Abstract
Area 17 in the neonate of numerous species receives projections from cortical areas that do not project to area 17 in the adult. To investigate if this were the case in the developing primate, we have made injections of retrograde tracers in area 17 of newborn monkeys (Macaca irus) and examined the areal distribution of labeled neurons. Neurons projecting to area 17 were found to be restricted to those cortical regions that project to area 17 in the adult. The projection to area 17 in the neonate did appear to be very different in that in the superior temporal sulcus there was a large contingent of labeled neurons in supragranular layers. This constitutes a transient projection because in the adult area 17 projecting neurons in this cortical region originate almost exclusively from infragranular layers. To test if a change in the laminar distribution of area 17 projecting neurons in extrastriate cortex is a general feature of postnatal development, we have computed in neonates and adults the proportion of area 17 afferent neurons in infra- and supragranular layers for each cortical region that projects to area 17. This revealed (i) that in the adult the laminar distribution of area 17 afferents is characteristic for each cortical area and (ii) that this distribution emerges during development from an immature state in which labeled neurons are more numerous in supragranular layers. These results show that there is an extensive remodeling of the neuronal circuitry connecting visual cortical areas during postnatal development in the monkey and that the transient connectivity of primate area 17 is very different from that observed in other mammalian species.
Full text
PDF

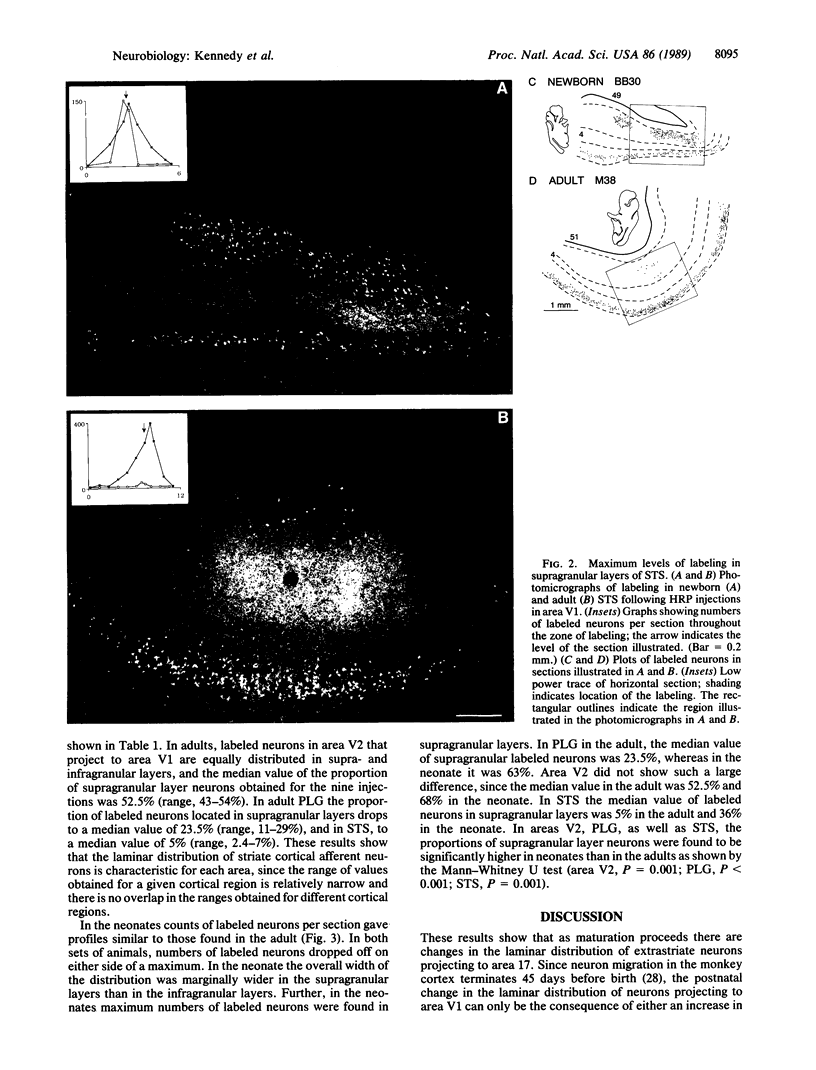
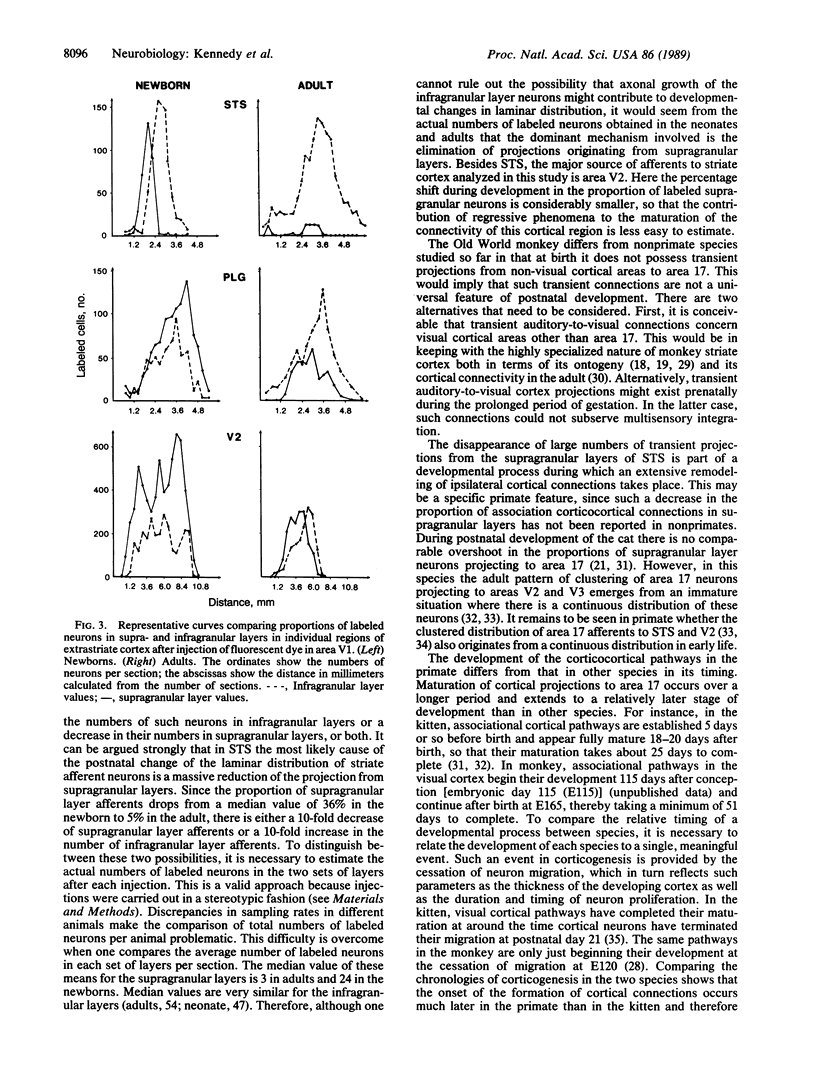

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chalupa L. M., Killackey H. P., Snider C. J., Lia B. Callosal projection neurons in area 17 of the fetal rhesus monkey. Brain Res Dev Brain Res. 1989 Apr 1;46(2):303–308. doi: 10.1016/0165-3806(89)90294-0. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Baumbach H. D., Lawson R. Callosal projections of the striate cortex in the neonatal rabbit. Exp Brain Res. 1981;42(2):122–126. doi: 10.1007/BF00236899. [DOI] [PubMed] [Google Scholar]
- Clarke S., Innocenti G. M. Organization of immature intrahemispheric connections. J Comp Neurol. 1986 Sep 1;251(1):1–22. doi: 10.1002/cne.902510102. [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Fawcett J. W., O'Leary D. D., Stanfield B. B. Regressive events in neurogenesis. Science. 1984 Sep 21;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Cusick C. G., Lund R. D. Modification of visual callosal projections in rats. J Comp Neurol. 1982 Dec 20;212(4):385–398. doi: 10.1002/cne.902120406. [DOI] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985 Sep 5;317(6032):58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Dehay C., Bullier J., Kennedy H. Transient projections from the fronto-parietal and temporal cortex to areas 17, 18 and 19 in the kitten. Exp Brain Res. 1984;57(1):208–212. doi: 10.1007/BF00231149. [DOI] [PubMed] [Google Scholar]
