Abstract
Extracellular matrix (ECM) molecules play critical roles in muscle function by participating in neuromuscular junction (NMJ) development and the establishment of stable, cytoskeleton-associated adhesions required for muscle contraction. Matrix metalloproteinases (MMPs) are neutral endopeptidases that degrade all ECM components. While the role of MMPs and their inhibitors, the tissue inhibitor of metalloproteinases (TIMPs), has been investigated in many tissues, little is known about their role in muscle development and mature function. TIMP-2−/− mice display signs of muscle weakness. Here, we report that TIMP-2 is expressed at the NMJ and its expression is greater in fast-twitch (extensor digitorum longus, EDL) than slow-twitch (soleus) muscle. EDL muscle mass is reduced in TIMP-2−/− mice without a concomitant change in fiber diameter or number. The TIMP-2−/− phenotype is not likely due to increased ECM proteolysis because net MMP activity is actually reduced in TIMP-2−/− muscle. Most strikingly, TIMP-2 co-localizes with β1 integrin at costameres in the wild-type EDL and β1 integrin expression is significantly reduced in TIMP-2−/− EDL. We propose that reduced β1 integrin in fast-twitch muscle may be associated with destabilized ECM-cytoskeletal interactions required for muscle contraction in TIMP-2−/− muscle; thus, explaining the muscle weakness. Given that fast-twitch fibers are lost in muscular dystrophies and age-related sarcopenia, if TIMP-2 regulates mechanotransduction in an MMP-independent manner it opens new potential therapeutic avenues.
Keywords: costamere, integrin, knockout, mechanotransduction, myogenesis, proMMP-2
Muscle contraction is dependent upon the coupling of synaptic transmission at the neuromuscular junction (NMJ) and subsequent activation of the myofibrillar contractile apparatus. Motor nerve terminals and the muscle fibers they innervate are physiologically adapted to the distinct functional demands they experience. The development of fast motor nerve terminal characteristics (Bewick et al., 2004) and fast-twitch muscle fibers (DiMario and Stockdale, 1997; Wigmore and Evans, 2002) emerges during the second postnatal week as pups progress from sedentary sucklings to active weight-bearing locomotion.
Basal lamina extracellular matrix (ECM) proteins, secreted both by motor neurons and muscle, play pivotal roles in NMJ maturation and lateral force transmission during muscle contraction. Nerve-derived agrin, a heparan sulfate proteoglycan that binds to laminins and integrins, is important for maintenance of acetylcholine receptor (AChR) clusters (Sanes et al., 1990). Like agrin, β1 integrins are expressed by both motor neurons and muscle; however, β1 integrins in muscle, but not motor neurons, are required for skeletal muscle innervation (Schwander et al., 2004). Integrins are the principal cell surface ECM receptors. α7β1, the major integrin found in adult skeletal muscle, is localized at the NMJ, myotendinous junction, and costameres, protein complexes overlying sarcomeric Z disks that regulate ECM-cytoskeletal force transmission (Ervasti, 2003; Samarel, 2005). Like agrin, the dystroglycan complex is required for stabilization of AChR clusters (Jacobson et al., 2001). Like integrins, dystroglycan is located at costameres. Agrin (VanSaun and Werle, 2000), integrins (Ratnikov et al., 2002), and dystroglycan (Yamada et al., 2001) are substrates for matrix metalloproteinases (MMPs).
Although a role for MMPs in myoblast fusion has long been recognized (Couch and Strittmatter, 1983), their physiological function in NMJ development and maintenance is less clear (Carmeli et al., 2004). MMP activity is regulated by interaction with tissue inhibitor of metalloproteinases (TIMPs). Of the four known TIMPs, TIMP-2 is of particular interest because it plays a role in MMP activation as well as MMP inhibition. At low TIMP-2 concentrations, MT1-MMP activates proMMP-2 while; at higher TIMP-2 levels, MT1-MMP function is blocked, thus preventing proMMP-2 activation (Butler et al., 1998). Although classically recognized for their MMP inhibitory activity, it is now well accepted that TIMPs function independent of MMP inhibition (Fernandez et al., 2003; Seo et al., 2003), in particular via interaction with α3β1 integrin (Seo et al., 2003; Pérez-Martínez and Jaworski, 2005).
Previously, we reported that TIMP-2−/− mice display motor deficits indicative of muscle weakness (Jaworski et al., 2006). The foundation of this phenotype was investigated by examining fast-twitch locomotor (extensor digitorum longus, EDL), slow-twitch locomotor (soleus), and mixed fast/slow non-locomotor (diaphragm) muscles for histological and biochemical alterations. Here, we report that TIMP-2 co-localizes with β1 integrin at costameres in the wild-type EDL. Furthermore, reduced muscle weight was observed only in TIMP-2−/− EDL and was associated with significantly decreased β1 integrin expression. Although muscle fibers appear histologically normal, we propose that mechanotransduction is altered in TIMP-2−/− mice due to reduced β1 integrin-mediated stabilization of ECM-cytoskeletal interactions. If TIMP-2 regulates mechanotransduction in an MMP-independent manner, it opens new therapeutic avenues for muscular dystrophies and age-related sarcopenia.
Materials and Methods
Animals
Mice bearing a targeted disruption of the TIMP-2 gene have been described elsewhere (Wang et al., 2000). TIMP-2−/− mice and wild-type littermates from heterozygous matings were used for most analyses, except the developmental western blots and gelatinase activity assay where muscles from entire litters of homozygous matings were pooled to obtain sufficient protein. Procedures that involved animals were in accordance with the institutional guidelines of the University of Vermont Animal Care and Use Committee.
Antibodies
Primary antibodies: rabbit anti-human TIMP-2 for western blots (1:1500; Chemicon; Temecula, CA), sheep anti-human TIMP-2 for immunohistochemistry (1:200; Biogenesis; Kingston, NH), rat anti-mouse β1 integrin (1:2000 for western blot; 1:250 for immunohistochemistry; Chemicon), goat anti-human actin (1:5000; Santa Cruz Biotechnology; Santa Cruz, CA), rabbit anti-rat neurofilament-145 (1:500; Chemicon), mouse anti-chicken α-tubulin (1:1000; Sigma; Saint Louis, MO). Secondary antibodies: Cy3-conjugated donkey anti-sheep (1:500), Cy2-conjugated goat anti-rabbit (1:100), HRP-conjugated donkey anti-sheep or anti-rabbit (1:3000) and donkey anti-goat (1:5000) were from Jackson ImmunoResearch (West Grove, PA). FITC-conjugated anti-rat IgG2a (1:100) was from Southern Biotech (Birmingham, AL).
