Abstract
In the Medicare program, increases in cost sharing by a supplemental insurer can exert financial externalities. We study a policy change that raised patient cost sharing for the supplemental insurer for retired public employees in California. We find that physician visits and prescription drug usage have elasticities that are similar to those of the RAND Health Insurance Experiment (HIE). Unlike the HIE, however, we find substantial “offset” effects in terms of increased hospital utilization. The savings from increased cost sharing accrue mostly to the supplemental insurer, while the costs of increased hospitalization accrue mostly to Medicare.
The elderly are the most intensive consumers of health care in the United States today. Individuals over age 65 consume 36 percent of health care in the US, despite representing only 13 percent of the population (Centers for Medicaid and Medicare Services 2005). The Medicare program that insures the nation's elderly (as well as the disabled) is the thirt largest expenditure item for the federal government, and is projected to exceed Social Security by 2024 (Centers for Medicaid and Medicare Services 2005a). This rapid growth in program expenditures was reinforced by the recent introduction of Medicare Part D, a new plan providing coverage for the outpatient prescription drugs used by Medicare beneficiaries.
The federal government has undertaken a variety of strategies to control Medicare program growth on the supply side, from the introduction of prospective reimbursement for hospitals to reductions in provider reimbursement rates. Yet Medicare spending growth has continued unabated. Recently, therefore, there has been a growing interest in demand-side approaches to controlling system costs, through higher patient costs which would induce more price sensitivity in medical spending.
Demand-side approaches, however, are complicated by the fact that Medicare beneficiaries are often covered by multiple insurers at once. Because Medicare already has quite substantial cost sharing, most enrollees have some form of supplemental coverage for their medical spending, provided by an employer, purchased on their own, or provided through state Medicaid programs. The incentives of the supplemental insurer and Medicare are not necessarily readily aligned. Indeed, there are long-standing concerns about the fiscal externality on Medicare from supplemental coverage: by insulating beneficiaries from costs, the policies increase utilization, thereby raising costs to Medicare (Adam Atherly 2001). In this paper, we focus on an additional, offsetting effect of supplemental coverage: if the additional utilization induced by supplemental insurance coverage prevents subsequent hospitalizations, then the net external cost of supplemental insurance is smaller than previously believed.
A necessary condition for such an externality is that changes in cost sharing affect individual utilization of health care. For the nonelderly, the question of the sensitivity of medical consumption to its price was addressed by the famous RAND Health Insurance Experiment (HIE), one of the most important pieces of social policy research of the postwar period. The RAND HIE randomized individuals across health insurance plans of differing generosity with respect to patient costs, and the results showed that higher patient payments significantly reduced medical care utilization, without any adverse health outcomes on average (Willard G. Manning et al. 1987; Joseph P. Newhouse 1993). However, the RAND HIE evidence is nearly 30 years old and may not be germane to Medicare because the elderly were excluded from this experiment. Therefore, our paper begins by analyzing the price sensitivity of medical care decisions among the elderly.
We next examine whether increased cost sharing for the elderly causes an “offset” in the form of medical costs elsewhere in the system. Such offsets may arise, for example, if patients respond to copayment increases by cutting back on maintenance drugs for chronic illness and, consequently, need to be hospitalized later. The HIE did test this “offset effect” for the nonelderly and found no evidence, for example, that higher outpatient cost sharing led to more use of inpatient services. But, as we noted, the HIE excluded the elderly, did not analyze prescription drug use.1
We examine policy changes put in place by the California Public Employees Retirement System (CalPERS) Board. Facing mounting fiscal pressure from health plan cost increases, CalPERS enacted a staggered set of copayment changes that allow us to carefully evaluate their impact on the medical care utilization of the elderly. To evaluate these policy changes, we have compiled (with the assistance of CalPERS) a comprehensive database of all medical utilization data2 for those enrolled continuously in several of the CalPERS plans from January 2000 through September 2003.
First, we find that both physician office visits and prescription drug utilization are modestly price sensitive among the elderly, with implied arc-elasticities that are similar to those found in the HIE for the nonelderly. Second, unlike the HIE, we find significant “offset” effects in terms of increased hospital utilization in response to the combination of higher copayments for physicians and prescription drugs. These offset effects are concentrated in the most ill populations. While our dataset precedes the implementation of Medicare Part D for prescription coverage, this finding has implications for the design of Medicare Part D, which currently includes 100 percent coinsurance (the so-called “donut hole”) for beneficiaries with relatively high levels of spending—who are likely to be similar to the chronically ill enrollees who experienced disproportionate offsets in our analysis.
Finally, we find evidence of a fiscal externality from increased cost sharing: the savings from increased cost sharing accrue mostly to the supplemental insurer, while the costs of increased hospitalization accrue mostly to Medicare. Similar incentive problems are likely to arise in an intertemporal setting, when different insurers are responsible for an individual's medical costs at different ages, as highlighted by Hanming Fang and Alessandro Gavazza (2007).
Our paper proceeds as follows. Section I provides some background on previous work in this area and on the policy change we are studying. Section II describes the data and empirical strategy. Section III presents our basic set of results on price sensitivity. Section IV presents evidence on the offset effect and then extends the results in a variety of directions. Section V concludes.
I. Background
A. Previous Work
There is a rich literature on the impact of copayments on utilization and we review this literature in great detail in Chandra, Gruber, and McKnight (2008). Of particular note is the RAND HIE, which is summarized in Manning et al. (1987) and Newhouse (1993). The HIE showed that medical services were modestly price responsive, with an overall estimated arc-elasticity of medical spending in the range of −0.2. Newhouse (1993) summarizes other important findings of the HIE, but two stand out for our purposes. First, the reduction in medical utilization was relatively uniform: for almost every category of care, utilization fell for both “effective” and “ineffective” care. Second, the study found no “offset effects”: there was no evidence that high coinsurance, by causing individuals to forgo efficacious preventive care, would raise costs through care later on.
More recent studies rely on natural experiments to assess price sensitivity of medical care decisions. These studies find price sensitivity in use of office visits (Daniel Cherkin, Louis Grothaus, and Edward Wagner 1989), emergency room use (Joe Selby, Bruce Fireman, and Bix Swain 1996), prescription drug use (Goldman et al. 2004; Pamela B. Landsman et al. 2005; Martin Gaynor, Jian Li, and William B. Vogt 2007), and overall spending (Matthew Eichner 1996). These studies have two important limitations. First, they are exclusively focused on the nonelderly, ignoring the elderly patients who are responsible for a massive, and growing, share of public health spending.3 Second, they do not proceed, as RAND did, to examine the implications of changing utilization for either health or utilization elsewhere in the medical system.4 One notable exception is Gaynor, Li, and Vogt (2007), which found evidence of a spending offset in the nonelderly population.
Two other papers have reported substantial effects of decreased prescription drug use on adverse events among the elderly, although neither paper has a strictly defined control group (in the first, identification is cross sectional and, in the second, identification is over time). John Hsu et al. (2006) find that Medicare beneficiaries whose pharmacy benefits were subject to a cap had 13 percent higher (nonelective) hospitalizations and 22 percent higher death rates than beneficiaries whose benefits were not capped. Robyn Tamblyn et al. (2001) find that the rate of emergency department hospitalizations for the elderly increased by 14.2 per 10,000 patient-months in the 17 months after patient cost sharing was instituted.
Of particular relevance to our analysis is the long-standing policy concern about the design of supplemental insurance for Medicare. In particular, because supplemental insurance insulates beneficiaries from Medicare's cost sharing, it likely increases their utilization of Medicare-covered services (so long as those services are not inelastically demanded). Atherly (2001) provides a review of the research history, observing that the literature consistently finds a positive correlation between supplemental insurance and Medicare expenditures. 5
B. The Institutional Setting
Our analysis focuses on changes in copayments for the health plans offered to public employees by the state of California through the CalPERS program. This program provides insurance to 1.2 million of California's active and retired civil servants and their dependents, making it the third largest purchaser of health insurance in the nation (CalPERS 2006). The coverage differs for individuals who are eligible for Medicare and those who are not. For Medicare beneficiaries, our population of interest, the state provides insurance designed to supplement the benefits provided by the Medicare program. Each year, during an open enrollment period, members are offered a choice of two state-run Preferred Provider Organizations (PPOs), PERSCare and PERS Choice, as well as a variety of state-sanctioned HMO choices that have changed over time.
