exercise such that bone density increases under higher loads and decreases under lower loading (Robling et al. 2006). This strategy makes for efficient resource allocation because tissues are built to be as strong as they need to be, not unlike “on demand” stocking of supplies in warehouses. But when tissues are subjected to forces that are too high, these regulatory mechanisms can also contribute to disease. Over-inflation of the lung triggers ventilator injury, a serious complication for patients on ventilators (Lionetti et al. 2005). Hypertension is a major risk factor for atherosclerosis, as well as aneurysm (Hahn and Schwartz 2009; Krishna et al. 2010).
Embryonic development also depends on sensing mechanical forces. Morphogenesis requires that each tissue assume its correct shape and size, specified through complex interactions among cell adhesion, cytoskeleton, soluble factors, and gene expression programs. It is almost impossible to envision how cells could determine the macroscopic shape and size of an organ without input from mechanical forces exerted on the tissue; certainly organ size or shape are not encoded directly in our genes. Recent evidence supports this view. In Drosophila embryos, pressure on the anterior foregut and stomodeal primordium from the extending germ band appears to activate β-catenin signaling and subsequent expression of mesenchymal genes such as Twist (Farge 2003). Migration of Drosophila border cells from the anterior region to the developing oocytes requires nuclear translocation of the transcription factor Mal-D, which appears to be induced by stretching of the cells (Somogyi and Rorth 2004). In mouse embryos, remodeling of the primitive yolk sac vascular plexus requires fluid shear stress from flowing blood (Lucitti et al. 2007). Correct looping of the outflow tract from the heart also depends on the shear stress from blood flowing out of the heart (Yashiro et al. 2007).
It is obvious that the structures that bear the force should be involved in sensing it. Every component of the mechanical linkages between the ECM, integrins, and the cytoskeleton is therefore a candidate mechanotransducer. Indeed, the participation of many components of the ECM-integrin-cytoskeleton linkage in mechanotransduction has been borne out by a wide range of experimental data and considerable insight has been obtained into molecular and biophysical mechanisms. This review will cover mechanotransduction by each of these components and their relationship to cell physiology.
CYTOSKELETAL CONNECTIONS
Formation of adhesions by integrins involves both binding to ECM proteins and linkage to the actin cytoskeleton, two processes that show highly cooperative behavior (Burridge and Chrzanowska-Wodnicka 1996). In culture, the adhesions have been divided into several types (Zaidel-Bar et al. 2004; Geiger and Yamada 2011). Focal adhesions are large, elongated structures, typically approximately 2 µm wide and 3–10 µm long, in which clustered integrins bind ECM fibrils on the outside of the cell and connect on the inside to contractile actomyosin stress fiber bundles (Fig. 1). Focal adhesions depend on activation of the small GTPase Rho and can be found distributed across the lower surface of the cell. Cells also contain focal complexes that have similar compositions but are smaller and circular, typically around 2 µm in diameter. They also connect to actomyosin and are force-dependent but are under actively protruding cell edges and require activation of Cdc42 or Rac instead of Rho. Migrating cells also contain very small nascent adhesions that form right behind protruding lamellipodial edges (Choi et al. 2008). These adhesions are below the spatial resolution of conventional light microscopes and are myosin-independent; instead, they require continual rearward-flowing actin, as occurs at active cell edges. Nascent adhesions appear to mature to focal complexes on association with actomyosin, whereas focal complexes mature to focal adhesions on association with large actin stress fibers. Other types of adhesions such as podosomes and fibrillar adhesions also link ECM proteins to actin, and some evidence suggests they can participate in mechanotransduction (Collin et al. 2008). However, less is known about mechanotransduction in these structures and they will not be discussed further here.
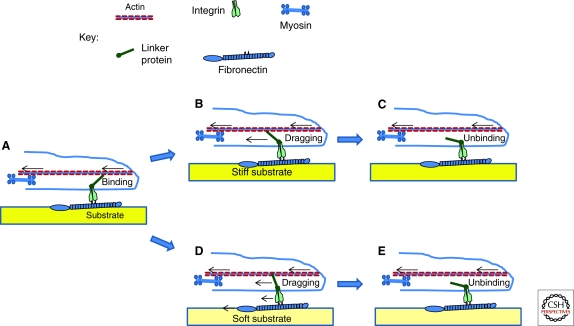
Figure 1.
A model for stiffness sensing. (A) The lamellipodial edge has actin that is moving backward because of a combination of the force from polymerization pushing against the plasma membrane and pulling force from myosin further back in the cell. Integrins are bound to fibronectin or other ECM proteins adsorbed to the substrate or incorporated into the insoluble matrix, and connect to the actin through linker proteins such as talin and vinculin. (B) Binding of the linker to the actin triggers a rapid increase in tension and, assuming slip bond behavior, dissociation of the linkage (C). (D–E) On a soft substrate, tension is applied in the same way but deformation of the substrate allows the fibronectin and integrin to move backward too. Thus, force build-up slows and the interaction is prolonged. One can propose variations on this theme. For example, if linkages are catch bonds, on soft substrata, the slow onset of tension may lead to a shorter lifetime for the bound state. The central idea is that the clutch mechanism allows the stiffness of the substratum to control the timing of the protein-protein interactions, which can lead to changes in signaling.