- Dehay C., Horsburgh G., Berland M., Killackey H., Kennedy H. Maturation and connectivity of the visual cortex in monkey is altered by prenatal removal of retinal input. Nature. 1989 Jan 19;337(6204):265–267. doi: 10.1038/337265a0. [DOI] [PubMed] [Google Scholar]
- Dehay C., Kennedy H., Bullier J., Berland M. Absence of interhemispheric connections of area 17 during development in the monkey. Nature. 1988 Jan 28;331(6154):348–350. doi: 10.1038/331348a0. [DOI] [PubMed] [Google Scholar]
- Dehay C., Kennedy H., Bullier J. Callosal connectivity of areas V1 and V2 in the newborn monkey. J Comp Neurol. 1986 Dec 1;254(1):20–33. doi: 10.1002/cne.902540103. [DOI] [PubMed] [Google Scholar]
- Dehay C., Kennedy H., Bullier J. Characterization of transient cortical projections from auditory, somatosensory, and motor cortices to visual areas 17, 18, and 19 in the kitten. J Comp Neurol. 1988 Jun 1;272(1):68–89. doi: 10.1002/cne.902720106. [DOI] [PubMed] [Google Scholar]
- Dehay C., Kennedy H. The maturational status of thalamocortical and callosal connections of visual areas V1 and V2 in the newborn monkey. Behav Brain Res. 1988 Aug;29(3):237–244. doi: 10.1016/0166-4328(88)90028-9. [DOI] [PubMed] [Google Scholar]
- Dreher B., Thong I. G., Shameem N., McCall M. J. Development of cortical afferents and cortico-tectal efferents of the mammalian (rat) primary visual cortex. Aust N Z J Ophthalmol. 1985 Aug;13(3):251–261. doi: 10.1111/j.1442-9071.1985.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Clarke S. Bilateral transitory projection to visual areas from auditory cortex in kittens. Brain Res. 1984 May;316(1):143–148. doi: 10.1016/0165-3806(84)90019-1. [DOI] [PubMed] [Google Scholar]
- Innocenti G. M., Frost D. O. The postnatal development of visual callosal connections in the absence of visual experience or of the eyes. Exp Brain Res. 1980;39(4):365–375. doi: 10.1007/BF00239301. [DOI] [PubMed] [Google Scholar]
- Jacobson M. Development of specific neuronal connections. Science. 1969 Feb 7;163(3867):543–547. doi: 10.1126/science.163.3867.543. [DOI] [PubMed] [Google Scholar]
- Keizer K., Kuypers H. G., Huisman A. M., Dann O. Diamidino yellow dihydrochloride (DY . 2HCl); a new fluorescent retrograde neuronal tracer, which migrates only very slowly out of the cell. Exp Brain Res. 1983;51(2):179–191. doi: 10.1007/BF00237193. [DOI] [PubMed] [Google Scholar]