Western blot analysis
Western blot analysis was performed as previously described (Lluri and Jaworski, 2005).
Immunohistochemistry
Immunohistochemistry was performed as previously described (Jaworski et al., 2006). Briefly, for whole mount immunohistochemistry, the perfusion fixed muscle was stained with rhodamine-conjugated α-bungarotoxin (BTX) (10 μg/ml; Molecular Probes; Eugene, OR) for 15 minutes, the tissue was rinsed with PBS, incubated in methanol, rinsed as before and blocked in PBS with 0.2 % Triton X-100, 2.0 % BSA, and 0.1 % sodium azide. The muscle was incubated with rabbit anti-rat neurofilament-145, diluted in blocking buffer, overnight at room temperature. After rinsing with blocking buffer, the muscle was incubated in Cy-2 conjugated goat anti-rabbit for 4 hours. After washing with PBS, the muscle was mounted onto gelatin coated glass slides and examined with a Nikon E800 microscope (Micro Video Instruments; Avon, MA) and images captured either with a Spot RT digital camera (Diagnostic Instruments; Sterling Heights, MI) or Nikon Eclipse C1confocal microscope. For muscle sections, the muscle was frozen in Tissue-Tek O.C.T. Compound (Sakura; Tokyo, Japan), 12 μm-thick cryosections were collected onto gelatin coated slides, and frozen at −20 °C until used. Sections were thawed at room temperature, hydrated with PBS, and immunohistochemistry performed as described above except that primary antibody was incubated at 4 °C overnight and secondary antibody was incubated for 2 hours.
Histochemical staining
ATPase, Period Acid Schiff, Masson Trichrome, NADH Diaphorase, cresyl violet, acid phosphatase, alkaline phosphatase, Oil O Red, and succinic dehydrogenase was performed according to standard techniques (Carson, 1997) under the auspices of the Fletcher Allen Health Care Department of Pathology (Burlington, VT).
FM1-43 Labeling
Endplates were stained with α-BTX (20 μM) for 15 minutes and rinsed with PBS prior to loading with FM1-43. Tissue whole-mounts were pinned onto a Sylgard (Dow Corning)-coated dish and loaded with FM1-43 (16.4 μM in normal saline; Molecular Probes) by depolarization with Kreb’s saline containing 60 mM KCl for 5 minutes. After rinsing with calcium deficient Kreb’s saline (e.g., Ca2+ omitted) for 5 minutes, the muscle was incubated in normal Kreb’s saline (1.8 mM Ca2+ and 1 mM Mg2+) for 5 minutes. TTX (3e-7 M) was present during imaging. Muscles were imaged on a BioRad Radiance MPD multiphoton system installed on an Olympus BX-51 upright microscope using a 60x-dipping lens (0.9 NA). Excitation was at 800 nm with a Coherent Chameleon tunable pulsed near-IR laser. Emission was detected using bandpass filters centered at 515 nm for FM1-43 and 645 nm for α-BTX.
Gross muscle properties
Mice were euthanized by cervical dislocation and weighed. The entire muscle (soleus and EDL) was carefully excised by cutting from tendon to tendon or by carefully cutting around the entire ribcage (diaphragm). Wet muscle weight (in mg) was normalized to body weight (in g) for diaphragm, and 10X mg tissue weight/g body weight for soleus and EDL. The soleus and EDL were then extended to their in vivo length, pinned to a Sylgard-coated dish, and fixed with 4% paraformaldehyde for 10 minutes at room temperature. After rinsing with PBS twice for 10 minutes, the muscle was frozen in OCT. Transverse (12 μm-thick) cryosections through the mid-belly of the muscle were thaw mounted onto gelatin coated slides and frozen at − 20 °C until used. Immunohistochemistry for β1 integrin, as described above, was used to reveal muscle fiber basement membrane. Digital images of three random fields (20X) were captured with the Spot RT camera. Fiber cross-sectional area was measured using software provided with the camera. To determine fiber number per cross-sectional area, a 100 × 100 μm box was created using the Spot software and the number of muscle fibers entirely within the box was manually counted.
Synapse elimination
Confocal microscopy was used to capture images of at least 50 random en face endplates labeled with neurofilament and α-BTX. These images were then examined using Adobe Photoshop (Adobe Systems Inc., San Jose, CA) to determine whether the endplate was multiply innervated. The number of monoinnervated and polyinnervated endplates was manually counted and the percent monoinnervation calculated relative to the total endplates with discernable innervation.
Gelatinase Assay
Muscles were homogenized in Tris-buffered saline (TBS; 50 mM Tris-HCl pH 7.4, 150 mM NaCl), lysates were spun at 14,000 rpm for 15 minutes at 4 °C, and the supernatant used for assays. Protein concentrations were determined by the method of (Bradford, 1976) using the Bio-Rad reagent and bovine serum albumin as a standard. Net gelatinolytic activity was determined using the EnzCheck Gelatinase Assay (Molecular Probes) according to manufacturer’s instructions. MMP activity is reported as the rate of fluorescence increase over 2 hours normalized to assay reagent containing DQ gelatin alone. The specificity of the assay was determined by the inclusion of the MMP inhibitor 1,10 phenanthroline (1mM) or a cocktail of protease inhibitors (phenyl methyl sulfonylfluoride [1 mM] to inhibit serine protease, N-ethylmaleimide and ε-amino-n-caproic acid [5 mM each] to inhibit cysteine proteases, pepstatin A [5 μg/ml] to inhibit acid proteases, and leupeptin [5 μg/ml] to inhibit serine and cysteine proteases).
DNA Microarray analysis
The soleus and EDL from P21 TIMP-2−/− and wild-type littermate controls were carefully excised from tendon to tendon and frozen at −80 °C until used. Both solei and EDL were pooled into one sample. Muscles from 4 independent litters were analyzed. It should be noted that the microarray was performed prior to the determination that TIMP-2 is differentially expressed in fast-twitch muscles. Muscle was homogenized in RNeasy lysis buffer (Qiagen; Valencia, CA) with cubic boron nitride-aluminum oxide abrasives. The lysate was passed through a Qiashredder column followed by total RNA extraction and DNase treatment according to manufacturer’s instructions. Gene chip expression synthesis and hybridization to Affymetrix mouse 430A Gene Chips utilized standard protocols.
Statistics
Data are presented as mean ± s.e.m.. Significant interactions were identified by paired t-test when littermates were compared and unpaired t-test when non-littermates were analyzed. Criterion for statistical significance is p < 0.05.