The genesis of the CalPERS policy change was the rapidly rising health care costs in the self-funded CalPERS plans. Cost increases resulted from increased provider prices (reflecting the nationwide trend toward tougher bargaining by providers with managed care organizations) and increased utilization, particularly of prescription drugs. In response to these pressures, the board of CalPERS instituted two key coverage changes for the Medicare population, in February 2001 for PPOs and in January 2002 for HMOs:
A rise in physician office visit copayments for the HMOs from $0 to $10 in 2002. There was no corresponding change for PPOs in 2001.
A rise in copayments for prescription drugs for all PPO plans from $5 for generics and $10 for name brands to $5 for generics, $15 for formulary name brands, and $30 for nonformulary name brand prescriptions, as well as a rise in mail order prescription copayments from $5 to $10/$25/$45, with a $1,000 stop-loss in 2001. HMOs had a similar rise in copayments for prescription drugs in 2002; for HMOs, however, copayments prior to the policy change were $1 for almost all enrollees and did not differ for generics and name brands.6
II. Data and Empirical Strategy
A. Data
For our analysis, we use a comprehensive database of all medical utilization data for four of the CalPERS plans between January 2000 and September 2003. We focus on a panel of Medicare supplemental plan members who were continuously enrolled in their plan during our sample period, noting that this sample is not necessarily representative of the Medicare population. The resulting dataset includes information on medical utilization by 70,912 members; 93 percent of these members were over the age of 65 in January 2000.
By selecting a sample of continuously enrolled individuals, we risk mismeasuring the population responsiveness of individuals to copayment changes. If individuals switch out of plans raising copayments, and the individuals who switch have a sensitivity of medical care utilization with respect to price that is higher (or lower) than average, then our estimated elasticities in this sample will be biased downward (or upward) in absolute value. Such switching, however, does not seem to be of sufficient importance to bias our results. We find, for example, that 92 percent of the members who were enrolled in our PPOs in February 2000 remained enrolled in the same plan in February 2001, despite the anticipated copayment increases in February 2001. Indeed, this 92 percent retention rate was slightly higher than the 91 percent retention rate, over the same period, at HMOs that were not expecting a copayment increase. Similarly, 92 percent of the members who were enrolled in our HMOs in February 2001 remained enrolled in the same plan in February 2002. Again, despite the January 2002 copayment increase, the HMO retention rate was slightly higher than the 90 percent retention rate for the PPOs over the same period.7 As a result, when we reestimate our models using the full sample of enrollees (including individuals who both enter and exit our plans), we get almost identical results to those presented here.8
For each individual, we measure office visit utilization by the number of medical encounters that occurred in an office or outpatient setting during the month. We measure drug utilization by the number of prescriptions filled during the month. We measure hospital utilization using an indicator for whether the individual spent any days in the hospital during the month.
In our analysis, we compute an average of each utilization measure for each plan in each month, which yields 180 observations in our final dataset (45 months × 4 plans). Thus, each of the 180 observations on a utilization measure in our final dataset reflects the average utilization per person among all continuously enrolled members of a single plan in a given month. In order to document the impact of the policy change on copayments, we also calculate average copayments and deductibles across all visits (or prescription drug purchases) for each plan in each month. Each of the 180 observations on copayments in our final dataset reflects the average copayment per visit (or prescription drug purchase) in a single plan in a single month.9
We analyze the impact of these policy changes on medical spending as well as utilization. Ideally, we would observe the payments associated with every medical encounter, and we would redo our analysis using these payments as the dependent variable. In practice, however, there is not any financial information on payments attached to HMO claims. We therefore pursue an alternative approach where we use available data on total payments from PPO claims to impute total payments to each claim based on the primary diagnosis category of the office visit (or the National Drug Code (NDC) of the prescription drug), and the average payment for an office visit with that diagnosis category (or for that specific drug) among all PPO claims. Following Medicare Part B policy, we assume that Medicare made 80 percent of the total payments for office visits and that the supplemental insurance plan paid the difference between the remaining 20 percent and any patient copayments. For drugs, we assume that the supplemental insurance plan made all payments above the patient copayments.
For hospitalizations, where Medicare's share of total payments is not a fixed percentage, we followed a different methodology. Total hospital payments are the sum of imputed supplemental insurance payments, imputed Medicare payments, and any actual cost sharing paid by the patient. We imputed the first component based on the diagnosis code of the hospitalization and the average insurance payment for hospitalizations with the same diagnosis in the PPO data. We imputed Medicare's payments based on the diagnosis code of the hospitalization and the average Medicare payment for hospitalizations with that diagnosis code, using the universe of Medicare claims in the state of California during our sample period.10 For cost sharing, we used the actual amounts that were reported in the data. We then constructed total hospital payments as the sum of these three payments.
A clear concern with our approach for physician and hospital visits is that we assign the average payments per diagnosis, whereas ideally we would like to use the marginal payments for the given admission. The bias from using average rather than marginal payments is unclear, but we have explored one exercise to assess its importance. Among those admitted to the hospital, we have regressed length of hospital stay on the policy dummy in our difference-in-difference framework. If the marginal patients admitted due to higher physician/drug copayments are very different from the average patient admitted, we should see a marginal change in the length of stay for hospital patients. In fact, there is no significant effect on length of stay, providing crude evidence that the marginal and average patients are not very different.
B. Empirical Strategy
Our analysis of this quasi-experimental change in CalPERS policy is fairly straightforward. We begin by estimating difference-in-difference models of the form
where UTIL is a measure of utilization (or out-of-pocket costs) for plan p in month t, α is a constant term, HIPAY is a dummy for increased copayments in plan p in month t (specifically, an interaction of an indicator variable for being in a plan where greater cost sharing is instituted and an indicator for being in the post-increase era), and δp and λt are plan and month fixed effects, respectively.11 In this model, the effect of the copayment change is identified by β, which measures the change in utilization in the plans with a copayment change relative to those without. For each type of utilization, we estimate two models of this type: one that separately identifies effects on PPOs (focusing on data from January 2000 to December 2001), and another that separately identifies effects on HMOs (focusing on data from February 2001 to September 2003). Regressions are weighted by the number of plan members who are continuously enrolled during our sample period in each plan.
There are two potential problems with this approach. First, utilization is likely autocorrelated within plans; this autocorrelation causes the standard errors in ordinary regressions to be understated. To address this issue, we estimate our regressions using generalized least squares (GLS) allowing for plan-specific autocorrelation as well as plan-specific heteroskedasticity.12
Second, there may be underlying trends in utilization, which can confound the estimation of the causal effects that we are interested in. A particularly worrisome source of such trends for the HMO analysis is the earlier PPO policy change. That is, our difference-in-difference analysis for the HMO policy change compares the change in utilization in the HMOs between the prepolicy period (February 2001–December 2001) and the postpolicy period (January 2002–September 2003) to the change in utilization in the PPOs over the same periods. If the policy change in the PPOs had immediate effects in February 2001, this is not a problem. But it is possible that utilization in the PPOs adjusted slowly to the PPO policy change, with full adjustment only by the end of 2001. If this were the case, absent any other change, there would be a negative utilization difference between 2001 and 2002 for the PPO, leading to a spurious positive difference-in-difference estimate of the effect of the 2002 policy change on HMO utilization. To deal with this concern, we present dynamic models of the policy change effect, estimating separate treatment effects for each quarter before and after the policy change.13 In this way, we can examine whether any changes in utilization in 2002 represent the effects of a slow-moving relative trend or a sharp break when the policy is put in place. If the dynamic model indicates the latter, it suggests that we are not just picking up the dynamic effects of the PPO policy change.
C. Means
Table 1 presents the means of the data. We show the mean utilization rate and copayments for each type of utilization for HMOs and PPOs in each year. Once again, we have no preperiod data for the PPO policy change for office visits due to data limitations. Several discontinuities that preview our ensuing difference-in-difference specifications are apparent in these tabulations. First, average copayments for an office visit jump from $0.14 in 2001 to $10.11 in 2002 for the HMOs, while they remain flat in the (control) PPO plans over time.14 Over the same period, average office visits fell by 0.03 per member per month in the HMOs (relative to an increase of 0.07 visits per member per month in the PPOs, which experienced no copayment increase). Thus, the means suggest a differential decline in office visits for HMO members at the time that office visit copayments increased for them.
Table 1.
