Summary
Twenty-six specimens obtained from twenty human orthotopic liver allografts 10–968 days after transplantation were studied by light microscopy, electron microscopy, and immunofluorescence. The main lesions consisted of mononuclear-cell infiltration around the portal tracts, centrilobular cholestasis, liver-cell atrophy and reticulin collapse, obliterative intimal thickening of hepatic arteries, and fibrosis. Moderate amounts of IgG and/or IgM and complement (β1C/β1A globulin or C'lq) were observed in four of the liver samples and smaller deposits were present in another five. A further three specimens contained IgG without complement. IgA was detected in only one of the samples. The immunoglobulins were found in the walls of the portal and central veins and of the sinusoids in all thirteen positive liver samples, in the walls of branches of the hepatic artery in three, and in the cytoplasm of some of the mononuclear cells infiltrating the portal tracts in nine of the specimens. Fibrinogen was seen in eight of the samples, usually in the spaces of Disse. Accumulations of immunoglobulins and complement were less frequent in liver than in kidney and heart allografts. These findings suggest that in the failure of human liver allografts cell-mediated immunity and non-immunological factors may be more important than humoral antibody.
Introduction
Morphological and immunopathological studies of human renal 1-9 and cardiac 10, 11 allografts have shown that circulating immunoglobulins and complement probably play an important part in the rejection of these organs. In this report we seek evidence of the same mechanism in hepatic allografts. Twenty-six specimens obtained from twenty orthotopic allogeneic liver grafts 10–968 days after transplantation were examined immunopathologically. The findings suggest that deposition of immunoglobulins and complement in human hepatic allografts is less frequent and less intense than in renal and cardiac allografts protected by similar immunosuppressive regimens.
Materials and Methods
Liver Specimens
Twenty-six liver specimens (table i) obtained from twenty hepatic allografts were studied by light and electron microscopy and by immunofluorescent techniques. Fourteen of the transplants, indicated by the letters OT, were from the University of Colorado Medical Center, and six, indicated by the letters OL, were from Addenbrooke's Hospital, Cambridge, and King's College Hospital, London. The commonest indications for liver replacement were primary hepatic malignancy and biliary atresia. Fifteen of the specimens were obtained by aspiration needle or by open surgical biopsy, four at removal of the graft (and replacement with a fresh allograft in three of the cases), and seven at necropsy. All the patients received prednisone and azathioprine. Seventeen were also treated with horse antilymphocyte globulin (a.l.g.). In four patients this was for 5–10 days only. The number of days after transplantation when the specimen was taken, together with additional clinical data, are given in table i. Morphologically normal liver tissue, obtained accidentally during percutaneous renal biopsy in two young patients with lipoid nephrosis, was used as a control for immunofluorescence.
TABLE I.
CLINICAL DATA ON 19 PATIENTS WITH ORTHOTOPIC HEPATIC ALLOGRAFTS
| Patient |
Donor |
HL-A group mismatches between donor and recipient |
Specimen | Time specimen taken after transplant (days) |
Clinical condition at time specimen collected |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Sex | Age | Disease | Sex | Age | ||||
| OT 8 | F | 1 yr. 7 mo. | Hepatoma | M | 1 yr. 6 mo. | None | Biopsy | 100 | Plasma-bilirubin 3 mg./100 ml. Biopsy taken during operation for resection of extrahepatic metastasis. Rejection episode at 21 days had been reversed. |