Results
Previously, we reported the presence of a motor deficit in TIMP-2−/− mice (i.e., decreased motor endurance, grip strength, hindlimb extension, and increased gait width) (Jaworski et al., 2006). The present studies were undertaken to more fully characterize the role of TIMP-2 in NMJ maturation. Three ages were investigated – postnatal day 3 (P3, prior to NMJ maturation), P21 (the period of maximum hyperkinesia in TIMP-2−/− mice), and adult (P90-150, when motor weakness is most pronounced in TIMP-2−/− mice).
TIMP-2 is expressed in pre-and post-synaptic NMJ components
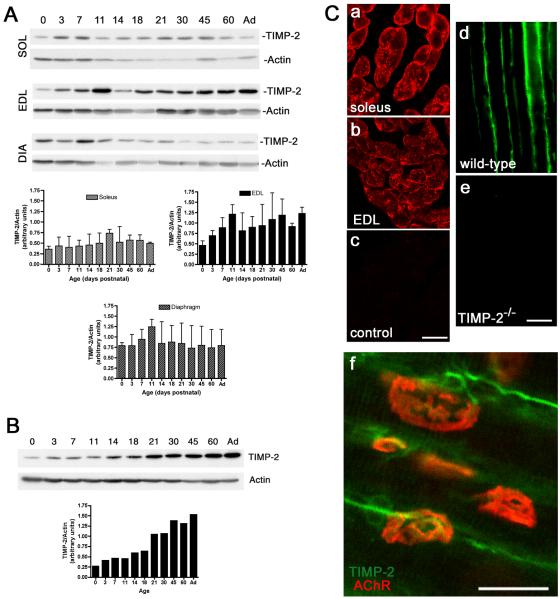
Previously, we reported that TIMP-2 expression increased during muscle development (Jaworski et al., 2006). Because all muscles of the lower extremity were pooled for this analysis it precluded the determination of whether TIMP-2 was differentially expressed in slow-twitch versus fast-twitch muscle fiber types. Adult muscle fiber types are identified as either type 1 (slow) or type 2 (fast) based upon contraction velocity and fatigue rate. Although most muscles contain both slow and fast fibers, one type predominates. To determine whether TIMP-2 is preferentially associated with a particular fiber type, its expression in soleus (slow-twitch locomotor muscle), extensor digitorum longus (EDL, fast-twitch locomotor muscle), and diaphragm (mixed slow/fast non-locomotor muscle) was examined by western blot analysis (Fig. 1A). TIMP-2 expression was largely unchanged during soleus and diaphragm development. In contrast, TIMP-2 expression, which was greater in the EDL than soleus or diaphragm, increased throughout development. Because TIMP-2 is also expressed in spinal motor neurons (Jaworski et al., 2006) and may be released at nerve terminals, TIMP-2 expression during spinal cord (i.e., lumbar enlargement) development was investigated to determine its contribution to NMJ maturation (Fig. 1B). TIMP-2 expression increased throughout spinal cord development and in the adult is greater than that expressed in muscle. Immunohistochemistry revealed that TIMP-2 associates with the myofiber basal lamina (Fig. 1Ca-e). TIMP-2 is expressed on motor neuron soma in the spinal cord (Jaworski et al., 2006) and by motor neuron axons at the NMJ (Fig. 1Cf). Therefore, the relative contribution of muscle-derived and nerve-derived TIMP-2 to the basal lamina is unknown.
Figure 1. TIMP-2 is expressed at the neuromuscular junction.
A) Western blot analysis of soleus (SOL), extensor digitorum longus (EDL), and diaphragm (DIA) (25 μg crude homogenate) at the indicated postnatal ages (Ad-adult). TIMP-2 expression was normalized to actin as a loading control. TIMP-2 expression is greatest in EDL and is increased during development. B) Western blot analysis of spinal cord lumbar enlargement (20 μg crude homogenate). TIMP-2 is more abundantly expressed in spinal cord than muscle. Like the EDL, TIMP-2 expression in spinal cord is developmentally up-regulated. C) Confocal photomicrographs of TIMP-2 expression in transverse sections of P21 soleus (a, c), EDL (b), and longitudinal sections of P14 sartorius (d, e). Antibody specificity is demonstrated by the lack of immunolabeling with secondary antibody alone (c) and in TIMP-2−/− muscle (e). In addition to labeling muscle basal lamina, TIMP-2 (green) labels motor neurons at the NMJ endplate (red, rhodamine-conjugated α- BTX) (f). Scale bars = 25 μm.
TIMP-2−/− mice display alterations in NMJ organization
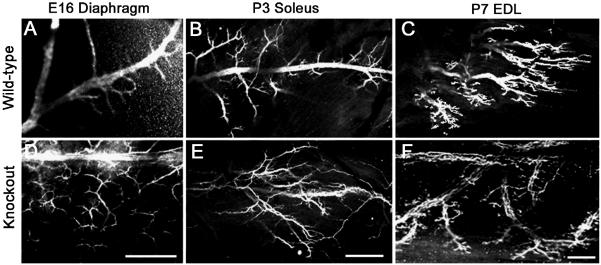
Inasmuch as TIMP-2 is expressed in motor neurons and muscle, NMJ cytoarchitecture was examined to determine the effect of TIMP-2 deletion on NMJ maturation. Previously, altered nerve branching was observed in the P7 soleus (Jaworski et al., 2006). To determine whether a similar phenotype was present in the P7 EDL and/or at younger ages, motor neuron terminal morphology was visualized immunohistochemically with an antibody to neurofilament-145. In contrast to wild-type muscles (Fig. 2A-C), TIMP-2−/− muscles showed increased intramuscular nerve branching and decreased fasciculation (Fig. 2D-F). The soleus and EDL were not examined at E16, but altered nerve branching was also present in the P7 soleus and P3 EDL (data not shown). Thus, the phenotype is present in both slow- and fast-twitch muscles. Interestingly, the acquisition of muscle fiber types occurs in the second postnatal week and the nerve branching phenotype is only apparent during the first two postnatal weeks.
Figure 2. Intramuscular nerve branching is increased in TIMP-2−/− mice.
Confocal micrographs show neurofilament-145 immunolabeling in TIMP-2−/− mice and wild-type littermate controls. A well-defined central nerve trunk with branches emanating from it is present in wild-type E16 diaphragm (A) and P3 soleus (B), while the P7 EDL shows a discretely organized endplate band (C). This is in stark contrast to the nerve branching in TIMP-2−/− mice. The altered TIMP-2−/− nerve morphology may be due to increased nerve branching (D) and/or axon defasciculation (E); thereby, resulting in a disorganized endplate region (F). Altered nerve branching is also present in the diaphragm at P3, but not P7, and in the P3 EDL and P7 soleus (data not shown). Data are representative of 3 embryos and 6 neonatal mice. Scale bar = 100 μm.