Means of Key Dependent Variables (By type of plan and year)
| PPOs |
HMOs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Prepolicy |
Postpolicy |
Prepolicy |
Postpolicy |
|||||
| 2000 | 2001 | 2002 | 2003 | 2000 | 2001 | 2002 | 2003 | |
| Office visits | ||||||||
| Average copayment per visit (in dollars) | — | $0.68 | $0.61 | $0.59 | — | $0.14 | $10.11 | $9.89 |
| Visits per member per month | — | 1.07 | 1.14 | 1.19 | — | 0.75 | 0.72 | 0.75 |
| Prescription drugs | ||||||||
| Average copayment per drug (in dollars) | $6.93 | $13.50 | $13.82 | $13.29 | $1.36 | $1.27 | $7.63 | $7.43 |
| Drugs per member per month | 1.98 | 2.07 | 2.21 | 2.44 | 1.27 | 1.43 | 1.34 | 1.50 |
| Hospitalizations | ||||||||
| Share of members with any hospital days during the month (×10,000) | 156.7 | 169.8 | 182.2 | 206.7 | 119.5 | 131.0 | 149.0 | 174.3 |
Average out-of-pocket payments for a prescription drug increased from $6.93 in 2000 to $13.50 in 2001 for PPOs. Over the same period, average drug utilization rose by 0.09 prescriptions per member per month in the PPOs, relative to an increase of 0.16 prescriptions per member per month in the HMOs, which had not yet experienced a drug copayment increase. These means, then, suggest the possibility of a relative decline in drug utilization resulting from the increased drug copayments for the PPOs in 2001. For the HMO policy change, the means show an increase in prescription drug copayments in 2002, and a decrease in prescriptions per member per month in the HMOs between 2001 and 2002, relative to an increase in the PPOs during the same period.
Finally, we show means for hospitalizations for each year and type of plan. All hospitalization rates in Table 1 and in later regression tables are multiplied by 10,000 in order to make the numbers easier to read. Hospitalization rates increase over time for both types of plans throughout the sample period, presumably reflecting the aging of our panel.
III. Basic Results
A. Office Visits
We begin our analysis by examining the effect of the 2002 copayment increase on office visits in the HMOs. This policy change increased copayments from a base of $0 to $10 for those in the supplemental plan. The results of our analysis are shown in Table 2. Each cell reports a coefficient and standard error (in parentheses).
Table 2.
Effects of 2002 HMO Office Visit Copayment Increase on Office Visit Utilization
| Copayment (Dollars per drug) | Utilization (Number of office visits per member per month) | |||||||
|---|---|---|---|---|---|---|---|---|
| Independent variable | (1) | (2) | (3) | (4) | ||||
| HIPAY | $10.06** (0.05) | –0.132** (0.018) | –0.095** (0.012) | |||||
| HIPAYt–4 | 0.016 (0.018) | |||||||
| HIPAYt–3 | 0.0002 (0.016) | |||||||
| HIPAYt–1 | 0.130** (0.016) | |||||||
| HIPAYt | –0.036** (0.016) | |||||||
| HIPAYt+1 | –0.094** (0.016) | |||||||
| HIPAYt+2 | –0.071** (0.016) | |||||||
| HIPAYt+3 | –0 .082** (0.021) | |||||||
| HIPAYt+4 | –0.101** (0.016) | |||||||
| HIPAYt+5 | –0.113** (0.016) | |||||||
| HIPAYt+6 | –0.029** (0.016) | |||||||
| N | 128 | 128 | 128 | 104 | ||||
Notes: Each column shows coefficients from a different regression; standard errors are reported in parentheses. The dependent variable is indicated on the column heading; the independent variable is indicated on the row label. Regressions control for plan and month fixed effects. They are estimated by GLS, allowing for plan-specific autocorrelation and plan-specific heteroskedasticity. Regressions include data from February 2001 through September 2003. Column 4 excludes data from the three months before and after the policy change, in order to eliminate the effects from any temporary shifts in the timing of care.
Denotes significance at the 5 percent level.
The first column shows the basic difference-in-difference estimate of the impact on copayments per visit. The statistically significant coefficient of 10.06 is virtually identical to the $10 increase we expect.
The second column of the table shows the difference-in-difference estimate of the impact on office visit utilization. There is a sizeable and highly statistically significant reduction of 0.132 office visits per member per month. Relative to the preperiod mean of 0.753, this is a 17.5 percent decline in office visits. While this appears to be a large response to a $10 increase in copayments, it is important to note that the $10 increase represents a very large percentage increase in out-of-pocket costs for patients, relative to the $0 copayment in the prepolicy period. The implied arc-elasticity of demand for office visits is −0.10, which is quite similar to the arc-elasticities produced by the RAND HIE for the nonelderly: Emmett B. Keeler and John E. Rolph (1988) report arc-elasticities for outpatient services that ranged from −0.17 to −0.31.15
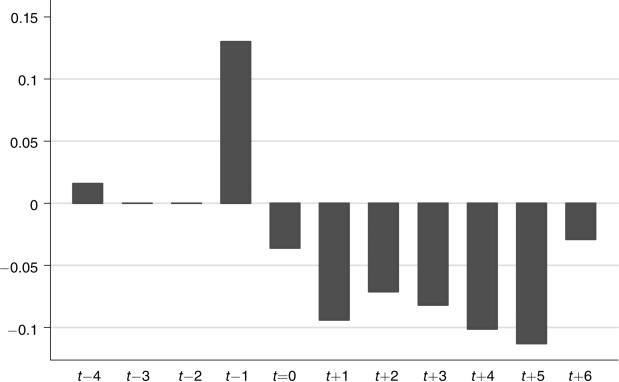
The third column explores the dynamic pattern of coefficients by showing the effects for each of the three quarters before, the quarter of, and all quarters after the policy change; the results are shown graphically in Figure 1. All coefficients for the dynamic model are measured relative to the omitted quarter, which is two quarters prior to the policy change. There is a large rise in office visits in the quarter before the policy change, which presumably reflects anticipation of the change, and then a large and immediate drop in utilization in the quarter after the policy change. This graph clearly suggests a causal impact of the copayment increase itself, and not a spurious trend in utilization.
Figure 1.
Effect of 2002 HMO Policy Change on Office Visits
Note: The bars show the point estimates from column 3 of Table 2.
Much of the large rise immediately before, and the fall immediately after, the copayment change likely represents a one-time shift in the timing of office visits, and not a fundamental change in utilization patterns in response to this higher patient cost. Thus, in the last column of the table, we show the difference-in-difference estimate excluding the quarters immediately before and after the policy change (the estimated change in copayments is almost identical when we excluded these quarters). There is a sizeable decline in the coefficient on office visits to −0.095, although it remains highly significant. The implied arc-elasticity is −0.07. We will use this specification as our baseline for the remaining results on office visits.
B. Prescription Drugs
There were large increases in copayments for prescription drugs in both 2001 (for PPOs) and 2002 (for HMOs), as described earlier. Unlike physician office visits, however, the exact magnitude of the copayment change for each member depends on the mix of drugs used, as the copayments changed differently for different types of drugs.
Table 3 shows estimated effects for the 2001 and 2002 policy changes. The effects of these changes on copayments per drug are shown in columns 1 and 5, respectively. For the 2001 policy change, we estimate a $7.25 rise in average copayment. For the 2002 policy change, the estimated change in out-of-pocket charges is slightly smaller at $6.74. However, these first-stage estimates confound two effects: the static effect of rising copayments, holding constant the prepolicy mix of drugs, and the dynamic effect of consumers shifting to less expensive drugs in response. We believe that the correct first-stage estimate would include only the first, static part of this response and ignore the second portion. To estimate this static copayment change, we measure utilization in the prepolicy period, multiply by both old and new copayments, and calculate the difference in copayments for this fixed set of drugs. Using this methodology, we get larger estimates of the copayment increases—$8.06 for the 2001 change and $7.26 for the 2002 change.
Table 3.