| Necropsy | 400 | Dead from carcinomatosis. | |||||||
| OT 9 | F | 1 yr. 9 mo. | Extrahepatic biliary atresia | F | 4 yr. | HL-A2 | Necropsy | 133 | Dead from liver failure caused by chronic rejection and septic infarct. |
| OT 12 | F | 1 yr. 4 mo. | Extrahepatic biliary atresia | M | 1 yr. 2 mo. | HL-A6, HL-A7, Te 11 | Necropsy | 105 | Dead from septic hepatic infarction. Rejection episode at 4 days had taken 70 days to reverse. |
| OT 13 | M | 2yr. | Extrahepatic biliaryatresia |
M | 3yr. | HL-A8, Te 11 | Resected graft 1 | 878 | Liver failure caused by chronic rejection. |
| M | 10 yr. | HL-A9, HL-A13 ± Te 6, Te 59 | Graft 2 at necropsy |
19 | Dead from bacterial peritonitis. Graft only given arterial blood supply. | ||||
| OT 14 | F | 16 yr. | Hepatoma | M | 27 yr. | HL-A2 | Resected graft 1 | 380 | Liver failure caused by chronic rejection. |
| OT 15 | M | 44 yr. | Hepatoma, cirrhosis | F | 20 yr. | Te 9 | Necropsy | 339 | Dead from carcinomatosis. Rejection episode at 6 days had been reversed. |
| OT 16 | M | 1 yr. 11 mo. | Extrahepatic biliaryatresia | M | 3yr. | None | Resected graft 1 | 68 | Liver failure caused by chronic rejection. |
| OT 19 | M | 4 yr. | Intrahepatic biliaryatresia | M | 10 yr. | HL-A2, HL-A3, HL-A7 | Biopsy | 968 | Normal liver function. Rejection episodes at 29 and 72 days had been reversed. |
| OT 22 | M | 33 yr. | Cirrhosis | M | 25 yr. | Te 12 | Resected graft | 10 | Extrahepatic biliary duct obstruction. |
| OT 23 | M | 15 yr. | Hepatoma | M | 6 yr. | HL-A6 | Necropsy | 143 | Dead from carcinomatosis. Rejection episode beginning at 7 days had been reversed. |
| OT 25 | M | 45 yr. | Hepatoma | M | 20 yr. | HL-A7 | Necropsy | 39 | Bile peritonitis caused by bile fistula. |
| OT27 | M | 11 yr. | Hepatolenticular degeneration | M | 11 yr. | Te 3 ±, Te9, Te 10, Te 12 | Biopsy | 210 | Normal liver function. |
| Biopsy | 514 | Normal liver function. Rejection episodes at 4 and 21 days had been reversed. | |||||||
| OT 29 | M | 6 yr. | Intrahepatic biliary atresia | M | 10 yr. | HL-A5, Tel 3 | Biopsy | 270 | Viral hepatitis. Plasma bilirubin 2·3 mg./100 ml. |
| OL 8 | M | 57 yr. | Cholangio-carcinoma | M | 52 yr. | 4b | Biopsy | 11 | Acute rejection episode. Plasma bilirubin 10mg./100 ml. |
| Biopsy | 46 | Plasma-bilirubin 2·6 mg./100 ml. Rejection episode at 7 days had been reversed. | |||||||
| OL 9 | F | 44 yr. | Hepatoma | M | 4 yr. | HL-A5, 4a | Biopsy | 240 | Good liver function. Rejection episode at 10 days had been reversed. |
| OL 10 | M | 56 yr. | Hepatoma, cirrhosis | M | 37 yr. | HL-A7 | Biopsy | 38 | Acute rejection episode. Plasma bilirubin 4 mg./100 ml. |
| Biopsy | 180 | ? rejection. ? cholangitis. Plasma bilirubin 8·7 mg./100 ml. Rejection episode at 38 days had been reversed. | |||||||
| OL 16 | M | 51 yr. | Cholangio-carcinoma | M | 12yr. | HL-A10, HL-A12 | Biopsy | 17 | Biliary peritonitis caused by bile fistula |
| Biopsy | 90 | Liver function good. Biopsy taken during operation for relief of duodenal obstruction. | |||||||
| OL 17 | M | 47 yr. | Cholangio-carcinoma | M | 23 yr. | HL-A2, 4b, LND | Biopsy | 45 | Liver function good. Biopsy taken during operation to deal with biliary leak. Possible rejection episode at 4 days had been reversed. |
| OL 19 | M | 28 yr. | Hepatoma | F | 20 yr. | HL-A5, 4a | Biopsy | 12 | Acute rejection episode. Plasma-bilirubin 20 mg./100 ml. |
| Biopsy | 21 | Rejection responding to treatment. Plasma bilirubin 15 mg./100 ml. | |||||||
Antisera Used for Immunofluorescent Studies
