Abstract
Importance of the field
Metabolic disease, which is associated with obesity and cardiovascular disease, is a worldwide epidemic. There continues to be a tremendous effort towards the development of therapies to curtail obesity and its associated pathophysiological sequelaes. The mitogen-activated protein kinases (MAPKs) have been implicated in metabolic disease suggesting that these enzymes, and those that regulate them, can potentially serve as therapeutic targets to combat this disease. The MAPK phosphatase-1 (MKP-1) mediates the dephosphorylation and inactivation of the MAPKs in insulin-responsive tissues. Therefore, the actions of MKP-1 may play an important role in the maintenance of metabolic homeostasis.
Areas covered in this review
The goal of this article will be to review the functional effects of MKP-1 in MAPK regulation with emphasis on its role in physiological and pathophysiological signaling functions that have been elucidated through the use of mouse genetics.
What the reader will gain
The reader will learn that MAPK inactivation through the effects of MKP-1 is essential for the maintenance of metabolic homeostasis. We will convey the idea that MKP-1 acts as a critical signaling node in MAPK-mediated regulation of cell signaling and metabolism.
Take home message
Pharmacological inactivation of MKP-1 may be of therapeutic value in the treatment of obesity and possibly other metabolic disorders.
Keywords: MAP kinase phosphatase, MAP Kinase, Obesity, Metabolic disease
Introduction
The mitogen activated protein kinase (MAPK) phosphatase (MKP) family of enzymes are established non-redundant regulators of MAPK signaling. The founding member and the most extensively studied of this family, MKP-1, has dynamic roles in the regulation of physiological processes such as innate and adaptive immunity, skeletal muscle regeneration and metabolism [1–5]. Here, we will highlight those studies that have examined the function of MKP-1 using approaches that employ mice lacking the expression of MKP-1. In particular, we will focus on work from this laboratory that has uncovered a role for MKP-1 in the regulation of metabolism. These studies have uncovered unanticipated opportunities for the possibility that pharmacological inactivation of MKP-1 may provide therapeutic benefit in obesity and other metabolic diseases.
Regulation of MKP-1 function: pathway and signaling specificity
The MAPK family of enzymes is ubiquitously expressed and is essential for cells to transduce information from the extracellular surface to the nucleus in order to evoke diverse cellular functions [6]. The MAPKs constitute the growth-factor responsive extracellular-signal regulated kinases 1 and 2 (ERK1/2) and the stress-responsive MAPKs, p38 MAPK and the c-Jun NH2-terminal kinases (JNK). MAPKs are activated by phosphorylation on a regulatory threonine and tyrosine residue in the activation loop by their upstream activators, the MAPK kinases [7]. Once activated, the MAPKs are inactivated by dephosphorylation of these same two residues by the MAPK phosphatases (MKPs) [8, 9].
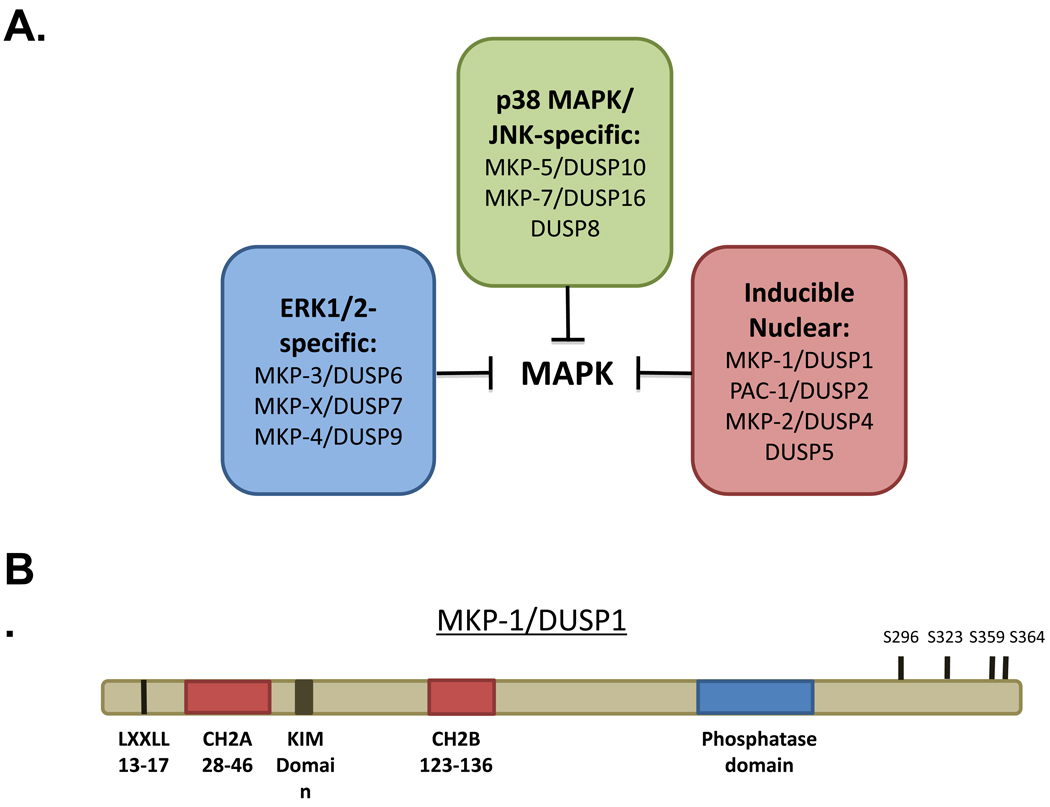
The MKP family consists of ten dual-specificity phosphatases (DUSPs) that are classified into three subgroups based on sub-cellular localization and substrate preference [8, 9]. MKP-1 was originally described as an ERK1/2-specific phosphatase, though it was later determined that it was able to dephosphorylate JNK as well as p38α/β MAPK [10–12]. Structurally, MKP-1 contains an NH2-terminus non-catalytic domain wherein a kinase interaction motif (KIM) resides (Figure 1B). The KIM, typically constitutes a basic cluster of residues, which is responsible for direct MKP-1 binding to the MAPKs [13]. MKP-1 binding to MAPK is sufficient to induce the activation of MKP-1 [14]. MKP-1 also contains a unique motif (LXXLL) that is required for its nuclear localization (Figure 1B) [15]. The cdc25 homology domains A (CH2A) and B (CH2B), as the name implies are regions, which bear similarity to domains found in the cdc25 phosphatase (Figure 1B). However, little function has been ascribed to these domains. Within the COOH terminus of MKP-1 resides the PTP domain, which conveys the properties for specific MAPK dephosphorylation (Figure 1B) [8]. Finally, at its extreme COOH terminus, MKP-1 contains several MAPK phosphorylation sites that have been proposed to control MKP-1 stability [16, 17].