Because TIMP-2 is expressed at the NMJ and proteases have been implicated as mediators of synapse elimination (Chang and Balice-Gordon, 1997), we sought to determine whether the neonatal increased nerve branching and dyskinesia in TIMP-2−/− mice at P21 was due to protracted synapse elimination. Motor neuron axons were examined using antibodies to neurofilament while the distribution of AChRs was examined using rhodamine-conjugated α-bungarotoxin (α-BTX). The relative percent of monoinnervated endplates in the diaphragm was comparable in TIMP-2−/− mice and wild-type littermates throughout the period of synapse elimination (i.e., P3, 7, and 14) (Fig. 3A). In addition, no obvious difference was observed in the number of motor axons at each polyinnervated endplate (Fig. 3B). A similar pattern was observed in the soleus and EDL (data not shown). Thus, the absence of TIMP-2 does not alter neonatal synapse elimination.
Figure 3. Synapse elimination is unaltered in TIMP-2−/− mice.

A) Synapse elimination was determined by quantitating the percent of monoinnervated endplates in the diaphragm. Monoinnervated endplates in wild-type controls and TIMP-2−/− littermates is similar at P3 (45.6 ± 0.5% vs. 43.3 ± 2.0%; p = 0.3, n = 3), P7 (74.4 ± 1.9% vs. 79.1 ± 4.2%; p = 0.3, n = 4), and P14 (93.7 ± 1.9% vs. 94.0 ± 1.3%; p = 0.9, n = 3). B) Confocal photomicrographs of P3 diaphragm demonstrate that the number of axons per polyinnervated endplate is also comparable in wild-type and TIMP-2−/− mice. C) Multiphoton micrograph demonstrating the nerve terminal with FM1-43 (green) and the AChR endplate region with rhodamine-conjugated α-BTX (red) indicates that pre- and post-synaptic NMJ components are appropriately aligned in the diaphragm of P21 wild-type and TIMP-2−/− mice. Scale bars = 50 μm (B), 25 μm (C).
During NMJ examination, it appeared as though all nerve terminals were apposed to an endplate and all endplates received innervation. Since neurofilament immunolabeling does not always extend to the endplate, the alignment between pre-and post-synaptic NMJ components was more accurately assessed using the styryl dye FM1-43 in conjunction with α-BTX. When FM1-43 is incubated with a neuronal preparation, it inserts into the outer leaflet of the plasma membrane and is internalized during vesicle retrieval after stimulation of exocytosis. After washing away the remaining external dye, fluorescently labeled synaptic vesicles within nerve terminals are visualized. Analysis of P21 diaphragm confirmed our initial observations that nerve terminals were appropriately aligned with endplates in TIMP-2−/− muscle (Fig. 3C). Since the TIMP-2 null dyskinesia is likely not due to pre-synaptic NMJ defects, our focus turned to muscle as the foundation for the movement phenotype.
Muscle cytoarchitecture is unaltered in TIMP-2−/− mice in vivo
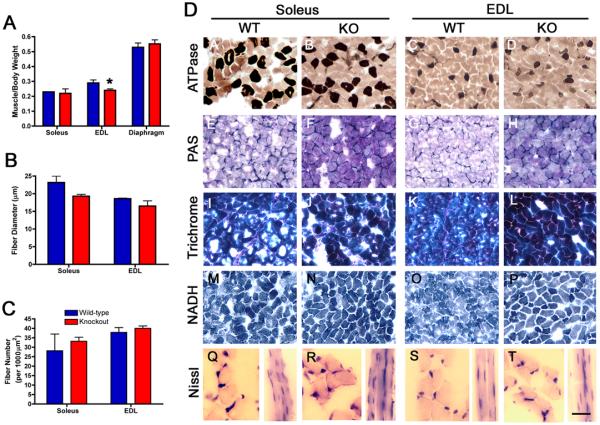
To determine the effect of TIMP-2 deletion on muscle, the soleus, EDL and diaphragm were examined for gross, histological, and biochemical alterations. Muscle weight was reduced at P21 in the TIMP-2−/− EDL, but not soleus or diaphragm (Fig. 4A). While there was a trend towards reduced muscle fiber diameter (Fig. 4B) and a compensatory increase in muscle fiber number (Fig. 4C), these did not reach statistical significance. Hence, the reduced muscle weight may be due to decreased water and/or protein content. Next, muscles were examined with a panel of histochemical stains to determine whether pathological alterations were present (Fig. 4D). ATPase, pH 4.3, showed no difference in either the proportion or distribution of slow-twitch fibers indicating the absence of myopathic and peripheral neuropathic alterations, respectively (Fig. 4DA-D). Periodic Acid Schiff (Fig. 4DE-H) and Masson Trichrome (Fig. 4DI-L) showed increased staining of basement membrane glycoproteins and collagen, respectively, in TIMP-2−/− soleus and EDL (discussed below, Fig. 5). NADH diaphorase, to identify mitochondria, Z-band material, and sarcoplasmic reticulum showed no differences (Fig. 4DM-P), suggesting the TIMP-2 null phenotype is not metabolic in nature. Nissl stain (i.e., cresyl violet) revealed that TIMP-2−/− muscle lack central nuclei, but it appeared as though the number of nuclei per fiber may be increased (Fig 4DQ-T). In addition to the lack of central nuclei, alkaline phosphatase, acid phosphatase, and Oil O Red staining showed a lack of regenerating fibers, inflammatory cells, and fat deposition, respectively (not shown), suggesting the lack of myopathic alterations at P21. Because the cytoarchitectural organization of TIMP-2−/− myofibers appears grossly normal, we examined TIMP-2−/− muscle for biochemical alterations as the foundation for the movement phenotype.
Figure 4. Muscle cytoarchitecture is normal in P21 TIMP-2−/− muscle.
A) Muscle weight, normalized to body weight, is reduced in TIMP-2−/− EDL (* p = 0.02), but not soleus (p = 0.5) or diaphragm (p = 0.5, n = 5). B) Fiber diameter is not reduced in soleus (wt: 21.4 ± 1.8, ko: 19.2 ± 0.5, p = 0.2) or EDL (wt: 18.4 ± 0.3, ko: 17.4 ± 1.2, p = 0.5, n = 3). C) The number of muscle fibers (per 10,000 μm2) is also not different in the soleus (wt: 28.2 ± 8.9, ko: 33.2 ± 2.2; p = 0.6) or EDL (wt: 37.9 ± 2.5, ko: 40 ± 1.3; p = 0.5). D) TIMP-2−/− muscles at P21 appear histologically normal; with the exception of increased basement membrane glycoproteins and collagen detected with Periodic Acid Schiff (PAS, E-H) and Masson Trichrome stain (I-L), respectively. ATPase at pH 4.3 shows no changes in slow-twitch muscle fiber number or distribution (A-D). In addition, no difference in NADH diaphorase is present (M-P). Examination of muscle cross sections reveals that TIMP-2−/− muscle lack central nuclei, but longitudinal sections appear to possess more nuclei per muscle fiber (Q-T). Scale bar = 50 μm (A-P), 25 μm (Q-T longitudinal sections), and 12.5 μm (Q-T cross sections).