The following antisera used for fluorescein labelling were kindly supplied by other investigators or purchased from commercial laboratories: antihuman IgG and antihuman C'lq (Dr. J. Morse and Dr. C. L. Christian 12); antihuman IgA (Dr. R. D. Rossen 13); antihuman β1C/β1A globulin (Hoechst Pharmaceuticals); anti-λ and anti-κ human light chains (Dr. E. R. Osserman 14); antihuman fibrinogen (Dr. F. Gorstein); anti-horse globulin (Hyland Division of Travenol Laboratories, Inc.). The globulin fractions were separated from these antisera and from normal rabbit, goat, and horse sera and were conjugated with fluorescein by methods previously described.15
Tissue Processing for Light and Electron Microscopy
Each specimen was divided into three parts. The first portion was fixed in 10% neutral formalin, embedded in paraffin wax, sectioned, and stained with hæmatoxylin and eosin, periodic-acid Schiff, Weigert's for elastic counter-stained with hæmatoxylin and van Gieson, methyl-green pyronin, Gordon and Sweet's silver-impregnation method for reticulin fibres, and Perls' prussian-blue method for iron. The second part was fixed in buffered osmium tetroxide, embedded in ‘Epon 812’, sectioned, stained with lead citrate, and examined in a ‘Philips EM 300’ electron microscope. The third part was quickly frozen in a mixture of alcohol and dry ice or in isopentane slush in liquid nitrogen. Frozen sections 4μ thick were cut in a cryostat and treated with fluorescein-conjugated antibodies by techniques already described.15
Results
Morphological Changes
The histology of the lesions observed in most of these human liver transplants has been described elsewhere.16, 17 The more striking morphological abnormalities are summarised in table ii. A number of identifiable factors affected the structural findings; these included rejection, viral hepatitis, proved extra-hepatic biliary-duct obstruction, and recurrent carcinoma in the allograft.
TABLE II.
HISTOPATHOLOGICAL LESIONS IN 26 LIVER ALLOGRAFT SPECIMENS
| Lesion | Patient and time (in days) when specimen was obtained |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OT 8 | OT 9 | OT 12 | OT 13 | OT 14 | OT 15 | OT 16 | OT 19 | OT 22 | OT 23 | OT 25 | OT 27 | OT 29 | OL 8 | OL 9 | OL 10 | OL 16 | OL 17 | OL 19 | ||||||||
| 100 | 400 | 133 | 105 | 878 | 19 | 380 | 339 | 68 | 968 | 10 | 143 | 39 | 210 | 514 | 270 | 11 | 46 | 240 | 38 | 180 | 17 | 90 | 45 | 12 | 21 | |
| Mononuclear cell infiltration in portal tracts .. .. .. | + | + | 0 | ± | + + | 0 | ± | + | + + | ± | + | ± | ± | + | ± | + + | ± | 0 | ± | 0 | + | + | ± | ± | ± | ± |
| Percentage of mononuclear cells with pyroninophilic cytoplasm | 5 | <5 | 0 | 0 | 40 | 0 | 0 | <5 | 40 | 0 | 20 | 0 | 0 | 30 | <5 | 5 | 10 | 0 | 0 | 0 | 10 | 10 | 5 | 0 | 0 | 0 |
| Hepatic arteries — intimal thickening .. .. .. | 0 | 0 | + + | 0 | + | 0 | + | 0 | + + + | + + | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic arteries—rupture of internal elastic lamina .. | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bileduct hyperplasia .. .. | + | + | 0 | + + | 0 | 0 | ± | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bileduct obstruction .. .. | 0 | + + | 0 | + + | 0 | 0 | 0 | + + + | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cholangitis .. .. .. | + | + + | 0 | 0 | 0 | 0 | + | + + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 |
| Centrilobular cholestasis .. | + | + + | + + | + | + + + | + | + + + | ± | + | 0 | + | + + + | ± | 0 | ± | ± | + | + + | 0 | 0 | ± | ± | 0 | + | + | + + |
| Focal areas of liver-cell necrosis | 0 | 0 | 0 | + | 0 | + + | + | 0 | + | 0 | 0 | ± | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 |
| Infarcts .. .. .. | 0 | 0 | 0 | + + | 0 | + + | 0 | 0 | + + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Centrilobular liver-cell atrophy and reticulin collapse .. | 0 | + | + | + | 0 | 0 | + + | + + | ± | 0 | 0 | + | + + | 0 | 0 | 0 | + | + | 0 | 0 | ± | 0 | 0 | + | 0 | 0 |