Figure 1.
Representation of the family of MAP kinase phosphatases (MKPs)/dual-specificity phosphatases (DUSPs). (A) The MKPs constitute a family of 10 enzymes that can be grouped into three categories depending upon either their substrate preference for the MAPKs and sub-cellular localizaiton. (B) Schematic of MKP-1 depicting the various structural and functional domains.
MKP-1 dephosphorylates all three MAPKs, with substrate preference for p38α/β MAPK ≥ JNK > Erk1/2 and this rank order of substrate preference correlates closely with the ability of MKP-1 to interact with each of these MAPKs [9, 10, 14]. An important component of MKP-1 functionality is its sub-cellular localization to the nucleus, which is directed through a domain within its NH2 terminus [15]. Nuclear residence of MKP-1 affords it the capacity to direct MAPK dephosphorylation in a restricted manner within this compartment. Work from our laboratory has demonstrated that the localization of MKP-1 to the nucleus is crucial to its function [4]. In addition to both its specificity for the MAPKs and nuclear localization, MKP-1 is rapidly induced in response to a plethora of stresses. The expression of MKP-1 is highly regulated at both the level of transcription and protein stability. The promoter of MKP-1 is highly responsive to stimuli including ultra violet radiation, oxidative stress, growth factors, inflammatory stimuli, fatty acids, glucocorticoids, and hypoxia [18–21]. The transcription of the MKP-1 gene has been shown in multiple contexts to be regulated by the MAPKs themselves [22–25]. MKP-1 is an immediate-early gene, and its transcript is relatively labile, with a half-life of about 1 hour, which can be stabilized upon treatment with oxidants [26, 27]. MKP-1 protein stability is also tightly regulated with a protein half-life also of about 1 hour, and it is a target for ubiquitination and degradation by the proteasome [16, 28, 29]. MKP-1 activity is positively and negatively regulated by post-translational modifications [16]. Tumor necrosis factor-α potentiates JNK activity by increasing free radical production, which results in reactive oxygen species oxidizing the catalytic cysteine of MKP-1 (as well as other MKPs) leading to its inactivation [30]. MKP-1 is also acetylated on Lysine 57 in response to stimulation by LPS, which has been suggested to increase its ability to bind and hence dephosphorylate the MAPKs [31].
The induction of MKP-1 protein expression is often, but not always, driven by the activation of the MAPKs themselves, particularly the stress-responsive MAPKs, thereby establishing a negative feedback loop on the MAPKs. The nature of the dynamic upregulation of MKP-1, and subsequent MAPK dephosphorylation, is an important mechanism through which the magnitude and temporal regulation of MAPK activity is controlled. MAPK activation and inactivation has long been appreciated to be a major determinant directing biological outcomes through either transient or sustained activation kinetics [32, 33]. Once the MAPKs have translocated to the nucleus, MKP-1 plays an essential role in setting the kinetics of deactivation of this pool of MAPKs [34], functioning to fine tune the activity of the MAPKs as they acquire proximity to their target substrates.
MKP-1 as a critical “node” in MAPK signaling
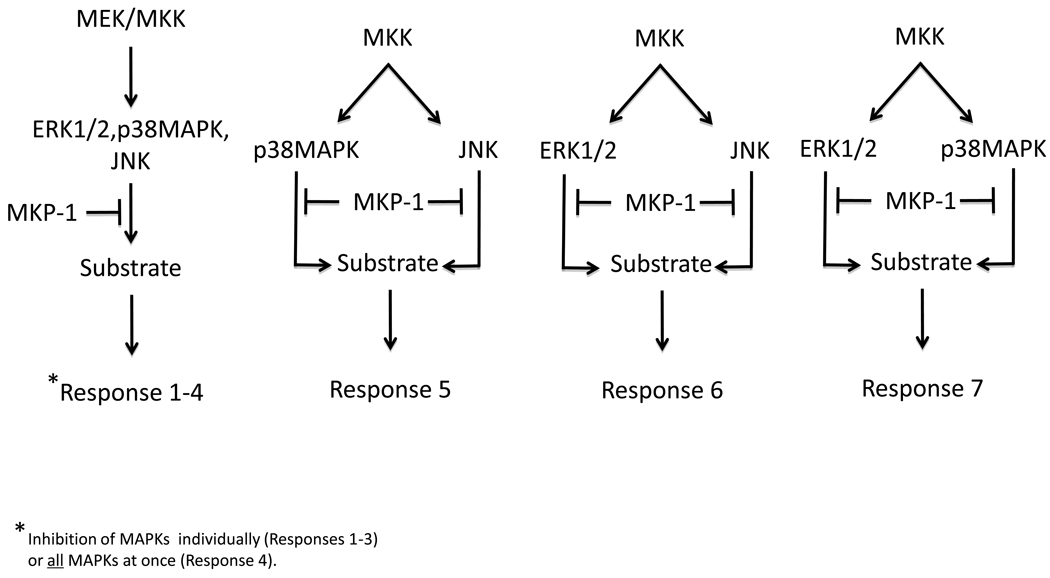
Much of the information we have learned about the MAPKs has been derived through studying the actions of individual MAPK modules [7]. Physiologically the MAPKs act simultaneously, and often on the same targets, to evoke a biological response. Therefore, MKP-1, which has the capacity to regulate multiple MAPKs simultaneously within the nucleus, likely represents a “critical node” in MAPK signaling (Figure 2). The concept of signaling networks and “critical nodes” in metabolism have been discussed [35, 36]. The general assignment of a signaling node is one in which several molecular isoforms exist and there is an involvement of divergent signaling pathways that are regulated in both a positive and negative manner. We suggest that MKP-1 satisfies these criteria and constitutes a bona fide “critical node” in the MAPK pathway. First, MKP-1 is one of several molecular isoforms (Figure 1A) and serves to receive signals from multiple MAPKs that recognize divergent inputs [33]. These characteristics endow MKP-1 with a prominent regulatory feature that if targeted, could result in the modulation of an integrated MAPK signaling response. We contend that the schematics often represented to explain the signaling effects of MKP-1, and other MKPs, should incorporate a representation of the potential for variable outputs of MAPK/MKP substrate phosphorylation (Figure 2).