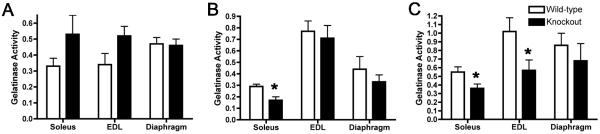
Figure 5. MMP activity increases as wild-type muscles age and is reduced in TIMP-2−/− muscle.
MMP activity was determined in P3 (A), P21 (B), and adult (C) muscle. A) At P3, there is a slight increase in MMP activity in TIMP-2−/− soleus and EDL, but this increase did not reach statistical significance (soleus: p = 0.14, EDL: p = 0.07, diaphragm: p = 0.8; n = 5). B) At P21, MMP activity is decreased in the soleus (* p = 0.02) and unchanged in the EDL (p = 0.6) and diaphragm (p = 0.4; n = 4). C) In the adult, MMP activity is decreased in both the soleus (* p = 0.04) and EDL (* p = 0.04), but not diaphragm (p = 0.5; n = 6).
TIMP-2 co-localizes with β1 integrin at costameres and β1 integrin expression is decreased only in TIMP-2−/− EDL
To determine whether the NMJ alterations in TIMP-2−/− muscle could be due to excessive MMP-mediated proteolysis of ECM molecules critical to NMJ development, net MMP activity was measured using a fluorescently caged gelatin (denatured collagen) substrate. Cleavage of this substrate by MMPs (e.g., both gelatinases and collagenases) in the muscle homogenate yields highly fluorescent peptides. The increase in fluorescence is proportional to proteolytic activity. Interestingly, MMP activity is increased during muscle development in wild-type mice (P3 to adult). This increase was greatest in the EDL (p = 0.005), least in the soleus (p = 0.023), and intermediate in the diaphragm (p = 0.017). No change in MMP activity was detected in TIMP-2−/− muscles at P3 (Fig. 5A). At P21, MMP activity was not altered in the TIMP-2−/− EDL (Fig. 5B), suggesting that the reduced muscle weight was not due to excessive proteolysis. Surprisingly, MMP activity was significantly decreased in adult TIMP-2−/− EDL and soleus (Fig. 5C). Therefore, excessive ECM proteolysis is likely not the foundation for the TIMP-2 null phenotype.
Since MMP activity was not increased in P21 TIMP-2−/− muscle, DNA microarray analysis was performed to identify genes whose expression is altered in TIMP-2−/− muscle, the period of maximum dyskinesia. β1 integrin was selected for further analysis for three reasons. First, it showed the greatest alteration (8.2-fold decrease) and most statistical significance (p=0.001) of the differentially regulated genes identified. Second, β1 integrins are known to play a role in NMJ maturation and mature muscle function (Schwander et al., 2004; Boppart et al., 2006). Third, TIMP-2 has been shown to exert MMP-independent functions via β1 integrins (Seo et al., 2003; Pérez-Martínez and Jaworski, 2005). To determine whether β1 integrin and TIMP-2 are co-localized in wild-type muscle, immunohistochemistry was performed. Both TIMP-2 and β1 integrin are abundantly expressed in the basal lamina at P21 (Fig. 6). Strikingly, TIMP-2 shows a very different expression pattern in the EDL and soleus. In the EDL, TIMP-2 co-localizes with β1 integrin at costameres (Figs. 6AA-C), while in the soleus, TIMP-2 is primarily expressed in capillary endothelial cells (Figs. 6AD-F) as revealed by immunoreactivity for von Willebrand factor (data not shown). In the adult, TIMP-2 is abundantly expressed in the basal lamina and capillary endothelial cells of the EDL (Figs. 6AG-I) and soleus (Figs. 6AJ-L). Costameric expression of both TIMP-2 and β1 integrin is not as prominent in the adult as at P21. To determine whether dysregulated β1 integrin expression could underlie the TIMP-2 null phenotype, the developmental regulation of β1 integrin was examined in wild-type and TIMP-2−/− EDL, the muscle in which both proteins co-localized. Western blot analysis revealed that the normal developmental down-regulation of β1 integrin expression occurred more precipitously in the TIMP-2−/− EDL (Fig. 6B). Furthermore, immunohistochemistry demonstrated that β1 integrin expression was more significantly reduced in the P21 TIMP-2−/− EDL (Fig. 6C), the muscle in which TIMP-2 expression is greatest (Fig. 1A) and in which TIMP-2 co-localizes with β1 integrin (Fig. 6A), than in the soleus. Taken together, these data suggest a potential relationship between TIMP-2 and β1 integrin in fast-twitch muscle.
Figure 6. β1 integrin expression is reduced in TIMP-2−/− fast-twitch muscle.
A) Immunohistochemistry was performed on perfusion fixed, longitudinal cryosections. TIMP-2 co-localizes with β1 integrin at costameres in P21 EDL (A-C) and to a lesser extent adult EDL (G-I). In the soleus, TIMP-2 and β1 integrin are both expressed in the basal lamina. In addition, TIMP-2 is abundantly expressed in capillary endothelial cells at P21 (D-F) and in the adult (J-L). Scale bar = 25 μm. B) Western blot analysis with 25 μg protein from wild-type and TIMP-2−/− EDL. Densitometry was performed and the relative expression of β1 integrin was normalized to α-tubulin (n=3). The developmental decline in β1 integrin expression that normally occurs in wild-type muscle is much more precipitous in TIMP-2−/− muscle. C) Immunohistochemistry confirms the western blot analysis. β1 integrin expression at P21 is significantly decreased in the EDL and only moderately decreased in the soleus (n=3). Scale bar = 50 μm.
Discussion
The present study was undertaken to further characterize the TIMP-2−/− phenotype (Jaworski et al., 2006). Here, we showed that, unexpectedly, MMP activity is reduced in adult TIMP-2−/− muscle. TIMP-2 co-localizes with β1 integrin at costameres in the EDL, but not soleus and β1 integrin expression is down-regulated in the TIMP-2−/− EDL. Hence, we propose that the TIMP-2−/− phenotype is due, at least in part, to decreased ECM compliance and mechanotransduction resulting in muscle weakness.