The linkage from integrins to actin is accomplished by a complex set of interactions (Liu et al. 2000; Wegener and Campbell 2008; Wickstrom et al. 2010; Zaidel-Bar and Geiger 2010). The best understood linkage is through talin, whose head domain binds sequences in β integrin cytoplasmic domains, whereas its tail domain binds actin both directly and indirectly through vinculin (Fig. 1A). Integrins can also associate with F-actin through α-actinin, which binds β tails directly as well as binding vinculin; through filamin, which binds directly to both integrin β tails and F-actin; and through the actin-binding protein actopaxin, which binds paxillin and ILK (Fig. 1B). Integrin β4 can associate with intermediate filaments but their relationship to mechanotransduction has not been much explored, thus, will not be discussed further.
CELLULAR RESPONSES TO FORCE
Forces on cells and tissues can be applied from external sources, as in stretching of artery walls by pressure from the blood, or by the cells’ own actomyosin (or other motors). Where they have been compared, the effects of internal versus external forces were similar, indicating that force per se across specific structures is what is sensed, independent of its origin. This point has been shown elegantly with focal adhesions, in which tension from the actin stress fibers could be replaced by a glass pipet pulling the cell body (Riveline et al. 2001). Stretching cells on an elastic substratum also has effects on the adhesions similar to the effects of stimulation of myosin activation, for example, focal adhesions enlarge and FAK is activated (Hamasaki et al. 1995; Sai et al. 1999). The following discussion therefore does not distinguish between endogenous and exogenous forces.
The major response of adhesions to force is strengthening or reinforcement, in which the adhesions enlarge or recruit new cytoskeletal proteins that help resist the applied force. Focal adhesions only form in the first place when connected to highly contractile actin stress fibers, and treatment with myosin inhibitors causes their rapid disassembly (Chrzanowska-Wodnicka and Burridge 1996). Focal complexes, though smaller and not associated with large actin bundles, are also myosin-dependent (Choi et al. 2008). Focal complexes form from smaller so-called nascent adhesions only when they connect to the actin-myosin network, and myosin inhibitors cause replacement of focal complexes with nascent adhesions. Measurement of traction forces exerted by cells on their substratum over a wide range of conditions revealed that force per unit area of focal adhesion/focal complex was constant at approximately 5.5 nN/μm2 (Balaban et al. 2001). Changes in adhesion size occurred rapidly after altering forces (seconds to minutes), strongly arguing for efficient homeostatic control mechanisms (Balaban et al. 2001). However, these responses to forces clearly occur at multiple levels and affect each component of the physical linkage between the ECM and the cytoskeleton. We therefore discuss each one, starting from outside the cell and working in.
ECM
Cells respond to force on integrin-mediated adhesions by remodeling the ECM. For example, cyclic stretching of fibroblasts and other cell types activates expression of genes for collagens (coll), fibronectin (FN), and metalloproteinases (MMPs), and stretched cells assemble a dense ECM that is enriched in collagen (Chiquet et al. 2003). Matrix assembly usually occurs in a directional manner according to the applied force. Collagen and fibronectin fibrils both tend to align along the same axis as the force (Nguyen et al. 2009). Fibronectin assembly itself is mechanically regulated (Zhong et al. 1998; Gao et al. 2003). Current models propose that FN initially binds through an integrin, then is subject to tension from actomyosin. Pulling on FN opens folded domains to reveal cryptic binding sites that promote its assembly into fibrils. Stretch-induced FN-FN binding was directly demonstrated (Zhong et al. 1998), as was extension of FN fibrils under contractile force from cells during matrix assembly (Baneyx et al. 2002). Conversely, inhibiting cell contractility causes loss of the FN matrix. There is also evidence that cells actively arrange their fibrillar collagens in a force-dependent manner (Canty et al. 2006).
Though less well studied, there are data suggesting that assembly of basement membranes consisting of laminins and type IV collagen is also sensitive to the mechanics of the environment (Streuli and Bissell 1990). These data indicate at least two levels of regulation. First, expression of genes for both ECM proteins and ECM-remodeling enzymes (metalloproteinases and inhibitors thereof, etc.) are turned on or off by exposure of cells to mechanical stresses or by modulation of actomyosin contractility (Chiquet et al. 2003). In many cases, this regulation appears to occur through signaling pathways downstream of the integrins themselves (Katsumi et al. 2004). Second, the polymerization or organization of ECM fibrils is influenced more directly by mechanical forces. Cells actively lay down their own matrix in an ordered fashion, and forces exerted through matrix receptors control these processes (Canty et al. 2006). The net result in most cases is production of ECM that can resist the applied forces (Isenberg and Tranquillo 2003; Solan et al. 2009). These processes are crucial in vascular smooth muscle subject to cyclic stretch from pumping blood, as failure to strengthen would result in aneurysms (Ghorpade and Baxter 1996).