| Fibrosis .. .. .. | + | + + | + | + | 0 | 0 | + + | 0 | + | ± | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 |
| Cirrhosis .. .. .. | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fat in centrilobular hepatocytes | 0 | 0 | 0 | 0 | 0 | + + | ± | + | 0 | 0 | + + | 0 | + | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metastases .. .. .. | + | + + | 0 | 0 | 0 | 0 | + | + + | 0 | 0 | 0 | + + + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
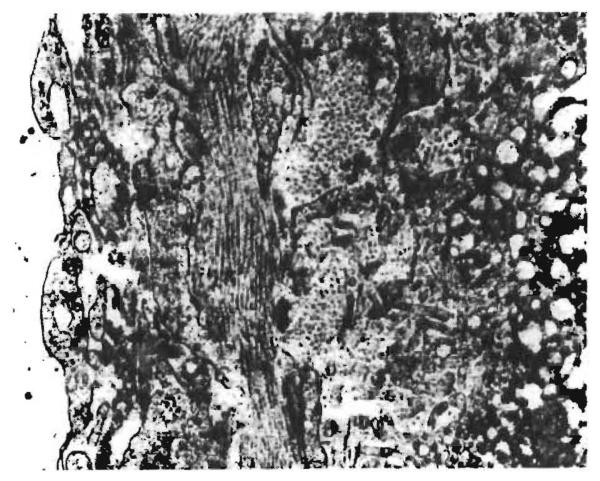
The differentiation of rejection from other causes of abnormalities was difficult. On the basis of findings in untreated and treated canine liver allografts,18-20 we assumed that dense infiltration of the portal tracts with large pyroninophilic lymphoid cells (fig. 1) indicated an active rejection process. This change was present in only two of the grafts, the 878-day specimen from OT 13 and the 68-day specimen from OT 16. Both livers also showed obliterative arterial lesions. Cellular infiltration of moderate severity was the major abnormality in a third liver (OT 27). Arterial intimal thickening was present in a biopsy taken 304 days later from this same graft.
Fig. 1. Hepatic allograft (OT 16) removed 68 days after transplantation.
Uncontrollable rejection followed early withdrawal of antilymphocyte globulin. Large number of infiltrating mononuclear cells with basophilic cytoplasm lies in a portal tract. (Hæmatoxylin and eosin; ×420.)
It was also assumed, on the basis of the same canine studies,18-20 that a milder infiltration of the portal tracts with mostly non-pyroninophilic mononuclear cells, together with prominent centrilobular bile stasis, atrophy of the centrilobular hepatocytes, collapse and condensation of the central reticulin, and accumulation of fibrin and/or collagen fibrils in the spaces of Disse (fig. 2) probably indicated residual damage following subsidence of a rejection episode, either spontaneously or as the result of treatment. These changes were present in ten of the specimens (OT 8 biopsy, OT 22, OT 23, OT 25, OL 8 both biopsies, OL 18 second biopsy, OL 17, OL 19 both biopsies) (tables i and ii).
Fig. 2. Wall of sinusoid from hepatic allograft (OT 27) 514 days after transplantation.
Fibrin and collagen occupy the space of Disse. The black granules are collections of glycogen in a hepatocyte. (Electron micrograph; ×12,000.)
In human and canine renal allografts recurrent clinical episodes of acute rejection or persistent chronic rejection are often associated with the development of intimal thickening of the arteries of the graft.11, 22 Similar changes were present in the small branches of the hepatic artery in six of the liver allografts. In most of the specimens the arterial narrowing was accompanied by fibrosis, cellular infiltration, and cholestasis; in two livers there was also a portal type of micronodular cirrhosis (OT 9, OT 14).
Extrahepatic bileduct obstruction, with or without cholangitis, affected five of the liver specimens. The second graft in patient OT 13 was only given an arterial blood-supply, and at necropsy 19 days later contained many large infarcts. One graft (OT 29) showed evidence of viral hepatitis. This diagnosis was supported by positive serological tests for Australia antigen.
Two of the biopsy specimens examined were normal and showed no evidence of either active or past rejection. In one of these grafts (OL 10) cellular infiltration and centrilobular cholestasis, liver-cell atrophy, and reticulin collapse subsequently developed. The other patient (OL 9) remains well with normal liver function 2 years 8 months after transplantation.
Metastases from the primary liver tumour were present in the specimens from four of the grafts (OT 8, OT 14, OT 15, and OT 23).