MMP-mediated proteolysis as the foundation for the TIMP-2−/− phenotype
Regulated ECM turnover is critical to appropriate tissue morphogenesis and a role for MMPs in myogenesis has long been proposed (Couch and Strittmatter, 1983). However, the MMPs involved and their exact contribution is yet to be elucidated. We first hypothesized that the TIMP-2−/− phenotype was due to excessive MMP-mediated ECM proteolysis. In the absence of compensation by the other three TIMPs, which we have not detected, one would predict that net MMP activity would be increased in TIMP-2−/− mice due to the lack of TIMP-2-mediated MMP inhibition. MMP activity was increased at P3, but did not reach statistical significance. However, the increase may be physiologically significant in that small changes in proteolysis can have profound effects on tissue integrity. In contrast to P3, net MMP activity was significantly decreased in adult TIMP-2−/− muscle. In addition to MMP inhibition, TIMP-2 is required for proMMP-2 activation via MT1-MMP (Butler et al., 1998). In the absence of TIMP-2, proMMP-2 activation is impaired (Caterina et al., 2000; Wang et al., 2000). Of the two gelatinases, MMP-2 and MMP-9, MMP-2 expression in muscle is greater (Nuttall et al., 2004). Thus, in the presence of reduced proMMP-2 activation, MMP-9 activity predominates. Because MMP-9 expression decreases between P7 and P14 (Nuttall et al., 2004), it likely contributes to the increased MMP activity at P3 and reduced MMP activity in adult muscle. We acknowledge the possibility that some MMPs may be up-regulated while others are down-regulated, resulting in no net increase in MMP activity. Up-regulation of MMP-3 would be of particular relevance because it has been reported to remove agrin from the synaptic basal lamina (VanSaun and Werle, 2000). If MMP-3 expression is increased, it could explain the alteration in endplate number and size observed in TIMP-2−/− muscle (Jaworski et al., 2006).
The role of MMPs in mature muscle function is also largely unknown (Carmeli et al., 2004). We report here, for the first time to our knowledge, an age-related increase in MMP activity that is greatest for fast-twitch muscle. Because fast-twitch fibers contain less collagen than slow-twitch fibers (Zimmerman et al., 1993), fast-twitch fibers are even more susceptible to the damaging effects of increased MMP activity. Many cell types contribute to the MMPs present in muscle, including motor neurons (Platt et al., 2003), Schwann cells (Muir and Manthorpe, 1992), myocytes (Demestre et al., 2005), satellite cells (Guerin and Holland, 1995), and fibroblasts (Ogawa et al., 2005). The cellular source(s) of increased MMPs is at present unknown, but warrants further investigation due to their potential role in muscle aging.
Decreased β1 integrin expression as the foundation for the TIMP-2−/− phenotype in fast-twitch muscle
Integrins play critical roles throughout myogenesis by serving as the principal cell surface ECM receptors (Mayer, 2003). Given that β1 integrins represent the largest class it is not surprising that β1 integrin−/− mice die during early embryogenesis (Stephens et al., 1995). Therefore, mice specifically deficient in β1 integrin in the nervous system (Graus-Porta et al., 2001) or muscle (Schwander et al., 2003) were generated. In contrast to mice lacking β1 integrin in muscle, which die immediately after birth with only poorly developed muscle fibers, TIMP-2−/− mice are viable and myofibers appears grossly normal. This is likely due to the fact that β1 integrin expression in TIMP-2−/− mice is unaltered prior to P14. Nonetheless, TIMP-2−/− mice share two neuromuscular phenotypes with muscle-deficient β1 integrin mice. Both β1 integrin−/− (Schwander et al., 2003) and TIMP-2−/− (Lluri and Jaworski, unpublished observation) myotube formation is dramatically reduced in vitro. In addition, β1 integrin−/− (Schwander et al., 2004) and TIMP-2−/− (Figure 2) mice both have increased intramuscular nerve branching. However, two observations suggest that this phenotype is not β1 integrin-mediated in TIMP-2−/− muscle. First, the altered nerve organization is only apparent in the first two postnatal weeks, the period when β1 integrin expression is comparable in wild-type and TIMP-2−/− muscle. Second, all three muscles examined possessed aberrant nerve organization even though decreased β1 integrin expression is primarily restricted to the EDL. Increased nerve branching is a common feature of mice deficient in a NMJ component (Gautam et al., 1999; Brandon et al., 2003; Wang et al., 2003; Schwander et al., 2004; Fu et al., 2005). While cell adhesion molecules, cytokines, and growth factors regulate axonal sprouting (English, 2003), the most powerful inducer of sprouting is paralysis (Tam and Gordon, 2003). In contrast to many of the aforementioned mice, TIMP-2−/− mice are not paralyzed. Inasmuch as agrin (Campagna et al., 1995) and β2 laminin (Patton, 2000) arrest neurite outgrowth and promote motor nerve terminal differentiation and both are MMP substrates (d’Ortho et al., 1997; VanSaun and Werle, 2000), their proteolysis could produce uncontrolled sprouting upon contact with muscle. Since net MMP activity in TIMP-2−/− muscle is greater only in neonates, increased nerve branching would be restricted to this developmental period. While we believe the altered nerve organization may be the consequence of increased proteolysis, we cannot rule out the contribution of decreased motor activity. Although TIMP-2−/− mice are not paralyzed, they do exhibit less activity prior to P14. When neonatal wild-type mice are placed on their backs they squirm until they turn over, while TIMP-2−/− pups just lie there motionless (Jaworski, unpublished observation). This is further substantiated by the observation that the number of spontaneously contracting myotubes and the rate at which myotubes contract is significantly reduced in TIMP-2−/− cultures relative to wild-type cultures (Lluri and Jaworski, unpublished observation). Finally, muscle inactivity is associated with increased mEPP amplitude (Wang et al., 2005) and mEPP amplitude is increased in P21 TIMP-2−/− EDL, but not soleus or diaphragm (Lluri, Parsons, and Jaworski, unpublished observation). Taken together, these data suggest a role for TIMP-2 in regulating muscle contractile properties.