Figure 2.
MKP-1 functions as a signaling node for the MAPKs. The MAPKs are dephosphorylated by MKP-1, and depending upon which MAPK is targeted, a combination of dephosphorylation events can occur. In addition, the substrates of the MAPKs can be phosphorylated by more than one MAPK, the combination of these phosphorylation events can evoke variable functional effects. The outcome of MKP-1 dephosphorylation of the MAPKs can result in a number of different functional outcomes from a particular phosphorylated MAPK substrate indicated hypothetically as “Responses 1–7”,.
Given the repertoire of MAPKs that are activated in response to a particular signal the result of the combined simultaneous inactivation of these MAPKs by MKP-1 can provide unique effects on any given MAPK substrate (Figure 2). This is particularly true if the MAPK substrate is phosphorylated on multiple residues, by more than one MAPK, and the effects of each of these phosphorylation sites yields distinct functional consequences (Figure 2). Hence, the actions of MKP-1 can in some instances manifest as either positive or negative signaling outcomes on a particular pathway (Figure 2), which can vary in different cell types either as a function of the relative activities of the various MAPKs and/or abundance of the MAPK substrate. Furthermore, due to the temporal nature of MKP-1 transcriptional activation the attenuation of an integrated MAPK response by MKP-1 serves as another measure through which signaling specificity is generated. For this reason it is difficult to predict whether the outcome of activation of a single MAPK module will necessarily be recapitulated by the inactivation of MKP-1. In some cases, inactivation of MKP-1 can be rescued by the inhibition of a single MAPK family member. These observations suggest that there are MAPK-dependent pathways that MKP-1 regulates that are controlled predominately by the actions of a single MAPK. However, whether such examples translate to the intact organism remains to be determined.
Physiological functions of MKP-1: Lessons learned from MKP-1 knock-out mice
Although it was initially realized that MKP-1 was capable of dephosphorylating the MAPKs when overexpressed, it was unclear as to whether its actions could be sub-served by other MKP family members. The generation of mice lacking the expression of MKP-1 was anticipated to resolve this issue. These initial studies indicated that mice lacking the expression of MKP-1 were neither born with developmental abnormalities nor did these mice exhibit any post-developmental phenotype [37]. Fibroblasts derived from mice lacking the expression of MKP-1 neither showed hyper-activation of ERK1/2 nor increased cell proliferation [37]. These observations led to the conclusion that MKP-1 plays a redundant role in the inactivation of the MAPKs.
Our laboratory sought to extend the examination of MKP-1 to determine its potential requirement as an essential negative regulator of the stress-responsive MAPKs and to ask whether under conditions of stress if an essential role for MKP-1 in the intact organism could be uncovered. These studies revealed that in fibroblasts derived from MKP-1-deficient mice that the level of ERK1/2 activation was equivalent to wild type cells, in agreement with those initially published [37]. In contrast, the stress-responsive MAPKs, p38α/β MAPK and JNK, were hyperactivated in these cells in response to growth factors and stress-inducing stimuli [34]. In addition, loss of MKP-1 resulted not in an increase in cell proliferation, as anticipated, but rather impaired proliferation due to increased apoptosis [34]. These results were the first to demonstrate that loss of MKP-1 is essential for the negative regulation of the stress-responsive MAPKs [34].
MKP-1 is a negative regulator of the inflammatory response
The earliest observations indicating an essential function for MKP-1 were derived from experiments focusing on the role of MKP-1 in macrophages. Several groups showed that MKP-1 plays an essential role in negatively regulating both p38α/β MAPK and JNK in the innate immune response [5, 38–40] (Figure 3). MKP-1 appears to be intimately coupled with the innate immune apparatus since its expression is regulated through the Toll-like Receptor 4 (TLR4) in macrophages, in a MyD88 and TRIF-dependent manner [5]. As a result of the loss of MKP-1, its key attenuating properties on the stress-responsive MAPKs are revealed, as MKP-1-deficient mice show an enhanced susceptibility to septic shock following LPS challenge [5, 38–40]. Interestingly, glucocorticoids, which are powerful anti-inflammatory agents, have been shown to be potent inducers of MKP-1 expression [41, 42]. Mice lacking the expression of MKP-1 are unresponsive to the anti-inflammatory actions of glucocorticoids suggesting that MKP-1 is a major mediator of the anti-inflammatory response driven by this agent [42]. These findings demonstrate the role of MKP-1 as an important regulator of innate immunity and the anti-inflammatory actions of the glucocorticoids. However, it appears that MKP-1 does not participate in all aspects of glucocorticoid-mediated inflammatory signaling since mice lacking MKP-1 still respond to the anti-allergic actions of the glucocorticoids [43]. More recently, MKP-1 has been suggested to be a positive mediator of B- and T-cell function [44] which, provides another example of how the actions of MKP-1 in the immune system are important, but also that within the hematopoietic system it can mediate both positive and negative effects.