Integrins
There is also evidence that integrins themselves may be mechanosensors. As discussed by Campbell and Humphries, integrins undergo complex conformational rearrangements that govern both affinity for extracellular matrix proteins and associations with cytoskeletal proteins (Calvete 2004; Wegener and Campbell 2008; Campbell and Humphries 2010). It would be surprising if these conformations were insensitive to forces between the extracellular ligand binding site and the cytoplasmic domains that bind cytoskeletal proteins. Experimental data, albeit indirect, support the idea that integrin conformation can be modulated by applied force (Puklin-Faucher et al. 2006; Friedland et al. 2009). Application of force to integrin α5β1 is required for conversion to a state that can be chemically cross-linked to the FN beneath the cell; inhibition of cell contractility blocks cross-linking but can be rescued by application of force from fluid shear stress (Friedland et al. 2009). This effect was associated with binding of α5β1 to the synergy site within FN (a second binding site within the 10th FN type III repeat, close to but distinct from the RGD sequence in the ninth type III repeat). These events are also correlated with protein tyrosine phosphorylation of focal adhesion kinase (see Geiger and Yamada 2011), suggesting that integrin conformational transitions regulate intracellular signaling. Studies of the leukocyte integrin LFA-1 (αLβ2) also suggest a role for force in integrin conformation (Jin et al. 2004; Zhu et al. 2008). It was proposed based largely on structural data and molecular dynamics simulations that forces that act either parallel or perpendicular to the plasma membrane can induce or stabilize the high affinity state.
Cytoskeleton
The adhesion-associated actin cytoskeleton rapidly remodels in response to changes in force; the best-studied case being adhesion reinforcement or strengthening as discussed earlier. These effects in fact represent a set of mechanisms that operate on different time frames. Experiments with optical tweezers showed that adhesions begin to recruit vinculin and increase their strength within seconds of applying force (Galbraith et al. 2002). Vinculin recruitment has now been attributed to the fact that vinculin-binding sites in the talin tail domain are concealed within bundles of α helices, which can open under tension (del Rio et al. 2009; Campbell and Humphries 2010). At only slightly longer times, entire adhesions in adherent cells lengthen under applied force, indicating the recruitment of not only vinculin but integrins and other proteins (Riveline et al. 2001). Tension applied to cells on elastic surfaces also triggers an increase in integrin activity (i.e., affinity) as measured by binding of soluble FN fragments (Katsumi et al. 2005; Thodeti et al. 2009). This result implies communication from the bound integrins that bear the force to the unbound integrins that convert to the high affinity state and bind soluble ligands. This signal appeared to be mediated by PI 3-kinase (PI3K) (Katsumi et al. 2005). Integrin activation was followed by new binding to the ECM and enlargement of the adhesions. Thus, it appears that the rapid responses to force that involve integrin and talin conformation, and the slower activation of PI3K and unoccupied integrins may be related. It is attractive to propose that force induces conformational changes in the bound integrins, which triggers FAK activation, which activates PI3K. PI 3-lipids then mediate activation of unbound integrins followed by their binding to the ECM to enlarge and strengthen the adhesions. All of these steps have been observed independently; however, it remains to be tested whether the entire pathway works in this manner.
Importantly, adhesion strengthening cannot be universal, because then adhesions would always be very difficult to break. This clearly is not the case, because cells migrate, which in many cases involves disassembling or breaking adhesions under force (Ballestrem et al. 2001). Indeed, vinculin recruitment and adhesion strengthening seen with laser tweezers were only observed at cells’ leading edges; quiescent sides of cells did not show this behavior (Nishizaka et al. 2000). Recent work has shed some light on the mechanism that controls adhesion strengthening versus disassembly under force. Development of a fluorescence-based tension sensor for vinculin showed that adhesion strengthening was associated with high force across this molecule, which was followed by recruitment of additional vinculin and enlargement of the adhesion, resulting in decreased force per vinculin (Grashoff et al. 2010). However, there was a population of focal adhesions at the trailing edge of migrating cells where force across vinculin was negligible. These adhesions showed centripetal sliding, a form of controlled force-dependent disassembly in which the whole adhesion appears to move toward the cell center because of differential rates of assembly versus disassembly at the adhesion’s distal versus proximal ends (Ballestrem et al. 2001). It was also shown that stabilization of adhesions under force requires vinculin. Together, these data suggest that, in adhesions that strengthen in response to force, vinculin bears force, whereas in adhesions that disassemble, force is transmitted by other linkages. The nature of the vinculin-independent links and the pathways that regulate adhesion strengthening versus disassembly are unknown.