Effects of Drug Copayment Increases On Drug Utilization
| 2001 Policy change |
2002 Policy change |
|||||||
|---|---|---|---|---|---|---|---|---|
| Independent variable | Copayment (Dollars per drug) |
Utilization ( Number of drugs per member per month) |
Copayment (Dollars per drug) |
Utilization (Number of drugs per member per month) |
||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| HIPAY | $7.25** (0.06) | –0.111** (0.020) | –0.048** (0.014) | $6.74** (0.09) | –0.276** (0.016) | –0.261** (0.021) | ||
| HIPAYt–5 | –0.030 (0.034) | |||||||
| HIPAYt–4 | –0.075** (0.020) | 0.005 (0.029) | ||||||
| HIPAYt–3 | –0.023 (0.021) | 0.016 (0.017) | ||||||
| HIPAYt–1 | 0.093** (0.021) | 0.039** (0.017) | ||||||
| HIPAYt | –0.101** (0.020) | –0.236** (0.016) | ||||||
| HIPAYt+1 | –0.073** (0.020) | –0.219** (0.016) | ||||||
| HIPAYt+2 | –0.082** (0.020) | –0.220** (0.016) | ||||||
| HIPAYt+3 | –0.052 (0.034) | –0.189** (0.016) | ||||||
| HIPAYt+4 | –0.320** (0.016) | |||||||
| HIPAYt+5 | –0.312** (0.016) | |||||||
| HIPAYt+6 | –0.269** (0.016) | |||||||
| N | 80 | 80 | 80 | 58 | 124 | 124 | 124 | 100 |
Notes: Each column shows coefficients from a different regression; standard errors are reported in parentheses. The dependent variable is indicated on the column heading; the independent variable is indicated on the row label. Regressions control for plan and month fixed effects. They are estimated by GLS, allowing for plan-specific autocorrelation and plan-specific heteroskedasticity. Columns 1–4 include data from January 2000 through November 2001, and columns 5–8 include data from March 2001 through September 2003. Columns 4 and 8 exclude data from the three months before and after the relevant policy change, in order to eliminate the effects of any temporary shifts in the timing of care.
Denotes significance at the 5 percent level.
The effects of these changes on the number of prescriptions are shown in columns 2 and 6, using the basic difference-in-difference specification. In each case, there is a negative and significant effect on the average number of prescriptions filled. For PPO enrollees in 2001, we estimate an arc-elasticity of approximately −0.08 of drug utilization with respect to its patient cost. For HMO enrollees in 2002, we estimate a similar arc-elasticity of −0.15, which is also quite similar to the arc-elasticity of demand for office visits and, again, to the arc-elasticities obtained from the RAND HIE for the nonelderly.16
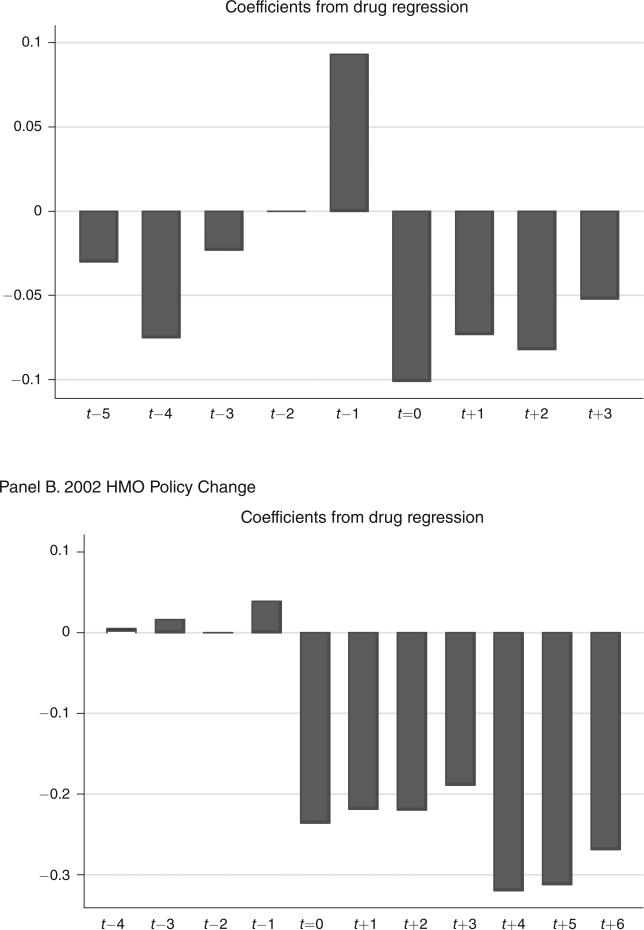
The dynamics of these responses are reported in columns 3 and 7 of Table 3 and are shown graphically in the two panels of Figure 2. The results clearly indicate that there were no preexisting trends toward less drug use. Thus, the estimated utilization decline appears to be a causal impact of the policy change.17 For the 2001 change, however, we once again see that there is an anticipation effect in the quarter before the policy change. To avoid such timing effects, we again drop the quarter before and after the policy change, and show the results in columns 4 and 8 of Table 3.18 In the case of the 2001 policy change, the estimated effect decreases by just over a half, so that the implied arc-elasticity falls to only −0.03.
Figure 2.
Effect of Drug Copayment Policy Changes on Drug Utilization
Note: The bars show the point estimates from columns 3 and 7 of Table 3.
Our experiment also allows us to explore the behavioral response to different types of drugs. By assembling a panel of three practicing physicians and two pharmacists, we classified each drug into one of three categories:19
Acute care drugs are those that, if not taken, will increase the probability of an adverse health event within a month or two (examples are anticonvulsants, antimalarials and anti-angials, coronary vasodilators, and thrombolytics);
Chronic care medications are designed to treat more persistent conditions that, if not treated, will result in a potentially adverse health event within the year (examples include analgesics, antivirals, ACE inhibitors, antigout medications, beta-blockers, hypertension drugs, statins, and glaucoma medications); and
Medications that, while necessary to improve patients' quality of life, will not result in an adverse health event if not taken, because they provide symptom relief as opposed to affecting the underlying disease process (examples are acne medications, antihistamines, motion sickness medications, cold remedies, relief of pain drugs).
We found substantial responsiveness for all three types of drugs, suggesting that members decreased their use of drugs that, according to our expert panel, were likely preventing short- and long-term adverse health events.
IV. The Offset Effect
A. Hospital Utilization
A major concern with increased patient cost sharing is the so-called “offset effect”: by raising the cost of going to the doctor or filling prescriptions, increased cost sharing may delay necessary care and increase hospitalizations. Indeed, the fact that individuals in our sample decreased utilization of drugs that were classified as “acute care” by our panel of experts (or “essential” by Tamblyn et al. 2001) increases this concern. The RAND HIE found no evidence of such offset effects, but there has been little subsequent investigation of this question, particularly for the elderly.
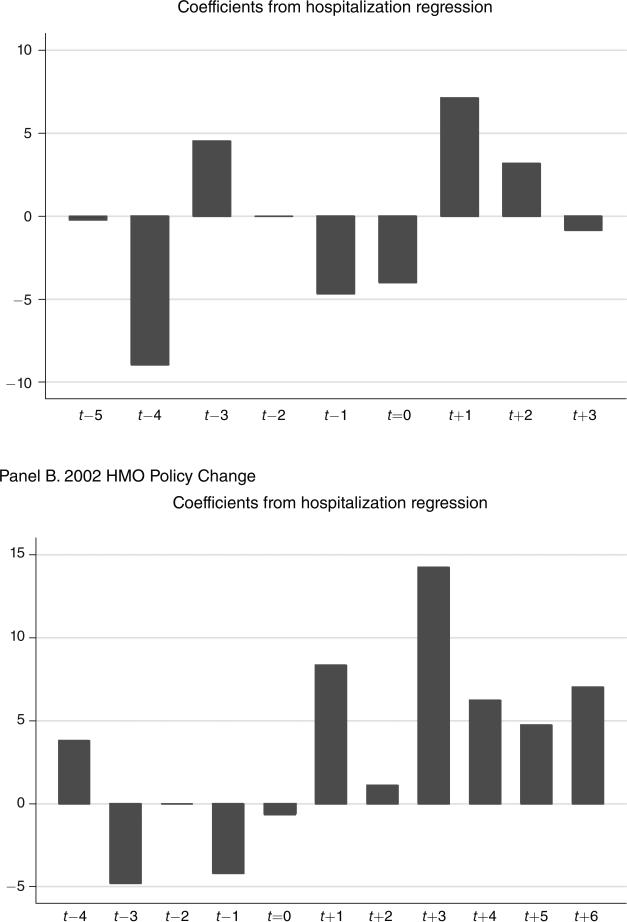
In Table 4 we explore the effect of both policy changes on hospital utilization. The difference-in-difference estimates of the impact of the two policy changes on hospital utilization are shown separately in columns 1 and 4. Interestingly, both coefficients are positive, although only that for the 2002 policy change is statistically significant. For the 2002 policy change, we find an increase in the probability of any hospital days during the month of 0.078 percent, relative to the prepolicy mean of 1.30 percent, or a rise of 6.0 percent. As the dynamic results show, both in Table 4 and graphically in Figure 3, these effects do not appear to reflect preexisting trends. However, the coefficients for the 2001 changes are small and statistically insignificant. The larger response in 2002 may be due to the rise in office visit copayments under the HMO plans and the larger reduction in prescription drug use. These results suggest some potential “offset” effect of the changes in coverage for physician and prescription services, in contrast to the RAND HIE. The finding of such an offset effect in the short run is quite striking, and it is likely that any offset effect would operate more strongly over time.