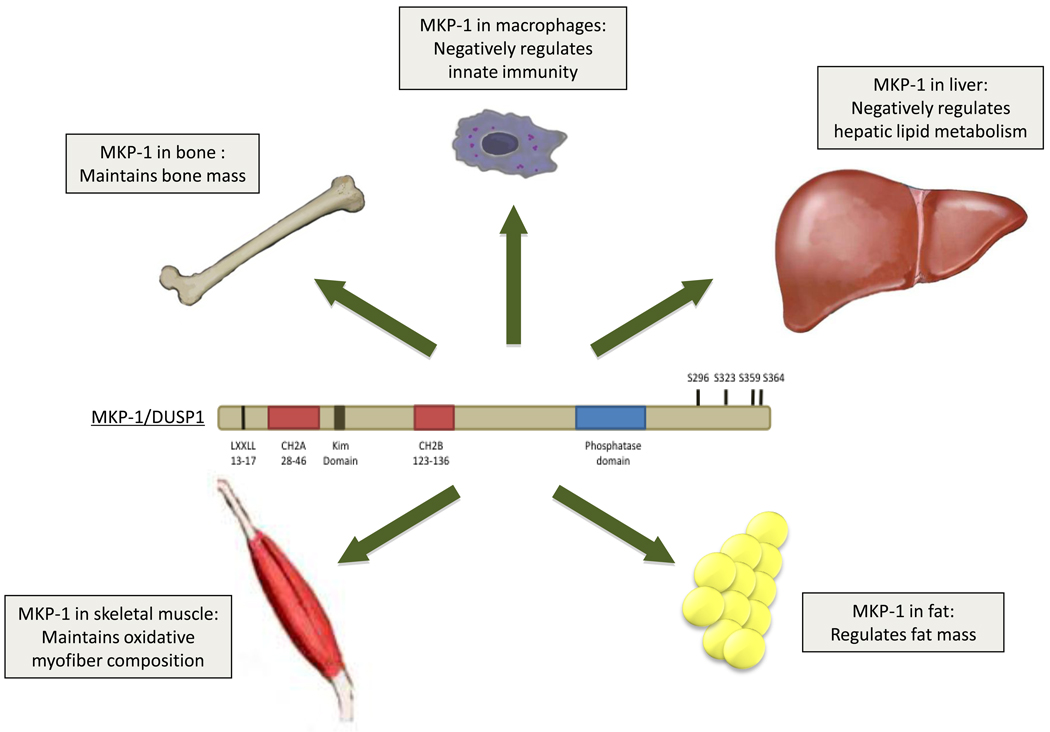
Figure 3.
Essential physiological roles of MKP-1. MKP-1 has been implicated in the regulation of innate immuninty in macrophages, regulation of metabolic homeostasis in the liver, skeletal muscle and fat. MKP-1 also has effects on the maintenance of bone mass.
MKP-1 in bone remodeling
The prominent function of MKP-1 in macrophages prompted our laboratory to investigate whether there was a role for MKP-1 in bone formation and function. Surprisingly, despite a pronounced requirement for MKP-1 in macrophages, the effects of MKP-1 on bone remodeling were subtle. Only in females was a decrease in bone mass observed [3] suggesting that MKP-1-deficient mice exhibited either increased macrophage-derived osteoclast or decreased osteoblast activity (Figure 3). MKP-1-deficient mice have significantly lower numbers of osteoclasts as compared to wild type animals, without an appreciable difference in the number of osteoblasts indicating that the reduced bone mass likely occurred through enhanced osteoclast rather than reduced osteoblast activity [3]. Consistent with this, MKP-1-deficient osteoclasts showed increased p38α/β MAPK and JNK activity and were appreciably more active in their bone absorptive capacity [3]. These results implied that MKP-1 negatively regulates osteoclast function and bone homeostasis.
MKP-1 in the maintenance of skeletal muscle function
Adult muscle growth and repair is promoted through the actions of the MAPKs, in particular p38α/β MAPK plays a prominent role in this process. How muscle growth and repair are regulated through the actions of the MKPs was unknown. We investigated the role of MKP-1 in adult regenerative myogenesis and found that while MKP-1 was capable of inactivating p38α/β MAPK in the population of cells responsible for regeneration (satellite cells), MKP-1-deficient mice were impaired in their ability to regenerate skeletal muscle in response injury [1]. These results suggest that MKP-1 positively regulates skeletal muscle regeneration despite the fact that it opposes the pro-myogenic actions of p38α/β MAPK in satellite cells (Figure 3). However, it also appears that the actions of MKP-1 on skeletal muscle regeneration are not solely a function of its effects in satellite cells since in response to muscle injury there is a profound inflammatory response, which likely contributes to the impaired muscle regenerative capacity [1]. In this instance, MKP-1 tissue-specific knock-out mice will be required to fully dissect the relative contribution of satellite cells and immune system effects to the overall skeletal muscle regenerative response.
MKP-1 in the control of metabolic homeostasis
The MAPKs have been shown to be involved in a multitude of metabolic processes including adipogenesis, insulin signaling, glucose uptake, fatty acid metabolism, lipogenesis, and energy expenditure [45–52]. Consistent with this, a role for the MKPs in metabolism is also emerging [2, 4, 53, 54]. Work from our group discovered that MKP-1-deficient mice are resistant to age and high-fat diet induced obesity [4]. The resistance to weight gain in the MKP-1-deficient mice was attributed to increased energy expenditure. Unexpectedly, measures of glucose homeostasis revealed that MKP-1-deficient mice were not insulin sensitive despite being leaner than wild type mice. The fact that MKP-1-deficient mice, though protected from obesity, were not insulin sensitive posed a conundrum. This issue was resolved by experiments which showed that MKP-1 controlled the nuclear pool of MAPKs that are involved in driving the expression of energy expenditure genes, while leaving the activity of the cytosolic MAPKs such as JNK, which influence insulin sensitivity through phosphorylation of the insulin-receptor substrate-1, unaffected [4]. These results revealed the functional importance of the nuclear restriction of MKP-1 dephosphorylation of the MAPKs and provided evidence in mice for the importance of the spatial regulation of the MAPK/MKP signaling module.
The most likely contributing factor for the reduced adiposity in MKP-1-deficient mice resides in the increased levels of energy expenditure observed in these mice [4]. Interestingly, a role for MKP-1 in adipogenesis has been suggested. In 3T3-L1 adipocytes, MKP-1 levels increased during adipocyte differentiation, concomitant with a decrease in ERK1/2 activity. Furthermore, reducing MKP-1 expression with antisense oligonucleotides decreased adipogenesis in an ERK1/2-dependent manner [55]. At odds with these reports, we found that adipocyte differentiation from mouse embryonic fibroblasts derived from wild-type and MKP-1-deficient mice occurred at equivalent rates [4]. It is conceivable that the ability of MKP-1 to regulate ERK1/2 activity in adipocytes may influence the activity of the master regulator of adipogenesis, the peroxisome proliferator-activated receptor γ (PPARγ), which is negatively regulated by ERK1/2 phosphorylation [56].
Although MKP-1 was originally identified as a stress-responsive gene in the liver [57, 58], its physiological function in this tissue was unknown. We found that hepatic steatosis is dramatically attenuated in mice lacking MKP-1 when mice are fed a high fat diet [4]. This effect was due to enhanced fatty acid metabolism since in MKP-1-deficient mice, ligand-induced activation of PPARα, which promotes fatty acid β-oxidation, was enhanced in a p38 MAPK-dependent manner [4]. Furthermore, a target of PPARα, carnitine palmitoyltransferase I–L, which is responsible for the transport of long-chain fatty acids into the mitochondria, was elevated in the liver after fasting in MKP-1-deficient mice [4]. These results suggest that in the liver, MKP-1 negatively regulates fatty acid metabolism by decreasing the sensitivity of PPARγ to bind ligand and become activated. Hence, pharmacological inactivation of MKP-1 may provide a unique way in which to limit hepatic steatosis in cases of obesity and other metabolic disorders.