There was no correlation between the severity of rejection and the degree of mismatching observed with tissue typing.
Immunofluorescent Findings
The controls were consistently negative. The findings in the twenty-six liver specimens are summarised in table iii. Eleven of the samples gave negative results with all the fluorescent antibodies and fluorescent horse globulins. However, in two of these grafts (OT 9 and OT 16) there was arterial narrowing and a further three (OT 23, OL 8 first biopsy, OL 10 second biopsy) showed morphological evidence suggestive of residual damage caused by rejection.
TABLE III.
LOCALISATION OF FLUORESCEIN-LABELLED ANTIBODIES IN 26 LIVER ALLOGRAFT SPECIMENS
| Patient | Time specimen taken after transplant (days) |
Deposits in liver specimens* |
Localisation of deposits within liver |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgA | C′ |
Fibrinogen | Walls of |
Cytoplasm of mononuclear cells |
|||||
| β1C/β1A | C′1q | Arteries | Portal and central veins |
Sinusoids | |||||||
| OT 25 | 39 | + + | + + | 0 | + + | + + | + | + | + | + | + |
| OT 14 | 380 | + + | + | 0 | 0 | + | + + | + | + | + | + |
| OT 8 | 100 | + | + | 0 | 0 | + + | 0 | 0 | + | + | + |
| OT 22 | 10 | + | + | 0 | + | + | 0 | 0 | 0 | + | + |
| OT 27 | 210 | + | ± | 0 | 0 | + | ± | 0 | + | + | + |
| OT 15 | 339 | + | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 |
| OL 8 | 46 | + | 0 | 0 | + | 0 | 0 | 0 | 0 | + | + |
| OL 17 | 45 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 |
| OT 13 | 878 | ± | + + | 0 | 0 | + + | 0 | 0 | + | 0 | 0 |
| OL 19 | 12 | ± | 0 | 0 | ± | 0 | + + | 0 | 0 | + | + |
| OL 19 | 21 | ± | ± | 0 | 0 | 0 | + | 0 | 0 | + | + |
| OT 27 | 514 | 0 | 0 | ± | 0 | 0 | + | + | 0 | + | 0 |
| OT 12 | 105 | 0 | + | 0 | 0 | + | 0 | 0 | + | 0 | + |
| OT 19 | 968 | 0 | 0 | 0 | 0 | 0 | + + | 0 | 0 | + | 0 |
| OL 9 | 240 | 0 | 0 | 0 | 0 | 0 | ± | 0 | + | + | 0 |
| OL 8 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OL 10 | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OL 10 | 180 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OL 16 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OL 16 | 90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OT 8 | 400 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OT 9 | 133 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OT 13 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OT 16 | 68 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OT 23 | 143 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OT 29 | 270 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Intensity of immunofluorescence graded from 0 to 1 + i.
The remaining fifteen specimens showed no significant binding of fluorescent antibody to x and λ light chains and equine globulins or to fluorescein-labelled horse globulins.
Nine (OT 25, OT 14, OT 8, OT 22, OT 27, OT 15, OT 8, OT 17, and OT 13) of the 15 specimens showed complement and IgG and/or IgM in the walls of blood-vessels. The sinusoids, small portal veins, and central veins (fig. 3) were most constantly affected, but in two grafts small hepatic artery branches (fig. 4) were also involved. The fluorescent pattern was interrupted linear, finely granular, or a combination of the two. Immunoglobulins were also present in the cytoplasm of infiltrating mononuclear cells (fig. 5) in eight of the nine liver samples. In eight of the specimens that contained immunoglobulins and complement there was morphological evidence of active or resolving rejection. Extrahepatic bileduct obstruction and metastases were the main findings in the ninth specimen. The deposits of immunoglobulins and complement present in the biopsy of one liver allograft (OT 8) 100 days after transplantation were no longer detectable in the necropsy specimen 300 days later.