Table 4.
Effects of Copayment Increases on the Probability That a Member Experiences Any Hospital Days during the Month
| 2001 Policy change |
2002 Policy change |
|||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| HIPAY | 3.69 (2.61) | 5.31** (2.70) | 7.79** (2.20) | 7.16** (2.29) | ||
| HIPAYt–5 | –0.22 (6.83) | |||||
| HIPAYt–4 | –8.96 (4.33) | 3.79 (5.00) | ||||
| HIPAYt–3 | 4.51 (4.43) | –4.81 (4.48) | ||||
| HIPAYt–1 | –4.67 (4.43) | –4.20 (4.48) | ||||
| HIPAYt | –4.01 (4.32) | –0.66 (4.31) | ||||
| HIPAYt+1 | 7.10 (4.32) | 8.33* (4.32) | ||||
| HIPAYt+2 | 3.17 (4.32) | 1.09 (4.32) | ||||
| HIPAYt+3 | –0.85 (4.94) | 14.22** (4.32) | ||||
| HIPAYt+4 | 6.21 (4.32) | |||||
| HIPAYt+5 | 4.72 (4.32) | |||||
| HIPAYt+6 | 7.00 (4.35) | |||||
| N | 96 | 96 | 72 | 128 | 128 | 104 |
Notes: Each column shows coefficients from a different regression; standard errors are reported in parentheses. The dependent variable is indicated in the column heading; the independent variable is indicated in the row label. Regressions control for plan and month fixed effects. They are estimated by GLS, allowing for plan-specific autocorrelation and plan-specific heteroskedasticity. Columns 1–3 include data from January 2000 through December 2001, and Columns 4–6 include data from February 2001 through September 2003. Columns 3 and 6 exclude data from the three months before and after the relevant policy change, in order to eliminate the effects of any temporary shifts in the timing of care. Note that the dependent variable is multiplied by 10,000.
Denotes significance at the 5 percent level.
Denotes significance at the 10 percent level.
Figure 3.
Effect of Policy Changes on Hospitalizations
Notes: The bars show the point estimates from column 2 and 5 of Table 4. Note that all values in this figure and in Table 4 are multiplied by 10,000.
We further explore this effect by moving from utilization measures to our payment measures.20 The results of this exercise are presented in the first panel of Table 5, which contains the same difference-in-difference regressions as in the earlier tables, but replaces utilization measures with expenditures. All of the results exclude the quarters before and after the policy change. For physician visits, we find a decline in imputed expenditures of $13.16, which is 14.1 percent of the base cost of $93.25 per person per month. For prescription drugs, we find a reduction in payments of $23.06 for the 2002 policy change, which is 32 percent of the base spending on drugs. Thus, in total, we find a reduction in office visit plus prescription drug payments of $36.22 for the 2002 policy change. At the same time, we find an increase in hospitalization payments of $7.23, which is 5.4 percent of baseline hospital expenditures. These results suggest that increases in hospital spending offset 20 percent of the savings from higher copayments for physicians and prescription drugs. While this offset is sizeable, it is unlikely to be enough to reverse any conclusions about the efficacy of copayment changes.
Table 5.
Effects of 2002 Copayment Increases on Medical Payments per Member per Month (By source of payment)
| 2002 Policy change |
||||
|---|---|---|---|---|
| (1) Office visit payments (Dollars) | (2) Drug payments (Dollars) | (3) Hospital payments (Dollars) | (4) Offset (Percent) | |
| All sources | –13.16** (1.18) | –23.06** (1.85) | 7.23** (2.60) | 20.0 |
| Payment source | ||||
| Medicare | –10.53** (0.95) | — | 5.58** (2.25) | 53.0 |
| Supplemental insurance | –11.24 (0.26) | –29.20** (1.67) | 1.49** (0.38) | 3.7 |
| N | 104 | 100 | 104 | |
Notes: Each cell shows the coefficient from a separate difference-in-difference regression; standard errors are reported in parentheses. The dependent variable is indicated in the column heading; the payment source is indicated in the row label. Regressions control for plan and month fixed effects. They are estimated by GLS, allowing for plan-specific autocorrelation and plan-specific heteroskedasticity. All regressions exclude data from the three months before and after the relevant policy change, in order to exclude the effects of any temporary shifts in the timing of care. There are four fewer observations used for the drug payment regressions, because months that were affected by timing shifts in drug purchases due to other policy changes were excluded. Specifically, the regressions exclude February 2001, due to unusually large, one-time decreases in drug purchases in the PPOs resulting from the February 2001 policy change. Column 4 shows the increase in hospital payments as a percentage of the decrease in office visit and drug payments. That is, it is equal to [–column 3/(column 1 + column 2)].
Denotes significance at the 5 percent level.
* Denotes significance at the 10 percent level.
B. Payer-Specific Offsets and the Fiscal Externality of Supplemental Insurance
Importantly, however, this offset does not operate equally for all payers. The next panel of Table 5 divides these responses into payments by Medicare and payments by the CalPERS supplemental insurance plans. Medicare, in this time period, did not cover prescription drugs, so the only effects on the Medicare program are lower physician costs and higher hospital costs.
The results of this division by payer are quite interesting. The physician savings accrue roughly equally to Medicare and to the supplemental insurer, despite the fact that Medicare pays 80 percent of the charges for physician visits and the supplemental insurer pays only 20 percent. This is because the supplemental insurer obtains all the benefit from the shifting of payments from insurer to patient. Thus, when the HMOs introduce the $10 physician copayment, there is a total reduction in spending on physicians of $13.16, of which Medicare receives roughly 80 percent (0.8 × $13.16 = $10.53). Meanwhile, the supplemental insurer obtains the other 20 percent of savings from reduced utilization (0.2 × 13.16 $2.63), plus the transfer of costs from the supplemental insurance plans to patient ($10 per visit, with an average of 0.86 visits per enrollee per month, equals $8.60), for a total of $11.23.
The savings from reduced prescription drug utilization, of course, accrue totally to the supplemental insurer. These savings are equal to the total reduction in dollars spent on prescription drugs, plus the cost-shift from the supplemental insurer to enrollees through the increased copayments. At the same time, Medicare bears most of the increased cost of hospitalizations. Of the $7.23 in extra hospital costs, Medicare pays $5.58, and the supplemental insurer pays only $1.49. Thus, on net, Medicare saves $10.53 on physician visits, but pays $5.58 more in hospitalization costs, so the offset effect for the Medicare program in isolation is 53 percent. At the same time, the offset for the supplemental insurer is close to zero, since the supplemental insurer pays relatively little of the hospital costs but receives half of the savings in physician visits and all of the savings on prescription drugs. Thus, while the offset is small overall, it is a major consideration for the Medicare program.
C. Heterogeneity in Offset
While this overall offset effect is fairly modest, there is heterogeneity in the offset that has important implications for insurance design. Table 6 explores heterogeneity by enrollee health status, as measured by the Charlson index and by the presence of a chronic illness. The Charlson index is a weighted sum of indicators for the presence of certain conditions, where the weights reflect relative increases in one-year mortality risk for a person; mortality risk increases with the index. To calculate the index, we use diagnosis codes from each individual's claims history to identify the presence of each of 19 conditions, and then apply the weights described in M. E. Charlson et al. (1987). We then run regressions separately for individuals with Charlson indices of 0 (i.e. individuals with none of the conditions), 1–3, and 4 or greater. We identify the chronically as those individuals with a diagnosis of hyptertension, high cholesterol, diabetes, asthma, arthritis, affective disorders, and gastritis, following definitions in Goldman et al. (2004).
Table 6.