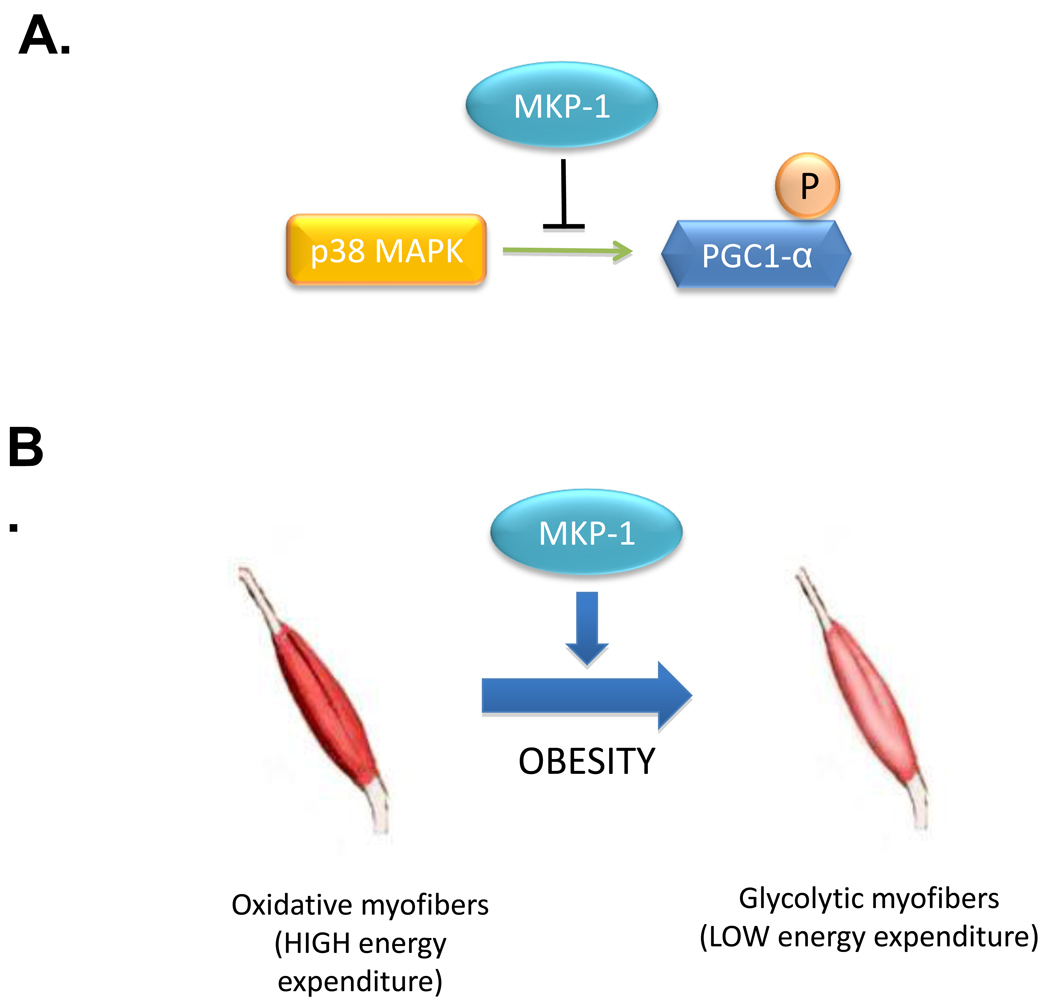
Skeletal muscle is a major site through which energy expenditure is regulated in the rodent [59–61]. We have shown that in skeletal muscle, MKP-1 regulates MAPK-dependent signaling which controls this process. Several lines of evidence suggest that MKP-1 negatively regulates energy expenditure in skeletal muscle. Mitochondrial oxidative phosphorylation is significantly enhanced in skeletal muscles of mice lacking the expression of MKP-1 [4] and this is accompanied by increased expression of oxidative myofibers [2]. This is particularly significant since the number of oxidative myofibers declines in obesity and switches to a more glycolytic composition [62, 63]. Glycolytic myofibers have reduced oxidative capacity and consume less energy. In mice lacking MKP-1, this pathophysiological switch from oxidative to glycolytic myofibers is attenuated during high fat feeding [4]. Therefore, mice lacking MKP-1 maintain both the composition of oxidative myofibers and levels of energy expenditure that otherwise decline after a high-fat diet in wild type mice. In obesity, MKP-1 levels of expression increase correlating with the loss of oxidative myofibers [4]. We have tied these changes in MKP-1 expression to reduced mitochondrial function through the actions of MKP-1 on p38 MAPK-mediated phosphorylation of the peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α). PGC-1α promotes energy expenditure by targeting genes that activate mitochondrial biogenesis [64, 65]. Under conditions of obesity MKP-1 is overexpressed in skeletal muscle resulting in the inhibition of p38 MAPK-mediated phosphorylation of PGC-1α, which facilitates its degradation and hence, reduces its activity (Figure 4) [2]. These results demonstrate that in skeletal muscle, MKP-1 underlies a basic mechanism for the control of energy expenditure by interfacing with MAPK-dependent events that regulate mitochondrial biogenesis.
Figure 4.
MKP-1 regulates MAPK-dependent signaling in metabolism. (A) The transcriptional co-activator, PGC-1α, is phosphorylated by p38 MAPK which promotes its stability and subsequent activity. MKP-1 dephosphorylates p38 MAPK resulting in reduced PGC-1α phosphorylation and function. (B) In obesity, MKP-1 is overexpressed in skeletal muscle resulting in reduced PGC-1α phosphorylation and activity. The loss of PGC-1α̣ activity impairs the capacity to maintain oxidative myofibers resulting in an increased susceptiblity of high energy consuming oxidative myofibers to switch to less energy consuming glycolytic myofibers.