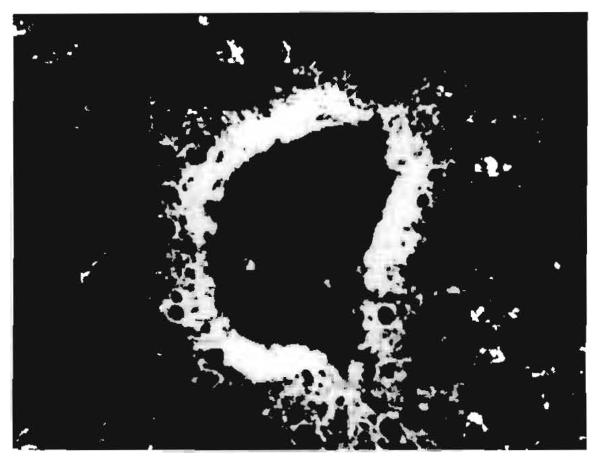
Fig. 3. Hepatic allograft (OT 14) 380 days after transplantation.
Localisation of fluorescent antibody to human IgG in the wall of a central vein. (×200.)
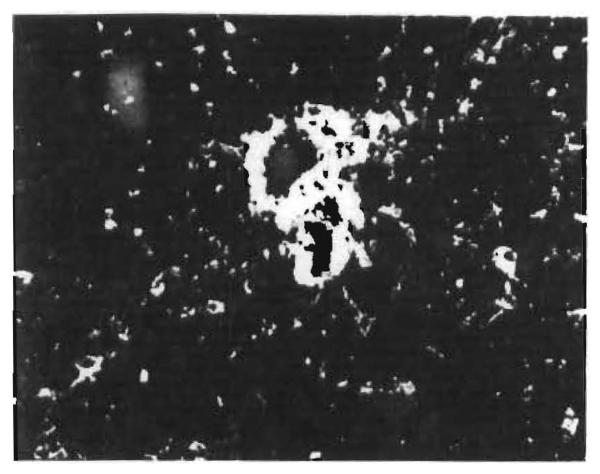
Fig. 4. Hepatic allograft (OT 25) 39 days after transplantation.
Localisation of fluorescent antibody to human IgG in the walls of a vein and of a small artery. (×250.)
Fig. 5. Hepatic allograft (OT 25) 39 days after transplantation.
The cytoplasm of infiltrating mononuclear cells is stained by fluorescent antibody to human IgG. (×300.)
A further three liver samples showed IgG in the walls of their veins and sinusoids but lacked complement (OT 15, OL 17, and OL 19). Morphological evidence of rejection was present in two of the specimens; the third was dominated by bileduct obstruction and cholangitis.
IgA was demonstrated in only one of the specimens (OT 27); small deposits were present in the walls of the small arteries and sinusoids. The morphological evidence of rejection in this graft included obliterative arterial lesions.
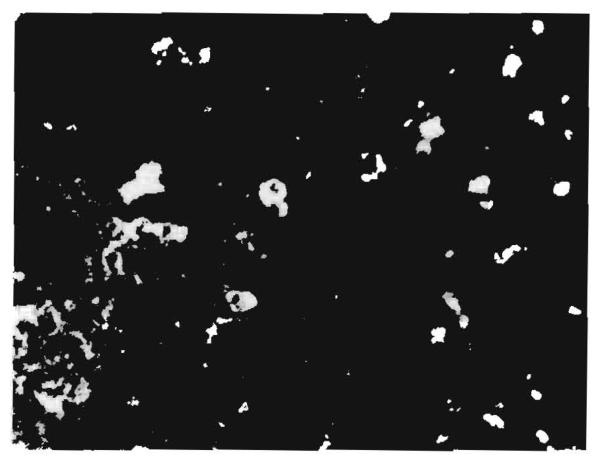
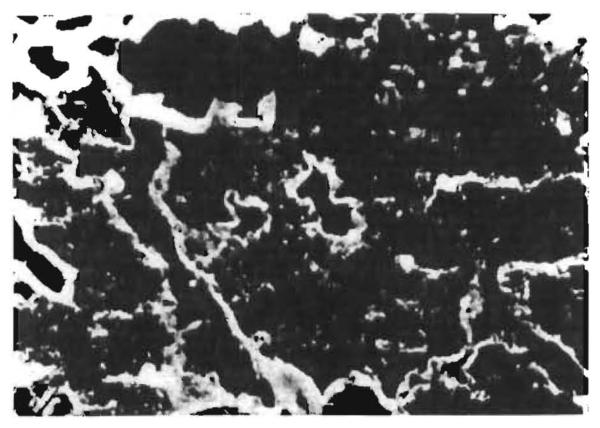
Eight of the fifteen specimens with positive findings by immunofluorescence showed fibrinogen in the walls of the sinusoids (fig. 6) and veins. The deposits were associated with immunoglobulins in six specimens and with morphological evidence of rejection in seven.