SIGNALING PATHWAYS AND GENE EXPRESSION
Application of tension to cells through their integrin-mediated adhesions, often by stretching cells on elastic substrata, regulates a wide range of signaling pathways, downstream genes and differentiation programs. These effects are of considerable physiological and pathological significance because the experimental system mimics stretching of tissues in vivo. Cardiac hypertrophy and remodeling of the smooth muscle layers in arteries represent two examples of integrin-dependent processes that are of particular clinical relevance (Brancaccio et al. 2006; Heerkens et al. 2007). Stretching cells triggers activation of signaling pathways that include MAP kinases, Rho GTPases, elevated cytoplasmic calcium, and generation of reactive oxygen among others, with some variations among cell types (Chiquet et al. 2003; Orr et al. 2006; Haga et al. 2007). These events lead to changes in gene expression for a variety of programs. Matrix remodeling genes are quite prominent, as are cell cycle genes and genes associated with differentiation toward contractile or mesenchymal phenotypes. A great many studies have catalogued the effects of stretch or other forces on gene expression and cellular phenotype (Chiquet et al. 2003; Orr et al. 2006).
Some of these signaling events are mediated by mechanisms associated with adhesion strengthening. For example, in smooth muscle cells and fibroblasts, stretch triggers integrin conversion to the high affinity state, which leads to increased integrin binding to the ECM (Katsumi et al. 2005). These newly bound integrins then signal to activate a variety of signaling pathways. One consequence of this mechanism is that, because different integrins signal differently, the downstream stretch pathways are modulated by the ECM beneath the cells. It has also been proposed that changes in conformation of proteins such as talin and vinculin under force may alter binding sites for signaling or adapter proteins, leading to downstream events. This has been shown for p130Cas, an important focal adhesion adapter protein (Sawada et al. 2006). Cas becomes a better substrate for Src family kinases after stretching cells on elastic substrata or after stretching Cas in vitro. Once phosphorylated on tyrosine residues, Cas recruits the SH2 domain adapter protein Crk, leading to recruitment of GEFs (GTPase exchange factors) and activation of the small GTPase Rap1, which is known to activate integrins (Reedquist et al. 2000). Crk can bind other GEFs such as DOCK180 (Hasegawa et al. 1996), suggesting that other small GTPases might also be activated through this pathway.
The focal adhesion protein zyxin appears to mediate an important subset of cellular responses to force. Zyxin is a LIM domain protein that binds the Ena/VASP proteins that, in turn, promote actin polymerization by binding the barbed ends of actin filaments and protecting them from capping protein (Renfranz and Beckerle 2002). Zyxin localization to focal adhesions shows complex force-dependence. Its localization to focal adhesions requires high contractility, indeed, it is one of the few proteins that localize strongly to focal adhesions but poorly to focal complexes (Zaidel-Bar et al. 2003). Zyxin’s dissociation rate from the adhesions increased within seconds of reducing contractile forces, suggesting that dissociation could be a relatively direct response to force, for example, through effects on protein conformation (Lele et al. 2006). However, stretching cells on elastic substrata, which presumably applied even higher forces, triggered movement of the zyxin out of the focal adhesions and onto actin stress fibers and into the nucleus (Cattaruzza et al. 2004; Yoshigi et al. 2005; Hirata et al. 2008). Ena/VASP proteins translocate between focal adhesions and actin filaments together with the zyxin and were required for force-dependent increases in actin polymerization at the site of zyxin/Ena/VASP targeting. In smooth muscle, zyxin moves to the nucleus in response to applied stretch and antisense oligonucleotides against zyxin altered stretch-induced changes in gene expression in these cells (Cattaruzza et al. 2004). These data therefore suggest a potentially important role for zyxin in transcriptional responses to force as well as the better documented regulation of actin polymerization.
Finally, matrix remodeling is a major target for mechanically regulated pathways. Cells under high stress generally assemble stiffer or stronger matrices (Isenberg and Tranquillo 2003; Balestrini and Billiar 2006). These changes appear to be due in part to effects of forces on gene expression and in part because of more direct effects on matrix assembly. For example, as discussed earlier, tension has direct effects on fibronectin conformation and fibrillogenesis (Zhong et al. 1998; Lemmon et al. 2009). Although, unlike fibronectin, fibrillar collagens spontaneously and efficiently polymerize in solution, their spatial arrangement in the ECM is actively controlled by the cells (Canty et al. 2006; Nguyen et al. 2009). This effect may be in part mechanical, with cells physically pulling collagen into oriented bundles. Direct effects of forces on TGFβ1 processing are also important for ECM assembly in some systems (Munger et al. 1999). TGFβ activation requires its separation from the latency-associated peptide (LAP) that maintains it in an inactive state. LAP has an RGD sequence that binds integrin αvβ6; this interaction mediates force-dependent disruption of the TGFβ1-LAP complex to release active TGFβ1 (Yang et al. 2007) (see also Sheppard and Munger 2011). TGFβ1 has numerous effects, including increasing the synthesis of collagenous matrices and differentiation of cells toward more contractile phenotypes (e.g., fibroblasts into myofibroblasts) (Gabbiani 2003; Schiller et al. 2004). Activation of TGFβ by integrin αvβ6 is associated with increased collagen production and tissue fibrosis in several disease models, strongly suggesting biological and perhaps clinical relevance (Nishimura 2009).