Pharmacological Targeting of MKP-1 in Metabolism
It may appear counterintuitive that inhibiting MKP-1, and hence increasing MAPK activity, may have beneficial metabolic effects in light of the fact that inhibition of the stress-responsive MAPK, JNK, has been suggested to be of therapeutic value for metabolic disease [66, 67]. Presumably, these different indications are attributed to the complex nature of both overlapping and non-overlapping signaling pathways regulated by the MAPKs and MKP-1, which further supports the idea that the concerted actions of MKP-1 as a MAPK signaling node can result in very unique outcomes (Figure 2).
Inhibition of MKP-1 activity in skeletal muscle may provide new ways in which to preserve the loss of oxidative myofibers and hence maintain whole body metabolism to curtail weight gain. In the diseased liver, the ability to inhibit MKP-1 activity may provide therapeutic benefit to ameliorate the development of hepatic steatosis and in so doing preserve hepatic function. Small molecule inhibitors to MKP-1 represent attractive therapeutic strategies for metabolic disease since MKP-1 in unstressed states is expressed at relatively low levels in insulin-responsive tissues. Whereas, in obesity and type II diabetes, MKP-1 is overexpressed [4, 68, 69]. Therefore, the actions of a small molecule inhibitor to MKP-1 will be expected to act predominately in those tissues overexpressing MKP-1. It will need to be determined whether MKP-1 is overexpressed in obesity and type II diabetes in humans. Interestingly, MKP-1 expression levels are repressed in humans following bariatric surgery [70] suggesting a correlation between MKP-1 expression and fat mass in humans. If so, this situation should provide MKP-1 the attributes that make it a good target for therapeutic intervention since only in those diseased tissues will the expression of MKP-1 be sufficiently high to exert an effect on the MAPK pathway.
There has been an apprehensive outlook towards inhibiting the MKPs due to concerns of toxicity and/or deleterious side effects because of the fact that one is broadly removing a ubiquitously expressed MAPK antagonist. However, it can be argued that this is unlikely to be the case because inhibition of MKP-1 in the mouse does not result in overt effects in unstressed animals [37]. In addition, specific inhibition of MKP-1 causes discrete upregulation of the nuclear pool of MAPKs [2, 4]. The restricted inhibition, and hence activation of the MAPKs to the nucleus, will likely reduce dramatically major side effects due to enhanced MAPK activation. In skeletal muscle, inactivation of MKP-1 pharmacologically, and hence upregulation of the nuclear pool of MAPKs would be expected to cause the activation of gene expression events that promote energy expenditure without affecting the cytosolic pools of MAPKs that interfere with insulin sensitivity. Secondary consequences of weight loss promoted through enhanced skeletal muscle energy expenditure would subsequently improve parameters of insulin sensitivity. Clearly, a substantial amount of work is required to definitively prove these outcomes in animal models and ultimately whether such affects are recapitulated in humans.
MKP-1 inhibitors have been identified through the efforts of high-throughput screens where small molecules exhibiting properties of high selectivity to the MKPs have been identified [71–73]. One of the drawbacks limiting progress towards successful high affinity MKP-1 inhibitor design and development rests in the fact that the PTP domains of the MKPs are all highly similar. Thus, the development of specific MKP-1 inhibitors still remains a challenge. To date, the crystallographic structure of MKP-1 has yet to be solved and therefore drug design based upon information at the atomic level is not yet possible. Efforts towards the design of MKP-1 inhibitors have utilized the crystal structure information for MKP-5 [74, 75], which also is an MKP that dephosphorylates the stress-responsive MAPKs [76]. In the absence of small molecule inhibitors to MKP-1, the use of anti-sense oligonucleotides or small interfering RNA technologies may provide a useful therapeutic benefit for metabolic diseases such as in the treatment of non-alcoholic fatty liver disease where MKP-1 inhibition has been shown to attenuate hepatic steatosis [4]. Whether MKP-1 inactivation truly represents an effective therapy for metabolic disease in humans at this juncture is obviously unclear. Deleterious effects of global MKP-1 inactivation have been reported to promote the activation of the innate immune system [5, 38, 39]. This effect alone may exacerbate metabolic disease. Other notable considerations are the potential affects of MKP-1 in the bone where its loss increases osteoclast activity [3], which could have negative affects on bone mass in the aging population. In some cases MKP-1 inactivation has been implied to promote [77], and in others, its inactivation impedes the pathogenesis of atherosclerosis [78, 79]. However, which of these effects on MKP-1 inhibition predominates when translated to the intact organism will require further investigation.
Conclusion
MKP-1 can now be defined as an essential and dynamic regulator of the MAPK pathway in the control of metabolic signaling. The next wave of research on MKP-1 will need to encompass an assessment of its effects in specific tissues in order to ascertain the site of action through which it exerts metabolic control. This is likely to be further complicated by the fact that MKP-1 acts as a critical node to control multiple MAPK signaling pathways. Nevertheless, the benefits of pharmacologically inhibiting MKP-1 in the treatment of metabolic disease are clearly appealing. Further studies should continue to provide support for the notion that MKP-1 is a potential therapeutic target for the treatment of metabolic disease.
Article highlights.
MKP-1 plays an essential role in the dephosphorylation and subsequent inactivation of the nuclear pool of MAPKs in insulin-responsive tissues in mice.
MKP-1 serves as a “critical node” to integrate MAPK signaling effects.
In obese mice MKP-1 is overexpressed suggesting that it is involved in the progression of dysfunctional metabolic signaling.
Mice lacking MKP-1 are resistant to diet-induced obesity due to increased energy expenditure. MKP-1 negatively regulates MAPK signaling through a pathway linked to mitochondrial function in skeletal muscle, which becomes dysregulated in obesity.
Pharmacological targeting of MKP-1 may serve as a therapeutic strategy for the treatment of metabolic diseases.
Acknowledgments
Declaration of interest
A.M. Bennett is supported by NIH R01 grants AR04625, DK75776 and DK57751 and R.J.R. Flach is supported by the Yale Liver Center Training grant T32 DK07356.