Heterogeneity in Effects of 2002 Copayment Increases on Payments per Member per Month (By type of plan member)
| 2002 HMO policy change |
2002 Offset effects |
|||||
|---|---|---|---|---|---|---|
| Type of plan member | Office visit payments (dollars) (1) | Drug payments (dollars) (2) | Hospital payments (dollars) (3) | Total offset (percent) (4) | Medicare offset (percent) (5) | Supplemental insurance offset (percent) (6) |
| Charlson index | ||||||
| 0 | –7.53** (0.87) | –11.78** (1.00) | 4.34** (1.78) | 22.5 | 61.0 | 2.8 |
| 1–3 | –11.05** (1.40) | –24.24** (1.33) | 12.89** (6.08) | 36.5 | 117.6 | 4.9 |
| 4+ | –13.68** (2.39) | –34.68** (2.28) | 85.93** (31.19) | 177.7 | 652.4 | 21.2 |
| Chronic condition | ||||||
| No chronic illness | –3.27** (1.09) | –8.19** (1.77) | –0.78 (4.19) | –6.8 | 42.7 | 1.7 |
| Any chronic illness | –15.19** (1.35) | –27.15** (1.76) | 18.25** (4.22) | 43.1 | 122.4 | 6.4 |
| N | 104 | 100 | 104 | |||
Notes: Each cell in columns 1–3 shows the coefficient from a separate difference-in-difference regression; standard errors are reported in parentheses. The dependent variable is indicated in the column heading; the subsample is indicated in the row label. Regressions control for plan and month fixed effects. They are estimated by GLS, allowing for plan-specific autocorrelation and plan-specific heteroskedasticity. All regressions exclude data from the three months before and after the relevant policy change, in order to eliminate the effects of any temporary shifts in the timing of care. There are four fewer observations used for the drug regressions, because we exclude February 2001, due to unusually large, one-time decreased drug purchases in the PPOs resulting from the February 2001 policy change. Column 4 shows the increase in total hospital payments as a percentage of the decrease in office visit and drug payments. That is, it is equal to [–column 3/(column 1 + column 2)]. Columns 5 and 6 show the same calculations for payments by Medicare and by supplemental insurance plans. Coefficients underlying the results in columns 5 and 6 are available from the authors upon request.
Denotes significance at the 5 percent level.
*Denotes significance at the 10 percent level.
Table 6 reports the results. The first three columns show the results for spending on office visits, prescription drugs, and hospitalizations. The fourth column shows the total offset effect, calculated directly from these first three columns. The fifth and six columns show the offset effect for Medicare and the supplemental insurer, respectively, which are calculated from payer-specific spending regressions.
We find a modest increase, in absolute value, in the physician and prescription drug effects by Charlson index, but the differential effect on hospital spending is even more striking. Indeed, the hospital spending effect is enormous for those who are unhealthiest by this measure, with hospital spending increasing by almost $2 for every $1 saved on other spending—and Medicare's hospital spending increasing by more than $6 for every $1 saved on physician spending!
A less parameterized measure, pursued in the second panel, is to examine these effects separately for those with and without a chronic illness. Once again, we find that there is little offset for those who are not chronically ill—but that the offset is quite large for the chronically ill. Overall, there is a 43 percent offset for the chronically ill—but it is 122 percent for the Medicare program alone. In Chandra, Gruber, and McKnight (2008), we show the results separately by chronic illness, and find that the offset effects are concentrated in those with hypertension, arthritis, diabetes, and affective disorders.21
In Chandra, Gruber, and McKnight (2008), we also examine in detail the plausibility of the timing and magnitude of the increases in hospitalizations. For instance, we note that our estimates imply that decreased prescription drug and office visit use only rarely translate into an additional hospitalization. Our baseline hospitalization results in column 6 of Table 4 reflect only 7 additional hospitalizations per month per 10,000 members (although hospitalization offsets are, of course, more prevalent among the chronically ill and those with high Charlson indices). In comparison, the coefficients from the office visit and prescription drug regressions suggest 950 fewer monthly office visits and 2,610 fewer monthly prescription drug purchases per 10,000 members. These magnitudes are consistent with Tamblyn et al. (2001), which reports that a reduction of 1,700 “essential” drugs per 10,000 members is associated with an increase of 7 adverse events (including acute care hospital admissions, long-term care admissions, and death) and 14 emergency department visits per 10,000 member-months (but over a longer time frame).
In addition, we explored the pattern of prescription drug cutbacks among the chronically ill population which, as shown in Table 6, experienced a differential offset. Specifically, we identified the classes of drugs that would be most relevant to the treatment of each chronic condition (e.g., statins for high cholesterol; antacids, acid blockers, and proton pump inhibitors for gastritis) and found that about 40 percent of the decrease in prescription drugs among chronically ill enrollees comes in the form of drugs that are intended to treat the chronic illness. This decrease in disease-specific drugs represents a direct channel to increasing the risk of hospitalization for the chronically ill, and increases our confidence that the increase in hospitalizations among the chronically ill could be driven by a decline in management of their chronic illness.
V. Conclusions and Implications
The rapid rise in both the share of our population that is elderly, and the spending per elderly person, has placed pressure on both public and private insurers to find new ways to control medical costs in this population. A number of approaches have been suggested, but at the fore-front is increased consumer cost-consciousness. While Medicare has relatively high copayments and deductibles, these “holes” are filled by supplementary insurance for most elderly persons. Because these supplemental insurers control the first-dollar exposure of the elderly, they can exert an important financial externality on Medicare.
A necessary condition for such an externality is price sensitivity among Medicare beneficiaries. Our results show that they are quite price sensitive in their use of office visits and prescription drugs, with implied arc-elasticities that are similar to those reported in the RAND HIE for the nonelderly.
Most interestingly, we document the first convincing evidence for an offset effect of higher copayments. While previous work has conjectured that such an offset might be present, the only convincing evidence, from the RAND HIE, rejected the notion of an offset, suggesting instead that physician and hospital care were complements. Overall, we find a rather modest offsetting rise in hospital care when physician and prescription drug copayments are raised. But we find substantial offsets for the sickest populations with chronic diseases, suggesting that, for chronically ill populations, there is little financial gain from higher copayments.
These findings have several important implications for optimal health insurance design. The first is that the fiscal externality associated with supplemental insurance coverage, which has been the subject of much policy discussion, may be more modest than originally presumed. This is because the increase in physician and drug spending arising from supplemental coverage is substantially offset (for the Medicare program) by the fall in hospital costs.22 Conversely, as we note in our paper, efforts by the supplemental insurer to increase patient cost sharing can impose a fiscal externality on the Medicare program. This finding highlights the general problem of sub-optimal incentives when there are multiple insurers providing coverage for different components of medical care. Similar incentive problems are likely to arise in an intertemporal setting, when different insurers are responsible for an individual's medical costs at different ages, as highlighted by Fang and Gavazza (2007).
Second, the results from RAND clearly implied that optimal health insurance would feature high patient coinsurance up to an income-related out-of-pocket limit. Our findings qualify that result, suggesting that optimal insurance would be tied to underlying health status, with chronically ill patients facing lower cost sharing. The cost-sharing increases in our data were sufficiently modest that income-related out-of-pocket limits alone would not accomplish this goal, so specific targeting of copayments may be required.
Finally, our findings have implications for the design of Medicare's new prescription drug benefit. One of the most controversial features of Medicare's new prescription drug benefit is the so-called “donut hole”: the gap in prescription drug coverage for beneficiaries with total drug costs that exceed $2,250 per year, until their drug costs exceed $5,100 per year. Our results suggest that the donut hole in coverage, by increasing coinsurance rates to 100 percent for some of the most chronically ill Medicare beneficiaries, could increase Medicare's costs. In particular, those beneficiaries who are affected by the donut hole are likely to be similar to the chronically ill subpopulations where we found that increased Medicare hospital spending exceeded any savings from reduced prescription drug and office visit utilization.23 Future research should carefully explore the full system-wide implications of this design feature of the Part D program.
Supplementary Material
Acknowledgments
We are grateful to two anonymous referees for very helpful comments, Kathy Donneson and Terrence Newsome from CalPERS for invaluable technical assistance, Dan Gottlieb and Weiping Zhou at Dartmouth Medical School for assistance with the Medicare data, Drs. Dhruv Bansal, Phoutie Bansal, Julie Bynum, Amy Richardson, and Ivy Tiu for assisting with the classification of prescription drugs, James deBenedetti, Michele Douglas, Will Manning, Doug Miller, April Omoto, Doug Staiger, and seminar participants at the Annual Health Economics Conference, the NBER, RAND, UC-Davis, University of Missouri, Wellesley College, and the Pharmaceutical Economics and Policy Council for helpful comments. Gruber acknowledges support from the Kaiser Family Foundation and the National Institute on Aging, and Chandra from NIA P01 AG19783-02, an NBER Aging Fellowship, and the Nelson Rockefeller Center at Dartmouth.
Footnotes
We were inspired to test for these offset effects by research on prescription drug utilization by the nonelderly. For this group, Dana Goldman, Geoffrey F. Joyce, and Pinar Karaca-Mandic (2006) show that higher copayments lead to lower utilization of maintenance drugs for chronic illness, which implies (although it has yet to be proved) that an offset effect may exist.