Muscle contraction is dependent upon interactions of the muscle cell basement membrane and the underlying cytoskeleton (Campbell and Stull, 2003; Grounds et al., 2005). The two main membrane-associated molecules responsible for force transmission are integrins and the dystroglycan/sarcoglycan complex. Integrins play a role in maintaining normal contractile function in skeletal (Lopez et al., 2005; Boppart et al., 2006), cardiac (Sarin et al., 2005), and smooth (Lehoux et al., 2005) muscle. In skeletal muscle, transgenic mice over-expressing the α7βX2 chain exhibit diminished exercise-induced muscle damage (Boppart et al., 2006) and diaphragms of α7-deficient mice show altered force transmission, compliance, and viscoelasticity (Lopez et al., 2005). TIMP-2−/− mice show behavioral signs of contractile dysfunction (Jaworski et al., 2006). Furthermore, we showed here that TIMP-2 co-localizes with β1 integrin at costameres in wild-type fast-twitch EDL muscle and β1 integrin expression is reduced in EDL from TIMP-2−/− mice. Taken together, our findings suggest a role for TIMP-2 in the maintenance of ECM-cytoskeletal interactions and, in turn, muscle force transduction especially in the EDL.
Although traditionally viewed as exerting their effects by regulating ECM integrity, evidence is mounting that MMPs and TIMPs also function in a protease-independent manner via direct interaction with integrins. Integrins have been shown to serve as a docking system for the proMMP-2/TIMP-2/MT1-MMP complex (Brooks et al., 1996; Hornebeck et al., 2002) and ADAM-12 regulates myogenic cell differentiation via α9β1 integrin (Eto et al., 2000; Lafuste et al., 2005). We (Pérez-Martínez and Jaworski, 2005), and others (Seo et al., 2003), have demonstrated that TIMP-2 binds to α3β1 integrin to exert its MMP-independent functions. These data combined with the decreased expression of β1 integrin mRNA in TIMP-2−/− muscle by microarray analysis suggest that TIMP-2 may not only directly activate integrin signal transduction, but also positively regulate β1 integrin transcription via a feed-forward, growth factor-like mechanism. Our current working model is that TIMP-2 plays a dual role in muscle function, both of which predominate in fast-twitch muscle fibers. In its traditional role, TIMP-2 inhibits MMP-mediated ECM proteolysis, which is greater in fast-twitch muscle. In the absence of TIMP-2, proMMP-2 activation, net MMP activity, and ECM compliance is decreased. Furthermore, TIMP-2 binds to integrins to stabilize ECM-cytoskeletal interactions in fast-twitch muscle. Loss of TIMP-2 destabilizes this interaction, resulting in decreased mechanotransduction that is further compounded by the increased ECM tensile strength, resulting in decreased muscle strength.
Acknowledgments
We wish to thank Drs. Sarah Locknar and Rodney Parsons for assistance with FM1-43 labeling and multiphoton microscopy, Drs. Felix Eckenstein and Rae Nishi for use of their confocal microscope, and Dr. Nishi for the use of the fluorescent microplate reader. We thank Tim Hunter, Scott Tighe, and Dr. Angie Watson for assistance with DNA microarray design, execution, and interpretation, respectively. The authors also thank Dr. Parsons for critical review of the manuscript. This work was supported by Grant NS045225 co-funded by NINDS and NCRR (DMJ). We acknowledge the University of Vermont Neuroscience Center of Biomedical Research Excellence for use of the Imaging and Physiology Core facilities (NIH NCRR IP20 RR16435). Densitometric analysis was performed in the VT Cancer Center DNA Analysis Facility and was supported, in part, by grant P30CA22435 from the NCI. DNA microarray analysis was performed in the VT Cancer Center DNA Analysis Facility and was supported, in part, by the Vermont Genetics Network through NIH Grant Number 1 P20 RR16462 from the BRIN program of the NCRR.
References
- Bewick GS, Reid B, Jawaid S, Hatcher T, Shanley L. Postnatal emergence of mature release properties in terminals of rat fast- and slow-twitch muscles. Eur. J. Neurosci. 2004;19:2967–2976. doi: 10.1111/j.0953-816X.2004.03418.x. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Burkin DJ, Kaufman SJ. α7β1 Integrin Regulates Mechanotransduction and Prevents Skeletal Muscle Injury. Am. J. Physiol. Cell Physiol. 2006 doi: 10.1152/ajpcell.00317.2005. E-pub. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Lin W, D’Amour KA, Pizzo DP, Dominguez B, Sugiura Y, Thode S, Ko CP, Thal LJ, Gage FH, Lee KF. Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. J. Neurosci. 2003;23:539–549. doi: 10.1523/JNEUROSCI.23-02-00539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, Crabbe T, Clements J, d’Ortho M-P, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. J. Biol. Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- Campagna JA, Ruegg MA, Bixby JL. Agrin is a differentiation-inducing “stop signal” for motoneurons in vitro. Neuron. 1995;15:1365–1374. doi: 10.1016/0896-6273(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Campbell KP, Stull JT. Skeletal muscle basement membrane-sarcolemma-cytoskeleton interaction minireview series. J. Biol. Chem. 2003;278:12599–12600. doi: 10.1074/jbc.R300005200. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- Carson FL. Histotechnology: A self-instructional text. ASCP Press; Chicago: 1997. [Google Scholar]
- Caterina JJ, Yamada S, Caterina NCM, Longenecker G, Holmbäck K, Shi J, Yermovsky AE, Engler JA, Birkedal-Hansen H. Inactivating mutation of the mouse Tissue Inhibitor of Metalloproteinases-2 (Timp-2) gene alters proMMP-2 activation. J. Biol. Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- Chang Q, Balice-Gordon RJ. Nip and tuck at the neuromuscular junction: a role for proteases in developmental synapse elimination. Bioessays. 1997;19:271–275. doi: 10.1002/bies.950190402. [DOI] [PubMed] [Google Scholar]
- Couch CB, Strittmatter WJ. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983;32:257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- d’Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur. J. Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- Demestre M, Orth M, Wells GM, Gearing AJ, Hughes RA, Gregson NA. Characterization of matrix metalloproteinases in denervated muscle. Neuropath. Appl. Neurobiol. 2005;31:545–555. doi: 10.1111/j.1365-2990.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Both myoblast lineage and innervation determine fiber type and are required for expression of the slow myosin heavy chain 2 gene. Dev. Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. [DOI] [PubMed] [Google Scholar]
- English AW. Cytokines, growth factors and sprouting at the neuromuscular junction. J. Neurocytol. 2003;32:943–960. doi: 10.1023/B:NEUR.0000020634.59639.cf. [DOI] [PubMed] [Google Scholar]