References
- 1.Shi H, Boadu E, Mercan F, et al. MAP kinase phosphatase-1 deficiency impairs skeletal muscle regeneration and exacerbates muscular dystrophy. FASEB J. 2010;24:2985–2997. doi: 10.1096/fj.09-150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth RJ, Le AM, Zhang L, et al. MAPK phosphatase-1 facilitates the loss of oxidative myofibers associated with obesity in mice. J Clin Invest. 2009;119:3817–3829. doi: 10.1172/JCI39054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson J, Cui W, Zhang Q, et al. Role of MKP-1 in osteoclasts and bone homeostasis. Am J Pathol. 2009;175:1564–1573. doi: 10.2353/ajpath.2009.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JJ, Roth RJ, Anderson EJ, et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metabolism. 2006;4:61–73. doi: 10.1016/j.cmet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Chi H, Barry SP, Roth RJ, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. PNAS. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 8.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 9.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 10.Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-actviated protein kinase in U397 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 11.Franklin CC, Srikanth S, Kraft AS. Conditional expression of mitogen-activated protein kinase phosphatase-1, MKP-1 is cytoprotective against UV-induced apoptosis. Proc Natl Acad Sci U S A. 1998;95:3014–3019. doi: 10.1073/pnas.95.6.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun H, Charles CH, Lau LF, et al. MKP-1 (3CH134), an intermediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 13.Tanoue T, Adachi M, Moriguchi T, et al. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–116. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- 14.Hutter D, Chen P, Barnes J, et al. Catalytic activation of mitogen-activated protein (MAP) kinase phosphatase-1 by binding to p38 MAP kinase: critical role of the p38 C-terminal domain in its negative regulation. Biochem J. 2000;352(Pt 1):155–163. [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JJ, Zhang L, Bennett AM. The noncatalytic amino terminus of mitogen-activated protein kinase phosphatase 1 directs nuclear targeting and serum response element transcriptional regulation. Mol Cell Biol. 2005;25:4792–4803. doi: 10.1128/MCB.25.11.4792-4803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brondello JM, Pouyssegur J, McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK- dependent phosphorylation. Science. 1999;286:2514–2517. doi: 10.1126/science.286.5449.2514. [DOI] [PubMed] [Google Scholar]
- 17.Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem. 2006;281:915–926. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Gorospe M, Yang C, et al. Role of mitogen-activated protein kinase phosphatase during cellular response to genotoxic stress. Journal of Biological Chemistry. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 19.Ryser S, Massiha A, Piuz I, et al. Stimulated initiation of mitogen-activated protein kinase phosphatase-1 (MKP-1) gene transcription involves the synergistic action of multiple cis-acting elements in the proximal promoter. Biochem J. 2004;378:473–484. doi: 10.1042/BJ20031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furst R, Schroeder T, Eilken HM, et al. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. Faseb J. 2006 doi: 10.1096/fj.06-6752com. [DOI] [PubMed] [Google Scholar]
- 21.Kassel O, Sancono A, Kratzschmar J, et al. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. Embo J. 2001;20:7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P, Li J, Barnes J, et al. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 23.Hu JH, Chen T, Zhuang ZH, et al. Feedback control of MKP-1 expression by p38. Cell Signal. 2007;19:393–400. doi: 10.1016/j.cellsig.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Tillo E, Comalada M, Farrera C, et al. Macrophage-Colony-Stimulating Factor-Induced Proliferation and Lipopolysaccharide-Dependent Activation of Macrophages Requires Raf-1 Phosphorylation to Induce Mitogen Kinase Phosphatase-1 Expression. J Immunol. 2006;176:6594–6602. doi: 10.4049/jimmunol.176.11.6594. [DOI] [PubMed] [Google Scholar]
- 25.Furst R, Zahler S, Vollmar AM. Dexamethasone-induced expression of endothelial mitogen-activated protein kinase phosphatase-1 involves activation of the transcription factors activator protein-1 and 3',5'-cyclic adenosine 5'-monophosphate response element-binding protein and the generation of reactive oxygen species. Endocrinology. 2008;149:3635–3642. doi: 10.1210/en.2007-1524. [DOI] [PubMed] [Google Scholar]
- 26.Kuwano Y, Kim HH, Abdelmohsen K, et al. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–4575. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. Embo J. 1985;4:3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charles CH, Abler AS, Lau LF. cDNA sequence of a growth-inducible immediate early gene and characterization of its encoded protein. Oncogene. 1992;7:187–190. [PubMed] [Google Scholar]
- 29.Noguchi T, Metz R, Chen L, et al. Structure, mapping, and expression of erp, a growth factor-inducible gene encoding a nontransmembrane protein tyrosine phosphatase, and effect of ERP on cell growth. Molecular and Celluar Biology. 1993;13:5195–5205. doi: 10.1128/mcb.13.9.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamata H, Honda S, Maeda S, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 31.Cao W, Bao C, Padalko E, et al. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson M, Mandl M, Keyse SM. Spatio-temporal regulation of mitogen-activated protein kinase (MAPK) signalling by protein phosphatases. Biochem Soc Trans. 2006;34:842–845. doi: 10.1042/BST0340842. [DOI] [PubMed] [Google Scholar]
- 34.Wu JJ, Bennett AM. Essential Role for Mitogen-activated Protein (MAP) Kinase Phosphatase-1 in Stress-responsive MAP Kinase and Cell Survival Signaling. J Biol Chem. 2005;280:16461–16466. doi: 10.1074/jbc.M501762200. [DOI] [PubMed] [Google Scholar]
- 35.Jordan JD, Landau EM, Iyengar R. Signaling Networks: The Origins of Cellular Multitasking. Cell. 2000;103:193–200. doi: 10.1016/s0092-8674(00)00112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 37.Dorfman K, Carrasco D, Gruda M, et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- 38.Zhao Q, Wang X, Nelin LD, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salojin KV, Owusu IB, Millerchip KA, et al. Essential Role of MAPK Phosphatase-1 in the Negative Control of Innate Immune Responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 40.Hammer M, Mages J, Dietrich H, et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasa M, Abraham SM, Boucheron C, et al. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22:7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham SM, Clark AR. Dual-specificity phosphatase 1: a critical regulator of innate immune responses. Biochem Soc Trans. 2006;34:1018–1023. doi: 10.1042/BST0341018. [DOI] [PubMed] [Google Scholar]