MATRIX RIGIDITY AND SPREAD AREA
Mechanical forces that originate in the cells’ own actomyosin are also modulated by the ECM. Cells sense the rigidity of the ECM that they are on or in, and adjust the tension that they exert accordingly (Choquet et al. 1997; Lo et al. 2000; Saez et al. 2005). Cells exert high traction forces on stiff matrices, coincident with formation of robust actin stress fibers and focal adhesions. On softer matrices, less force is exerted, and actin cables and focal adhesions are less well developed. The signaling consequences of matrix rigidity have been studied extensively and in many respects resemble the responses to stretch. For example, FAK phosphorylation, integrin activation, and activity of MAP kinases and Rho GTPases are all regulated by matrix stiffness (Klein et al. 2009; Michael et al. 2009; Provenzano et al. 2009; Pasapera et al. 2010). These similarities provide additional support for the idea that forces across integrins and focal adhesions trigger similar effects whether the forces originate inside or outside the cell.
Matrix rigidity also regulates differentiation. Interestingly, mesenchymal stem cells tend to differentiate toward lineages whose stiffness in vivo matches the stiffness of the artificial environment. For example, stiff matrices promote osteogenic differentiation, in keeping with the rigidity of bone, whereas very soft matrices favor neurogenic differentiation in keeping with the softness of brain (Engler et al. 2006). Matrix stiffness also modulates phenotypes of cancer cells. Tissue stiffness, determined mainly by the ECM, increases during progression of breast and other (though not all) cancers; breast cancer cells in stiff 3D matrices show more aggressive behavior such as luminal filling, invasion, and epithelial-mesenchymal transition (EMT) (Levental et al. 2009; Provenzano et al. 2009). By contrast, growing the same cells in soft matrix typical of normal tissue promotes a differentiated, epithelial, less invasive phenotype. These results strongly suggest that mechanical consequences of matrix remodeling are a causal factor in cancer progression. They are also in keeping with the general notion that the mesenchymal phenotype is associated with a higher force regime than that of epithelial cells.
Finally, it should be noted that traction force can be modulated by controlling the area over which a cell can spread (Reinhart-King et al. 2005; Tolic-Norrelykke and Wang 2005). Confining cells to small areas decreases focal adhesions, actin stress fibers, myosin phosphorylation, and cell contractility. These changes are also associated with decreased FAK phosphorylation. When mesenchymal stem cells were grown on islands of different sizes, small islands favored adipogenic differentiation whereas large islands favored osteogenic differentiation (McBeath et al. 2004). Thus, consequences of altered cell spreading may occur in part through effects on the forces across the focal adhesions.
MEDITATIONS ON MECHANISM
The dominant view of mechanotransduction at adhesions and elsewhere is that forces across proteins or membranes alter the energy landscape to cause conformational changes (Orr et al. 2006; Vogel 2006). Typically, proteins unfold to reveal novel sites for binding or phosphorylation, which alters signaling. This simple view most likely contains a good deal of truth and appears relevant to numerous situations. However, focal adhesions are dynamic structures whose components are rapidly exchanging even under ostensibly static conditions (Schlessinger and Geiger 1983; Ballestrem et al. 2001; Lele et al. 2006). Matrix rigidity sensing must be more complex.
Although it seems intuitively obvious that hard materials can support more tension than soft ones, the detailed molecular mechanisms by which cells sense physical properties of the ECM and regulate their contractile forces are largely unknown. The commonly used materials show something close to ideal elastic behavior, meaning that force increases in proportion to the displacement (Hooke’s law) (Pelham and Wang 1997; Tan et al. 2003). Thus, in principle, cells on more compliant surfaces could generate high tension by pulling the material a greater distance. That this does not happen suggests an active sensing mechanism rather than a simple inability to build up high forces. In the language of engineering, a stress sensor is unlikely. Indeed, a careful study of rigidity sensing using elastic pillars showed that when stiffness was varied over a wide range, the force that cells exerted increased with stiffness but the distance cells deformed the pillars was nearly constant (Saez et al. 2005). This result would seem to argue for some version of strain sensing. Overall, it would seem that cells actively sense compliance; doing so implies that they apply force to the material and “measure” the resultant displacement.
One possibility is that the sensing mechanisms may lie in the “clutch” that governs force transmission between actin and integrins (Brown et al. 2006; Hu et al. 2007). As described in more detail by Huttenlocher and Horwitz, actin polymerizing at the leading edge flows backward because of the resistance from the plasma membrane (Huttenlocher and Horwitz 2011). Myosin-generated tension pulls on the actin and contributes to rearward movement. As it flows over the adhesions, linker proteins such as vinculin and talin bind the actin filaments and resist the rearward forces, causing more force to be applied to the plasma membrane and leading to forward protrusion. There are two interesting and surprising aspects of this system. First, talin, vinculin, and other linker proteins move backward at speeds that are intermediate between that of actin and the integrins (which are immobile) (Hu et al. 2007). Their binding kinetics must therefore be quite rapid; dissociation rates may also change under force, though to what extent they are slip bonds (increased dissociation under force) or catch bonds (decreased dissociation under force) is unknown. Second, the strength of the connection between the actin and the integrins is variable, hence the notion of a clutch mechanism that controls force transmission.