Fig. 6. Hepatic allograft (OT 19) 968 days after transplantation.
Localisation of fluorescent antibody to human fibrinogen in the walls of sinusoids. (×550.)
Discussion
The purpose of this study was to establish whether immunoglobulins, complement, and fibrinogen were deposited in orthotopic liver allografts of patients treated with immunosuppressive agents, and to correlate the sites of localisation with the histological changes observed by light and electron microscopy.
Similar studies of kidney and heart allografts have already provided information which is useful in assessing possible pathogenetic mechanisms responsible for damaging the grafts. In kidney transplants appreciable deposits of immunoglobulins and complement in glomeruli and vessels were found in over half of long-surviving grafts.5 In nearly all cardiac transplants studied, extensive deposits of immunoglobulins and complement were seen in the sarcolemma, muscle fibres, and coronary arteries.10, 11 These observations suggested that kidney and heart allografts provoked strong humoral antibody responses in addition to delayed hypersensitivity, and provided evidence which linked deposition of immunoglobulins and complement with histological lesions and deterioration of graft function.
In contrast, our study of twenty-six specimens obtained at various times from nineteen human liver allograft recipients showed that binding of immunoglobulins and complement was less frequent and less intense than in kidney or hearts transplanted to patients who were treated with comparable immunosuppressive regimens. In fact, moderate amounts of immunoglobulins and complement were localised in the sinusoids and vessel walls in only four specimens. In five additional specimens only slight and focal binding was seen. The localisation of fluorescent antibodies to fibrinogen in the walls of sinusoids and vessels, observed in eight instances, probably corresponded to the fibrin deposits seen by electron microscope in Disse's space and in vessel walls.
These findings are in keeping with our previous studies with orthotopic liver transplantation in the dog.20 In five animals, which received no immunosuppressive therapy, there were no deposits of IgG or complement in vessel walls at 4 days post-transplantation, and at 8 days only one allograft showed such deposits, whereas all the specimens had advanced morphological evidence of rejection. Paronetto et al.,22 in their studies on untreated canine recipients of auxiliary liver allografts, also found that gammaglobulin did not appear in the walls of hepatic arteries until the second week after transplantation, but their conclusion was that humoral antibodies played a significant role in the destruction of allogeneic liver grafts. In rat auxiliary liver allografts, Lee and Edgington have showed somewhat earlier and more prominent immunoglobulin deposits.24
There are several possible explanations for the lack of correlation between the immunofluorescent findings and the morphological appearance of the allografts in some of the cases in this study. First, we cannot exclude the possibility that humoral factors may have participated in the production of tissue injury, because their presence in detectable amounts may be shortlived, as suggested by studies of sequential biopsies in human renal transplants 6 and also by observations on OT 8. In addition, the lesions in liver grafts do not progress uniformly, and sampling discrepancies may be frequent. However, although these two possibilities may deserve some consideration in explaining the absence of immunoglobulins and complement in rejecting liver allografts, other mechanisms are probably of greater importance. The findings suggest that the major injury is produced by cell-mediated immunity, since infiltrating mononuclear cells are frequently seen around the portal tracts and in Disse's space. Non-immunological factors such as sepsis, the use of hepatotoxic drugs, and viral hepatitis may also contribute to the liver damage.
Acknowledgments
We thank Dr. Paul Terasaki and Dr. Valerie Joysey for the leucocyte grouping.
This work was supported by U.S. Public Health Service grants AI-10334, AI-04527, AI-10176, AI-AM-08898, AM-07772, HE-09110, AM-14928, by grants from the U.S. Veterans Administration, by grants RR-00051 and RR-00069 from the General Clinical Research Center Program of the Division of Research Resources, National Institutes of Health, by the Medical Research Council of Great Britain, the Wates Foundation, and by the Consiglio Nazionale delle Ricerche, Italy, grants 115 815 1496 and 115 815 4677.