All claims information used was de-identified prior to receipt, for both CalPERS and study researchers.
There are studies that focus on elders, as reviewed by Thomas Rice and Karen Y. Matsuoka (2004), but virtually all of these studies are either cross-sectional comparisons of elders with and without generous drug coverage, or simple before/after comparisons of drug copayment changes (which suffer from the problem of uncontrolled trends in drug utilization). The one exception is Richard E. Johnson et. al. (1997), who studied an increase in copayments for one Medicare HMO, using a different Medicare HMO as a control. This study finds no consistent impact of changes in copayments on drug utilization.
Recent work by Goldman, Joyce, and Karaca-Mandic (2006) argues that higher copayments for prescription drugs leads to more hospitalizations, but this claim is based on combining the fact that higher copayments lead to less drug utilization with an estimate of the relationship between drug utilization and hospitalization. The latter parameter is obtained by comparing hospitalization rates among those who do and do not comply with their drug regimes. This may not reflect the reduction in drug use due to copayments so much as the type of individuals who do and do not comply.
Estimates of the magnitude of this externality on Medicare range from zero (John Wolfe and John Goddeeris 1991) to 72 percent increases in Medicare charges among unhealthy individuals (Nelda McCall et al. 1991). However, none of the existing papers is able to fully surmount the important selection problem in the holding of Medigap policies documented by Susan Ettner (1997). Studies such as Lee Lillard and Jeanette Rogowski (1995) use work history as an instrument for employer-provided Medigap coverage, but work history is itself likely correlated with demand for medical care and health. Moreover, none of these studies assesses the impact of varying copayments for the elderly, only the aggregate impact of having or not having supplemental insurance.
The copayment was $1 for one of our HMOs and $5 for the other, but the former HMO represents 98 percent of our continuously enrolled HMO sample.
Both plans experienced 92 percent retention rates over the one-year period between February 2002 and February 2003, when neither experienced a copayment change. This fact increases our confidence that the plans did not experience unusual attrition as a result of the policy changes.
One exception to this statement is our results for hospitalizations. In the full sample, we get a small negative effect on overall hospitalizations for the 2002 change, rather than the small positive effect reported below. But this appears to reflect preexisting trends in the full sample of data, and our key results on hospitalizations for the chronically ill are very similar. All the full-sample results are available from the authors upon request.
When we analyze the 2002 copayment increase for office visits, we exclude office visits data from 2000. One of the plans changed their coding of office visits between 2000 and 2001, generating a discontinuity in the data that is unrelated to any policy changes. To avoid any spurious results based on this reporting change, we simply exclude all office visit data prior to 2001.
We used a 100 percent sample of Medicare Part A payments for patients whose residence was in California at the time of the hospitalization; Medicare patients who reside in other states but were in California at the time of hospitalization were excluded. For this sample of patients, we constructed average Medicare payments by principal diagnosis code for hospitalizations that occurred between January 2000 and September 2003. In order to reduce sampling error associated with rare diagnoses, diagnosis codes were first aggregated to levels indicated by the Clinical Classification System (CCS). To capture payments made to physicians for in-hospital services rendered during these hospital stays, we used a 20 percent random sample of the Part A sample above for whom we had Part B claims. We further restricted these claims to those whose dates corresponded to a hospitalization (including the dates of admission and discharge) and whose service place was an overnight inpatient visit. We merged payments for these services with the corresponding Part A hospitalization. This analysis was performed at the Center for the Evaluative Clinical Sciences at Dartmouth Medical School, and the relevant SAS software programs are available from the authors on request.
Because we use a fixed panel of enrollees, there are no time-varying differences between HMO and PPO enrollees. Any fixed differences are captured by the plan fixed effects in our regressions. We chose to perform our analysis at the plan-month level in order to be as conservative as possible about our standard errors.
This is a nontrivial issue that we have explored from a variety of angles. The most natural solution to our problem would be to follow the insights of Marianne Bertrand, Esther Duflo, and Sendhil Mullainathan (2004) and cluster on the four plans. However, the approach relies critically on the asymptotic justification that the number of clusters goes to infinity; with too few clusters the cluster-robust standard errors are biased downward severely. Empirically, we found that this approach produced standard errors that were 0 percent to 35 percent smaller than those obtained from our GLS procedure. Alternatively, if we ignore the potential autocorrelation and cluster on plan-by-month (45 months × 4 plans = 180 clusters), we obtain standard errors that are similar, though 0 percent to 20 percent smaller, to those obtained from the more conservative GLS procedure.
In addition, we tested the sensitivity of our 2002 policy change results to excluding data from the first three months after the 2001 policy change went into effect (February, March, and April of 2001) and found that the results were unaffected by this exclusion.
The fact that the average copays in our data are not precisely $0 for the HMOs in 2001 or for the PPOs throughout the sample period may reflect the fact that some of the encounters that we identify as “office visits” were misclassified, or it may reflect some minor misreporting of copayments in the data.
Following Keeler and Rolph (1988), our arc-elasticities are calculated as ((Q2 – Q1)/(Q1 + Q2/2)/(P2 – P1)/(P1 + P2)/2).
We obtain this elasticity by noting that there is a reduction in prescriptions filled of 0.276, relative to the pre-period mean for that group of 1.39. There is a $7.26 rise in copayments for this population relative to a prepolicy mean of $1.28. Using the alternative estimates of the copayment increase, which incorporate both the static and dynamic effects of policy change, the implied elasticities remain −0.08 for the 2001 policy change and −0.15 for the 2002 policy change. Therefore, the use of the more sophisticated (and correct) method of estimating copayment changes does not substantively alter our results.
We also examined switching from formulary to generics for both our policy changes. For the PPO (2001) policy change, the difference-in-difference estimate of the utilization of nonformulary (retail) drugs is a fall of −0.041 (SE = 0.005 drugs per member per month (on a base of 0.17). For drugs on the formulary, the reduction was −0.028 (SE = 0.007). Here, the base use was 1.08 drugs per month. There was an increase in the use of generics of 0.032 (SE = 0.005) on a preperiod base of 0.72 drugs per month. In contrast, for the HMO policy change, the use of both generics and formulary drugs fell: formulary utilization fell by 0.166 (SE = 0.016) drugs per member per month (on a base of 0.51 drugs per month), and generic use fell by 0.064 per month (SE = 0.009) on a base of 0.72 drugs per month. This likely reflects the fact that copayments for the PPOs rose for nongeneric drugs but remained constant for generic drugs, while copayments rose for all types of drugs for the HMOs.
If HMO enrollees began stockpiling prescription drugs as much as eight months prior to the January 2002 policy change (because the decision to increase copayments was made in April of 2001), we would see this behavior in dynamic graphs estimated at the quarterly level (which are reported in Figure 2, Panel B). In none of the pretreatment quarters do we find evidence of significant increases in utilization, as would be the case if drugs were being stockpiled.
Consistent with the clinical literature on expert physician panels, our panel exhibited considerable variance of opinion, especially in the distinction between acute and chronic care drugs. We classified a drug as belonging to a certain class if the majority of experts believed that it was in that class. During their deliberations, our panels had access to the Physician Desk Reference and the Internet to inform their choices.
It is important to recall that our payment information is not measured directly in our data but rather is imputed, so that our conclusions about payment results are somewhat more uncertain than our utilization conclusions.
In Chandra, Gruber, and McKnight (2008), we also report results for heterogeneity by income, by age, and by intensity of spending in 2000 (terciles of the overall spending distribution in the period before any policy changes). Briefly, we find that there are relatively flat effects across income groups. As age increases, we find similar or even smaller effects on physician and prescription drug spending—but much larger effects on hospital spending. As a result, the implied offset is less than 20 percent for those 65–74, but over 40 percent for those 85 and older. There is relatively little variation in physician spending effects by spending tercile, and a rising reduction in prescription drug spending by tercile. Most striking, however, is the hospitalization effect: the effect is negative for the first tercile, small and positive for the second tercile, and very large for the third tercile. The resulting offset is 50 percent overall for the top tercile of spenders, with an increase in Medicare spending of $1.19 for each $1.00 in physician savings.