These observations suggest that if the adhesions moved backward because of stretching of the substratum, the force balance and timing of these interactions would be altered. For example, molecules of talin or vinculin could remain bound to both actin and integrin while bearing force. The altered timing could shift the kinetics of signaling events within the adhesions (Fig. 1). Hypothetically, stretched talin might bind a kinase that triggers downstream signals. On rigid surfaces, the interaction would be shorter lived and phosphorylation of substrates would be low, whereas on soft surfaces, the interaction would be prolonged and phosphorylation would be higher. This model is highly speculative and lacks any direct proof. However, though the details are almost certainly incorrect, it points toward the types of model needed to explain these data, which are essentially dynamic. That is, the difference between soft and stiff substrates is the rate at which forces build in response to cell-generated tension. Cells may sense the distance moved in a fixed period of time or the time required to move a fixed distance. But what is sensed must be the kinetics of events following application of force.
Solving this fascinating problem will require a detailed understanding of the effects of forces on molecular dynamics within the adhesions. Biophysical studies of molecular events within the adhesions, coupled with knowledge of the forces at the molecular level and their effects on protein interactions will be needed to unravel the mystery. These types of studies are underway and a more detailed understanding of mechanisms of force sensing is likely to be achieved in the foreseeable future.
CONCLUSIONS
It would be a major oversimplification to suggest that effects of matrix rigidity, externally applied strain, modulation of myosin activation and spread area are identical. Indeed, there is clear evidence to the contrary (Klein et al. 2009). However, there are distinct similarities, which are strong enough to suggest a unifying theme that reoccurs in multiple biological settings in which forces across focal adhesions are altered. Thus, conditions that give rise to high traction forces, large actin stress fibers and integrin clustering into large focal adhesions appear to result in a specific set of signals that are distinct from conditions in which traction force is low, actin is organized into smaller bundles, and focal adhesions are smaller. The high traction/large focal adhesion state requires a high density of matrix ligands on or in a stiff matrix and high forces across the adhesions. Thus, the high force/large focal adhesion state can be shifted to a low force/small adhesion state by multiple means: decreasing activation of myosin in the cells, spread area, matrix rigidity or integrin binding, and clustering. It therefore seems likely that focal adhesions themselves are a major determinant of the signaling output. The extent of integrin clustering could be an important variable that governs signal output. Focal adhesion stability could also govern signaling. Small adhesions are more dynamic (Rottner et al. 1999; Choi et al. 2008) and newly formed adhesions have high levels of active Src (Schlaepfer et al. 1998), high levels of tyrosine phosphorylation (Zamir et al. 1999), and promote high Rac and Cdc42 activity (DeMali et al. 2003). By contrast, large, Rho-dependent adhesions are less dynamic, and older focal adhesions have less active Src, and promote high Rho activity.
Evidence suggests that FAK mediates some of these differences in signaling. For example, FAK−/− cells cannot distinguish between stiff and soft surfaces during cell migration (Lo et al. 2000). Loss of FAK also diminishes cells’ ability to modulate cell proliferation in response to spread area (Pirone et al. 2006). However, large focal adhesions show other quantitative differences in composition relative to focal complexes. For example, as discussed earlier, zyxin is much more abundant in large focal adhesions, and this localization is force-dependent (Cattaruzza et al. 2004; Zaidel-Bar et al. 2004; Yoshigi et al. 2005; Lele et al. 2006; Hirata et al. 2008). By contrast, levels of phosphotyrosine are higher in small focal complexes. These differences are likely to influence signaling output as well.
That said, it is worth repeating that changes in matrix stiffness, externally applied forces, spread area and myosin activation do not yield identical outcomes. There are significant differences in the kinetics of cell interactions with ECM in these different circumstances, as well as fully independent effects. Changing myosin phosphorylation probably affects tension in cell structures other than focal adhesions and actin stress fibers. External stretch applies forces to structures other than the adhesions and actin cables, including the plasma membrane. The mechanisms by which cells sense these aspects of their environment are beginning to be unraveled. At present, it seems likely that differences in kinetic aspects of adhesion-cytoskeleton dynamics form the basis for these sensing mechanisms. Development of new biophysical tools is bringing these questions into the realm of the solvable. Understanding these aspects of adhesion biology is likely to shed light on diverse problems from stem cell differentiation and cancer metastasis to morphogenesis.
ACKNOWLEDGMENTS
I thank Brenton Hoffman for helpful comments on the manuscript. This work was supported by a USPHS grant ROI 47214 to M.A.S.