- Ervasti JM. Costameres: the Achilles’ heel of Herculean muscle. J. Biol. Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin α9β1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J. Biol. Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]
- Fernandez CA, Butterfield C, Jackson G, Moses MA. Structural and functional uncoupling of the enzymatic and angiogenic inhibitory activities of tissue inhibitor of metalloproteinase-2 (TIMP-2): loop 6 is a novel angiogenesis inhibitor. J. Biol. Chem. 2003;278:40989–40995. doi: 10.1074/jbc.M306176200. [DOI] [PubMed] [Google Scholar]
- Fu AK, Ip FC, Fu WY, Cheung J, Wang JH, Yung WH, Ip NY. Aberrant motor axon projection, acetylcholine receptor clustering, and neurotransmission in cyclin-dependent kinase 5 null mice. Proc. Natl. Acad. Sci. USA. 2005;102:15224–15229. doi: 10.1073/pnas.0507678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, DeChiara TM, Glass DJ, Yancopoulos GD, Sanes JR. Distinct phenotypes of mutant mice lacking agrin, MuSK, or rapsyn. Dev. Brain Res. 1999;114:171–178. doi: 10.1016/s0165-3806(99)00013-9. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. b1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Sorokin L, White J. Strength at the extracellular matrix-muscle interface. Scand. J. Med. Sci. Sports. 2005;15:381–391. doi: 10.1111/j.1600-0838.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- Guerin CW, Holland PC. Synthesis and secretion of matrix-degrading metalloproteases by human skeletal muscle satellite cells. Dev. Dyn. 1995;202:91–99. doi: 10.1002/aja.1002020109. [DOI] [PubMed] [Google Scholar]
- Hornebeck W, Emonard H, Monboisse JC, Bellon G. Matrix-directed regulation of pericellular proteolysis and tumor progression. Semin. Cancer Biol. 2002;12:231–241. doi: 10.1016/s1044-579x(02)00026-3. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Cote PD, Rossi SG, Rotundo RL, Carbonetto S. The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J. Cell Biol. 2001;152:435–450. doi: 10.1083/jcb.152.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Soloway P, Caterina J, Falls WA. Tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice display motor deficits. J. Neurobiol. 2006;66:82–94. doi: 10.1002/neu.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuste P, Sonnet C, Chazaud B, Dreyfus PA, Gherardi RK, Wewer UM, Authier FJ. ADAM12 and α9β1 integrin are instrumental in human myogenic cell differentiation. Mol. Biol. Cell. 2005;16:861–870. doi: 10.1091/mbc.E04-03-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation. 2005;111:643–649. doi: 10.1161/01.CIR.0000154548.16191.2F. [DOI] [PubMed] [Google Scholar]
- Lluri G, Jaworski DM. Regulation of TIMP-2, MT1-MMP, and MMP-2 expression during C2C12 differentiation. Muscle Nerve. 2005;32:492–499. doi: 10.1002/mus.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MA, Mayer U, Hwang W, Taylor T, Hashmi MA, Jannapureddy SR, Boriek AM. Force transmission, compliance, and viscoelasticity are altered in the α7-integrin-null mouse diaphragm. Am. J. Physiol. Cell Physiol. 2005;288:C282–289. doi: 10.1152/ajpcell.00362.2003. [DOI] [PubMed] [Google Scholar]
- Mayer U. Integrins: redundant or important players in skeletal muscle? J. Biol. Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- Muir D, Manthorpe M. Stromelysin generates a fibronectin fragment that inhibits Schwann cell proliferation. J. Cell Biol. 1992;116:177–185. doi: 10.1083/jcb.116.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004;563:129–134. doi: 10.1016/S0014-5793(04)00281-9. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Nikawa T, Furochi H, Kosyoji M, Hirasaka K, Suzue N, Sairyo K, Nakano S, Yamaoka T, Itakura M, Kishi K, Yasui N. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am. J. Physiol. Cell Physiol. 2005;289:C697–707. doi: 10.1152/ajpcell.00565.2004. [DOI] [PubMed] [Google Scholar]
- Patton BL. Laminins of the neuromuscular system. Microsc. Res. Tech. 2000;51:247–261. doi: 10.1002/1097-0029(20001101)51:3<247::AID-JEMT5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Platt CI, Krekoski CA, Ward RV, Edwards DR, Gavrilovic J. Extracellular matrix and matrix metalloproteinases in sciatic nerve. J. Neurosci. Res. 2003;74:417–429. doi: 10.1002/jnr.10783. [DOI] [PubMed] [Google Scholar]
- Pérez-Martínez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J. Neurosci. 2005;25:4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnikov BI, Rozanov DV, Postnova TI, Baciu PG, Zhang H, DiScipio RG, Chestukhina GG, Smith JW, Deryugina EI, Strongin AY. An alternative processing of integrin αv subunit in tumor cells by membrane type-1 matrix metalloproteinase. J. Biol. Chem. 2002;277:7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H2291–2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J. Cell Biol. 1990;111:1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin V, Gaffin RD, Meininger GA, Muthuchamy M. Arginine-glycine-aspartic acid (RGD)-containing peptides inhibit the force production of mouse papillary muscle bundles via α5β1 integrin. J. Physiol. 2005;564:603–617. doi: 10.1113/jphysiol.2005.083238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. β1 integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Schwander M, Shirasaki R, Pfaff SL, Muller U. β1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. J. Neurosci. 2004;24:8181–8191. doi: 10.1523/JNEUROSCI.1345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- Tam SL, Gordon T. Mechanisms controlling axonal sprouting at the neuromuscular junction. J. Neurocytol. 2003;32:961–974. doi: 10.1023/B:NEUR.0000020635.41233.0f. [DOI] [PubMed] [Google Scholar]
- VanSaun M, Werle MJ. Matrix metalloproteinase-3 removes agrin from synaptic basal lamina. J. Neurobiol. 2000;43:140–149. [PubMed] [Google Scholar]
- Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZZ, Washabaugh CH, Yao Y, Wang JM, Zhang L, Ontell MP, Watkins SC, Rudnicki MA, Ontell M. Aberrant development of motor axons and neuromuscular synapses in MyoD-null mice. J. Neurosci. 2003;23:5161–5169. doi: 10.1523/JNEUROSCI.23-12-05161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigmore PM, Evans DJ. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 2002;216:175–232. doi: 10.1016/s0074-7696(02)16006-2. [DOI] [PubMed] [Google Scholar]
- Yamada H, Saito F, Fukuta-Ohi H, Zhong D, Hase A, Arai K, Okuyama A, Maekawa R, Shimizu T, Matsumura K. Processing of β-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum. Mol. Genet. 2001;15:1563–1569. doi: 10.1093/hmg/10.15.1563. [DOI] [PubMed] [Google Scholar]
- Zimmerman SD, McCormick RJ, Vadlamudi RK, Thomas DP. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J. Appl. Physiol. 1993;75:1670–1674. doi: 10.1152/jappl.1993.75.4.1670. [DOI] [PubMed] [Google Scholar]