- 43.Maier JV, Brema S, Tuckermann J, et al. Dual specificity phosphatase 1 knockout mice show enhanced susceptibility to anaphylaxis but are sensitive to glucocorticoids. Mol Endocrinol. 2007;21:2663–2671. doi: 10.1210/me.2007-0067. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Reynolds JM, Chang SH, et al. MKP-1 Is Necessary for T Cell Activation and Function. Journal of Biological Chemistry. 2009;284:30815–30824. doi: 10.1074/jbc.M109.052472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bost F, Aouadi M, Caron L, et al. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Bost F, Aouadi M, Caron L, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- 47.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 48.Puigserver P, Rhee J, Lin J, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 49.Xiong Y, Collins QF, An J, et al. p38 Mitogen-activated Protein Kinase Plays an Inhibitory Role in Hepatic Lipogenesis. J Biol Chem. 2007;282:4975–4982. doi: 10.1074/jbc.M606742200. [DOI] [PubMed] [Google Scholar]
- 50.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somwar R, Koterski S, Sweeney G, et al. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J Biol Chem. 2002;277:50386–50395. doi: 10.1074/jbc.M205277200. [DOI] [PubMed] [Google Scholar]
- 52.Barger PM, Browning AC, Garner AN, et al. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 53.Emanuelli B, Eberle D, Suzuki R, et al. Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:3545–3550. doi: 10.1073/pnas.0712275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Yang Q, Shen M, et al. Dual specificity MAPK phosphatase 3 activates PEPCK gene transcription and increases gluconeogenesis in rat hepatoma cells. J Biol Chem. 2005;280:36013–36018. doi: 10.1074/jbc.M508027200. [DOI] [PubMed] [Google Scholar]
- 55.Sakaue H, Ogawa W, Nakamura T, et al. Role of MAPK phosphatase-1 (MKP-1) in adipocyte differentiation. J Biol Chem. 2004;279:39951–39957. doi: 10.1074/jbc.M407353200. [DOI] [PubMed] [Google Scholar]
- 56.Hu E, Kim J, Sarraf P, et al. Inhibition of Adipogenesis Through MAP Kinase-mediated phosphorylation of PPARg. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 57.Mohn KL, Laz TM, Hsu J-C, et al. The immediate-early growth response in regenerating liver and insulin- stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Molecular and Celluar Biology. 1991;11:381–390. doi: 10.1128/mcb.11.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mohn KL, Laz TM, Melby AE, et al. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Journal of Biological Chemistry. 1990;265:21914–21921. [PubMed] [Google Scholar]
- 59.Bassel-Duby R, Olson EN. Signaling Pathways in Skeletal Muscle Remodeling. Annu Rev Biochem. 2006 doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- 60.Zurlo F, Larson K, Bogardus C, et al. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zierath JR, Hawley JA. Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol. 2004;2:e348. doi: 10.1371/journal.pbio.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- 63.Simoneau JA, Veerkamp JH, Turcotte LP, et al. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J. 1999;13:2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 64.Handschin C, Spiegelman BM. Peroxisome Proliferator-Activated Receptor {gamma} Coactivator 1 Coactivators, Energy Homeostasis, and Metabolism. Endocr Rev. 2006 doi: 10.1210/er.2006-0037. er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 65.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bennett BL, Satoh Y, Lewis AJ. JNK: a new therapeutic target for diabetes. Current Opinion in Pharmacology. 2003;3:420–425. doi: 10.1016/s1471-4892(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 67.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 68.Reddy ST, Nguyen JT, Grijalva V, et al. Potential role for mitogen-activated protein kinase phosphatase-1 in the development of atherosclerotic lesions in mouse models. Arterioscler Thromb Vasc Biol. 2004;24:1676–1681. doi: 10.1161/01.ATV.0000138342.94314.64. [DOI] [PubMed] [Google Scholar]
- 69.Chin S, Ramirez S, Greenbaum LE, et al. Blunting of the immediate-early gene and mitogenic response in hepatectomized type 1 diabetic animals. Am J Physiol Endocrinol Metab. 1995;269:E691–E700. doi: 10.1152/ajpendo.1995.269.4.E691. [DOI] [PubMed] [Google Scholar]
- 70.Dankel SN, Fadnes DJ, Stavrum AK, et al. Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss. PLoS One. 2010;5:e11033. doi: 10.1371/journal.pone.0011033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vogt A, Tamewitz A, Skoko J, et al. The Benzo[c]phenanthridine Alkaloid, Sanguinarine, Is a Selective, Cell-active Inhibitor of Mitogen-activated Protein Kinase Phosphatase-1. Journal of Biological Chemistry. 2005;280:19078–19086. doi: 10.1074/jbc.M501467200. [DOI] [PubMed] [Google Scholar]
- 72.Lazo JS, Nunes R, Skoko JJ, et al. Novel benzofuran inhibitors of human mitogen-activated protein kinase phosphatase-1. Bioorganic & Medicinal Chemistry. 2006;14:5643–5650. doi: 10.1016/j.bmc.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 73.Bakan A, Lazo JS, Wipf P, et al. Toward a molecular understanding of the interaction of dual specificity phosphatases with substrates: insights from structure-based modeling and high throughput screening. Curr Med Chem. 2008;15:2536–2544. doi: 10.2174/092986708785909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tao X, Tong L. Crystal structure of the MAP kinase binding domain and the catalytic domain of human MKP5. Protein Sci. 2007;16:880–886. doi: 10.1110/ps.062712807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong DG, Yoon TS, Kim JH, et al. Crystal structure of the catalytic domain of human MAP kinase phosphatase 5: structural insight into constitutively active phosphatase. J Mol Biol. 2006;360:946–955. doi: 10.1016/j.jmb.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 76.Tanoue T, Moriguchi T, Nishida E. Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J Biol Chem. 1999;274:19949–19956. doi: 10.1074/jbc.274.28.19949. [DOI] [PubMed] [Google Scholar]
- 77.Zakkar M, Chaudhury H, Sandvik G, et al. Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ Res. 2008;103:726–732. doi: 10.1161/CIRCRESAHA.108.183913. [DOI] [PubMed] [Google Scholar]
- 78.Shen J, Chandrasekharan UM, Ashraf MZ, et al. Lack of mitogen-activated protein kinase phosphatase-1 protects ApoE-null mice against atherosclerosis. Circ Res. 2010;106:902–910. doi: 10.1161/CIRCRESAHA.109.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imaizumi S, Grijalva V, Priceman S, et al. Mitogen-activated protein kinase phosphatase-1 deficiency decreases atherosclerosis in apolipoprotein E null mice by reducing monocyte chemoattractant protein-1 levels. Mol Genet Metab. 2010;101:66–75. doi: 10.1016/j.ymgme.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]