Footnotes
Editors: Richard Hynes and Kenneth Yamada
Additional Perspectives on Extracellular Matrix Biology available at www.cshperspectives.org
REFERENCES
- Aumailley M, Has C, Tunggal L, Bruckner-Tuderman L 2006. Molecular basis of inherited skin-blistering disorders, and therapeutic implications. Expert Rev Mol Med 8: 1–21 [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Schwartz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahula D, Safran S, Beshadsky A, Addadi L, et al. 2001. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3: 466–472 [DOI] [PubMed] [Google Scholar]
- Balestrini JL, Billiar KL 2006. Equibiaxial cyclic stretch stimulates fibroblasts to rapidly remodel fibrin. J Biomech 39: 2983–2990 [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B 2001. Marching at the front and dragging behind: Differential αVβ3-integrin turnover regulates focal adhesion behavior. J Cell Biol 155: 1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, Vogel V 2002. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci 99: 5139–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G 2006. Integrin signalling: The tug-of-war in heart hypertrophy. Cardiovasc Res 70: 422–433 [DOI] [PubMed] [Google Scholar]
- Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW 2006. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci 119: 5204–5214 [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M 1996. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12: 463–518 [DOI] [PubMed] [Google Scholar]
- Calvete JJ 2004. Structures of integrin domains and concerted conformational changes in the bidirectional signaling mechanism of αIIbβ3. Exp Biol Med (Maywood) 229: 732–744 [DOI] [PubMed] [Google Scholar]
- Campbell I, Humphries M 2010. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol doi:10.1101/cshperspect.a004994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, Huffman A, O’Toole ET, Kadler KE 2006. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem 281: 38592–38598 [DOI] [PubMed] [Google Scholar]
- Cattaruzza M, Lattrich C, Hecker M 2004. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension 43: 726–730 [DOI] [PubMed] [Google Scholar]
- Chiquet M, Renedo AS, Huber F, Fluck M 2003. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol 22: 73–80 [DOI] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR 2008. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol 10: 1039–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP 1997. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88: 39–48 [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 133: 1403–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N 2008. Self-organized podosomes are dynamic mechanosensors. Curr Biol 18: 1288–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP 2009. Stretching single talin rod molecules activates vinculin binding. Science 323: 638–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K 2003. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol 15: 572–582 [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689 [DOI] [PubMed] [Google Scholar]
- Farge E 2003. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol 13: 1365–1377 [DOI] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D 2009. Mechanically activated integrin switch controls α5β1 function. Science 323: 642–644 [DOI] [PubMed] [Google Scholar]
- Gabbiani G 2003. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 200: 500–503 [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Sheetz MP 2002. The relationship between force and focal complex development. J Cell Biol 159: 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Craig D, Lequin O, Campbell ID, Vogel V, Schulten K 2003. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci 100: 14784–14789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Yamada KM 2011. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol doi:10.1101/cshperspect.a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorpade A, Baxter BT 1996. Biochemistry and molecular regulation of matrix macromolecules in abdominal aortic aneurysms. Ann N Y Acad Sci 800: 138–150 [DOI] [PubMed] [Google Scholar]
- Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466: 263–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga JH, Li YS, Chien S 2007. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech 40: 947–960 [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA 2009. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10: 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki K, Mimura T, Furuya H, Morino N, Yamazaki T, Komuro I, Yazaki Y, Nojima Y 1995. Stretching mesangial cells stimulates tyrosine phosphorylation of focal adhesion kinase pp125FAK. Biochem Biophys Res Comm 212: 544–549 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Kiyokawa E, Tanaka S, Nagashima K, Gotoh N, Shibuya M, Kurata T, Matsuda M 1996. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol 16: 1770–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerkens EH, Izzard AS, Heagerty AM 2007. Integrins, vascular remodeling, and hypertension. Hypertension 49: 1–4 [DOI] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Sokabe M 2008. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci 121: 2795–2804 [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR 2011. Integrins in cell migration. Cold Spring Harb Perspect Biol doi:10.1101/cshperspect.a005074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM 2007. Differential transmission of actin motion within focal adhesions. Science 315: 111–115 [DOI] [PubMed] [Google Scholar]
- Isenberg BC, Tranquillo RT 2003. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng 31: 937–949 [DOI] [PubMed] [Google Scholar]
- Jin M, Andricioaei I, Springer TA 2004. Conversion between three conformational states of integrin I domains with a C-terminal pull spring studied with molecular dynamics. Structure 12: 2137–2147 [DOI] [PubMed] [Google Scholar]
- Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA 2005. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem 280: 16546–16549 [DOI] [PubMed] [Google Scholar]
- Katsumi A, Orr AW, Tzima E, Schwartz MA 2004. Integrins in mechanotransduction. J Biol Chem 279: 12001–12004 [DOI] [PubMed] [Google Scholar]
- Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK 2009. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol 19: 1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna SM, Dear AE, Norman PE, Golledge J 2010. Genetic and epigenetic mechanisms and their possible role in abdominal aortic aneurysm. Atherosclerosis doi: 101016/jatherosclerosis201002008. [DOI] [PubMed] [Google Scholar]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE 2006. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol 207: 187–194 [DOI] [PubMed] [Google Scholar]
- Lemmon CA, Chen CS, Romer LH 2009. Cell traction forces direct fibronectin matrix assembly. Biophys J 96: 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Recchia FA, Ranieri VM 2005. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care 11: 82–86 [DOI] [PubMed] [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH 2000. Integrin cytoplasmic domain-binding proteins. J Cell Sci 113: 3563–3571 [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL 2000. Cell movement is guided by the rigidity of the substrate. Biophys J 79: 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME 2007. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134: 3317–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS 2004. Cell shape, cytoskeletal tension and RhoA regulate stem cell lineage commitment. Dev Cell 6: 483–495 [DOI] [PubMed] [Google Scholar]