Addendum
We have lately examined five additional orthotopic human liver allografts, 9 days, 1 month, 2½ months (two cases), and 3 years 5 months after transplantation (T. E. S.). The results accord with the reported findings. Even the 3 years 5 months allograft only showed minimal amount of fibrinogen products in the wall of a few sinusoids. Likewise in the other allografts immunoglobulins, complement, and fibrinogen were either absent or present in minimal amount only.
REFERENCES
- 1.Porter KA, Andres GA, Calder MW, Dossetor JB, Hsu KC, Rendall JM, Seegal BC, Starzl TE. Lab. Invest. 1968;18:159. [PubMed] [Google Scholar]
- 2.Lindquist RR, Guttmann RD, Merrill JP, Dammin GJ. Am. J. Path. 1968;53:851. [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie IFC, Whittingham S. Lancet. 1968;ii:1313. doi: 10.1016/s0140-6736(68)91815-1. [DOI] [PubMed] [Google Scholar]
- 4.Rosenau W, Lee JC, Najarian JS. Surgery Gynec. Obster. 1969;128:62. [PubMed] [Google Scholar]
- 5.Andres GA, Accinni L, Hsu KC, Penn I, Porter KA, Rendall JM, Seegal BC, Starzl TE. Lab. Invest. 1970;22:588. [PMC free article] [PubMed] [Google Scholar]
- 6.Rowlands DT, Burkholder PM, Bossen EH, Lin HH. Am. J. Path. 1970;61:177. [PMC free article] [PubMed] [Google Scholar]
- 7.Hume DM, Sterling WA, Weymouth RJ, Siebel HR, Madge GE, Lee HM. Transplant. Proc. 1970;2:361. [Google Scholar]
- 8.Pasternack A, Linder E. Acta path. microbiol. stand. B. 1971;79:1. doi: 10.1111/j.1699-0463.1971.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 9.McPhaul JJ, Dixon FJ, Brettschneider L, Starzl TE. New Engl. J. Med. 1970;282:412. doi: 10.1056/NEJM197002192820802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossen RD, Butler WT, Reisberg MA, Brooks DK, Leachman RD, Milam JD, Mittal KK, Montgomery JR, Nora JJ, Rochelle DG. J. Immun. 1971;106:171. [PubMed] [Google Scholar]
- 11.Ellis RJ, Lillehei CW, Zabriskie JB. J. Am. med. Ass. 1970;211:1505. [PubMed] [Google Scholar]
- 12.Morse JH, Christian CL. J. exp. Med. 1964;119:195. doi: 10.1084/jem.119.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossen RD, Morgan C, Hsu KC, Butler WT, Rose HM. J. Immun. 1968;100:706. [PubMed] [Google Scholar]
- 14.Tischendorf FW, Osserman EF. J. Immun. 1969;102:172. [PubMed] [Google Scholar]
- 15.Strauss AJL, Seegal BC, Hsu KC, Burkholder PM, Nastuk WL, Osserman KE. Proc. Soc. exp. Biol. Med. 1960;105:184. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: 1969. [Google Scholar]
- 17.Starzl TE, Porter KA, Brettschneider L, Penn I, Bell P, Putnam CW, McGuire RL. Surgery Gynet. Obstet. 1969;128:327. [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Marchioro TL, Porter KA, Taylor PD, Faris TD, Herrmann TJ, Hlad CJ, Waddell WR. Surgery, St. Louis. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Marchioro TL, Porter KA. In: Advances in Surgery. Welch C, editor. Chicago: 1966. p. 295. [PMC free article] [PubMed] [Google Scholar]
- 20.Groth CG, Porter KA, Otte JB, Daloze PN, Marchioro TL, Brettschneider L, Starzl TE. Surgery, St. Louis. 1968;63:658. [PMC free article] [PubMed] [Google Scholar]
- 21.Porter KA, Thomson WB, Owen K, Kenyon JR, Mowbray JF, Peart WS. Br. med. J. 1963;ii:639. doi: 10.1136/bmj.2.5358.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter KA, Calne RV, Zukowski CF. Lab. Invest. 1964;13:809. [PubMed] [Google Scholar]
- 23.Paronetto F, Horowitz RE, Sicular A, Burrows L, Kark AE, Popper H. Transplantation. 1965;3:303. doi: 10.1097/00007890-196505000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Edgington TS. Am. J. Path. 1968;52:649. [PMC free article] [PubMed] [Google Scholar]