Goldman and Tomas Philipson (2007) raise the important, related point that optimal health insurance design should not merely consider own-price elasticities of demand for medical care, but also cross-price elasticities. In the context of supplemental insurance, it is plausible that the cost-sharing structure chosen by the supplemental insurer affects both the “insured good” (such as office visits) and “other services” (such as hospitalizations) that impose differential costs on Medicare. Thus, the net external effect of supplemental insurance on Medicare costs may be composed of two parts: the increase in utilization of the affected service, due to a decrease in the beneficiary's out-of-pocket costs, and an offsetting decrease in utilization of a substitute service (or an increase in utilization of a complementary service).
In principle, the introduction of Medicare Part D may have decreased the problem of multiple insurers being responsible for different aspects of care, by giving Medicare more control over the cost sharing structure for prescription drugs. In practice, the reliance on private insurers to provide Medicare Part D plans, with varying cost sharing structures, diminishes this potential benefit from Part D.
Contributor Information
Amitabh Chandra, Kennedy School of Government, Harvard University, 79 JFK Street, Cambridge, MA 02138, and NBER Amitabh_Chandra@Harvard.Edu.
Jonathan Gruber, Department of Economics, MIT, 50 Memorial Drive E52-355, Cambridge, MA 02142, and NBER gruberj@mit.edu.
Robin McKnight, Department of Economics, Wellesley College, 106 Central Street, Wellesley, MA 02481, and NBER rmcknigh@wellesley.edu.
REFERENCES
- Atherly Adam. Supplemental Insurance: Medicare's Accidental Stepchild. Medical Care Research and Review. 2001;58(2):131–61. doi: 10.1177/107755870105800201. [DOI] [PubMed] [Google Scholar]
- Bertrand Marianne, Duflo Esther, Mullainathan Sendhil. How Much Should We Trust Differences-in-Differences Estimates? Quarterly Journal of Economics. 2004;19(1):249–75. [Google Scholar]
- CalPERS PERSCare and PERSChoice: The Road to Financial Recovery. 2000 http://www.calpers.ca.gov/health/plan/care-choice/PERSCareChoice.pdf.
- CalPERS Facts at a Glance. 2006 http://www.calpers.ca.gov/eip-docs/about/facts/health.pdf.
- Centers for Medicare and Medicaid Services . Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds Annual Report of the Board of Trustees. U.S. Department of Health and Human Services; 2005. http://www.cms.hhs.gov/ReportsTrustFunds/downloads/tr2005.pdf. [Google Scholar]
- Centers for Medicaid and Medicare Services National Health Expenditures. 2005 www.cms.hhs.gov/NationalHealthExpendData/04_NationalHealthAccountsAgePHC.asp.
- Chandra Amitabh, Gruber Jonathan, McKnight Robin. Patient Cost-Sharing, Hospitalization Offsets, and the Design of Optimal Health Insurance for the Elderly. National Bureau of Economic Research Working Paper 12972. 2008.
- Charlson ME, Pompei P, Ales KL, McKenzie CR. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. Journal of Chronic Disease. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Cherkin Daniel, Grothaus Louis, Wagner Edward. The Effect of Office Visit Copayments on Utilization in a Health Maintenance Organization. Medical Care. 1989;27:1036–45. doi: 10.1097/00005650-198911000-00005. [DOI] [PubMed] [Google Scholar]
- Eichner Matthew. Incentives, Price Expectations and Medical Expenditures: An Analysis of Claims Under Employer-Provided Health Insurance. 1996. Unpublished.
- Ettner Susan. Adverse Selection and the Purchase of Medigap Insurance by the Elderly. Journal of Health Economics. 1997;16:543–62. doi: 10.1016/s0167-6296(97)00011-8. [DOI] [PubMed] [Google Scholar]
- Fang Hanming, Gavazza Alessandro. Dynamic Inefficiencies in Employment-Based Health Insurance Systems: Theory and Evidence. National Bureau of Economic Research Working Paper 13371. 2007. [DOI] [PubMed]
- Gaynor Martin, Li Jian, Vogt William B. Substitution, Spending Offsets, and Prescription Drug Benefit Design. Forum for Health Economics & Policy. 2007;10(2) Article 4: http://www.bepress.com/fhep/10/2/4.
- Goldman Dana P., Joyce Geoffrey F., Escarce Jose J., Pace Jennifer E., Colomom Matthew D., Laouri Marianne, Landsman Pamela B., Teutsch Steven M. Pharmacy Benefits and the Use of Drugs by the Chronically Ill. Journal of the American Medical Association. 2004;291:2344–501. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- Goldman Dana, Joyce Geoffrey, Karaca-Mandic Pinar. Varying Pharmacy Benefits with Clinical Status: The Case of Cholesterol-Lowering Therapy. American Journal of Managed Care. 2006;12:21–28. [PubMed] [Google Scholar]
- Goldman Dana, Philipson Tomas. Integrated Insurance Design in the Presence of Multiple Medical Technologies. American Economic Review. 2007;97(2):427–32. doi: 10.1257/aer.97.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu John, Price Mary, Huang Jie, Brand Richard, Fung Vicki, Hui Rita, Fireman Bruce, Newhouse Joseph P., Selby Joseph V. Unintended Consequences of Caps on Medicare Drug Benefits. New England Journal of Medicine. 2006;354:2349–59. doi: 10.1056/NEJMsa054436. [DOI] [PubMed] [Google Scholar]
- Johnson Richard E., Goodman Michael J., Hornbrook Mark C., Eldredge Michael B. The Impact of Increasing Patient Prescription Drug Cost Sharing on Therapeutic Classes of Drugs Received and on the Health Status of Elderly HMO Members. Health Services Research. 1997;32(1):103–22. [PMC free article] [PubMed] [Google Scholar]
- Keeler Emmett B., Rolph John E. The Demand for Episodes of Treatment in the Health Insurance Experiment. Journal of Health Economics. 1988;7:337–67. doi: 10.1016/0167-6296(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Landsman Pamela B., Yu Winnie, Liu Xiao Feng, Tuetsch Steven M., Berger Mark L. Impact of 3-Tier Pharmacy Benefit Design and Increased Consumer Cost-Sharing on Drug Utilizaiton. American Journal of Managed Care. 2005;11:621–28. [PubMed] [Google Scholar]
- Lillard Lee, Rogowski Jeanette. Does Supplemental Private Insurance Increase Medicare Costs? RAND Labor and Population Program Working Paper 95–16. 1995.
- Manning Willard G., Newhouse Joseph P., Duan Naihua, Keeler Emmett B., Leibowitz Arleen. Health Insurance and the Demand for Medical Care: Evidence from a Randomized Experiment. American Economic Review. 1987;77(3):251–77. [PubMed] [Google Scholar]
- McCall Nelda, Rice Thomas, Boismier James, West Richard. Private Health Insurance and Medical Care Utilization: Evidence from the Medicare Population. Inquiry. 1991;28:276–87. [PubMed] [Google Scholar]
- Newhouse Joseph. Free for All: Lessons from the RAND Health Insurance Experiment. Harvard University Press; Cambridge, MA: 1993. [Google Scholar]
- Rice Thomas, Matsuoka Karen Y. The Impact of Cost-Sharing on Appropriate Utilization and Health Status: A Review of the Literature on Seniors. Medical Care Research and Review. 2004;61(4):415–52. doi: 10.1177/1077558704269498. [DOI] [PubMed] [Google Scholar]
- Selby Joe, Fireman Bruce, Swain Bix. Effect of a Co-payment on Use of the Emergency Department in a Health Maintenance Organization. New England Journal of Medicine. 1996;334:635–41. doi: 10.1056/NEJM199603073341006. [DOI] [PubMed] [Google Scholar]
- Tamblyn Robyn, Laprise Rejean, Hanley James A., Abrahamowicz Michael, Scott Susan, Mayo Nancy, Hurley Jerry, Grad Roland, Latimer Eric, Perreault Robert, McLeod Peter, Huang Allen, Larochelle Pierre, Mallet Louise. Adverse Events Associated With Prescription Drug Cost–Sharing Among Poor and Elderly Persons. Journal of the American Medical Association. 2001;285(4):421–29. doi: 10.1001/jama.285.4.421. [DOI] [PubMed] [Google Scholar]
- Wolfe John, Goodeeris John. Adverse Selection, Moral Hazard and Wealth Effects in the Medigap Insurance Market. Journal of Health Economics. 1991;10:433–59. doi: 10.1016/0167-6296(91)90024-h. [DOI] [PubMed] [Google Scholar]
This article has been cited by
- 1.Çağatay Koç. Disease-Specific Moral Hazard and Optimal Health Insurance Design for Physician Services. Journal of Risk and Insurance. 2010 no-no. [CrossRef] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.