- Michael KE, Dumbauld DW, Burns KL, Hanks SK, Garcia AJ 2009. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol Biol Cell 20: 2508–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. 1999. The integrin αvβ6 binds and activates latent TGFb1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328 [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Liang R, Woo SL, Burton SD, Wu C, Almarza A, Sacks MS, Abramowitch S 2009. Effects of cell seeding and cyclic stretch on the fiber remodeling in an extracellular matrix-derived bioscaffold. Tissue Eng Part A 15: 957–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura SL 2009. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol 175: 1362–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizaka T, Shi Q, Sheetz MP 2000. Position-dependent linkages of fibronectin- integrin-cytoskeleton. Proc Natl Acad Sci 97: 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA 2006. Mechanisms of mechanotransduction. Dev Cell 10: 11–20 [DOI] [PubMed] [Google Scholar]
- Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM 2010. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 188: 877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Wang YL 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci 94: 13661–13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone DM, Liu WF, Ruiz SA, Gao L, Raghavan S, Lemmon CA, Romer LH, Chen CS 2006. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J Cell Biol 174: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Keely PJ 2009. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28: 4326–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin-Faucher E, Gao M, Schulten K, Vogel V 2006. How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation. J Cell Biol 175: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedquist KA, Ross E, Koops EA, Wolthuis RMF, Zwartkruis FJT, vanKooyk Y, Salmon M, Buckley CD, Bos JL 2000. The small GTPase Rap1 mediates CDS31-induced integrin activation. J Cell Biol 148: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart-King CA, Dembo M, Hammer DA 2005. The dynamics and mechanics of endothelial cell spreading. Biophys J 89: 676–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfranz PJ, Beckerle MC 2002. Doing (F/L)PPPPs: EVH1 domains and their proline-rich partners in cell polarity and migration. Curr Opin Cell Biol 14: 88–103 [DOI] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwartz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD 2001. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153: 1175–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Castillo AB, Turner CH 2006. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8: 455–498 [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV 1999. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol 9: 640–648 [DOI] [PubMed] [Google Scholar]
- Saez A, Buguin A, Silberzan P, Ladoux B 2005. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys J 89: L52–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai X, Naruse K, Sokabe M 1999. Activation of pp60(src) is critical for stretch-induced orienting response in fibroblasts. J Cell Sci 112: 1365–1373 [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP 2006. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127: 1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper W 2009. Collateral circulation: Past and present. Basic Res Cardiol 104: 5–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL 1992. Reactivity of small blood vessels in hypertension: Relation with structural changes. State of the art lecture. Hypertension 19: II1–9 [DOI] [PubMed] [Google Scholar]
- Schiller M, Javelaud D, Mauviel A 2004. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci 35: 83–92 [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T 1998. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: Summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol 18: 2571–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J, Geiger B 1983. The dynamic interrelationships of actin and vinculin in cultured cells. Cell Motil 3: 399–403 [DOI] [PubMed] [Google Scholar]
- Sheppard D, Munger J 2011. Integrin crosstalk/TBG-beta regulation. Cold Spring Harb Perspect Biol doi:10.1101/cshperspect.a005017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan A, Dahl SL, Niklason LE 2009. Effects of mechanical stretch on collagen and cross-linking in engineered blood vessels. Cell Transplant 18: 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi K, Rorth P 2004. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev Cell 7: 85–93 [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ 1990. Expression of extracellular matrix components is regulated by substratum. J Cell Biol 110: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS 2003. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci 100: 1484–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE 2009. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res 104: 1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolic-Norrelykke IM, Wang N 2005. Traction in smooth muscle cells varies with cell spreading. J Biomech 38: 1405–1412 [DOI] [PubMed] [Google Scholar]
- Uitto J 2009. Progress in heritable skin diseases: Translational implications of mutation analysis and prospects of molecular therapies*. Acta Derm Venereol 89: 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V 2006. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct 35: 459–488 [DOI] [PubMed] [Google Scholar]
- Wegener KL, Campbell ID 2008. Transmembrane and cytoplasmic domains in integrin activation and protein-protein interactions (review). Mol Membr Biol 25: 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström SA, Lange A, Montanez E, Fassler R 2010. The ILK/PINCH/parvin complex: The kinase is dead, long live the pseudokinase! EMBO J 29: 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS 2007. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol 176: 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Shiratori H, Hamada H 2007. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450: 285–288 [DOI] [PubMed] [Google Scholar]
- Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC 2005. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol 171: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Geiger B 2010. The switchable integrin adhesome. J Cell Sci 123: 1385–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B 2003. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci 116: 4605–4613 [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Cohen M, Addadi L, Geiger B 2004. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans 32: 416–420 [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z 1999. Molecular diversity of cell-matrix adhesions. J Cell Sci 112: 1655–1669 [DOI] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin A, Burridge K 1998. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol 141: 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA 2008. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell 32: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]