- Kennedy H., Bullier J. A double-labeling investigation of the afferent connectivity to cortical areas V1 and V2 of the macaque monkey. J Neurosci. 1985 Oct;5(10):2815–2830. doi: 10.1523/JNEUROSCI.05-10-02815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H., Dehay C., Bullier J. Organization of the callosal connections of visual areas V1 and V2 in the macaque monkey. J Comp Neurol. 1986 May 15;247(3):398–415. doi: 10.1002/cne.902470309. [DOI] [PubMed] [Google Scholar]
- Lund R. D., Chang F. L., Land P. W. The development of callosal projections in normal and one-eyed rats. Brain Res. 1984 May;316(1):139–142. doi: 10.1016/0165-3806(84)90018-x. [DOI] [PubMed] [Google Scholar]
- Luskin M. B., Shatz C. J. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985 Dec 22;242(4):611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Malpeli J. G., Baker F. H. The representation of the visual field in the lateral geniculate nucleus of Macaca mulatta. J Comp Neurol. 1975 Jun 15;161(4):569–594. doi: 10.1002/cne.901610407. [DOI] [PubMed] [Google Scholar]
- Maunsell J. H., van Essen D. C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983 Dec;3(12):2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. M., Hegarty E., Barbas H., Carson K. A., Gower E. C., Knapp A. G., Moss M. B., Mufson E. J. Additional factors influencing sensitivity in the tetramethyl benzidine method for horseradish peroxidase neurohistochemistry. J Histochem Cytochem. 1980 Nov;28(11):1255–1259. doi: 10.1177/28.11.6159394. [DOI] [PubMed] [Google Scholar]
- Miller M. W., Vogt B. A. The postnatal growth of the callosal connections of primary and secondary visual cortex in the rat. Brain Res. 1984 Jun;316(2):304–309. doi: 10.1016/0165-3806(84)90319-5. [DOI] [PubMed] [Google Scholar]
- Olavarria J., Van Sluyters R. C. Organization and postnatal development of callosal connections in the visual cortex of the rat. J Comp Neurol. 1985 Sep 1;239(1):1–26. doi: 10.1002/cne.902390102. [DOI] [PubMed] [Google Scholar]
- Price D. J., Blakemore C. Regressive events in the postnatal development of association projections in the visual cortex. Nature. 1985 Aug 22;316(6030):721–724. doi: 10.1038/316721a0. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rockel A. J., Hiorns R. W., Powell T. P. The basic uniformity in structure of the neocortex. Brain. 1980 Jun;103(2):221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Salin P. A., Bullier J., Kennedy H. Convergence and divergence in the afferent projections to cat area 17. J Comp Neurol. 1989 May 22;283(4):486–512. doi: 10.1002/cne.902830405. [DOI] [PubMed] [Google Scholar]
- Shipp S., Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985 May 23;315(6017):322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Functional specialisation in the visual cortex of the rhesus monkey. Nature. 1978 Aug 3;274(5670):423–428. doi: 10.1038/274423a0. [DOI] [PubMed] [Google Scholar]
- Zeki S., Shipp S. The functional logic of cortical connections. Nature. 1988 Sep 22;335(6188):311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]